Abstract
AIB1 (amplified in breast cancer 1), also called SRC-3 and NCoA-3, is a member of the p160 nuclear receptor co-activator family and is considered an important oncogene in breast cancer. Increased AIB1 levels in human breast cancer have been correlated with poor clinical prognosis. Overexpression of AIB1 in conjunction with members of the epidermal growth factor receptor (EGF/HER) tyrosine kinase family, such as HER2, is associated with resistance to tamoxifen therapy and decreased disease-free survival. A number of functional studies in cell culture and in rodents indicate that AIB1 has a pleiotropic role in breast cancer. Initially AIB1 was shown to have a role in the estrogen-dependent proliferation of breast epithelial cells. However, AIB1 also affects the growth of hormone-independent breast cancer and AIB1 levels are limiting for IGF-1-, EGF- and heregulin-stimulated biological responses in breast cancer cells and consequently the PI3 K/Akt/mTOR and other EGFR/HER2 signaling pathways are controlled by changes in AIB1 protein levels. The cellular levels and activity of AIB1 are in turn regulated at the levels of transcription, mRNA stability, post-translational modification, and by a complex control of protein half life. In particular, AIB1 activity as well as its half-life is modulated through a number of post-translational modifications including serine, threonine and tyrosine phosphorylation via kinases that are components of multiple signal transduction pathways. This review summarizes the possible mechanisms of how dysregulation of AIB1 at multiple levels can lead to the initiation and progression of breast cancer as well as its role as a predictor of response to breast cancer therapy, and as a possible therapeutic target.
Keywords: AIB1, HER2, EGFR, Estrogen
Introduction
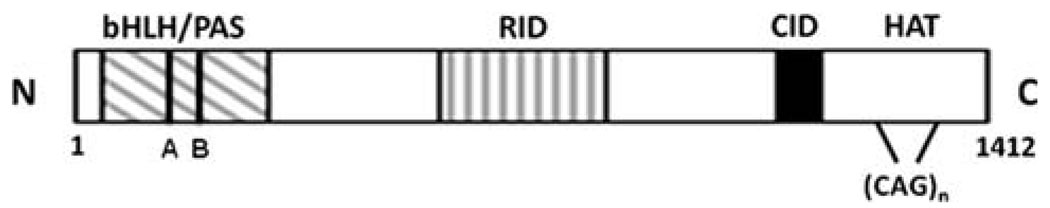
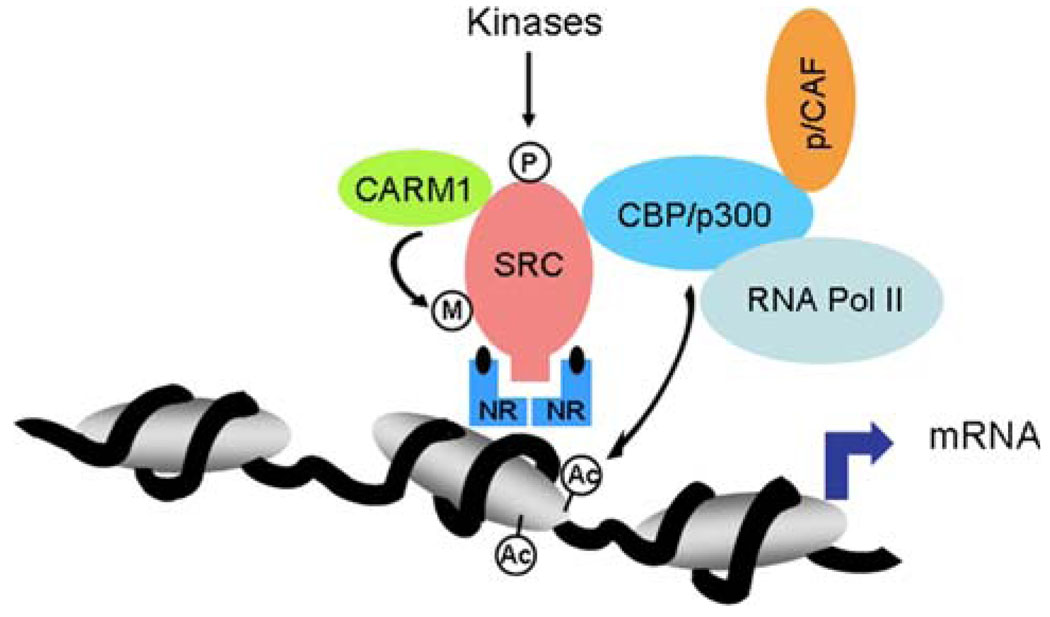
Since the discovery in 1997 that the AIB1 gene is often amplified in breast cancer, there has been extensive research on the role of AIB1 in breast cancer [1]. AIB1, a member of the nuclear coactivator (NCoA-3) and p160 steroid receptor co-activator (SRC) family, which includes SRC-1 [2] and TIF-2 [3], was discovered independently by several groups, and given various names; AIB1 (amplified in breast cancer 1) [1], SRC-3 (steroid receptor co-activator-3) [4], ACTR (activator of thyroid hormone and retinoid receptor) [5], RAC-3 (receptor associated co-activator-3) [6], and TRAM-1 (thyroid hormone receptor activating molecule) [7]. The mouse homologue of AIB1 is p/CIP (p/300/CBP interacting protein) [8]. The function of AIB1 as a transcriptional coactivator has been reviewed previously [9] and only a brief overview of this function is presented here. AIB1 is a transcriptional co-activator that promotes the transcriptional activity of multiple nuclear receptors such as the estrogen receptor [1, 4,5] and a number of other transcription factors, including E2F-1, AP-1, NFκB, and STAT6 [10–13]. Three domains common to all SRC family members are involved in protein-protein interactions; an amino-terminal basic helix-loop-helix (bHLH)/Per/Arnt/Sim (PAS) domain, an internal nuclear receptor interaction domain (RID), and a carboxylterminal CREB-binding protein (CBP)/p300 interaction domain (CID; Fig. 1). In addition, there is a stretch of 26–30 glutamine repeats that juxtaposes the CBP binding domain (Fig. 1) which may play a role in AIB1 function in breast cancer (see next section). After AIB1 interacts with ligandbound nuclear receptors, via its RID, it recruits other transcriptional cofactors and the basal transcriptional machinery. Full AIB1 co-activator function also requires the recruitment of the histone acetyltransferases CBP/p300 and p/CAF [5]. Acetylation of histones by these acetyltransferases modifies chromatin structure, facilitates access of transcription factors to gene promoters and leads to enhanced gene expression [14]. AIB1 can also help transcription factors interact with other transcriptional cofactors, a role that is regulated by enzyme-dependent methylation and phosphorylation (Fig. 2). In addition to its roles in promoting transcription, AIB1 can function as a transcriptional repressor of inflammatory cytokine-encoding mRNAs [15].
Fig. 1.
Structural and functional domains of AIB1. Basic-helix-loop-helix (bHLH)/per-arnt-sim (PAS), receptor interaction domain (RID), CBP/p300 interaction domain (CID), histone acetyltranferase domain (HAT). A region containing multiple glutamine (CAG) repeats is indicated
Fig. 2.
Model showing a transcriptional complex of AIB1 and its methylation by the CARM1 methyltransferase and phosphorylation by multiple kinases. CARM1, coactivator-associated arginine methyltransferase 1; RNA Pol II, RNA polymerase II; CBP/p300, CREB binding-protein/E1A binding-protein p300; NR, nuclear receptor; P, phosphorylation; Ac, acetylation; M, methylation
Evidence linking AIB1 overexpression to breast cancer risk and prognosis
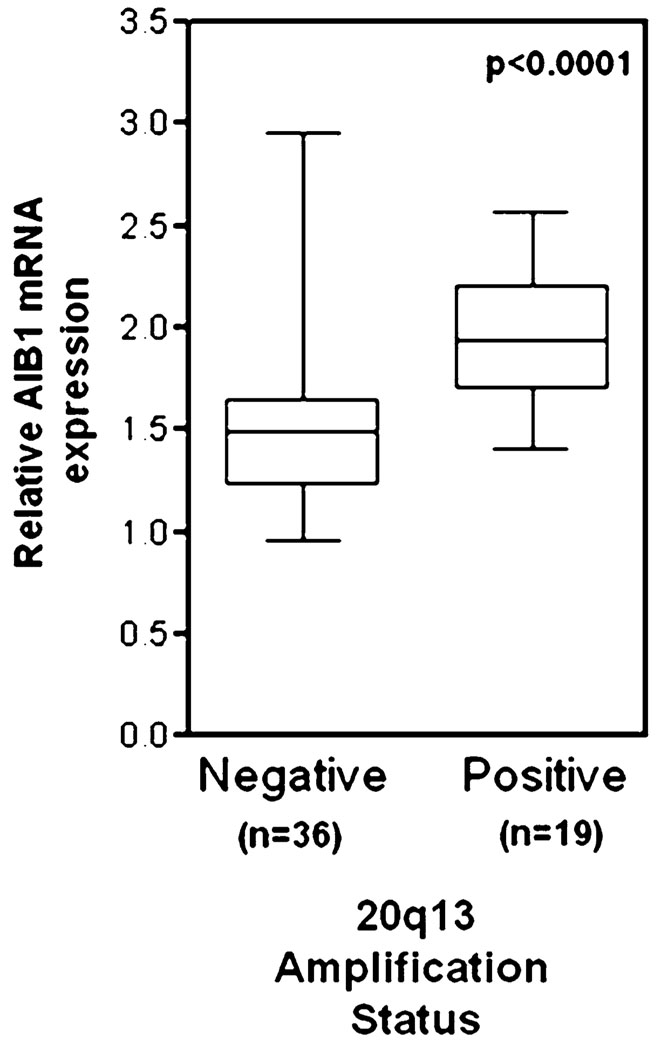
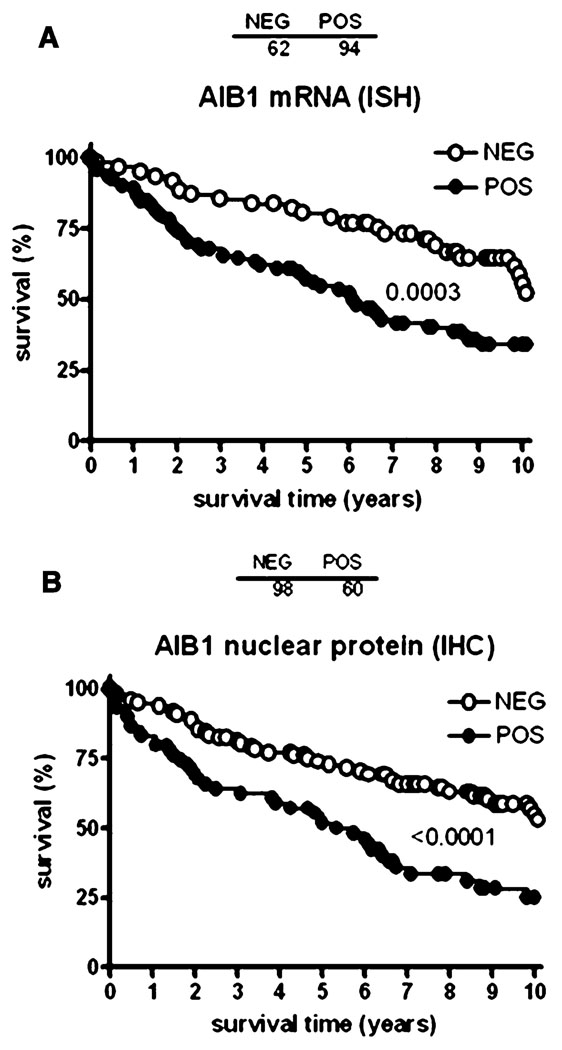
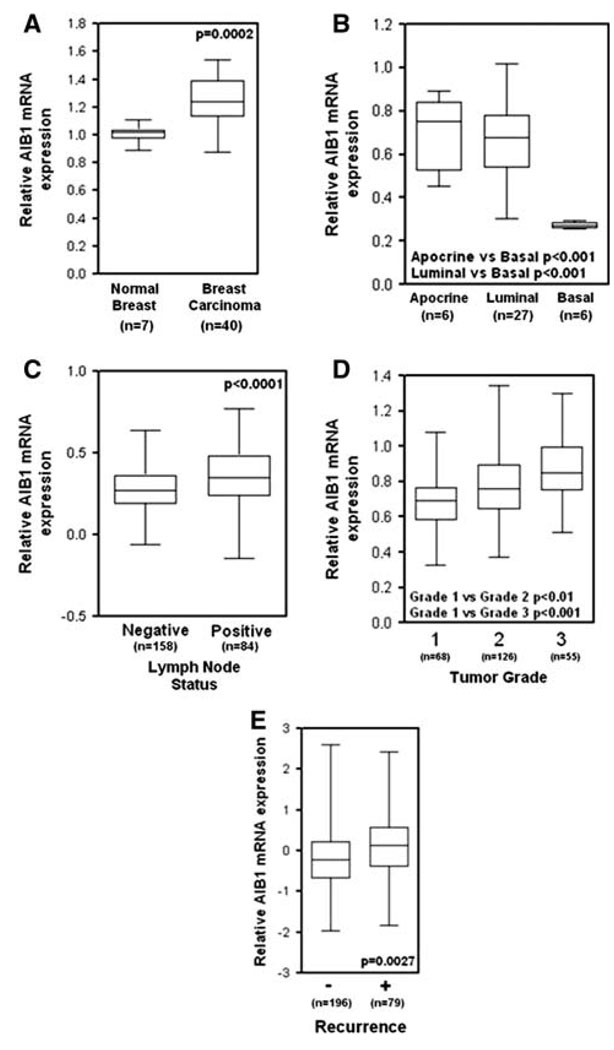
Amplification of the AIB1 mRNA and protein has been shown in 2–10% of breast cancer samples [1, 16–19] and our reanalysis of Oncomine 3.6 microarray data (www.oncomine.org) from human breast cancer clinical samples from Ginestier et al. [20] shows that abnormally high AIB1 mRNA levels are associated with 20q13 amplification (Fig. 3). However, increased amounts of AIB1 mRNA have been found in 31–64% of human breast tumors [1, 21, 22] indicating that AIB1 transcript levels can be increased in breast tumors by mechanisms other than amplification of the gene. Although some studies have shown that AIB1 amplification is not associated with worse disease outcome, other studies indicate that high levels of AIB1 correlate with shorter disease-free interval [23, 24] and that AIB1 levels are higher in invasive higher grade tumors [25]. To further investigate the prognostic significance of AIB1 mRNA levels in breast cancer, we analyzed AIB1 mRNA levels using a tissue microarray, provided by CBCTR of the National Cancer Institute/NIH, that we have utilized previously for other analyzes [26] (Fig. 4a), and found that high levels of AIB1 mRNA measured by in situ hybridization (ISH) are predictive of worse outcome (Fig. 4a). In addition, we analyzed microarray data from human breast cancer samples from several studies (Fig. 5). Re-analysis of data from Richardson et al., [27] shows that AIB1 mRNA levels are significantly higher in human breast carcinomas than in normal breast tissue (Fig. 5a) and data from Farmer et al. [28] shows that AIB1 mRNA levels are higher in luminal and apocrine-type breast cancer than in basal-type breast cancer (Fig. 5b). Analysis of data from Ivshina et al. [29] shows that AIB1 mRNA level expression is higher in lymph node positive and high grade breast cancer (Fig. 5c, d) and analysis of a study by van de Vijver et al. [30] also reveals an association between high AIB1 mRNA levels and breast cancer recurrence (Fig. 5e). Overall, the preponderance of the published data and this analysis supports an association of high levels of AIB1 mRNA with the development of breast cancer.
Fig. 3.
AIB1 mRNA expression is significantly higher in human breast tumors with gene amplification of 20q13 (data obtained from Ginestier et al. [20] and analyzed by Oncomine 3.6 at www.oncomine.org). The median, lower and upper quartile (box) and extremes (whiskers) are shown
Fig. 4.
Analysis of the levels of AIB1 mRNA (A) by in situ hybridization (ISH) or AIB1 protein by immunohistochemistry (IHC) on a tissue micoarray of breast cancer samples from patients with known overall survival times provided by CBCTR of the National Cancer Institute [26]
Fig. 5.
AIB1 mRNA expression in breast relative to the phenotype. Data are from Oncomine 3.6 and are presented as “box and whisker plots” showing the median, upper and lower 25% (boxed) and the upper and lower extreme values (bars). a AIB1 mRNA expression is significantly higher in human breast carcinoma as compared with normal breast tissue (from Richardson et al. [27]). b AIB1 mRNA expression is significantly lower in basal type human breast carcinoma as compared with luminal and apocrine type breast carcinoma (from Farmer et al. [28]). c AIB1 mRNA expression is significantly higher in lymph node positive and d high grade human breast carcinoma as compared with normal breast tissue (from Ivshina et al. [29]). e AIB1 mRNA expression is significantly higher in early recurrence breast cancers (5 years; from van de Vijver et al. [30])
High nuclear levels of AIB1 protein have been reported in 10–16% of breast cancer patients [31]. Interestingly, cytosolic staining with AIB1 was also reported in these studies although it is not known how the relative cytoplasmic to nuclear ratio of AIB1 expression relates to disease outcome and therapeutic response. Irrespective of subcellular location of AIB1 protein, the results of some studies indicate that AIB1 mRNA levels may not always predict AIB1 protein levels. For example, List et al. [22], using imunohistochemistry (IHC), found smaller differences in nuclear AIB1 staining, between breast cancer samples and normal tissue samples, than expected on the basis of prior studies on AIB1 mRNA levels. The difference in the relative levels of AIB1 mRNA and protein in tumors could be either due to the threshold sensitivity of detection of mRNA (PCR based methods) versus protein detection by IHC or due to the complex control of AIB1 protein degradation (see “Regulation of AIB1 mRNA and protein levels” of this review). In addition, recent evidence suggests that translation of AIB1 mRNA might be dysregulated in breast cancers (see “Regulation of AIB1 mRNA and protein levels”). To study the relationship of AIB1 protein with AIB1 mRNA in tumors at various disease stages and outcome, we have compared the AIB1 nuclear protein levels measured by IHC with AIB1 mRNA levels measured by in situ hybridization in 94 breast cancer samples on a tissue microarray, provided by CBCTR of the National Cancer Institute/NIH, that we have utilized previously for other analyzes [26]. We observed that high AIB1 nuclear protein and high AIB1 mRNA levels were well correlated and predicted reduced survival rates (Fig. 4 a, b).
The AIB1 gene harbors a number of polymorphisms and some of these have been associated with reduced breast cancer risk [32]. In addition there is a polyglutamine stretch in the C-terminus of AIB1 that has variable length between 26 and 30 amino acids (Fig. 1). However, various reports show conflicting data as to the association of the repeat length with breast cancer risk. Specifically, studies have asked whether AIB1 polyglutamine length is associated with the mutation status of the tumor suppressor proteins BRCA1 and BRCA2, proteins that have roles in multiple cellular functions including cell cycle progression, DNA repair, and transcriptional regulation (reviewed in [33]). Some reports have shown that AIB1 poly-glutamine length correlates with an increased breast cancer risk in women with BRCA1 mutations [34, 35], whereas other studies did not find an increased risk for breast cancer in women with either BRCA1 or BRCA2 mutations [36, 37]. Polymorphisms in the CAG repeat region of AIB1 have also been associated with a more aggressive phenotype in ovarian cancer [38].
Evidence linking AIB1 to estrogen and progesterone effects in breast cancer
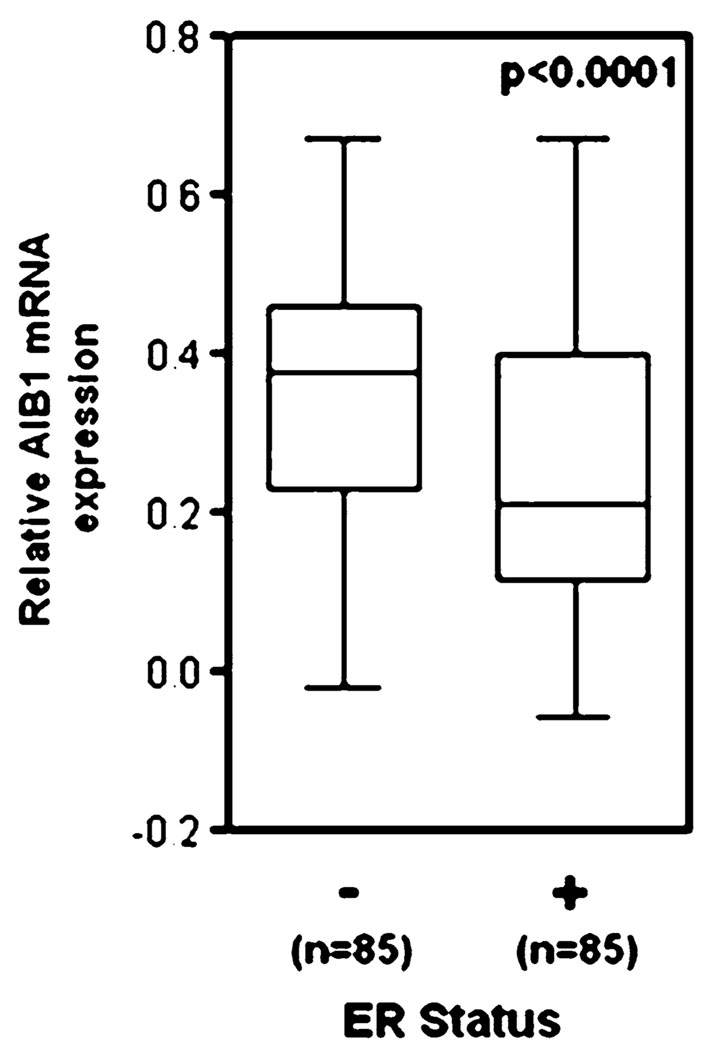
The estrogen receptor-α (ERα) and progesterone receptor (PR) status of breast cancer is an important consideration for breast cancer therapy and prognosis [39] and AIB1 is a co-activator of ERα and PR activity [40]. However, collectively, the clinical data suggest that increased AIB1 protein levels do not correlate with ERα or PR positivity [24]. One study showed that AIB1 gene amplification in breast tumor samples correlated with increased expression of both ER and PR [41]. In another study, AIB1 mRNA overexpression in breast tumor samples was associated with loss of both ER and PR expression [21]. Our oncomine 3.6 re-analysis of data from Sotiriou et al. [42] reveals that ER-α negative breast cancer is associated with higher AIB1 mRNA levels than ERα positive breast cancer (Fig. 6). The discrepancy between these reports may be due to the differences in the role and regulation of AIB1 and the hormone receptors at different stages of breast disease. Clinical data notwithstanding, initial functional studies, that reported an association between breast cancer and elevated AIB1 mRNA/protein levels, also demonstrated that AIB1 mediates the effects of estrogen on ERα dependent gene expression [1, 22], thus suggesting a mechanism for how AIB1 influences the growth of hormone-dependent breast cancers. As predicted from this mechanistic model, depletion of AIB1 protein levels in ERα-positive MCF-7 human breast cancer cells results in decreased estrogen-stimulated proliferation and survival in culture [10, 43] and a decreased growth of MCF-7 xenografts in mice [43].
Fig. 6.
AIB1 mRNA expression is significantly lower in ERα-positive human breast carcinoma as compared with normal breast tissue (from Sotiriou et al. [42] and analyzed by Oncomine 3.6)
Role of AIB1 in anti-estrogen therapy resistance
AIB1 appears to play a major role in breast cancer resistance to anti-estrogen therapy. Since the 1970s, tamoxifen has been the standard endocrine therapy for women with ER positive breast cancer. Tamoxifen is a non-steroidal estrogen receptor antagonist that competes with estrogen for binding to ERα, resulting in the inhibition of ERα-mediated transcription and estrogen-dependent cell growth [44]. Two types of resistance to tamoxifen have been recognized, intrinsic and acquired. Intrinsic resistance is associated with those 50% of ERα-positive breast cancer patients that do not respond to tamoxifen therapy [45]. In acquired resistance, patients treated with tamoxifen for long periods of time often acquire resistance to therapy. Both types of resistances have been associated with the hormone-independent activation of ERα through cross-talk with growth factor signaling pathways [46]. Convincing clinical studies have shown that high levels of HER family member proteins have been associated with relapse after tamoxifen therapy in breast cancer patients that have high AIB1 protein expression [47–49].
While simple overexpression of AIB1 alone can increase the agonist properties of tamoxifen in breast cancer cell lines [50], the transition from hormone-dependent to hormone-independent cancer, resulting from hormone-independent ERα activation, may also be explained by increased growth factor-induced signaling through receptor tyrosine kinases such as the human epidermal growth factor receptor (HER) family members, which include HER1 (EGFR), HER2 (erbB2), and HER3 (erbB3; reviewed in [51]). Fleming et al. [52] observed that high protein expression of both HER2 and SRC-1, a p160 co-activator closely related to AIB1, is associated with resistance to tamoxifen therapy in breast cancer. Multiple studies have sought to identify the molecular mechanism causing this hormone-independent ERα activation, where tamoxifen becomes an ERα agonist in breast cancer cells with high protein levels of both AIB1 and HER2. However, an additional hypothesis linking growth factor signaling and AIB1 is that growth factor-stimulated receptor tyrosine kinase signaling may result in resistance to tamoxifen due to the enhancement of the agonist properties of tamoxifen in breast cancer cells [53]. This proposed mechanism has been corroborated by an in vitro study using ERα-positive MCF-7 breast cancer cells engineered to overexpress HER2. This study demonstrated that tamoxifen stimulates proliferation and induces ERα-dependent gene expression. Both of these effects result from the HER2-driven phosphorylation of AIB1 (via extracellular signal-regulated kinase (ERK) −1/2), which enhances AIB1 co-activator function [54]. More recently it has been shown that a balance between AIB1 and the transcriptional repressor PAX2 controls the estrogen induced expression of HER2 in breast cancer cells [55]. Tamoxifen resistance develops when AIB1 is high and PAX2 low thus inducing high HER2 expression [55].
Other endocrine therapies approved by the FDA exist for the treatment of ERα-positive breast cancer, including fulvestrant (Faslodex), a complete ERα antagonist that binds to ERα causing its subsequent degradation (reviewed in [56]). It was approved in 2004 for the treatment of postmenopausal women with metastatic breast cancer who had received prior anti-estrogen therapy. It has not yet been determined whether protein levels of AIB1 and HER2 affect the clinical outcome of breast cancer patients on fulvestrant. Aromatase inhibitors including letrozole, anastrozole, and exemestane, which block the conversion of the adrenal steroids testosterone and androstenedione into estrogen, are another FDA-approved therapeutic option for postmenopausal women with ERα-positive breast cancer (reviewed in [57]). However, breast tumors that have high protein expression of HER2 and AIB1 may also become resistant to aromatase inhibitors [58, 59].
The molecular mechanism for aromatase resistance appears to have at least some elements in common with tamoxifen resistance in that AIB1 is recruited to estrogen-dependent promoters in a hormone-independent manner in both types of drug resistance. One study showed that treatment of aromatase-expressing MCF-7 cells with androstenedione results in increased recruitement of AIB1 to the pS2 estrogen-responsive promoter, which is inhibited by the aromatase inhibitor letrozole [59]. However, when HER2 is overexpressed in these cells, AIB1 is recruited, along with ERα, to the pS2 promoter even in the presence of letrozole. It should also be noted that AIB1 mRNA levels are increased in MCF-7 breast cancer cells with anti-estrogens (ICI 182,780 and tamoxifen), whereas AIB1 mRNA levels are decreased by estrogen [60]. This data might suggest that during anti-estrogen therapy, AIB1 levels are increased and can contribute to resistance by enhancing hormone independent proliferative pathways. In summary, these data suggest a major role for AIB1 in anti-estrogen resistance and that it may be useful to assess the expression of HER2 and AIB1 when deciding upon the proper clinical regimen.
AIB1 in hormone -independent breast cancer
Even though AIB1 levels have been shown to be limiting for ERα-positive breast cancer growth (See “Evidence linking AIB1 overexpression to breast cancer risk and prognosis”), substantial evidence indicates that AIB1 has roles in tumorigenesis other than as a co-activator for ERα-dependent transcription. These other studies provide convincing evidence that AIB1 can stimulate the growth of breast cancer cell lines through estrogen-independent mechanisms. Overexpression of AIB1 in ERα-positive breast cancer cell lines has been shown to increase proliferation even in the presence of the ER antagonist ICI 182,780 [10]. More convincingly, overexpression of AIB1 promotes the growth of ERα-negative breast cancer cells by increasing the expression of E2F1-induced gene products such as E2F1, cyclin E, and cyclin-dependent kinase 2, all of which promote cell proliferation [10]. In one study, growth factor stimulation resulted in the release of E2F1 from retinoblastoma protein (Rb), allowing E2F1 to bind DNA and activate transcription of its target genes. AIB1 was recruited to E2F binding sites on DNA via its interaction with E2F1 and co-activated E2F-dependent transcription [10]. Thus, the ability of AIB1 to co-activate E2F-dependent gene expression was hypothesized to be a hormone-independent mechanism by which AIB1 could promote breast tumor growth. Consistent with this hypothesis, it was subsequently shown that anchorage-independent growth of MCF10A human mammary epithelial cells, achieved by AIB1 overexpression, required AIB1 to interact with E2F1 [61].
AIB1 has also been shown to promote the growth and survival of breast cancer cells by acting as a co-activator of growth factor-stimulated activating protein 1 (AP-1), the transcription factor complex that contains Jun and Fos family members [11], and of NFκB-dependent transcription [12], which increases the expression of cell cycle and anti-apoptotic genes [62]. Inhibition of AP-1-dependent transcription in MCF-7 breast cancer cells was shown to result in inhibition of cell proliferation [63]. In breast cancer cells, NFκB has been shown to promote cell proliferation and survival [64–66]. Thus, the increased AIB1 protein levels in many human breast tumor cells can have multiple roles in tumor progression that are either dependent on, or independent of its original association with ERα-positive breast cancer. These transcriptional interactions ultimately lead to AIB1 rate limiting effects in several growth factor signaling pathways, which are discussed in the next section, that are critical to the initiation and progression of human breast cancer.
AIB1 controls different growth factor activated signaling pathways
Tumor cells depend on a diverse set of signaling pathways for growth and survival. The importance of AIB1 in the regulation of multiple growth factor activated pathways has been shown by a number of studies. The signaling response induced by insulin-like growth factor (IGF)-1, results in tyrosine phosphorylation of the IGF-1 receptor, recruitment of insulin receptor substrate (IRS) proteins, and activation of the phosphatidylinositol 3-kinase (PI3 K)/Akt/mammalian target of rapamycin (mTOR) pathway (reviewed in [67]). AIB1 was initially shown to be a factor involved in IGF-1 signaling from two independent studies involving mice with a gene deletion of AIB1 (p/CIP), both of which found reduced serum IGF-1 levels [68, 69]. On the other hand, IGF-1 serum levels were increased in transgenic mice that overexpressed AIB1 [70]. However, these effects on IGF-1 serum levels do not fully account for the regulation of the IGF-1 signaling pathway by AIB1. For example, IGF-1 receptor protein expression is increased in the mammary glands of AIB1-Δ3 (an alternatively-spliced isoform of AIB1 that lacks the N-terminal bHLH and PASA domains transgenic mice), but there is no change in serum IGF-1 levels [71]. In addition, cells derived from AIB1 knockout mice have an inherent deficiency in their biological response to IGF-1 stimulation; cultured hepatocytes and embryonic fibroblasts from AIB1 knockout mice are unresponsive to IGF-1-stimulated DNA synthesis [68]. Similarly, small-interfering RNA (siRNA)-mediated AIB1 knockdown resulted in decreased IGF-1-stimulated anchorage-independent proliferation of MCF-7 human breast cancer cells and IGF-I-dependent anti-anoikis [72].
In addition to regulating IGF-1 levels, AIB1 regulates the expression of other proteins involved in the IGF-1 signaling pathway. Torres-Arzayus et al. [73] have shown that inhibition of mTOR with the rapamycin analog RAD001 (Novartis) prevented mammary hyperplasia and hypertrophy originally induced by the overexpression of the AIB1 transgene in the mouse mammary gland. In addition, RAD001 treatment inhibited the growth of tumor xenografts in mice from epithelial cells derived from AIB1-induced mammary tumors [73]. Therefore, the PI3 K/Akt/mTOR pathway has a role in AIB1-mediated tumorigenesis, but it is unclear whether this is solely due to increased IGF-1 levels or if other mechanisms are involved.
AIB1 was also identified as being involved in v-Ha-Ras-mediated tumorigenesis and transformation. AIB1 knockout mice harboring the v-Ha-Ras transgene, driven by the mouse mammary tumor virus (MMTV) promoter, were utilized to study the role of AIB1 in Ras-mediated mammary tumorigenesis [74]. The tumor latency in v-HA-Ras transgenic mice crossed with AIB1−/− mice increased as compared with AIB1+/+-ras mice in both virgin and multiparous animals, and tumor development was completely abolished in ovariectomized animals [74]. Therefore, an involvement of AIB1 in Ras-dependent tumorigenesis in mammary epithelial cells was partially hormone-dependent. Loss of AIB1 decreased the incidence, growth, and metastasis of v-HA-Ras-induced mammary tumors. Interestingly, AIB1 was also shown to enhance v-Ha-Ras-induced transformation of mouse embryonic fibroblasts [75]. These studies suggest that AIB1 modulates the IGF-1 signaling pathway by regulating the expression of multiple genes encoding proteins that participate in this pathway.
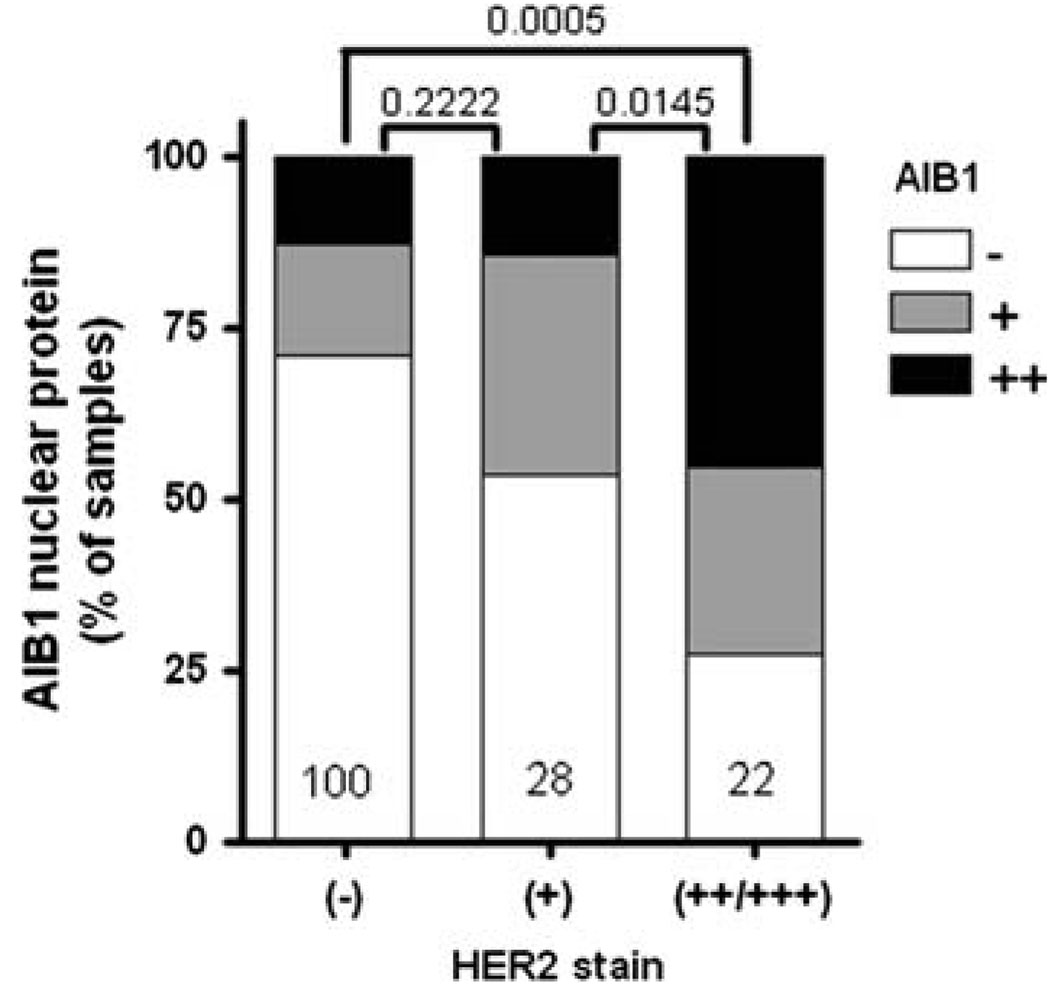
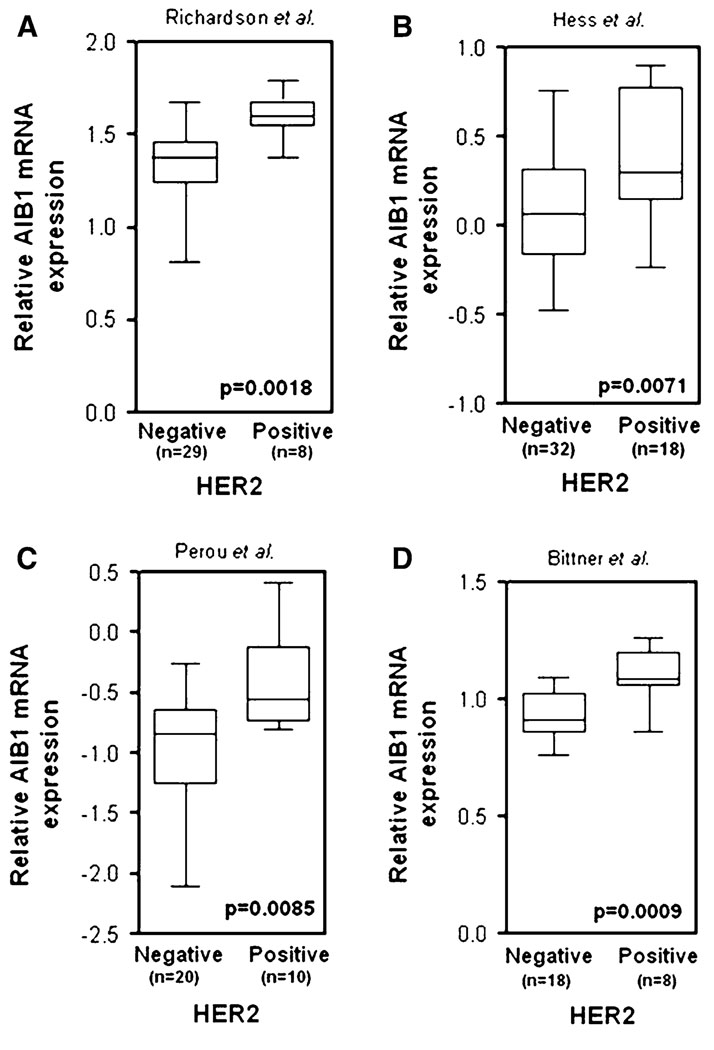
There is emerging evidence that AIB1 could also be functionally involved in regulating the activity of the HER/erbB family of transmembrane receptor tyrosine kinases. This family of receptors includes EGFR/HER1/erbB1, HER2/erbB2/neu, HER3/erbB3, and HER4/erbB4, which can form either homodimers or heterodimers with each other (reviewed in [76]). A functional relationship between AIB1 and the HER/erbB family members has been shown to be clinically relevant being that HER/erbB family members are frequently activated in human breast cancers and are the target of drugs that have been successfully used for cancer therapy (reviewed in [77]). Multiple studies have demonstrated that AIB1 mRNA and protein expression in breast cancer is associated with an increase in the protein expression of HER2 [21, 47, 48]. We have also found this association in our analysis of a tissue microarray, provided by CBCTR of the National Cancer Institute/NIH, that we have utilized previously for other IHC analyzes [26] (Fig. 7). In addition, our analysis of data from four studies [27, 78–80] shows that high AIB1 mRNA expression in breast cancer clinical samples correlates with high HER2 expression (Fig. 8). A role for AIB1 in the regulation of the HER2 pathway was recently elucidated, where AIB1 was shown to be required for HER2-mediated mammary tumorigenesis in a mouse model for breast cancer through regulation of HER2 phosphorylation and signaling [81]. Generation of neu/HER2 transgenic mice with either loss of one or both copies of the AIB1 gene resulted in reduced or complete abolition of mammary tumor development, respectively. The importance of AIB1 in HER2-mediated mammary tumorigenesis may be comparable with its importance in the v-Ha-Ras transgenic mouse model where it was shown that with loss of both copies of the AIB1 gene, mammary tumors still developed albeit at a reduced rate [74].
Fig. 7.
High expression of AIB1 protein is associated with high HER2+ staining in a significant portion of breast cancer samples. For this study we analyzed a tissue micoarray of breast cancer samples provided by CBCTR of the National Cancer Institute [26]. HER2 status was provided by the NCI
Fig. 8.
AIB1 mRNA expression is significantly higher in human breast carcinoma with high HER2 expression as compared with normal breast tissue (from a Richardson et al. [27], b Hess et al. [78], c Perou et al. [79], and d Bittner et al. [80] and analyzed by Oncomine 3.6)
EGFR is another family member of HER2 that has been shown to be affected by the level of AIB1 expression. This was observed in MDA-MB-231 breast cancer cells where a reduction of AIB1 levels by siRNA-mediated knockdown resulted in decreased EGF-stimulated EGFR tyrosine phosphorylation, signaling, and biological responses [82]. Our laboratory has also observed that EGF-stimulated EGFR phosphorylation was decreased in mammary epithelial cells from AIB1 knockout mice (unpublished data). These data suggest that increased AIB1 expression in breast cancer cells could enhance EGFR signaling as a result of HER2 overexpression and enable cells to be more resistant to drugs that target HER2 activity. Thus, our finding that AIB1 affects the signaling capacity of EGFR in cancer cells suggests that AIB1 may play an important role in EGFR-mediated oncogenic processes, which may have potential therapeutic applications for current therapies that are used for treating HER2-overexpressing breast cancer.
HER2 is overexpressed in 20–30% of breast cancer patients and is correlated with reduced disease-free and overall survival [83, 84]. Trastuzumab (Herceptin), a humanized monoclonal antibody directed against an extracellular region of the HER2 protein, was the first HER2-targeted therapy approved by the FDA for the treatment of HER2-overexpressing metastatic breast cancer (reviewed in [85]). Additionally, trastuzumab therapy improves disease-free and overall survival of patients with early-stage HER2-positive breast cancer in combination with chemotherapy [86–88]. Not all HER2-overexpressing breast cancers, however, respond to trastuzumab therapy or may acquire resistance during treatment [89], and breast cancers with normal HER2 levels also respond at the same rate [90]. Resistance to trastuzumab treatment has been attributed to the activation of IGF-IR signaling [91, 92], decreased p27kip1 expression [93–95], and the presence of truncated forms of circulating HER2 [96]. The studies reviewed here suggest that measurements of AIB1 protein levels in breast tumors may be a useful diagnostic tool for predicting treatment outcome. Since AIB1 enhances IGF-1 signaling, overexpression of AIB1 in HER2-overexpressing breast cancer cells may contribute to trastuzumab resistance through activation of IGF-IR. It will be important to determine the correlation of high AIB1 and HER2 protein expression with the clinical response to trastuzumab therapy. The ability of AIB1 to enhance the activity of multiple signal transduction pathways involved in cancer, including HER/erbB and IGF-IR, supports the possibility that AIB1 could be a target, or predictive marker for cancer therapy. There are many potential ways to target AIB1 in cancer cells, including a reduction in AIB1 expression through RNA interference-mediated knockdown of AIB1 protein levels or through inhibition of AIB1’s co-activator function by disrupting its interaction with CBP/p300.
Functional studies in mice linking AIB1 to breast cancer
Mouse model studies have increased our understanding of AIB1’s roles in mammary tumorigenesis. Transgenic mice expressing high levels of the human AIB1 transgene, under the transcriptional control of the MMTV LTR, developed mammary hyperplasia and tumors of the mammary gland [70]. Interestingly, the AIB1 transgene encoded protein was detected in other mouse tissues such as lung, pituitary, and uterus, where tumors also developed. The conclusion drawn form this study, that AIB1 protein levels may be a factor in mammary tumorigenesis, was reinforced by a study showing that mice mammary glands over-expressing AIB1, but to a lesser extent (2.5-fold vs. 7.6-fold, at the mRNA level), do not develop tumors but do develop mammary hyperplasia [97]. Another mouse model study that gave similar results, i.e. only a partial progression to mammary tumors, used a human AIB1 isoform AIB1-Δ3, and a different promoter, from cytomegalovirus (CMV) [71]. This AIB1-Δ3 isoform is a more potent transcriptional co-activator than full-length AIB1 [71]. These transgenic mice developed ductal hypertrophy of the mammary gland, along with increased proliferation of mammary epithelial cells, but did not develop mammary tumors [71]. The lack of tumor formation in the AIB1-Δ3 transgenic mice was explained by lower AIB1 protein levels than in the transgenic mice containing the entire human AIB1 protein [70]. Kuang et al. [74] showed that v-Ha-ras mammary gland tumor incidence was dramatically reduced in AIB1−/− mice as compared with wild-type AIB+/+ and heterozygous AIB1+/− mice. Taken together, these human and animal studies provide overwhelming evidence that AIB1 overexpression plays an important role in human breast cancer. In the next sections we will examine the signaling pathways that AIB1 influences in the breast cancer cells and the potential mechanisms of regulation of AIB1 in breast cancer.
Regulation of AIB1 mRNA and protein levels
Total AIB1 protein expression is regulated at the DNA (gene amplification and transcription), RNA (translation) and protein (stability) levels. Transcription of the AIB1 gene is controlled by regulatory sequences within the −250 to +350 base pair region of its promoter (relative to the translation initiation site), a region which contains binding sites for two transcription factors, E2F1 and Sp1 [61, 98]. Since AIB1 is a transcriptional co-activator for E2F1-dependent gene transcription, the finding of an E2F1 binding site in the AIB1 promoter suggested that AIB1 can self-regulate [61, 98]. Interestingly, the evidence confirming this prediction of positive self-regulation, via AIB1 stimulation of E2F1-dependent transcription from the AIB1 promoter, showed that it did not require the E2F binding site, but rather the Sp1 binding site and the binding of Sp1 to this site [98]. Total AIB1 mRNA levels are also increased when MCF-7 breast cancer cells are treated with anti-estrogens (ICI 182,780 and tamoxifen), all-trans-retinoic acid or TGF-β and are decreased by estrogen treatments [60]. These effects are associated with increased or decreased transcription [60]. In addition, the translation of AIB1 mRNA can be regulated by endogenous microRNAs, which inhibit translation by binding to the 3′-untranslated regions of target mRNAs [99]. Specifically, the microRNA Mir-17-5p inhibits the translation of AIB1 mRNA, causing a decrease in AIB1 protein levels [100]. The level of this microRNA is low in breast cancer cell lines with high levels of AIB1 protein [100].
AIB1 protein levels are also regulated by proteasomal degradation pathways (reviewed in [101]). The particular pathway utilized may depend on the stimulus and/or specific post-translational modifications of AIB1. In the ubiquitinmediated proteosome degradation pathway, ubiquitin molecules are attached to proteins by E3 ligases, resulting in their degradation by the 26S proteosome, in an ATP-dependent process [102]. For example, the E3 ubiquitin ligase E6-associated protein (E6-AP) can interact with AIB1 in MCF-7 cells, suggesting that E6-AP may target AIB1 for proteosome-mediated degradation [103]. Another E3 ligase, SCFFbw7α, has been shown to ubiquitinate lysine residues 723 and 786 in AIB1 following GSK3-mediated phosphorylation of two AIB1 serine residues (505 and 509), leading to increased AIB1 proteosomal degradation [104]. Additionally, methylation of AIB1 by the methyltransferase CARM1 results in increased AIB1 degradation [105]. However, it is not known if the CARM1-driven degradation of AIB1 is through ubiquitin-targeted proteosomal degradation. Increased AIB1 turn-over by the 20S proteosome regulator REGγ in an ubiqutin- and ATP-independent manner occurs in MCF-7 cells [106]. REGγ also interacts with AIB1 in MCF-7 cells and modulation of REGγ levels affects AIB1 levels without affecting SRC-1 levels [106]. Additionally, atypical PKC was shown to phosphorylate AIB1 and to inhibit its proteosomal degradation, by inhibiting the association of AIB1 with the C8 subunit of the 20S core proteasome, in an estrogen-dependent manner [107]. Consistent with this, we found an association between AIB1 and the C8 subunit of the 20S core proteasome in our laboratory by using MS/MS analysis to identify proteins that co-immunoprecipitate with AIB1, from total cell lysates of MCF-7 cells (our unpublished results).
Regulation of AIB1 function/activity
The co-activator function of AIB1 is regulated by multiple cellular signaling pathways. This regulation is primarily via post-translational modifications of specific amino acids, which have distinct but often related effects; these modifications affect AIB1 co-activator function by affecting AIB1 protein-protein interactions, AIB1 sub-cellular localization, and AIB1 stability. AIB1 is modified by methylation [105, 108], sumoylation [109], and acetylation [5], however, the most studied modification of AIB1 is phosphorylation. The first evidence linking AIB1 activation to its serine and threonine phosphorylation was found in extracts from MCF-7 breast cancer cells. The extracted AIB1 could also be used as an in vitro substrate for phosphorylation by extracellular signal-regulated kinase 2 (ERK2) [110]. Subsequently, AIB1 was shown to be phosphorylated by different kinases and on multiple serine and threonine sites [75, 110]. The identity of individual AIB1 phosphorylation sites was determined by analyzing recombinant AIB1 produced in sf9 insect cells [75]. The individual phosphorylation sites included multiple serine residues (S505, S543, S857, S860, and S867) and a single threonine residue (T24) [75]. Multiple kinases such as ERK, JNK, p38MAPK, GSK3, and PKA were also shown to phosphorylate AIB1 at these sites in vitro [75]. AIB1 was shown to be phosphorylated in response to estrogen in MCF-7 cells and in response to a TNF-α in HeLa cervical carcinoma cells [12, 75]. Subsequently, estrogen-induced AIB1 phosphorylation on serine 857 was shown to be dependent on IκB kinase (IKK)-α [111]. More recently we have demonstrated that AIB1 is phosphorylated at a C-terminal tyrosine residue (Y1357) and this phosphorylation can be induced by estrogen, EGF and IGF through Abl kinase [112]. Interestingly, high levels of Y-1357 phospho-AIB1 are found in HER2/Neu induced mammary tumors [112] and in human tumors (unpublished data) suggesting that Abl kinase activation of AIB1 may play a functional role in mammary tumorigenesis. This raises the possibility that Abl kinase inhibitors such as imatinib (Gleevec) may be useful in a defined therapeutic or preventive setting in breast cancer.
The phosphorylation of AIB1 has multiple functional consequences. AIB1 phosphorylation is required for binding to other transcription cofactors such as CBP/p300 and for its ability to fully function as a transcriptional co-activator in the tumorigenic process [75, 110, 112]. AIB1 phosphorylation also regulates its proteosomal degradation. For example, glycogen synthase kinase-3 (GSK-3) was shown to phosphorylate AIB1 on serine 505 in MCF-7 cells, resulting in its proteosomal degradation [104]. GSK-3 is a serine/threonine kinase that acts downstream of Akt and is inhibited when phosphorylated by Akt [113]. Thus, AIB1 is phosphorylated on multiple serine residues and on at least one tyrosine residue by kinases that are activated by hormone, growth factor or cytokine signaling. Overall the published data suggest that AIB1’s potential to participate in cross-talk between signaling pathways may be greater than that previously thought; instead of receiving cross-talk signals only from serine/threonine kinases, AIB1 might also participate in crosstalk between steroid receptors and tyrosine kinase receptors.
Conclusion
Multiple studies have demonstrated that AIB1 overexpression provides a growth advantage for breast cancer cells and promotes the development of mammary tumors in mice. AIB1 overexpression is associated with the progression of breast cancer and other epithelial cancers. Since high protein levels of AIB1 and HER2 predicts worse clinical outcome, and resistance to tamoxifen therapy, the level of expression of both of these proteins may provide an important prognostic indicator whether patients should be treated with tamoxifen or aromatase inhibitors.
References
- 1.Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM, Meltzer PS. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277(5328):965–968. doi: 10.1126/science.277.5328.965. doi:10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- 2.Onate SA, Tsai SY, Tsai MJ, O’Malley BW. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270(5240):1354–1357. doi: 10.1126/science.270.5240.1354. doi:10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- 3.Voegel JJ, Heine MJ, Zechel C, Chambon P, Gronemeyer H. TIF2, a 160 kDa transcriptional mediator for the liganddependent activation function AF-2 of nuclear receptors. EMBO J. 1996;15(14):3667–3675. [PMC free article] [PubMed] [Google Scholar]
- 4.Suen CS, Berrodin TJ, Mastroeni R, Cheskis BJ, Lyttle CR, Frail DE. A transcriptional coactivator, steroid receptor coactivator-3, selectively augments steroid receptor transcriptional activity. J Biol Chem. 1998;273(42):27645–27653. doi: 10.1074/jbc.273.42.27645. doi:10.1074/jbc.273.42.27645. [DOI] [PubMed] [Google Scholar]
- 5.Chen H, Lin RJ, Schiltz RL, Chakravarti D, Nash A, Nagy L, Privalsky ML, Nakatani Y, Evans RM. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell. 1997;90:569–580. doi: 10.1016/s0092-8674(00)80516-4. doi:10.1016/S0092-8674(00)80516-4. [DOI] [PubMed] [Google Scholar]
- 6.Li H, Gomes PJ, Chen JD. RAC3, a steroid/nuclear receptor-associated coactivator that is related to SRC-1 and TIF2. Proc Natl Acad Sci USA. 1997;94:8479–8484. doi: 10.1073/pnas.94.16.8479. doi:10.1073/pnas.94.16.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeshita A, Cardona GR, Koibuchi N, Suen CS, Chin WW. TRAM-1, A novel 160-kDa thyroid hormone receptor activator molecule, exhibits distinct properties from steroid receptor coactivator-1. J Biol Chem. 1997;272(44):27629–27634. doi: 10.1074/jbc.272.44.27629. doi:10.1074/jbc.272.44.27629. [DOI] [PubMed] [Google Scholar]
- 8.Torchia J, Rose DW, Inostroza J, Kamei Y, Westin S, Glass CK, Rosenfeld MG. The transcriptional co-activator p/CIP binds CBP and mediates nuclear-receptor function. Nature. 1997;387:677–684. doi: 10.1038/42652. doi:10.1038/42652. [DOI] [PubMed] [Google Scholar]
- 9.Liao L, Kuang SQ, Yuan Y, Gonzalez SM, O’Malley BW, Xu J. Molecular structure and biological function of the cancer-amplified nuclear receptor coactivator SRC-3/AIB1. J Steroid Biochem Mol Biol. 2002;83(1–5):3–14. doi: 10.1016/s0960-0760(02)00254-6. [DOI] [PubMed] [Google Scholar]
- 10.Louie MC, Zou JX, Rabinovich A, Chen HW. ACTR/AIB1 functions as an E2F1 coactivator to promote breast cancer cell proliferation and antiestrogen resistance. Mol Cell Biol. 2004;24(12):5157–5171. doi: 10.1128/MCB.24.12.5157-5171.2004. doi:10.1128/MCB.24.12.5157-5171.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan J, Yu CT, Ozen M, Ittmann M, Tsai SY, Tsai MJ. Steroid receptor coactivator-3 and activator protein-1 coordinately regulate the transcription of components of the insulinlike growth factor/AKT signaling pathway. Cancer Res. 2006;66(22):11039–11046. doi: 10.1158/0008-5472.CAN-06-2442. doi:10.1158/0008-5472.CAN-06-2442. [DOI] [PubMed] [Google Scholar]
- 12.Wu RC, Qin J, Hashimoto Y, Wong J, Xu J, Tsai SY, Tsai MJ, O’Malley BW. Regulation of SRC-3 (pCIP/ACTR/AIB-1/RAC-3/TRAM-1) Coactivator activity by I kappa B kinase. Mol Cell Biol. 2002;22(10):3549–3561. doi: 10.1128/MCB.22.10.3549-3561.2002. doi:10.1128/MCB.22.10.3549-3561.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arimura A, vn Peer M, Schroder AJ, Rothman PB. The transcriptional co-activator p/CIP (NCoA-3) is up-regulated by STAT6 and serves as a positive regulator of transcriptional activation by STAT6. J Biol Chem. 2004;279(30):31105–31112. doi: 10.1074/jbc.M404428200. doi:10.1074/jbc.M404428200. [DOI] [PubMed] [Google Scholar]
- 14.Clayton AL, Hazzalin CA, Mahadevan LC. Enhanced histone acetylation and transcription: a dynamic perspective. Mol Cell. 2006;23(3):289–296. doi: 10.1016/j.molcel.2006.06.017. doi:10.1016/j.molcel.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 15.Yu C, York B, Wang S, Feng Q, Xu J, O’Malley BW. An essential function of the SRC-3 coactivator in suppression of cytokine mRNA translation and inflammatory response. Mol Cell. 2007;25(5):765–778. doi: 10.1016/j.molcel.2007.01.025. doi:10.1016/j.molcel.2007.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuny M, Kramar A, Courjal F, Johannsdottir V, Iacopetta B, Fontaine H, Grenier J, Culine S, Theillet C. Relating genotype and phenotype in breast cancer: an analysis of the prognostic significance of amplification at eight different genes or loci and of p53 mutations. Cancer Res. 2000;60(4):1077–1083. [PubMed] [Google Scholar]
- 17.Iwase H, Omoto Y, Toyama T, Yamashita H, Hara Y, Sugiura H, Zhang Z. Clinical significance of AIB1 expression in human breast cancer. Breast Cancer Res Treat. 2003;80(3):339–345. doi: 10.1023/A:1024916126532. doi:10.1023/A:1024916126532. [DOI] [PubMed] [Google Scholar]
- 18.Shibata A, Hayashi Y, Imai T, Funahashi H, Nakao A, Seo H. Somatic gene alteration of AIB1 gene in patients with breast cancer. Endocr J. 2001;48(2):199–204. doi: 10.1507/endocrj.48.199. doi:10.1507/endocrj. 48.199. [DOI] [PubMed] [Google Scholar]
- 19.Kirkegaard T, McGlynn LM, Campbell FM, Müller S, Tovey SM, Dunne B, Nielsen KV, Cooke TG, Bartlett JM. Amplified in breast cancer 1 in human epidermal growth factor receptor—positive tumors of tamoxifen-treated breast cancer patients. Clin Cancer Res. 2007;13(5):1405–1411. doi: 10.1158/1078-0432.CCR-06-1933. doi:10.1158/1078-0432.CCR-06-1933. [DOI] [PubMed] [Google Scholar]
- 20.Ginestier C, Cervera N, Finetti P, Esteyries S, Esterni B, Adelaide J, Xerri L, Viens P, Jacquemier J, Charafe-Jauffret E, et al. Prognosis and gene expression profiling of 20q13-amplified breast cancers. Clin Cancer Res. 2006;12(15):4533–4544. doi: 10.1158/1078-0432.CCR-05-2339. doi:10.1158/1078-0432.CCR-05-2339. [DOI] [PubMed] [Google Scholar]
- 21.Bouras T, Southey MC, Venter DJ. Overexpression of the steroid receptor coactivator AIB1 in breast cancer correlates with the absence of estrogen and progesterone receptors and positivity for p53 and HER2/neu. Cancer Res. 2001;61(3):903–907. [PubMed] [Google Scholar]
- 22.List HJ, Reiter R, Singh B, Wellstein A, Riegel AT. Expression of the nuclear coactivator AIB1 in normal and malignant breast tissue. Breast Cancer Res Treat. 2001;68(1):21–28. doi: 10.1023/a:1017910924390. doi:10.1023/A:1017910924390. [DOI] [PubMed] [Google Scholar]
- 23.Zhao C, Yasui K, Lee CJ, Kurioka H, Hosokawa Y, Oka T, Inazawa J. Elevated expression levels of NCOA3, TOP1, and TFAP2C in breast tumors as predictors of poor prognosis. Cancer. 2003;98(1):18–23. doi: 10.1002/cncr.11482. doi:10.1002/cncr.11482. [DOI] [PubMed] [Google Scholar]
- 24.Harigopal M, Heymann J, Ghosh S, Anagnostou V, Camp RL, Rimm DL. Estrogen receptor co-activator (AIB1) protein expression by automated quantitative analysis (AQUA) in a breast cancer tissue microarray and association wit patient outcome. Breast Cancer Res Treat. 2009;115(1):77–85. doi: 10.1007/s10549-008-0063-9. doi:10.1007/s10549-008-0063-9. [DOI] [PubMed] [Google Scholar]
- 25.Hudelist G, Czerwenka K, Kubista E, Marton E, Pischinger K, Singer CF. Expression of sex steroid receptors and their co-factors in normal and malignant breast tissue: AIB1 is a carcinoma-specific co-activator. Breast Cancer Res Treat. 2003;78(2):193–204. doi: 10.1023/a:1022930710850. doi:10.1023/A:1022930710850. [DOI] [PubMed] [Google Scholar]
- 26.Henke RT, Eun Kim S, Maitra A, Paik S, Wellstein A. Expression analysis of mRNA in formalin-fixed, paraffinembedded archival tissues by mRNA in situ hybridization. Methods. 2006;38(4):253–262. doi: 10.1016/j.ymeth.2005.11.013. doi:10.1016/j.ymeth.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 27.Richardson AL, Wang ZC, De Nicolo A, Lu X, Brown M, Miron A, Liao X, Iglehart JD, Livingston DM, Ganesan S. X chromosomal abnormalities in basal-like human breast cancer. Cancer Cell. 2006;9(2):121–132. doi: 10.1016/j.ccr.2006.01.013. doi:10.1016/j.ccr.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 28.Farmer P, Bonnefoi H, Becette V, Tubiana-Hulin M, Fumoleau P, Larsimont D, Macgrogan G, Bergh J, Cameron D, Goldstein D, et al. Identification of molecular apocrine breast tumours by microarray analysis. Oncogene. 2005;24(29):4660–4671. doi: 10.1038/sj.onc.1208561. doi:10.1038/sj.onc.1208561. [DOI] [PubMed] [Google Scholar]
- 29.Ivshina AV, George J, Senko O, Mow B, Putti TC, Smeds J, Lindahl T, Pawitan Y, Hall P, Nordgren H, et al. Genetic reclassification of histologic grade delineates new clinical subtypes of breast cancer. Cancer Res. 2006;66(21):10292–10301. doi: 10.1158/0008-5472.CAN-05-4414. doi:10.1158/0008-5472.CAN-05-4414. [DOI] [PubMed] [Google Scholar]
- 30.van de Vijver MJ, He YD, van’t Veer LJ, Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347(25):1999–2009. doi: 10.1056/NEJMoa021967. doi:10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 31.Iwase H. Molecular action of the estrogen receptor and hormone dependency in breast cancer. Breast Cancer. 2003;10(2):89–96. doi: 10.1007/BF02967632. doi:10.1007/BF02967632. [DOI] [PubMed] [Google Scholar]
- 32.Burwinkel B, Wirtenberger M, Klaes R, Schmutzler RK, Grzybowska E, Försti A, Frank B, Bermejo JL, Bugert P, Wappenschmidt B, et al. Association of NCOA3 polymorphisms with breast cancer risk. Clin Cancer Res. 2005;11(6):2169–2174. doi: 10.1158/1078-0432.CCR-04-1621. doi:10.1158/1078-0432.CCR-04-1621. [DOI] [PubMed] [Google Scholar]
- 33.Deng CX. BRCA1: cell cycle checkpoint, genetic instability, DNA damage response and cancer evolution. Nucleic Acids Res. 2006;34(5):1416–1426. doi: 10.1093/nar/gkl010. doi:10.1093/nar/gkl010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rebbeck TR, Wang Y, Kantoff PW, Krithivas K, Neuhausen SL, Godwin AK, Daly MB, Narod SA, Brunet JS, Vesprini D, et al. Modification of BRCA1- and BRCA2-associated breast cancer risk by AIB1 genotype and reproductive history. Cancer Res. 2001;61(14):5420–5424. [PubMed] [Google Scholar]
- 35.Kadouri L, Kote-Jarai Z, Easton DF, Hubert A, Hamoudi R, Glaser B, Abeliovich D, Peretz T, Eeles RA. Polyglutamine repeat length in the AIB1 gene modifies breast cancer susceptibility in BRCA1 carriers. Int J Cancer. 2004;108(3):399–403. doi: 10.1002/ijc.11531. doi:10.1002/ijc.11531. [DOI] [PubMed] [Google Scholar]
- 36.Hughes DJ, Ginolhac SM, Coupier I, Barjhoux L, Gaborieau V, Bressac-de-Paillerets B, Chompret A, Bignon YJ, Uhrhammer N, Lasset C, et al. Breast cancer risk in BRCA1 and BRCA2 mutation carriers and polyglutamine repeat length in the AIB1 gene. Int J Cancer. 2005;117(2):230–233. doi: 10.1002/ijc.21176. doi:10.1002/ijc. 21176. [DOI] [PubMed] [Google Scholar]
- 37.Spurdle AB, Antoniou AC, Kelemen L, Holland H, Peock S, Cook MR, Smith PL, Greene MH, Simard J, Plourde M, et al. The AIB1 polyglutamine repeat does not modify breast cancer risk in BRCA1 and BRCA2 mutation carriers. Cancer Epidemiol Biomarkers Prev. 2006;15(1):76–79. doi: 10.1158/1055-9965.EPI-05-0709. doi:10.1158/1055-9965.EPI-05-0709. [DOI] [PubMed] [Google Scholar]
- 38.Li AJ, Lerner DL, Gapuzan ME, Karlan BY. AIB1 polymorphisms predict aggressive ovarian cancer phenotype. Cancer Epidemiol Biomarkers Prev. 2005;14(12):2919–2922. doi: 10.1158/1055-9965.EPI-05-0540. doi:10.1158/1055-9965.EPI-05-0540. [DOI] [PubMed] [Google Scholar]
- 39.Soerjomataram I, Louwman MW, Ribot JG, Roukema JA, Coebergh JW. An overview of prognostic factors for long-term survivors of breast cancer. Breast Cancer Res Treat. 2008;107(3):309–330. doi: 10.1007/s10549-007-9556-1. doi:10.1007/s10549-007-9556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reiter R, Wellstein A, Riegel AT. An isoform of the coactivator AIB1 that increases hormone and growth factor sensitivity is overexpressed in breast cancer. J Biol Chem. 2001;276(43):39736–39741. doi: 10.1074/jbc.M104744200. doi:10.1074/jbc.M104744200. [DOI] [PubMed] [Google Scholar]
- 41.Bautista S, Valles H, Walker RL, Anzick S, Zeillinger R, Meltzer P, Theillet C. In breast cancer, amplification of the steroid receptor coactivator gene AIB1 is correlated with estrogen and progesterone receptor positivity. Clin Cancer Res. 1998;4(12):2925–2929. [PubMed] [Google Scholar]
- 42.Sotiriou C, Wirapati P, Loi S, Harris A, Fox S, Smeds J, Nordgren H, Farmer P, Praz V, Haibe-Kains B, et al. Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst. 2006;98(4):262–272. doi: 10.1093/jnci/djj052. [DOI] [PubMed] [Google Scholar]
- 43.List HJ, Lauritsen KJ, Reiter R, Powers C, Wellstein A, Riegel AT. Ribozyme targeting demonstrates that the nuclear receptor coactivator AIB1 is a rate-limiting factor for estrogen-dependent growth of human MCF-7 breast cancer cells. J Biol Chem. 2001;276(26):23763–23768. doi: 10.1074/jbc.M102397200. doi:10.1074/jbc.M102397200. [DOI] [PubMed] [Google Scholar]
- 44.Jordan VC, Dowse LJ. Tamoxifen as an anti-tumour agent: effect on oestrogen binding. J Endocrinol. 1976;68(02):297–303. doi: 10.1677/joe.0.0680297. doi:10.1677/joe.0.0680297. [DOI] [PubMed] [Google Scholar]
- 45.Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339(22):1609–1618. doi: 10.1056/NEJM199811263392207. doi:10.1056/NEJM1998 11263392207. [DOI] [PubMed] [Google Scholar]
- 46.Osborne CK, Shou J, Massarweh S, Schiff R. Crosstalk between estrogen receptor and growth factor receptor pathways as a cause for endocrine therapy resistance in breast cancer. Clin Cancer Res. 2005;11(2 Pt 2) 865s–870s. [PubMed] [Google Scholar]
- 47.Osborne CK, Bardou V, Hopp TA, Chamness GC, Hilsenbeck SG, Fuqua SA, Wong J, Allred DC, Clark GM, Schiff R. Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst. 2003;95(5):353–361. doi: 10.1093/jnci/95.5.353. [DOI] [PubMed] [Google Scholar]
- 48.Kirkegaard T, McGlynn LM, Campbell FM, Muller S, Tovey SM, Dunne B, Nielsen KV, Cooke TG, Bartlett JM. Amplified in breast cancer 1 in human epidermal growth factor receptor—positive tumors of tamoxifen-treated breast cancer patients. Clin Cancer Res. 2007;13(5):1405–1411. doi: 10.1158/1078-0432.CCR-06-1933. doi:10.1158/1078-0432.CCR-06-1933. [DOI] [PubMed] [Google Scholar]
- 49.Dihge L, Bendahl PO, Grabau D, Isola J, Lovgren K, Ryden L, Ferno M. Epidermal growth factor receptor (EGFR) and the estrogen receptor modulator amplified in breast cancer (AIB1) for predicting clinical outcome after adjuvant tamoxifen in breast cancer. Breast Cancer Res Treat. 2007;109(2):255–262. doi: 10.1007/s10549-007-9645-1. doi:10.1007/s10549-007-9645-1. [DOI] [PubMed] [Google Scholar]
- 50.Reiter R, Oh AS, Wellstein A, Riegel AT. Impact of the nuclear receptor coactivator AIB1 isoform AIB1-Delta3 on estrogenic ligands with different intrinsic activity. Oncogene. 2004;23(2):403–409. doi: 10.1038/sj.onc.1207202. doi:10.1038/sj.onc.1207202. [DOI] [PubMed] [Google Scholar]
- 51.Silva CM, Shupnik MA. Integration of steroid and growth factor pathways in breast cancer: focus on signal transducers and activators of transcription and their potential role in resistance. Mol Endocrinol. 2007;21(7):1499–1512. doi: 10.1210/me.2007-0109. doi:10.1210/me.2007-0109. [DOI] [PubMed] [Google Scholar]
- 52.Fleming FJ, Myers E, Kelly G, Crotty TB, McDermott EW, O’Higgins NJ, Hill AD, Young LS. Expression of SRC-1, AIB1, and PEA3 in HER2 mediated endocrine resistant breast cancer; a predictive role for SRC-1. J Clin Pathol. 2004;57(10):1069–1074. doi: 10.1136/jcp.2004.016733. doi:10.1136/jcp.2004.016733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Massarweh S, Schiff R. Unraveling the mechanisms of endocrine resistance in breast cancer: new therapeutic opportunities. Clin Cancer Res. 2007;13(7):1950–1954. doi: 10.1158/1078-0432.CCR-06-2540. doi:10.1158/1078-0432.CCR-06-2540. [DOI] [PubMed] [Google Scholar]
- 54.Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, Schiff R. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96(12):926–935. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- 55.Hurtado A, Holmes KA, Geistlinger TR, Hutcheson IR, Nicholson RI, Brown M, Jiang J, Howat WJ, Ali S, Carroll JS. Regulation of ERBB2 by oestrogen receptor-PAX2 determines response to tamoxifen. Nature. 2008;456(7222):663–666. doi: 10.1038/nature07483. doi:10.1038/nature07483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patel RR, Sharma CG, Jordan VC. Optimizing the antihormonal treatment and prevention of breast cancer. Breast Cancer. 2007;14(2):113–122. doi: 10.2325/jbcs.966. doi:10.2325/jbcs.966. [DOI] [PubMed] [Google Scholar]
- 57.Lin NU, Winer EP. Advances in adjuvant endocrine therapy for postmenopausal women. J Clin Oncol. 2008;26(5):798–805. doi: 10.1200/JCO.2007.15.0946. doi:10.1200/JCO.2007.15.0946. [DOI] [PubMed] [Google Scholar]
- 58.Belosay A, Brodie AM, Njar VC. Effects of novel retinoic acid metabolism blocking agent (VN/14–1) on letrozole-insen-sitive breast cancer cells. Cancer Res. 2006;66(23):11485–11493. doi: 10.1158/0008-5472.CAN-06-2168. doi:10.1158/0008-5472.CAN-06-2168. [DOI] [PubMed] [Google Scholar]
- 59.Shin I, Miller T, Arteaga CL. ErbB receptor signaling and therapeutic resistance to aromatase inhibitors. Clin Cancer Res. 2006;12(3 Pt 2) doi: 10.1158/1078-0432.CCR-05-2352. 1008s–1012s. doi:10.1158/1078-0432.CCR-05-2352. [DOI] [PubMed] [Google Scholar]
- 60.Lauritsen KJ, List HJ, Reiter R, Wellstein A, Riegel AT. A role for TGF-beta in estrogen and retinoid mediated regulation of the nuclear receptor coactivator AIB1 in MCF-7 breast cancer cells. Oncogene. 2002;21(47):7147–7155. doi: 10.1038/sj.onc.1205943. doi:10.1038/sj.onc.1205943. [DOI] [PubMed] [Google Scholar]
- 61.Louie MC, Revenko AS, Zou JX, Yao J, Chen HW. Direct control of cell cycle gene expression by proto-oncogene product ACTR, and its autoregulation underlies its transforming activity. Mol Cell Biol. 2006;26(10):3810–3823. doi: 10.1128/MCB.26.10.3810-3823.2006. doi:10.1128/MCB.26.10. 3810-3823.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441(7092):431–436. doi: 10.1038/nature04870. doi:10.1038/nature 04870. [DOI] [PubMed] [Google Scholar]
- 63.Liu Y, Lu C, Shen Q, Munoz-Medellin D, Kim H, Brown PH. AP-1 blockade in breast cancer cells causes cell cycle arrest by suppressing G1 cyclin expression and reducing cyclin-dependent kinase activity. Oncogene. 2004;23(50):8238–8246. doi: 10.1038/sj.onc.1207889. doi:10.1038/sj.onc.1207889. [DOI] [PubMed] [Google Scholar]
- 64.Biswas DK, Shi Q, Baily S, Strickland I, Ghosh S, Pardee AB, Iglehart JD. NF-kappa B activation in human breast cancer specimens and its role in cell proliferation and apoptosis. Proc Natl Acad Sci USA. 2004;101(27):10137–10142. doi: 10.1073/pnas.0403621101. doi:10.1073/pnas.0403621101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Singh S, Shi Q, Bailey ST, Palczewski MJ, Pardee AB, Iglehart JD, Biswas DK. Nuclear factor-kappaB activation: a molecular therapeutic target for estrogen receptor-negative and epidermal growth factor receptor family receptor-positive human breast cancer. Mol Cancer Ther. 2007;6(7):1973–1982. doi: 10.1158/1535-7163.MCT-07-0063. doi:10.1158/1535-7163.MCT-07-0063. [DOI] [PubMed] [Google Scholar]
- 66.Boehm JS, Zhao JJ, Yao J, Kim SY, Firestein R, Dunn IF, Sjostrom SK, Garraway LA, Weremowicz S, Richardson AL, et al. Integrative genomic approaches identify IKBKE as a breast cancer oncogene. Cell. 2007;129(6):1065–1079. doi: 10.1016/j.cell.2007.03.052. doi:10.1016/j.cell.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 67.Laviola L, Natalicchio A, Giorgino F. The IGF-I signaling pathway. Curr Pharm Des. 2007;13(7):663–669. doi: 10.2174/138161207780249146. doi:10.2174/138161207780249146. [DOI] [PubMed] [Google Scholar]
- 68.Wang Z, Rose DW, Hermanson O, Liu F, Herman T, Wu W, Szeto D, Gleiberman A, Krones A, Pratt K, et al. Regulation of somatic growth by the p160 coactivator p/CIP. Proc Natl Acad Sci USA. 2000;97(25):13549–13554. doi: 10.1073/pnas.260463097. doi:10.1073/pnas.260463097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu J, Liao L, Ning G, Yoshida-Komiya H, Deng C, O’Malley BW. The steroid receptor coactivator SRC-3 (p/CIP/RAC3/AIB1/ACTR/TRAM-1) is required for normal growth, puberty, female reproductive function, and mammary gland development. Proc Natl Acad Sci USA. 2000;97(12):6379–6384. doi: 10.1073/pnas.120166297. doi:10.1073/pnas.120166297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Torres-Arzayus MI, De Mora JF, Yuan J, Vazquez F, Bronson R, Rue M, Sellers WR, Brown M. High tumor incidence and activation of the PI3 K/AKT pathway in transgenic mice define AIB1 as an oncogene. Cancer Cell. 2004;6(3):263–274. doi: 10.1016/j.ccr.2004.06.027. doi:10.1016/j.ccr.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 71.Tilli MT, Reiter R, Oh AS, Henke RT, McDonnell K, Gallicano GI, Furth PA, Riegel AT. Overexpression of an N-terminally truncated isoform of the nuclear receptor coactivator amplified in breast cancer 1 leads to altered proliferation of mammary epithelial cells in transgenic mice. Mol Endocrinol. 2005;19(3):644–656. doi: 10.1210/me.2004-0106. doi:10.1210/me.2004-0106. [DOI] [PubMed] [Google Scholar]
- 72.Oh A, List HJ, Reiter R, Mani A, Zhang Y, Gehan E, Wellstein A, Riegel AT. The nuclear receptor coactivator AIB1 mediates insulin-like growth factor I-induced phenotypic changes in human breast cancer cells. Cancer Res. 2004;64(22):8299–8308. doi: 10.1158/0008-5472.CAN-04-0354. doi:10.1158/0008-5472.CAN-04-0354. [DOI] [PubMed] [Google Scholar]
- 73.Torres-Arzayus MI, Yuan J, DellaGatta JL, Lane H, Kung AL, Brown M. Targeting the AIB1 oncogene through mammalian target of rapamycin inhibition in the mammary gland. Cancer Res. 2006;66(23):11381–11388. doi: 10.1158/0008-5472.CAN-06-2316. doi:10.1158/0008-5472.CAN-06-2316. [DOI] [PubMed] [Google Scholar]
- 74.Kuang SQ, Liao L, Zhang H, Lee AV, O’Malley BW, Xu J. AIB1/SRC-3 deficiency affects insulin-like growth factor I signaling pathway and suppresses v-Ha-ras-induced breast cancer initiation and progression in mice. Cancer Res. 2004;64(5):1875–1885. doi: 10.1158/0008-5472.can-03-3745. doi:10.1158/0008-5472.CAN-03-3745. [DOI] [PubMed] [Google Scholar]
- 75.Wu RC, Qin J, Yi P, Wong J, Tsai SY, Tsai MJ, O’Malley BW. Selective Phosphorylations of the SRC-3/AIB1 Coactivator Integrate Genomic Reponses to Multiple Cellular Signaling Pathways. Mol Cell. 2004;15(6):937–949. doi: 10.1016/j.molcel.2004.08.019. doi:10.1016/j.molcel. 2004.08.019. [DOI] [PubMed] [Google Scholar]
- 76.Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19(13):3159–3167. doi: 10.1093/emboj/19.13.3159. doi:10.1093/emboj/19. 13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5(5):341–354. doi: 10.1038/nrc1609. doi:10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 78.Hess KR, Anderson K, Symmans WF, Valero V, Ibrahim N, Mejia JA, Booser D, Theriault RL, Buzdar AU, Dempsey PJ, et al. Pharmacogenomic predictor of sensitivity to preoperative chemotherapy with paclitaxel and fluorouracil, doxorubicin, and cyclophosphamide in breast cancer. J Clin Oncol. 2006;24(26):4236–4244. doi: 10.1200/JCO.2006.05.6861. doi:10.1200/JCO.2006.05.6861. [DOI] [PubMed] [Google Scholar]
- 79.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093. doi:10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 80.Bittner RM. International Genomics Consortium expO dataset- breast samples. International Genomics Consortium. 2006 http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE2109.
- 81.Fereshteh MP, Lahusen T, Kim SE, Xu J, O’Malley BW, Wellstein A, Furth PA, Riegel AT. The nuclear receptor coactivator Amplified in Breast Cancer-1 is required for Neu (ErbB2/HER2) activation, signaling and mammary tumorigenesis in mice. Cancer Res. 2008;68(10):3697–3706. doi: 10.1158/0008-5472.CAN-07-6702. doi:10.1158/0008-5472.CAN-07-6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lahusen T, Fereshteh M, Oh A, Wellstein A, Riegel AT. Epidermal growth factor receptor tyrosine phosphorylation and signaling controlled by a nuclear receptor coactivator, amplified in breast cancer 1. Cancer Res. 2007;67(15):7256–7265. doi: 10.1158/0008-5472.CAN-07-1013. doi:10.1158/0008-5472.CAN-07-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. doi: 10.1126/science.3798106. doi:10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 84.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244(4905):707–712. doi: 10.1126/science.2470152. doi:10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 85.Nahta R, Esteva FJ. Trastuzumab: triumphs and tribulations. Oncogene. 2007;26(25):3637–3643. doi: 10.1038/sj.onc.1210379. doi:10.1038/sj.onc.1210379. [DOI] [PubMed] [Google Scholar]
- 86.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–1684. doi: 10.1056/NEJMoa052122. doi:10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 87.Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL, Pusztai L, Green MC, Arun BK, Giordano SH, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23(16):3676–3685. doi: 10.1200/JCO.2005.07.032. doi:10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 88.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–1672. doi: 10.1056/NEJMoa052306. doi:10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 89.Nahta R, Esteva FJ. Herceptin: mechanisms of action and resistance. Cancer Lett. 2006;232(2):123–138. doi: 10.1016/j.canlet.2005.01.041. doi:10.1016/j.canlet. 2005.01.041. [DOI] [PubMed] [Google Scholar]
- 90.Paik S, Kim C, Wolmark N. HER2 status and benefit from adjuvant trastuzumab in breast cancer. N Engl J Med. 2008;358(13):1409–1411. doi: 10.1056/NEJMc0801440. doi:10.1056/NEJMc0801440. [DOI] [PubMed] [Google Scholar]
- 91.Lu Y, Zi X, Zhao Y, Mascarenhas D, Pollak M. Insulinlike growth factor-I receptor signaling and resistance to trastuzumab (Herceptin) J Natl Cancer Inst. 2001;93(24):1852–1857. doi: 10.1093/jnci/93.24.1852. doi:10.1093/jnci/93.24.1852. [DOI] [PubMed] [Google Scholar]
- 92.Nahta R, Yuan LX, Zhang B, Kobayashi R, Esteva FJ. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res. 2005;65(23):11118–11128. doi: 10.1158/0008-5472.CAN-04-3841. doi:10.1158/0008-5472.CAN-04-3841. [DOI] [PubMed] [Google Scholar]
- 93.Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL. Herceptin-induced inhibition of phosphati-dylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res. 2002;62(14):4132–4141. [PubMed] [Google Scholar]
- 94.Le XF, Claret FX, Lammayot A, Tian L, Deshpande D, LaPu-shin R, Tari AM, Bast RC., Jr The role of cyclin-dependent kinase inhibitor p27Kip1 in anti-HER2 antibody-induced G1 cell cycle arrest and tumor growth inhibition. J Biol Chem. 2003;278(26):23441–23450. doi: 10.1074/jbc.M300848200. doi:10.1074/jbc.M300848200. [DOI] [PubMed] [Google Scholar]
- 95.Nahta R, Takahashi T, Ueno NT, Hung MC, Esteva FJ. P27(kip1) down-regulation is associated with trastuzumab resistance in breast cancer cells. Cancer Res. 2004;64(11):3981–3986. doi: 10.1158/0008-5472.CAN-03-3900. doi:10.1158/0008-5472.CAN-03-3900. [DOI] [PubMed] [Google Scholar]
- 96.Anido J, Scaltriti M, Bech Serra JJ, Santiago Josefat B, Todo FR, Baselga J, Arribas J. Biosynthesis of tumorigenic HER2 C-terminal fragments by alternative initiation of translation. EMBO J. 2006;25(13):3234–3244. doi: 10.1038/sj.emboj.7601191. doi:10.1038/sj.emboj.7601191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Avivar A, Garcia-Macias MC, Ascaso E, Herrera G, O’Connor JE, de Mora JF. Moderate overexpression of AIB1 triggers pre-neoplastic changes in mammary epithelium. FEBS Lett. 2006;580(22):5222–5226. doi: 10.1016/j.febslet.2006.08.057. doi:10.1016/j.febslet.2006.08.057. [DOI] [PubMed] [Google Scholar]
- 98.Mussi P, Yu C, O’Malley BW, Xu J. Stimulation of steroid receptor coactivator-3 (SRC-3) gene overexpression by a positive regulatory loop of E2F1 and SRC-3. Mol Endocrinol. 2006;20(12):3105–3119. doi: 10.1210/me.2005-0522. doi:10.1210/me.2005-0522. [DOI] [PubMed] [Google Scholar]
- 99.Cullen BR. Transcription and processing of human microRNA precursors. Mol Cell. 2004;16(6):861–865. doi: 10.1016/j.molcel.2004.12.002. doi:10.1016/j.molcel.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 100.Hossain A, Kuo MT, Saunders GF. Mir-17-5p regulates breast cancer cell proliferation by inhibiting translation of AIB1 mRNA. Mol Cell Biol. 2006;26(21):8191–8201. doi: 10.1128/MCB.00242-06. doi:10.1128/MCB. 00242-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lonard DM, O’Malley BW. SRC-3 transcription-coupled activation, degradation, and the ubiquitin clock: is there enough coactivator to go around in cells? Sci Signal. 2008;1(13):e16. doi: 10.1126/stke.113pe16. doi:10.1126/stke.113pe16. [DOI] [PubMed] [Google Scholar]
- 102.Hoeller D, Hecker CM, Dikic I. Ubiquitin and ubiquitinlike proteins in cancer pathogenesis. Nat Rev Cancer. 2006;6(10):776–788. doi: 10.1038/nrc1994. doi:10.1038/nrc1994. [DOI] [PubMed] [Google Scholar]
- 103.Mani A, Oh AS, Bowden ET, Lahusen T, Lorick KL, Weissman AM, Schlegel R, Wellstein A, Riegel AT. E6AP mediates regulated proteasomal degradation of the nuclear receptor coactivator amplified in breast cancer 1 in immortalized cells. Cancer Res. 2006;66(17):8680–8686. doi: 10.1158/0008-5472.CAN-06-0557. doi:10.1158/0008-5472.CAN-06-0557. [DOI] [PubMed] [Google Scholar]
- 104.Wu R, Feng Q, Lonard D, Omalley B. SRC-3 Coactivator Functional Lifetime Is Regulated by a Phospho-Dependent Ubiquitin Time Clock. Cell. 2007;129(6):1125–1140. doi: 10.1016/j.cell.2007.04.039. doi:10.1016/j.cell.2007.04.039. [DOI] [PubMed] [Google Scholar]
- 105.Naeem H, Cheng D, Zhao Q, Underhill C, Tini M, Bedford MT, Torchia J. The activity and stability of the transcriptional coactivator p/CIP/SRC-3 are regulated by CARM1-dependent methylation. Mol Cell Biol. 2007;27(1):120–134. doi: 10.1128/MCB.00815-06. doi:10.1128/MCB.00815-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li X, Lonard DM, Jung SY, Malovannaya A, Feng Q, Qin J, Tsai SY, Tsai MJ, O’Malley BW. The SRC-3/AIB1 coactivator is degraded in a ubiquitin- and ATP-independent manner by the REGgamma proteasome. Cell. 2006;124(2):381–392. doi: 10.1016/j.cell.2005.11.037. doi:10.1016/j.cell.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 107.Yi P. Atypical Protein Kinase C Regulates Dual Pathways for Degradation of the Oncogenic Coactivator SRC-3/AIB1. Mol Cell. 2008;29(4):465–476. doi: 10.1016/j.molcel.2007.12.030. doi:10.1016/j.molcel.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Feng Q, Yi P, Wong J, O’Malley BW. Signaling within a coactivator complex: methylation of SRC-3/AIB1 is a molecular switch for complex disassembly. Mol Cell Biol. 2006;26(21):7846–7857. doi: 10.1128/MCB.00568-06. doi:10.1128/MCB.00568-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wu H, Sun L, Zhang Y, Chen Y, Shi B, Li R, Wang Y, Liang J, Fan D, Wu G, et al. Coordinated regulation of AIB1 transcriptional activity by sumoylation and phosphorylation. J Biol Chem. 2006;281(31):21848–21856. doi: 10.1074/jbc.M603772200. doi:10.1074/jbc.M603772200. [DOI] [PubMed] [Google Scholar]
- 110.Font de Mora J, Brown M. AIB1 is a conduit for kinasemediated growth factor signaling to the estrogen receptor. Mol Cell Biol. 2000;20(14):5041–5047. doi: 10.1128/mcb.20.14.5041-5047.2000. doi:10.1128/MCB.20.14.5041-5047. 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Park KJ, Krishnan V, O’Malley BW, Yamamoto Y, Gaynor RB. Formation of an IKKalpha-dependent transcription complex is required for estrogen receptor-mediated gene activation. Mol Cell. 2005;18(1):71–82. doi: 10.1016/j.molcel.2005.03.006. doi:10.1016/j.molcel.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 112.Oh AS, Lahusen JT, Chien CD, Fereshteh MP, Zhang X, Dakshanamurthy S, Xu J, Kagan BL, Wellstein A, Riegel AT. Tyrosine phosphorylation of the nuclear receptor coactivator AIB1/SRC-3 is enhanced by Abl kinase and is required for its activity in cancer cells. Mol Cell Biol. 2008;28(21):6580–6593. doi: 10.1128/MCB.00118-08. doi:10.1128/MCB.00118-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004;29(2):95–102. doi: 10.1016/j.tibs.2003.12.004. doi:10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]