Abstract
Background
In December 2009, when the H1N1 influenza pandemic appeared to be subsiding, public health officials and unvaccinated individuals faced the question of whether continued H1N1 immunization was still worthwhile.
Purpose
To delineate what combinations of possible mechanisms could generate a third pandemic wave and then explore whether vaccinating the population at different rates and times would mitigate the wave.
Methods
As part of ongoing work with the Office of the Assistant Secretary of Preparedness and Response at the USDHHS during the H1N1 influenza pandemic, the University of Pittsburgh Models of Infectious Disease Agents Study team employed an agent-based computer simulation model of the Washington, DC metropolitan region to delineate what mechanisms could generate a “third pandemic wave” and explored whether vaccinating the population at different rates and times would mitigate the wave. This model included explicit representations of the region's individuals, school systems, workplaces/commutes, households, and communities.
Results
Three mechanisms were identified that could cause a pandemic third wave: substantially increased viral transmissibility from seasonal forcing (changing influenza transmission with changing environmental conditions, i.e., seasons) and progressive viral adaptation, an immune escape variant, and changes in social mixing from holiday school closures. Implementing vaccination for these mechanisms even during the down-slope of the fall epidemic wave significantly mitigated the third wave. Scenarios showed the gains from initiating vaccination earlier, increasing the speed of vaccination, and prioritizing population subgroups based on Advisory Committee on Immunization Practices recommendations.
Conclusions
Additional waves in an epidemic can be mitigated by vaccination even when an epidemic appears to be waning.
INTRODUCTION
In December of 2009, when the H1N1 influenza pandemic appeared to be subsiding, public health officials and unvaccinated individuals faced the question of whether continued H1N1 immunization was still worthwhile. The limited time between the Northern Hemisphere's first "epidemic wave" in the spring of 2009 and the second epidemic wave in September–December 2009 left little time to surmount developmental, regulatory, and logistic hurdles involved in bringing the vaccine from its conception to the population. Despite massive efforts, large supplies of vaccine were still available and much of the population had not yet been vaccinated as the second wave subsided. This same issue will likely occur in the future unless technologic advances help compress the novel influenza strain vaccine development timeline.
Central to the decision of whether to continue vaccination is the probability of vaccination mitigating a possible third epidemic wave occurring in January–March 2010. First, public health agencies were diverting staff and other resources to vaccination clinics, and these were resources unavailable for other programs and priorities. Continued prioritization of the H1N1 vaccination program had to be justified on the grounds of a likely positive benefit to population health. Second, individuals receiving vaccinations also expended time—and sometimes out-of-pocket costs. So, if the threat of disease had passed, these expenditures were unwarranted. Third, acceptance of the H1N1 vaccination had been poor even when available during the fall 2009 among some communities and populations.1, 2 Continued urgings by public health officials when the threat of disease was substantially diminished would likely lessen their credibility with those groups.
Historically, there is evidence for multiple wave occurrences. Multiple waves had occurred during previous influenza pandemics in 1918 and 1957.3–5 The 1918 pandemic began with a herald wave in the spring, followed by a second surge of cases in October and then a third surge in February of 1919. The 1957 pandemic also included several pandemic waves: a series of small outbreaks over the summer of 1957 followed by a higher peak in October after children returned to school and then another wave of illness that afflicted the elderly in January and February of 1958. At the peak of the 1957 U.S. pandemic, fewer than half of the approximately 60 million doses produced had been delivered. This second surge subsided until January and February 1958, when another wave of illness afflicted the elderly. The 1968–1970 pandemic also had multiple waves: an initial milder wave in early 1968 followed by a more severe second wave in late 1968, early 1969.6
The mechanisms for these additional waves are still unclear. Moreover, epidemic waves are usually presented as aggregated national data. So the question remains, is it possible for a region that has experienced one or two previous waves to experience an additional wave, or did the previously observed third waves represent regions of the country experiencing the different waves of the epidemic at different time periods? If the former scenario occurred, then several possible mechanisms for a third wave exist:
Seasonal forcing
Environmental changes (temperature, humidity, less sunlight or other factors) accompanying the transition from autumn to winter may increase the transmissibility of the virus or susceptibility to influenza.
Changes in social mixing
Changes in social contact patterns may expose individuals who are still susceptible to infection to infectious individuals. Travel during the winter holidays, schools closing and then re-opening, and relaxation in concerns over influenza transmission may contribute to such changes. Holiday travel may increase contact among susceptible individuals (e.g., the elderly) and individuals (e.g., children) more likely to be infectious.
Progressive viral adaptation with increased human-to-human transmissibility
Viral mutation can result in a modified virus that has increased transmissibility.7
Emergence of new immune escape variant
Viral mutation can progress to the degree that a new virus variant emerges against which previously infected individuals are either only partially immune or not immune at all.
In December 2009, as part of ongoing work with the Office of the Assistant Secretary of Preparedness and Response (ASPR) at the USDHHS during the H1N1 influenza pandemic, the University of Pittsburgh Models of Infectious Disease Agents Study (MIDAS) team employed an agent-based computer simulation model (ABM) of the Washington, DC metropolitan region (which included five metropolitan statistical areas) to delineate what combinations of these possible mechanisms could generate a third pandemic wave and then explored whether vaccinating the population at different rates and times would mitigate the wave. This model included explicit representations of the region's individuals, school systems, workplaces/commutes, households, and communities and incorporated a Susceptible–Exposed–Infectious–Recovered (SEIR) disease framework. This model incorporated many methods from other previously published MIDAS simulation models.8, 9 Separate scenarios explored the impact of changing the rates of vaccination and vaccinating Advisory Committee on Immunization Practices (ACIP) priority groups first (versus anyone who wanted the vaccine), an issue that arose during the 2009 H1N1 pandemic.10
MATERIALS AND METHODS
Model Structure and Synthetic Census-Based Population
A previously published study provides details on the DC metropolitan region model, which encompassed the following five census metropolitan statistical areas11:
Baltimore–Towson Metropolitan Statistical Area
Washington–Arlington–Alexandria, DC–VA–MD–VA Metropolitan Statistical Area
Winchester, VA–WV Metropolitan Statistical Area
Lexington Park, MD Micropolitan Statistical Area
Culpeper, VA Micropolitan Statistical Area.
The model consisted of a total of 7,414,562 computer "agents", mirroring the actual population of the DC metropolitan region. Each agent served as a virtual person, complete with a set of assigned socio-demographic characteristics and daily behaviors (e.g., age, gender, occupation, household location, household membership, school assignment for students and teachers, work location assignment for employed adults, work status as employed or unemployed, and disease status).12
The count of households by size of households was as follows: 753,909 (26.4%) households had only a single occupant, 888,571 (31.1%) had two occupants, 490,984 (17.2%) had three occupants, 418,073 (14.6%) had four occupants, 189,606 (6.6%) had five occupants, 74,900 (2.6%) had six occupants, 43,146 (1.5%) had seven or more occupants, Over one third (36.9%) of households had children with 448, 099 (42.5% of households with children) having one child, 397,789 (37.7%) having two children, 148.116 (14.0%), 43,959 (4.2%) having four children, and the rest having five or more children. A majority of the households, 53.6%, had two adults while 33.8% had one adult and the rest had three or more.
Each simulation day agents moved amongst the region’s workplaces, schools, and community locations, similar to the movements of actual people. The day of the week and agent characteristics governed their movement and interaction with each other. A method modified from that developed by Beckman, et al. helped extract the agent population from U.S. Census Bureau’s Public Use Microdata files (PUMs) and Census aggregated data.12, 13 The following data sources generated school and workplace locations and assignments: U.S. Department of Education National Center for Education Statistics (public schools data), private data vendor (private schools), U.S. Census Standard Tabulation Product (STP64) commuting pattern data, and ESRI Business Analyst (InfoUSA business data).
Disease Parameters and Model Calibration
Previous MIDAS models provided disease parameters and assumptions.8, 9, 11, 14–18 In each individual, disease progressed through an underlying SEIR disease model. At the start of each simulation run, individuals who have already been vaccinated or infected (recently or remotely) began recovered (R) from and therefore immune to infection. All other individuals were initially susceptible (S) to influenza. The start of each simulation run involved introducing an infectious seed (a set of 100) randomly chosen infectious agents) to generate the epidemic. Every susceptible individual who contacted an infectious individual had a probability of contracting influenza. 8, 19 Table 1 lists these probabilities. A newly infected agent then progressed into the exposed (E) state where the agent remained for the duration of the disease's incubation period and then to the infectious state (I) where the person could infect others. Agents remained in the infectious state for a period of 4–7 days.20 Half of infectious patients manifested symptoms, while the other half remained asymptomatic but could still transmit disease. An infected agent remained infectious for the duration of the infectious period before transitioning into the recovered state (R).
TABLE 1.
Model transmission and person-to-person contact parameter values
| Transmission parameters | |||
|---|---|---|---|
| Contact group | Infected | Susceptible | Transmission probability18a |
| Household | Adult | Adult | 0.4 |
| Household | Child | Adult | 0.3 |
| Household | Adult | Child | 0.3 |
| Household | Child | Child | 0.6 |
| Elementary School | Student | Student | 0.0435 |
| Middle School | Student | Student | 0.0375 |
| High School | Student | Student | 0.0315 |
| Workplace | Adult | Adult | 0.0575 |
| Hospital | HCW | HCW | 0.0575 |
| Hospital | HCW | Patient | 0.01 |
| Hospital | Patient | HCW | 0.01 |
| Community | All | Child | 0.0048 |
| Community | All | Adult | 0.0048 |
| Social network parameters | |||
|---|---|---|---|
| Name | Participant | Contacts per day (M)b |
Social network |
| Classroom | Teachers | 15 | School |
| Classroom | Students | 15 | School |
| School outside of classrooms |
Students | 13.5 | School |
| School Outside of school | Student | 16.2 | Community |
| Weekend activity | Student | 24.1 | Community |
| Per office | Worker | 8 | Workplace |
| Per firm | Worker | 2 | Workplace |
| Community | All (including students) |
32.4 | Community |
| Per Hospital/Clinic | HCW | 2 | Workplace |
| ward | |||
| Per Hospital/Clinic building |
HCW | 8 | Workplace |
| Doctor seeing Patient | HCW that sees patients |
30 | Workplace |
Represent the transmission probability for both symptomatic and asymptomatic infections
Estimated by ABM model
Initial model calibration utilized the approach employed by Ferguson et al., Halloran, et al., and Lee, et al., and targeted an epidemic with an R0 of 1.4 (AR = 33%) as seen in the 1957–1958 pandemic.8, 9, 11, 15, 19 Additional runs used an R0 of 1.2 and 1.7, corresponding to attack rates of 19% and 38% respectively. The base-case scenario used the following assumptions from previous studies: 20% of working adults work on weekends, 50% of symptomatic students and workers stayed home with no community contacts unless they saw doctor, and 40% of symptomatic patients visited a clinic or emergency department.8, 9, 21–25
Modeling Possible Third-Wave Mechanisms
Modeling the third-wave mechanisms entailed adjusting the following model components in various degrees, ways, and combinations:
Daily Reproductive rate (R0): The reproductive rate is the expected number of additional new cases that a single infectious individual would generate if he or she entered a fully susceptible population, and can be adjusted to different levels each day.26 The R0 is directly related to the transmissibility of the virus.
-
Cross-Protection (x): Cross-protection is the degree to which a previously infected individual is immune to subsequent infection. When cross-protection is 100%, all previously infected individuals remain Recovered and are immune to additional infection.
Lowering the level of this variable allows previously infected individuals to be infected again. If x% is the degree of cross-protection then each previously infected individual has a (1–x)% chance of being infected during an effective contact with an infectious individual.
Opening and closing locations: Schools and workplaces can be opened and closed each day. When they are closed, agents that normally go to these locations and mix with each other instead stay at home and increase their community contacts by 20%.24
The third-wave mechanisms were as follows:
-
Seasonal Forcing: Seasonal forcing entails a gradual increase in viral transmissibility as time progresses from the autumn to the winter. The exact mechanism and degree to which this may happen is unclear. To model gradually changing viral transmissibility, an equation was selected that would generate a gradually sloping curve and contain parameters which can allow us to alter the amplitude and slope of the curve. Previous studies used sine/cosine functions to model seasonality.7, 27 Similarly, the current study employed the following sine wave function of time (t) is:
where:
-
○
A, the amplitude, is the peak deviation of the function from its center position
-
○
ω, the angular frequency, specifies how many oscillations occur in a unit time interval, (in radians per unit of time)
-
○
φ, the phase, specifies where in its cycle the oscillation begins at t = 0
For model simulations Y(t) represents the transmission multiplier correction that describes the increase or decrease in seasonality as a function of time. The cycle (angular frequency) was the number of days in 1 year (ω = 1/365), the amplitude (A) was the increase/decrease from the initial R0 level to the maximum R0 level and the phase shift (φ) is the time (in days) that the seasonality multiplier begins to increase/decrease.
-
○
Changes in Social Mixing: Closing and opening schools and workplaces allowed for the simulation of vacations and holidays that would affect agent movement and social contacts. For the purposes of simulating the November (Thanksgiving) and the winter holidays, simulation runs assumed that the epidemic began September 1, 2009, correlating to the second wave of the 2009 H1N1 pandemic. Additional scenarios explored the effects of increasing the amount of social mixing during the December holidays, a time when contact between school-aged children and the elderly increases sharply. This simulated a relaxation of voluntary social distancing during the heart of the first wave.
Viral Adaptation: The influenza virus progressively changes so that it is more transmissible (increasing R0), overcomes existing immunity (loss of cross-protection), or both.
Immune Escape Variant: Either the original virus mutates substantially to become an effectively different virus or a second completely distinct virus enters into the population. This mechanism can both increase R0 and decrease cross-protection.
Vaccination
Vaccination schedules were then implemented in each of the third-wave scenarios to determine whether vaccination could mitigate the third wave. Each newly vaccinated susceptible (S) individual had a probability (i.e., vaccine efficacy) of becoming recovered (R). Efficacy of the one-dose vaccine was 80% for those aged ≥10 years and 50% for those aged <10 years. Efficacy after a second vaccine dose was 80% for those aged <10 years.28, 29 Additional sensitivity scenarios explored the effect of ranging vaccine efficacy down to 50%. Each dose took 2 weeks after administration to achieve its effects. While the recommended interval between the first and second dose of vaccine is approximately 4 weeks (21 or more days is considered acceptable) for children aged 6 months to 9 years, the ideal timeframe may vary on an individual basis.30 Moreover, some children will never receive a second dose of the vaccine.
Different scenarios commenced vaccination at different points in the epidemic (8 weeks before the fall wave peak, 4 weeks prior to the peak, and concurrently with the peak). Sensitivity analyses explored changing the rates at which vaccination occurred (vaccination of the population was completed after 30, 90, and 180 days). The base case assumed 50% vaccine coverage; additional simulations ranged coverage down to 30% and 10% to span the values reported during the H1N1 pandemic.1 Separate scenarios also explored prioritizing versus not prioritizing the ACIP recommended priority groups.10
Computational Specifics
The ABM was programmed in C++. Simulations were performed at the Pittsburgh Supercomputing Center on Axon, an Intel Xeon-based Infiniband cluster.
RESULTS
All of the presented epidemic curves are the averages of 20 simulation runs that resulted in epidemics (i.e., the virus persists in the population for at least 20 days and infects at least 1,000 individuals) after seeding the population with 100 randomly infected individuals. The continuous lines in the figures represent 4-day moving average trend lines, which smooth out irregular patterns produced by the weekend effect (i.e., students and workers having different weekend contact patterns).
Third-Wave Scenarios
The simulations assumed that the seeded epidemic was a second wave, such as occurred with H1N1 in the fall of 2009. The following combinations of mechanisms generated an additional epidemic wave thereafter:
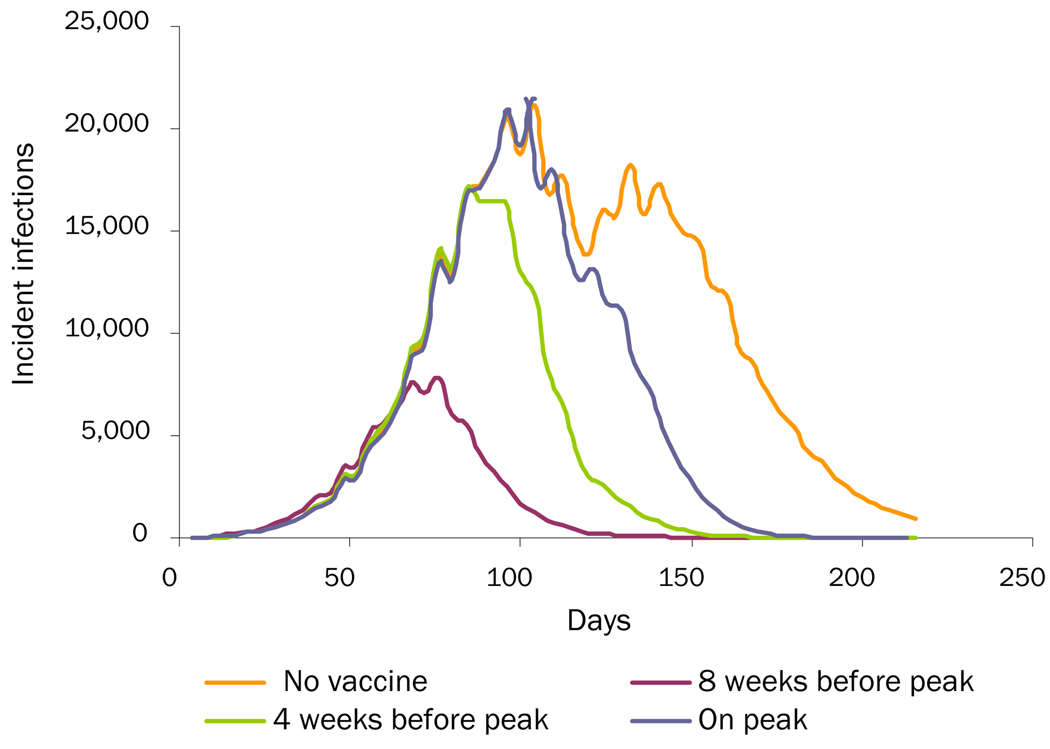
Increasing Viral Transmissibility: Baseline scenarios began the influenza virus as a R0 = 1.2 (AR = 19%) strain which then changes to a R0 = 2.0 on Day 120, as this is the lower limit estimated for the 2009 H1N1 pandemic.31 Additional scenarios began the influenza virus as R0= 1.4 and R0= 1.7 strains.
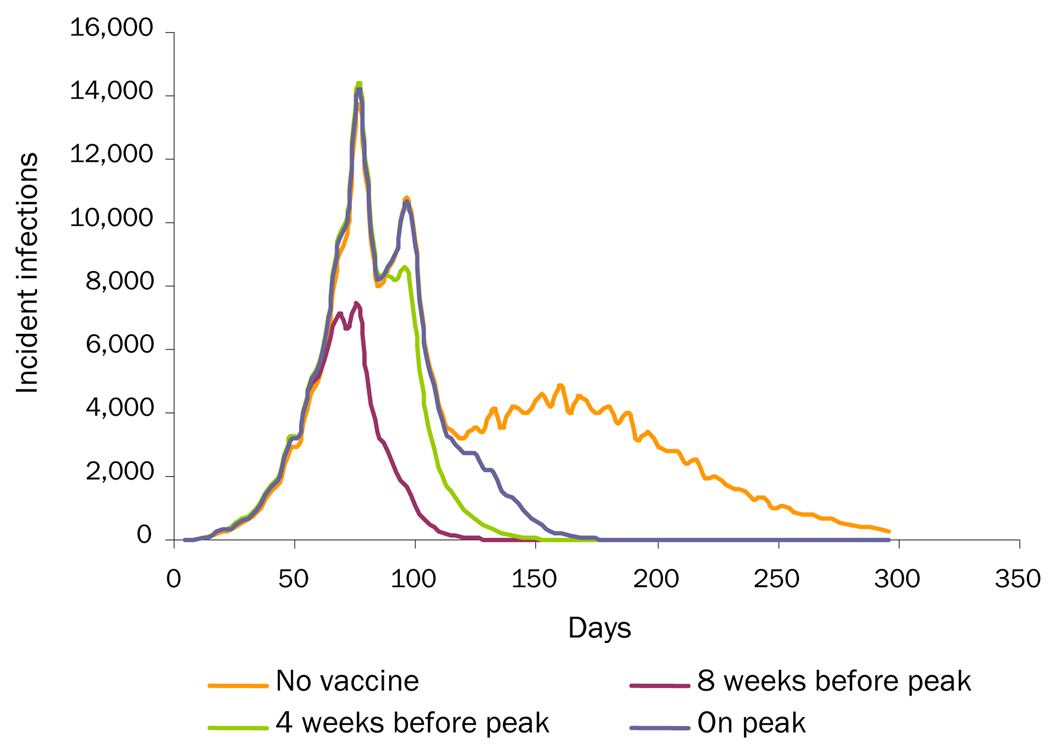
Loss of Cross-Protection: the influenza virus begins as a R0 = 1.2. On Day 120, a new strain, against which the individuals have 25% immunity, enters the population. This new strain also has a R0 = 1.2. Additional scenarios began the influenza virus as R0= 1.4 and R0= 1.7 strains.
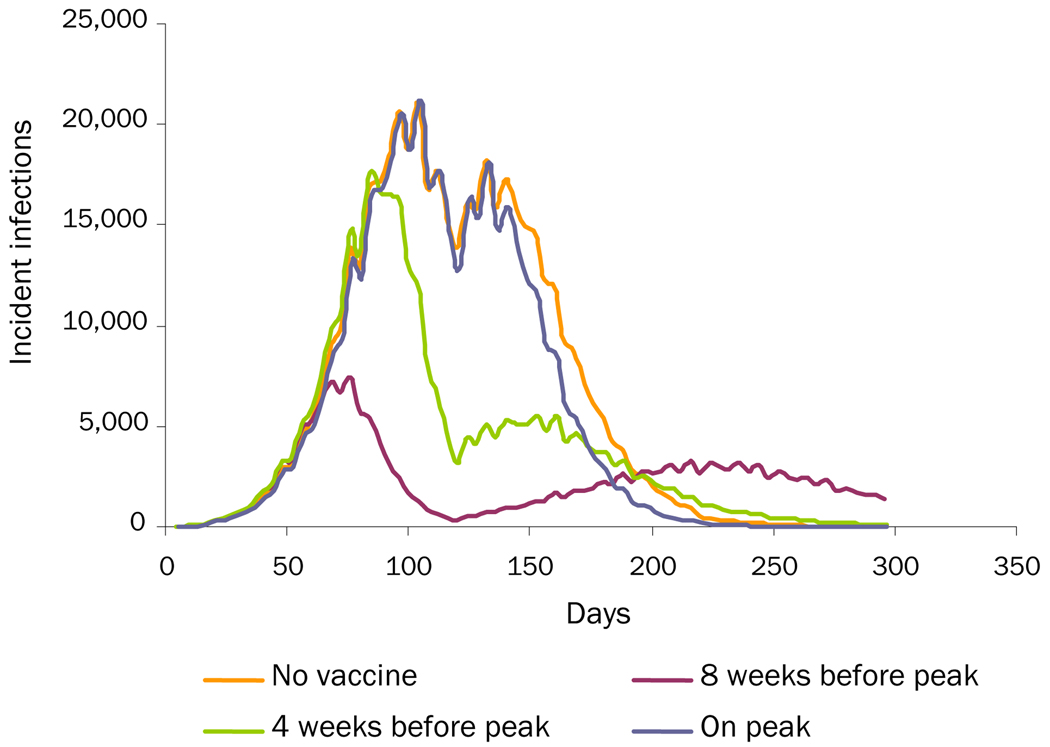
Change in Social Mixing: the influenza virus has a R0 = 1.2. Assuming a September 1 start of the epidemic, schools close during Thanksgiving (Wednesday, November 25, 2009, through Sunday, November 30, 2009) and the winter holidays (from Saturday, December 19, 2009 through Sunday, January 4, 2010). During school closure, students continue to mix with other students and adults in the community. Additional scenarios began the influenza virus as R0= 1.4 and R0= 1.7 strains.
Table 2 shows the effects of the various third-wave scenarios of overall and age-specific attack rates. Increasing the initial R0 to 1.4 and 1.7 increased the overall attack rate but decreased the additional wave attack rates, since increasing the R0 consumes susceptible individuals faster, leaving fewer susceptible individuals to maintain an additional wave. For the increased-viral-transmissibility scenario, an unmitigated epidemic that starts at R0=1.4 then increases to R0=2.0 generates an overall attack rate of 31.0% (0.5% additional wave) and one that starts at R0=1.7 generates an overall attack rate of 38.2% (0.1% additional wave). For the loss-of-cross-protection scenario, an unmitigated epidemic that starts at R0=1.4 generates an overall attack rate of 45.0% (14.6% additional wave) and one that starts at R0=1.7 generates an overall attack rate of 56.1% (18.0% additional wave). For the changes-in-social-mixing scenario, an unmitigated epidemic that starts at R0=1.4 generates an overall attack rate of 28.23% (0.26% additional wave) and one that starts at R0=1.7 generates an overall attack rate of 37.43% (0.04% additional wave).
TABLE 2.
Results from 90-day vaccination scenarios with no prioritization of ACIP groups
| Attack rate by age group (years) |
|||||||
|---|---|---|---|---|---|---|---|
| Total attack rate (%) |
≤ 4 (%) |
5–18 (%) |
19–24 (%) |
25–49 (%) |
50–64 (%) |
≥ 65 (%) |
|
| Increasing-viral-transmissibility | |||||||
| scenarios | |||||||
| No vaccination | 24.9 | 39.0 | 44.0 | 26.0 | 19.0 | 16.0 | 14.0 |
| Vaccination initiated: | |||||||
| 8 weeks before peak | 3.0 | 5.0 | 6.0 | 3.0 | 2.0 | 2.0 | 1.0 |
| 4 weeks before peak | 9.2 | 15.0 | 19.0 | 9.0 | 6.0 | 5.0 | 5.0 |
| At peak | 15.3 | 25.0 | 30.0 | 15.0 | 11.0 | 9.0 | 8.0 |
| Loss-of-cross-protection scenarios | |||||||
| No vaccination | 26.7 | 43.0 | 51.0 | 26.0 | 19.0 | 16.0 | 14.0 |
| Vaccination initiated: | |||||||
| 8 weeks before peak | 7.5 | 12.0 | 16.0 | 7.0 | 5.0 | 4.0 | 4.0 |
| 4 weeks before peak | 13.0 | 21.0 | 27.0 | 12.0 | 9.0 | 7.0 | 6.0 |
| At peak | 20.7 | 34.0 | 41.0 | 20.0 | 14.0 | 12.0 | 10.0 |
| Change-in-social-mixing scenarios | |||||||
| No vaccination | 26.7 | 22.0 | 24.0 | 14.0 | 10.0 | 8.0 | 7.0 |
| Vaccination initiated: | |||||||
| 8 weeks before peak | 2.6 | 4.0 | 5.0 | 2.0 | 2.0 | 1.0 | 1.0 |
| 4 weeks before peak | 6.0 | 10.0 | 11.0 | 6.0 | 4.0 | 4.0 | 3.0 |
| At peak | 7.2 | 12.0 | 12.0 | 8.0 | 5.0 | 5.0 | 4.0 |
SEs for all attack rates were < 1%.
Of note, for two of the scenarios, the age distribution of cases changed from the initial wave (younger) to the additional wave (older). In the changing-viral-transmissibility scenario, the age distribution of cases for the initial wave versus the next wave was as follows: 11.7% vs 10.7% (aged ≤4 years), 40.4% vs 32.2% (aged 5–18 years), 6.2% vs 7.1% (aged 19–24 years), 27.6% vs 32.9% (aged 25–49 years), 9.1% vs 11.02% (aged 50–64 years), and 4.9% vs 5.9% (aged ≥65 years). In the loss-of-cross-protection scenario, the age distribution of cases for the initial wave versus the next wave was as follows: 11.8% vs 11.3% (aged ≤4 years), 40.5% vs 37.8% (aged 5– 18 years), 6.2% vs 6.5% (aged 19–24 years), 27.5% vs 29.4% (aged 25–49 years), 9.1% vs 9.8% (aged 50–64 years), and 4.9% vs 5.2% (aged ≥65 years). This is primarily because children, who have high levels of mixing, are infected early in the epidemic, leaving an increased number of older individuals as susceptibles. However, for the changing-social-mixing scenario, the age distribution grew slightly younger: 11.7% vs 11.7% (aged ≤4 years), 34.7% vs 39.5% (aged 5–18 years), 7.1% vs 6.3% (aged 19–24 years), 30.9% vs 28.1% (aged 25–49 years), 10.2% vs 9.4% (aged 50–64 years), and 5.4% vs 5.0% (aged ≥65 years). This is because changes in social-mixing patterns are more likely to affect school-aged children who are high mixers, particularly at schools.32
The Effects of Vaccination in Mitigating Third Waves
Table 2 and Figures 1–3 show the effects of vaccination on the different "third-wave" scenarios. As can be seen, vaccination is effective in mitigating all three additional wave scenarios, even when vaccination takes as long as 180 days to complete (assuming 50% vaccine coverage). In all cases, initiating vaccination earlier and increasing the level of vaccination can further decrease the overall attack rate. Figure 2 (the loss-of-cross-protection scenario) illustrates how vaccination can mitigate the intensity of an additional wave caused by a new virus strain against which the vaccine has only 75% of baseline vaccine efficacy. Table 2 shows results when ACIP-recommended groups are not prioritized for immunization. (Simulation runs that vaccinated ACIP priority groups first, especially school-aged children who are high mixers, achieved greater mitigation of an additional wave: an up to 0.5% additional 0%–1% decrease in the overall attack rates.) Table 2 shows results when it takes 3 months to achieve target coverage of the population. Decreasing this time to 30 days results in an additional 1.1%–2.8% decrease in the increased-viral-transmission scenario, a 1.1%–2.4% decrease in the loss-of-cross-protection scenario, and a 0.2%–1.1% decrease in the change-in-social-mixing scenario in the overall attack rates. Increasing the time to 180 days boosts the attack rates by 1.9%–2.6% in the increased-viral-transmission scenario, 2.2%–3.9% in the loss-of-cross-protection scenario, and a 0.5%–1.5% in the change-in-social-mixing scenario.
Figure 1.
Increased-Viral-Transmissibility Scenario: Comparing Three Vaccination-Timing Scenarios with No Vaccination
Figure 3.
Change-in-Social-Mixing Scenario: Comparing Three Vaccination-Timing Scenarios with No Vaccination
Figure 2.
Loss-of-Cross-Protection Scenario: Comparing Three Vaccination-Timing Scenarios with No Vaccination
Ranging vaccine efficacy down to 50% and vaccine coverage down to 30% did not significantly affect the current results; however, further decreasing vaccine coverage to 10% did decrease the effectiveness of the vaccination program: increasing (compared to when vaccine coverage was 30%) the overall attack rate by 5.41% and the additional wave attack rate by 5.35% in the increased-viral-transmissibility scenario, 4.3% and 4.1% in the loss-of-cross-protection scenario, and 0.68% and 0.90% in the change-in-social-mixing scenario, respectively.
Vaccination in higher R0 scenarios (i.e., 1.4 and 1.7) had some varying effects. For the increased-viral-transmissibility scenario, since increasing the initial R0 substantially reduced the size of the additional wave, vaccination had little effect. For the loss-of-cross-protection scenario, initiating vaccinating 4 weeks prior to the first peak (completing the vaccination program in 3 months) in an epidemic that starts at R0=1.4 then increases to R0=2.0 cuts the overall attack rate from 45.0% to 25.5% (the additional wave from 14.6% to 3.3%). In one that starts at R0=1.7, vaccination cuts the overall attack rate from 56.1% to 45.0% (the additional wave from 18.0% to 12.4%). For the changes-in-social-mixing scenario, initiating the same type of vaccination program in an epidemic that starts at R0=1.4 cuts the overall attack rate from 28.23% to 21.51% (the additional wave from 0.26% to 0.01%). In one that starts at R0=1.7, vaccination cuts the overall attack rate from 37.43% to 32.34% (the additional wave from 0.04% to 0%).
DISCUSSION
The study delineated circumstances under which vaccination after an epidemic peak still confers substantial benefit. A vaccination program initiated too late to affect an epidemic's initial wave could still mitigate a possible additional wave even when the virus adapts or mutates considerably. This supports continuing a vaccination program during the waning of an epidemic, as in December 2009–January 2010. Although vaccinating a population before an epidemic begins is ideal, public health decision makers should not rule out initiating a vaccination program when they know that the vaccine will not arrive in time. Study results also suggest that adhering to vaccinating ACIP priority individuals first, even late in a pandemic, may be beneficial.
Covering an entire population before an epidemic begins is very difficult, as getting new vaccines developed, tested, approved, distributed, and administered in a very short time frame is a prodigious task. This was clearly in evidence during the 2009 H1N1 influenza pandemic. Despite swift decision making in the spring of 2009, the first vaccines did not arrive until October 2009 after the fall wave had started in August–September 2009. Vaccine production and arrival was much more prompt than in past pandemics. Inactivated influenza vaccines were first available in the U.S. in 1945 and production still requires 3–6 months after strain selection, which makes the production of a new monovalent vaccine in time for use in a pandemic challenging.33 Historical records suggest that vaccination in previous pandemics was limited due to vaccine shortages prior to the epidemic peak combined with poor vaccine uptake.34 In 1957, the new monovalent vaccine was available in small amounts by the end of August and not widely available until after October when the epidemic had already had peaked.3 Similarly, in 1968–1969, the vaccine strain was identified only 4 months before the peak of the epidemic in the U.S..34 Therefore, until the vaccine development process is substantially compressed, public health decision makers may face similar situations in the future.
Although this study showed the potential benefit of continuing a vaccination program, the exact expected value cannot be quantified since the likelihood of the proposed additional wave mechanisms is not known. Previous pandemics have demonstrated that additional waves can occur, but the causes of these waves remain unclear. Social-mixing data are limited. Failure to see additional waves in the 1937 H1N1 Manchester epidemic may have been due to the social distancing measures (e.g., quarantine) implemented.35 Studies suggest that seasonal forcing alone can cause approximately a 20% increase in transmissibility.36 Moreover, it is not clear how much of the impact of seasonal forcing is on the host versus the pathogen.37 In simulation experiments, viral adaptation and mutation were able to readily generate additional waves. However, to date, studies have not revealed convincing evidence of notable mutation during previous pandemics. There is a scant amount of molecular data on the evolution of the 1918 H1N1 strain during its first decade.38, 39 Although extensive phylogenetic analysis of the H2N2 1957 pandemic strain lineage revealed that the original strain diverged into two distinct co-circulating clades within 8– 10 years of its introduction, it has not shown that any of these changes occurred in 1957–1958.40, 41 Given that the 2009 H1N1 virus received nearly all of its genes (except PB1) from viruses of recent non-human origin, it would not be surprising to see evolutionary rates exceeding those observed for seasonal H3N2 and H1N1.42, 43 On the other hand, no notable antigenic shift has been detected so far in the evolution of H1N1 (2009), suggesting that reassortment of the surface glycoproteins similar to that observed during the first years of H2N2 is less likely.
Limitations
All computer models are simplifications of reality and can never account for every possible factor or interaction. Rather than make decisions, computer models provide information to decision makers about possible scenarios and relationships. An influenza pandemic and the resulting circumstances may not necessarily conform to the data and assumptions that the model drew from referenced sources or previously published models.
Conclusion
This study identified potential mechanisms for a multiple-wave epidemic and demonstrated how vaccination can mitigate additional waves, thereby supporting the continuation of a vaccination program even when an epidemic appears to be waning, as in December 2009–January 2010. Although vaccinating a population before an epidemic begins is ideal, public health decision makers should not rule out initiating a vaccination program even though they know that the vaccine will not arrive in time to affect the initial wave of an epidemic.
Uncertainties remain for decision making by public health officials on the question of dedicating resources and credibility to the H1N1 vaccination program. However, these simulations do lend support for continuation of the program and to continued public education on the benefits of receiving the vaccine.
ACKNOWLEDGEMENT
This study was supported by the National Institute of General Medical Sciences Models of Infectious Disease Agent Study (MIDAS) grant 1U54GM088491-0109. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
RKZ has received a research contract for employer vaccination from Medimmune, one of the many manufacturers of influenza vaccine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No other financial disclosures were reported by the authors of this paper.
REFERENCES
- 1.CDC. Interim Results: Influenza A (H1N1) 2009 Monovalent Vaccination Coverage—U.S., October–December 2009. 2010 [PubMed] [Google Scholar]
- 2.CDC. Interim Results: Influenza A(H1N1) 2009 Monovalent and Seasonal Influenza Vaccination Coverage Among Health-Care Personnel—U.S., August 2009–January 2010. 2010 [PubMed] [Google Scholar]
- 3.Henderson DA, Courtney B, Inglesby TV, Toner E, Nuzzo JB. Public health and medical responses to the 1957–58 influenza pandemic. Biosecur Bioterror. 2009;7(3):265–273. doi: 10.1089/bsp.2009.0729. [DOI] [PubMed] [Google Scholar]
- 4.Rios-Doria D, Chowell G. Qualitative analysis of the level of cross-protection between epidemic waves of the 1918–1919 influenza pandemic. J Theor Biol. 2009;261(4):584–592. doi: 10.1016/j.jtbi.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 5.Kilbourne ED. Influenza pandemics of the 20th century. Emerg Infect Dis. 2006;12(1):9–14. doi: 10.3201/eid1201.051254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller MA, Viboud C, Balinska M, Simonsen L. The signature features of influenza pandemics—implications for policy. N Engl J Med. 2009;360(25):2595–2598. doi: 10.1056/NEJMp0903906. [DOI] [PubMed] [Google Scholar]
- 7.Shi P, Keskinocak P, Swann JL, Lee BY. Modelling seasonality and viral mutation to predict the course of an influenza pandemic. Epidemiol Infect. 2010:1–10. doi: 10.1017/S0950268810000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferguson NM, Cummings DA, Fraser C, Cajka JC, Cooley PC, Burke DS. Strategies for mitigating an influenza pandemic. Nature. 2006;442(7101):448–452. doi: 10.1038/nature04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halloran ME, Ferguson NM, Eubank S, Longini IM, Jr, Cummings DA, Lewis B, et al. Modeling targeted layered containment of an influenza pandemic in the U.S. Proc Natl Acad Sci U S A. 2008;105(12):4639–4644. doi: 10.1073/pnas.0706849105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Center for Immunization and Respiratory Diseases C. Use of Influenza A (H1N1) 2009 Monovalent Vaccine Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. Atlanta: 2009. [PubMed] [Google Scholar]
- 11.Lee BY, Brown ST, Cooley PC, Zimmerman RK, Wheaton WD, Zimmer SM, et al. A Computer Simulation of Employee Vaccination to Mitigate an Influenza Epidemic. Am J Prev Med. 2009 doi: 10.1016/j.amepre.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wheaton WD, Cajka JC, Chasteen BM, Wagener DK, Cooley PC, Ganapathi L, et al. Synthesized population databases: A U.S. geospatial database for agent-based models. RTI Press. 2009 doi: 10.3768/rtipress.2009.mr.0010.0905. Publication No. MR-0010-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beckman RJ, Baggerly K, McKay M. Creating synthetic baseline populations. Transportation Research Part A: Policy and Practice 14 January. 1996;30(6):415–429. [Google Scholar]
- 14.Eubank S, Guclu H, Kumar VS, Marathe MV, Srinivasan A, Toroczkai Z, et al. Modelling disease outbreaks in realistic urban social networks. Nature. 2004;429(6988):180–184. doi: 10.1038/nature02541. [DOI] [PubMed] [Google Scholar]
- 15.Ferguson NM, Cummings DA, Cauchemez S, Fraser C, Riley S, Meeyai A, et al. Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature. 2005;437(7056):209–214. doi: 10.1038/nature04017. [DOI] [PubMed] [Google Scholar]
- 16.Germann TC, Kadau K, Longini IM, Jr, Macken CA. Mitigation strategies for pandemic influenza in the U.S. Proc Natl Acad Sci U S A. 2006;103(15):5935–5940. doi: 10.1073/pnas.0601266103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Longini IM, Jr, Halloran ME, Nizam A, Yang Y. Containing pandemic influenza with antiviral agents. Am J Epidemiol. 2004;159(7):623–633. doi: 10.1093/aje/kwh092. [DOI] [PubMed] [Google Scholar]
- 18.Longini IM, Jr, Nizam A, Xu S, Ungchusak K, Hanshaoworakul W, Cummings DA, et al. Containing pandemic influenza at the source. Science. 2005;309(5737):1083–1087. doi: 10.1126/science.1115717. [DOI] [PubMed] [Google Scholar]
- 19.Lee BY, Brown ST, Cooley P, Potter MA, Wheaton WD, Voorhees RE, et al. Simulating School Closure Strategies to Mitigate an Influenza Epidemic. J Public Health Manag Pract. 2009 doi: 10.1097/PHH.0b013e3181ce594e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ling LM, Chow AL, Lye DC, Tan AS, Krishnan P, Cui L, et al. Effects of early oseltamivir therapy on viral shedding in 2009 pandemic influenza A (H1N1) virus infection. Clin Infect Dis. 2010;50(7):963–969. doi: 10.1086/651083. [DOI] [PubMed] [Google Scholar]
- 21.Rothberg MB, Rose DN. Vaccination versus treatment of influenza in working adults: a cost-effectiveness analysis. Am J Med. 2005;118(1):68–77. doi: 10.1016/j.amjmed.2004.03.044. [DOI] [PubMed] [Google Scholar]
- 22.Lee BY, Tai JH, Bailey RR, Smith KJ. The timing of influenza vaccination for older adults (65 years and older) Vaccine. 2009;27(50):7110–7115. doi: 10.1016/j.vaccine.2009.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beigi RH, Wiringa AE, Bailey RR, Assi TM, Lee BY. Economic value of seasonal and pandemic influenza vaccination during pregnancy. Clin Infect Dis. 2009;49(12):1784–1792. doi: 10.1086/649013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bureau of Labor Statistics. American Time Use Survey—2008 Results. U.S. Department of Labor; 2009. [Google Scholar]
- 25.Lee BY, Tai JH, Bailey RR, Smith KJ, Nowalk AJ. Economics of influenza vaccine administration timing for children. Am J Manag Care. 2010;16(3):e75–e85. [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson RMMR, Anderson B. Infectious Diseases of Humans. Oxford University Press; 1992. [Google Scholar]
- 27.Casagrandi R, Bolzoni L, Levin SA, Andreasen V. The SIRC model and influenza A. Math Biosci. 2006;200(2):152–169. doi: 10.1016/j.mbs.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 28.National Institute of Allergy and Infectious Disease. Early Results: In Children, 2009 H1N1 Influenza Vaccine Works Like Seasonal Flu Vaccine.: NIH. 2009
- 29.National Institute of Allergy and Infectious Disease. Updated Results: In Youngest Children, a Second Dose of 2009 H1N1 Influenza Vaccine Elicits Robust Immune Response: NIH. :2009.
- 30.CDC. Recommendations for Vaccine against 2009 H1N1 Influenza Virus. 2009 Available from: http://www.cdc.gov/h1n1flu/vaccination/public/vaccination_qa_pub.htm.
- 31.WHO. Transmission dynamics and impact of pandemic influenza A (H1N1) 2009 virus. Weekly Epidemiological Record. 2009:46. [PubMed]
- 32.Olson DR, Simonsen L, Edelson PJ, Morse SS. Epidemiological evidence of an early wave of the 1918 influenza pandemic in New York City. Proc Natl Acad Sci U S A. 2005;102(31):11059–11063. doi: 10.1073/pnas.0408290102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bridges CB, Katz JM, Levandowski RA, Cox NJ. In: Vaccines. Fifth ed. Plotkin S, Orenstein W, Offit P, editors. Elsevier; 2008. pp. 259–290. [Google Scholar]
- 34.Murray R. Production and testing in the USA of influenza virus vaccine made from the Hong Kong variant in 1968–69. Bull WHO. 1969;41(3):495–496. [PMC free article] [PubMed] [Google Scholar]
- 35.Martin AE. A serological investigation into the epidemiology of influenza with particular reference to sporadic cases. Journal of Hygiene. 1940;40(1):104–114. doi: 10.1017/s0022172400027674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaman J, Pitzer VE, Viboud C, Grenfell BT, Lipsitch M. Absolute humidity and the seasonal onset of influenza in the continental U.S. PLoS Biol. 2010;8(2):e1000316. doi: 10.1371/journal.pbio.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hope-Simpson RE. The role of season in the epidemiology of influenza. J Hyg (Lond) 1981;86(1):35–47. doi: 10.1017/s0022172400068728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen J, Ma J, Wang Q. Evolutionary trends of A(H1N1) influenza virus hemagglutinin since 1918. PLoS One. 2009;4(11):e7789. doi: 10.1371/journal.pone.0007789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelson MI, Viboud C, Simonsen L, Bennett RT, Griesemer SB, St George K, et al. Multiple reassortment events in the evolutionary history of H1N1 influenza A virus since 1918. PLoS Pathog. 2008;4(2):e1000012. doi: 10.1371/journal.ppat.1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindstrom SE, Cox NJ, Klimov A. Genetic analysis of human H2N2 and early H3N2 influenza viruses, 1957–1972: evidence for genetic divergence and multiple reassortment events. Virology. 2004;328(1):101–119. doi: 10.1016/j.virol.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 41.Nakao HNK, Nakajima S. Location on the evolutionary trees of the non-structural protein (NS) and neuraminidase (NA) genes of late human influenza A (H2N2) viruses: parental viruses of the NS and NA genes of Hong Kong influenza A (H3N2) viruses. J Gen Virol. 1993;74(Aug):1667–1672. doi: 10.1099/0022-1317-74-8-1667. [DOI] [PubMed] [Google Scholar]
- 42.Shinde V, Bridges CB, Uyeki TM, Shu B, Balish A, Xu X, et al. Triple-reassortant swine influenza A (H1) in humans in the U.S., 2005–2009. N Engl J Med. 2009;360(25):2616. doi: 10.1056/NEJMoa0903812. [DOI] [PubMed] [Google Scholar]
- 43.Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56(1):152–179. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]