Abstract
The concept of liver transplantation is a relatively recent one. The first descriptions of liver replacement in experimental animals were published less than 25 years ago,1,2 and the first attempt at clinical liver transplantation was not made until March 1, 1963.3 The pace of clinical trials remained limited until 1980, the year when the new immunosuppressive agent cyclosporine was introduced. In the following article, the way in which immunosuppress: on was developed before and after 1980 will be described, as well as the influence of other nonoperative factors that conspired to make liver transplantation practical. Surgical technical advances will be considered elsewhere in this issue of Seminars.
IMMUNOSUPPRESSION
Truly long survival after liver transplantation between outbred mongrel dogs was demonstrated more than 20 years ago in ten dogs under treatment with azathioprine who lived for 4 postoperative months.4 After this time, their drug therapy was discontinued. A number of these animals lived for long periods,5 and one did not die until more than 10 years later. A short time later, similar results were obtained with heterologous antilymphocyte serum (ALS) and its globulin derivative (ALG).6
The number of animals that survived chronically in these investigations was less than 10% of the total. Nevertheless, proof of the feasibility of liver replacment under these difficult laboratory conditions was the great stimulus for the first clinical trials. The first human liver recipient to survive for at least 1 year was operated on in the summer of 1967,7 and the longest survival of a patient is now 15½ years. This recipient, whose original disease was biliary atresia with an incidental hepatoma, was treated with azathioprine, and ALG.
IMMUNOSUPPRESSION BEFORE CYCLOSPORINE
Renal Transplantation
All of the immunosuppressive regimens that have made whole liver transplantation feasible were worked out with the simpler model of renal transplantation (Table 1), beginning in Boston in 1962 with the use of azathioprine as the sole or principal immunosuppressive agent.8 There were no long survivors, and since that time, it has been recognized that cadaver organ transplantation could rarely, if ever, be successful using azathioprine alone.
TABLE 1.
Immunosuppressive Drug Regimens and Adjuncts Initially Developed for Kidney Transplantation and Applied Later for Extrarenal Organs
| Agents |
Year Described or Reported |
Place | Deficiencies |
Used for Livers |
|---|---|---|---|---|
| Azathioprine | 19628 | Boston | Ineffective, dangerous | No |
| Azathioprine-steroids | 19639 | Denver | Suboptimal | Yes |
| Thoracic duct drainage as adjunct | 196321* | Stockholm | Nuisance: requires 20 to 30 days pretreatment |
Yes |
| ALG as adjunct | 19666 | Denver | Suboptimal | Yes |
| Cyclophosphamide substitute for azathioprine |
197020 | Denver | No advantage except for patients with azathioprine toxicity |
Yes |
| Total lymphoid irradiation | 197923 198424 |
Palo Alto, Minneapolis |
Dangerous; extensive preparation; not quickly reversible |
Yes |
| Cyclosporine alone | 1978–197927 | Cambridge | Suboptimal | Yes |
| Cyclosporine-steroids | 198029 | Denver | Under evaluation | Yes |
| Monoclonal ALG as adjunct | 198117 | Boston | Under evaluation | Yes |
It was not realized until much later that pretreatment for 3 to 4 weeks before transplantation was a necessary condition.22
In 1962 and 1963, it was demonstrated in renal transplant recipients that azathioprine and steroids had at least additive, if not synergistic, actions.9 This so-called double-drug therapy was adopted in three other centers10–12 and became the gold standard worldwide by 1964. However, satisfactory results then13 and for more than a decade were obtained only with living related donors. The morbidity and mortality from the transplantation of cadaveric kidneys were excessive and the rate of graft function at 1 year hovered at the 50% range for many years.14
Although the addition of ALG as a third and short-term immunosuppressive adjunct6,15 improved the results of renal transplantation in most centers in which this expedient was tried, the usefulness of ALG was limited. The drug could not be standardized, it had a number of undesirable side effects, and its discontinuance often was followed by rejection.5,15
There has been a resurgence of interest in ALG therapy, since it is now possible to raise potent and highly standardized antilymphoid antibodies with the monoclonal antibody techniques of Kohler and Milstein.16 The first trials with this improved product were carried out by Cosimi et al17 about 5 years ago using monoclonal antibodies raised against mature T-lymphocytes (T3). These studies and others that have followed have shown that otherwise intractable rejections of renal homografts often can be reversed with good monoclonal preparations.18,19 However, if maintenance therapy is being provided with azathioprine and prednisone, there is a very high probability of recurrence of rejection when the course of monoclonal therapy is completed.17–19
Other variations in immunosuppression between 1962 and 1979 are summarized in Table 1, including the substitution of cyclophosphamide for azathioprine,20 and the use of thoracic duct drainage21,22 or total lymphoid irradiation23,24 as an alternative to ALG for lymphoid depletion. None of these techniques has had a major impact on clinical transplantation.
Liver Transplantation
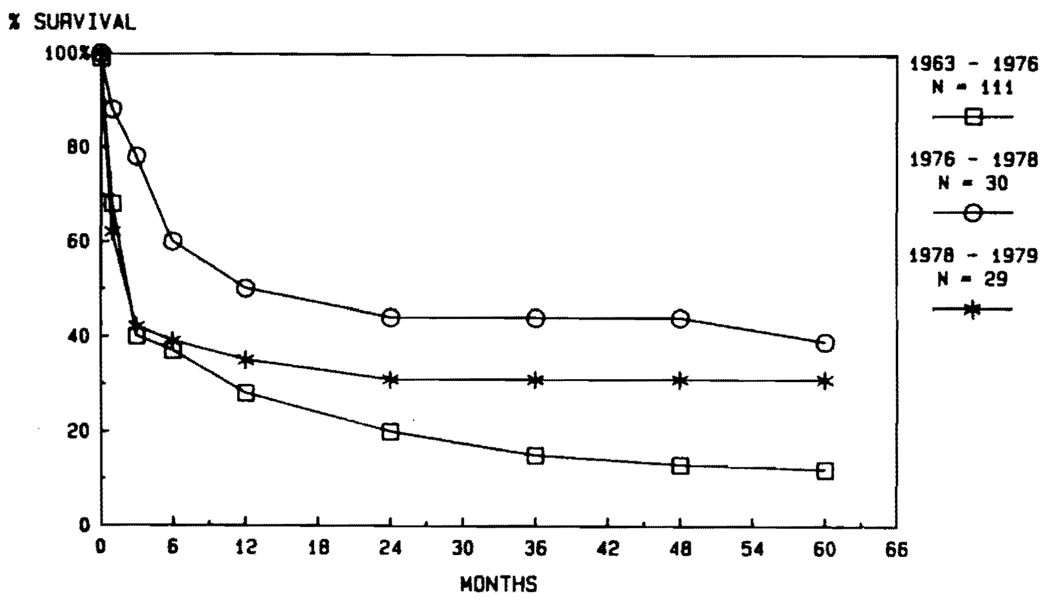
Most of our liver recipients from 1963 through 1979 had triple-drug immunosuppression with azathioprine, prednisone, and ALG. In some, cyclophosphamide was substituted for azathioprine, and in a few others, lymphoid depletion was achieved with thoracic duct drainage instead of ALG. Details of these variations are summarized elsewhere.25 None of the variations influenced survival after liver transplantation (Fig. 1). In the first trials from 1963 to 1976, only about one third of the patients lived for as long as 1 year. In a smaller second series of 30 patients treated form 1976 to 1978, the 1-year survival rose to 50%, but this improvement could not be sustained in the next 29 cases (Fig. 1).
FIG. 1. Results obtained over a 16-year period using the conventional immunosuppression regimens without cyclosporine, as shown in Table 1.
Note the failure to improve the results despite the acquisition of considerable technical experience.
IMMUNOSUPPRESSION WITH CYCLOSPORINE
In 1976, Borel et al26 reported studies in rodents of a new immunosuppressive agent called cyclosporin A. In late 1979, Calne and his associates27 reported the first major clinical experience with this drug.
Renal Transplantation
Calne et al27 noted that cyclosporin A had prolonged graft survival in almost half of the recipients of 32 kidneys, two livers, and two pancreases with no other immunosuppressive drug, an unprecedented achievement with a single agent. The publication was one of the most important in the history of clinical transplantation. Nevertheless, it contained three pieces of information so troubling that further clinical trials were jeopardized. First, three of the first 34 recipients had developed malignant lymphomas. Second, none of the kidney recipients had normal graft function. Third, there had been a high patient mortality. These adverse findings have been explained or minimized in subsequent trials.
Development of Lymphomas
The incidence of lymphomas in Calne and associates’27 first 32 kidney recipients raised the possibility that cyclosporin A had a unique capacity to produce lymphomas in humans, thus vitiating its value. Fortunately, the lymphoma threat became less and less ominous as information about the etiology and appropriate treatment of these lesions emerged. It became obvious that the lymphomas were caused by primary or secondary infection with the Epstein-Barr virus.28,29 Although it was speculated that lymphomas that produced a single immunoglobulin (monoclonality) already had become autonomous,28 this doctrine was overthrown.30 In a large number of patients followed by us, it was demonstrated that all of the cyclosporine lymphomas that developed early postoperatively could be expected to disappear spontaneously if immunosuppressive therapy was stopped, and often if treatment was only lightened. The regression occurred whether the lesions were polyclonal or monoclonal. In recipients of kidneys, livers, and hearts, reduction or discontinuance of immunosuppression was not necessarily followed by loss of the transplanted organ. Of seven of our kidney recipients in whom therapy with cyclosporine and steroids was stopped or drastically reduced, four retained their grafts, which have continued to function for 1½ to 3½ years subsequently. After the tumors had disappeared, immunosuppressive therapy at lower doses was reinstituted. Similar involution of lymphomas has been seen in several liver recipients.
The development of de novo malignancies in immunosuppressed patients is not unique to cyclosporine. It has been a well-known complication of therapy with azathioprine and prednisone (with or without ALG) since the 1960s.31,32 With conventional immunosuppression, there has been an extremely high incidence of epithelial cancers, which have outnumbered the lymphomas by a ratio of about 4 to 1.33 Under cyclosporine-steroid therapy, there has been little or no increase in the incidence of the epithelial tumors. Thus, the risk of the development of malignancies is probably considerably less with cyclosporine than with conventional immunosuppression, even if one considers the lymphomas to be true tumors, a concession that may not be valid.34
Cyclosporine Nephrotoxicity and Its Prevention
Of the kidney recipients first reported by Calne et al,27 none had normal renal function, a finding that they attributed to universal cyclosporin A nephrotoxicity. In retrospect, part of the problem was failure to distinguish rejection from drug toxicity.29 Nevertheless, many subsequent reports, including our own,29,35,36 have shown that nephrotoxicity is the most limiting side effect of cyclosporine.
The full exploitation of the drug was not possible without combining it with other agents, of which prednisone was the most important.29,36 By this polypharmacy approach, it was possible to control rejection better even though smaller and less nephrotoxic doses of cyclosporine were used. Since then, other drugs have been proposed or tried in modifications of the “pharmacologic cocktail” concept,37,38 but cyclosporine and steroids constitute the basic combination, to which other agents can be added. For example, cyclosporine and steroids can provide the baseline therapy to which one of the monoclonal ALG preparations that are undergoing preliminary clinical trials may be added. Our present opinion is that monoclonal ALG should be used to “rescue” patients in whom rejection cannot be controlled with cyclosporine-steroid therapy or in whom there are severe limitations for one reason or another to the amounts of cyclosporine that can be safely given. Such limitations are particularly important in applying knowledge about immunosuppression obtained from the kidney transplant model to the transplantation of other organs, such as the heart and liver, since secondary renal failure is common in patients with cardiac and hepatic disease, thereby complicating the use of cyclosporine.
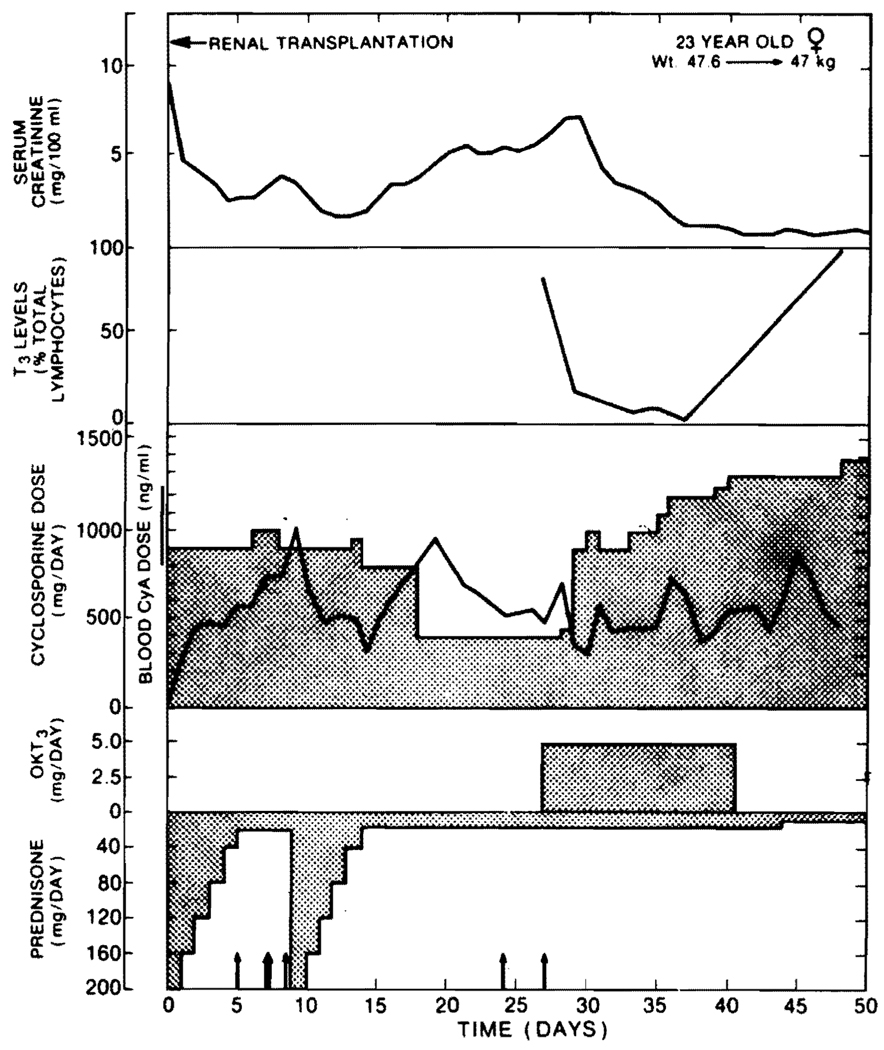
OKT3 monoclonal antibody therapy has been used in a number of our kidney recipients. If rejection has developed despite cyclosporine-steroid therapy, reversal with OKT3 antibody usually has been striking (Fig. 2). With the first dose of the monoclonal ALG, the circulating T-lymphocytes are practically eliminated (Fig. 2). Recurrence of rejection after the monoclonal antibody course has been completed has been far less common using baseline therapy with cyclosporine-steroids than previously reported with azathioprine-prednisone maintenance.17–19
FIG. 2. Course of a recipient of a kidney graft who developed inexorable renal rejection despite good blood levels of cyclosporine and despite a second burst of high-dose steroid therapy.
The rejection was immediately reversed with OKT3 therapy and with good function for the ensuing 8 months. Note the prompt reduction in circulating T-lymphocytes.
High Patient Mortality
The heavy mortality with the first use of cyclosporine27 apparently was a reflection of a learning experience in which cytotoxic drugs and steroids often were combined with cyclosporine with lethal effects. Even in our first trials with the far safer cyclosporine-steroid combination, the 1-year patient mortality following renal transplantation was 13.6%,39 but in the following year, the 1-year mortality was reduced to 2%.40 Since then, most groups using cyclosporine-steroid therapy have had a mortality of less than 5%. Cyclosporine-steroid therapy has been the safest of the therapeutic regimens yet tried.
Liver Transplantation
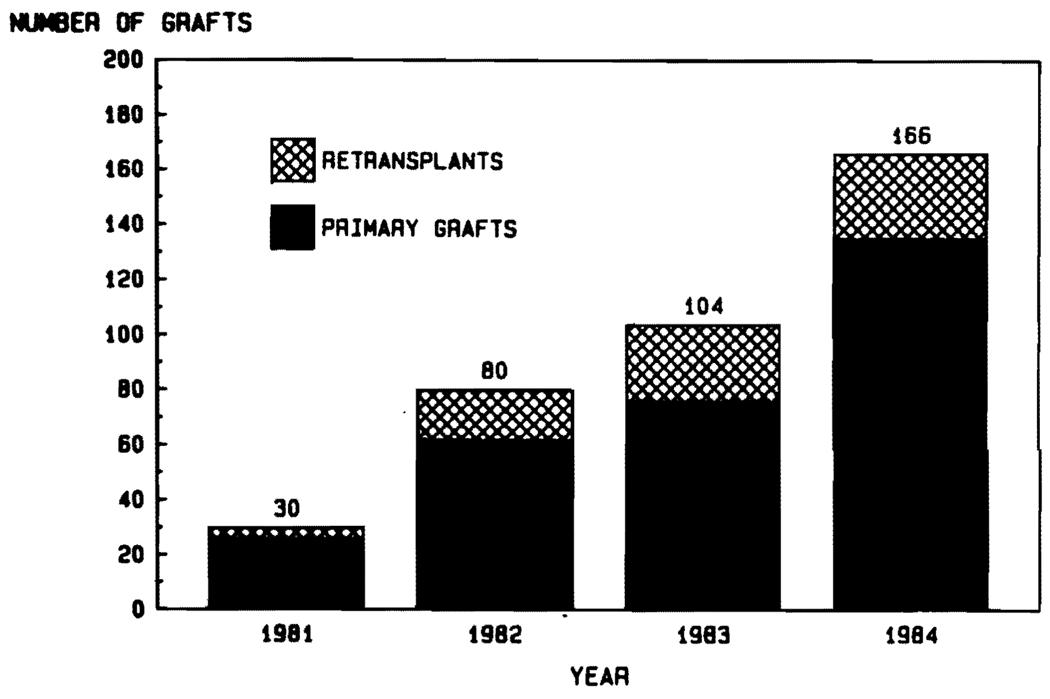
In 1980, cyclosporine and prednisone were used to treat 12 patients undergoing liver replacement. Two other liver recipients died on the operating table for a total patient pool in that year of 14, of whom 11 (78%) lived out the first year. If one counted only those who actually survived the operation to be able to receive drug therapy, the success rate was 11 of 12. (91.7%). These improved results became known in 1981,41 and almost immediately a remarkable effect was seen on the case numbers. Increments occurred year-by-year until in 1984 a total of 166 orthotopic liver transplantations were performed at the University of Pittsburgh (Fig. 3). Increased activity in other centers throughout the world has been documented elsewhere.42
FIG. 3. Increasing numbers of liver transplantations at the University of Pittsburgh between 1981 and 1984.
Note the significant number of retransplantations.
Our early trials of cyclosporine-steroid therapy were conducted without knowing what the cyclosporine blood levels were. The clinical judgment in managing such patients reflected a deliberate effort to balance the possibilities of rejection against those of nephrotoxicity.36
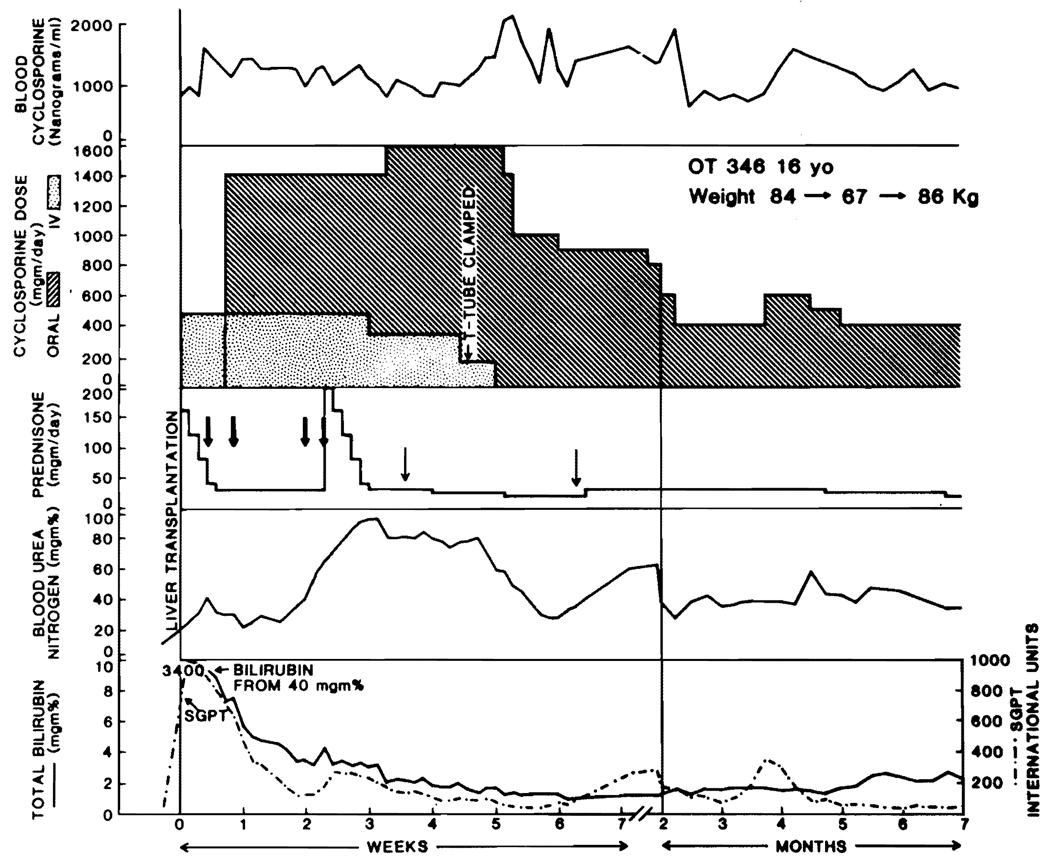
When techniques became available for assessment of whole blood or plasma cyclosporine concentration using radioimmunoassay (RIA) or highperformance liquid chromatography (HPLC), as described elsewhere in this issue of Seminars, it became popular to rely heavily on the results of these tests for management decisions. Recipients of liver transplantation have benefited from this practice, since intestinal absorption of cyclosporine after liver transplantation is unpredictable, as discussed elsewhere in this issue of Seminars. To assure adequate cyclosporine blood concentrations, it frequently has been necessary to administer the drug both intravenously and by mouth for several days, weeks, or even months postoperatively (Fig. 4). As absorption improves with the oral route, the intravenous doses are slowly reduced. Nevertheless, blind faith in the cyclosporine blood levels cannot be used to replace good clinical judgment, since toxicity of the drug —including that affecting the liver as well as the kidney —does not have an absolute correlation with the blood level.
FIG. 4. The use of cyclosporine and steroids.
Note that the cyclosporine initially is given intravenously (IV) and that the IV therapy is continued long after the drug is begun orally. The switch from double-route cyclosporine therapy to the oral route alone is carefully monitored with cyclosporine blood levels. Note the seeming increase in enteral absorption after clamping of the T-tube, the insistence on maintaining high blood levels of cyclosporine despite obvious low-grade nephrotoxocity, and the intensification of steroid therapy with either a cycle or intermittent bolus administration with suspicion of rejection. Large arrows: methylprednisolone sodium succinate; small arrows: hydrocortisone sodium succinate. (Reproduced with permission from Starzl et al.42)
NONRELEVANCE OF TISSUE TYPING
Antigen matching at the A, B, or DR loci has had little influence on the results after cadaveric renal transplantation and has not even been attempted for liver recipients. This kind of tissue matching probably will not play a significant role in further developments in liver transplantation.
A surprising finding has been the remarkable resistance of the liver to hyperacute rejection.43,44 There has been no obvious penalty with transplantation of livers to recipients whose sera contain the cytotoxic antigraft antibodies that almost invariably lead to immediate loss of kidney grafts. Furthermore, many liver transplantations have been and are being carried out across the ABO blood group barriers that frequently (although not invariably) cause hyperacute rejection of kidneys as the consequence of antigraft isoagglutinins.13 These observations have simplified enormously the logistic problems of liver transplantation.
CURRENT INDICATIONS FOR TRANSPLANTATION
The indications for liver replacement in the developmental phase of this field have been documented elsewhere25 and will not be mentioned here. Subsequent to the beginning of the cyclosporine era, 244 patients underwent this procedure between March 1980 and July 1, 1984. In Tables 2 and 3 are shown the principal indications for these operations. In about 10% of cases there were multiple pathologic diagnoses, such as the incidental presence of primary hepatic malignancies in livers with a variety of underlying chronic diseases.
TABLE 2.
Indications for Liver Transplantation in 140 Adults, 3/1/80 – 7/1/84
| Indication | Number | Percent |
|---|---|---|
| Acute hepatic necrosis | 3 | 2.1 |
| Budd-Chiari syndrome | 5 | 3.6 |
| Cirrhosis | 46 | 32.9 |
| Inborn errors of metabolism | 11 | 7.9 |
| Alpha1-antitrypsin deficiency | 6 | 4.3 |
| Wilson’s disease | 3 | 2.1 |
| Tyrosinemia | 1 | 0.7 |
| Primary biliary cirrhosis | 36 | 25.7 |
| Primary hepatic tumors | 13 | 9.3 |
| Secondary biliary cirrhosis | 5 | 3.6 |
| Sclerosing cholangitis | 19 | 13.6 |
| Other | 2 | 1.4 |
TABLE 3.
Indications for Liver Transplantation in 104 Children, 3/1/80 – 7/1/84
| Indication | Number | Percent |
|---|---|---|
| Biliary atresia | 56 | 53.8 |
| Budd-Chiari syndrome | 1 | 1.0 |
| Cirrhosis | 10 | 9.6 |
| Familial cholestasis | 7 | 6.7 |
| Inborn errors of metabolism | 23 | 22.1 |
| Alpha1-antitrypsin deficiency | 15 | 14.4 |
| Wilson’s disease | 4 | 3.8 |
| Tyrosinemia | 3 | 2.9 |
| Neonatal hepatitis | 3 | 2.9 |
| Secondary biliary cirrhosis | 1 | 1.0 |
| Sclerosing cholangitis | 1 | 1.0 |
| Other | 2 | 1.6 |
The profile of diseases in pediatric patients (less than 18 years old) has been different from that in adults. In adults, postnecrotic cirrhosis has been the most important reason for proceeding (Table 2). Other common diseases in adults have been primary biliary cirrhosis and sclerosing cholangitis (Table 2). In children, more than half of all the transplantations have been done for biliary atresia, the only other large group being a heterogenous collection of inborn errors of metabolism (Table 3). The inborn errors, if they are hepatic-based, are cured permanently by liver replacement, since the phenotype of the new liver remains that of the original donor.5,25
FACTORS INFLUENCING SURVIVAL
Cyclosporine
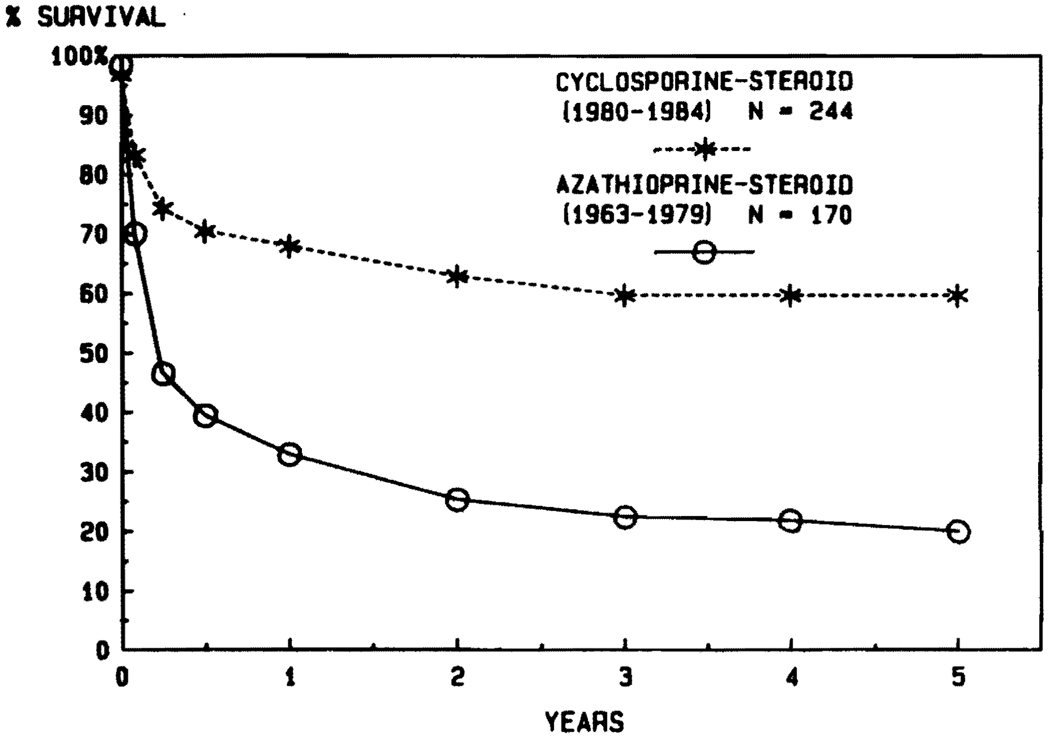
From 1963 through 1979, 170 patients were treated with conventional immunosuppression. The chances of living for a year after liver transplantation were only about one in three (Fig. 5). Subsequently, 244 liver recipients were provided with cyclosporine-steroid therapy between March 1980 and July 1, 1984, allowing follow-ups of 1 to more than 5 years. The chances of 1-year survival were more than doubled. Actuarial projections beyond 1 year indicate that these gains will be sustained for at least half a decade (Fig. 5). The results that followed the introduction of cyclosporine (Fig. 5) are appreciably greater than in the years that preceded its use (Fig. 1), indicating that cyclosporine per se was a major factor in achieving the improved results.
FIG. 5.
Marked improvement in results of liver transplantation after the introduction of cyclosporine-steroid therapy in ealry 1980.
Age
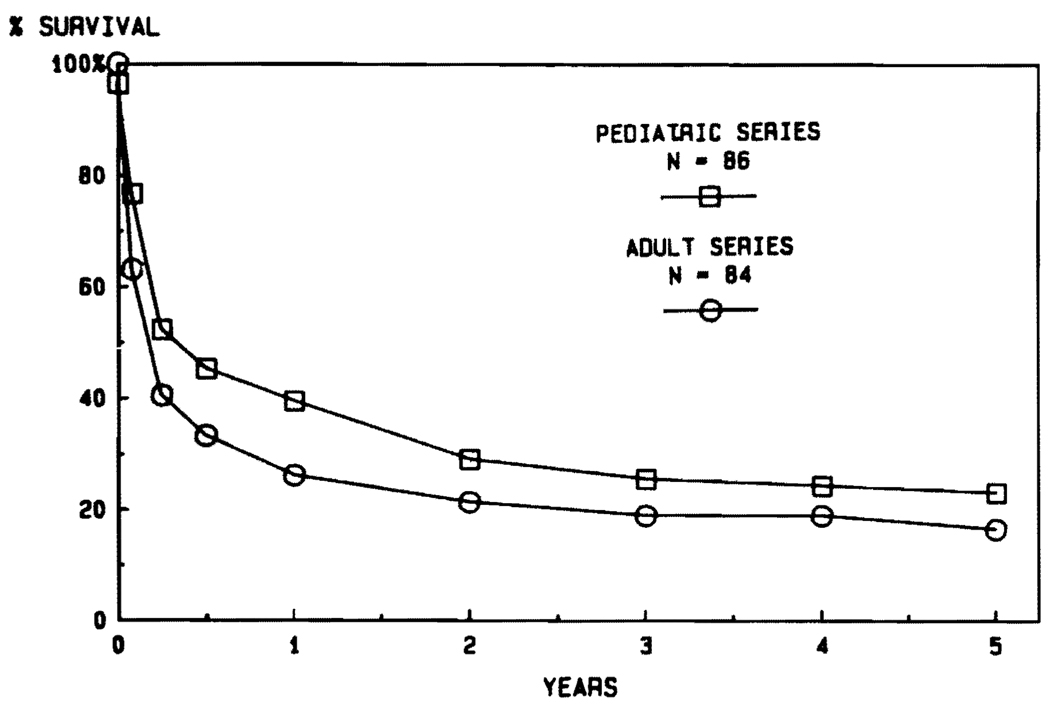
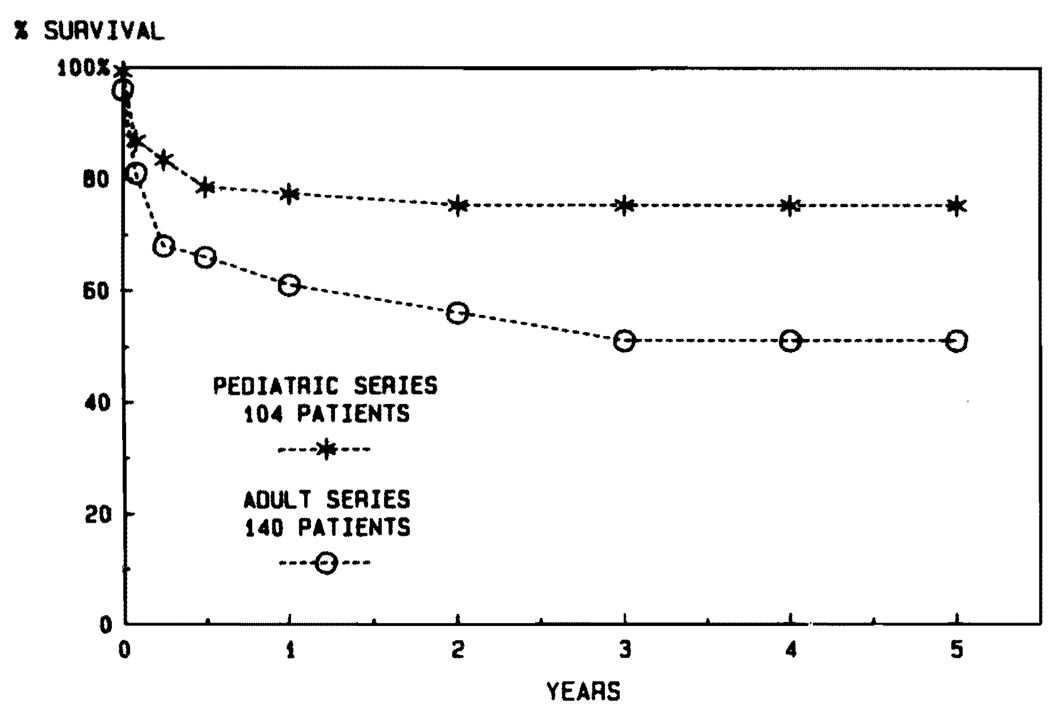
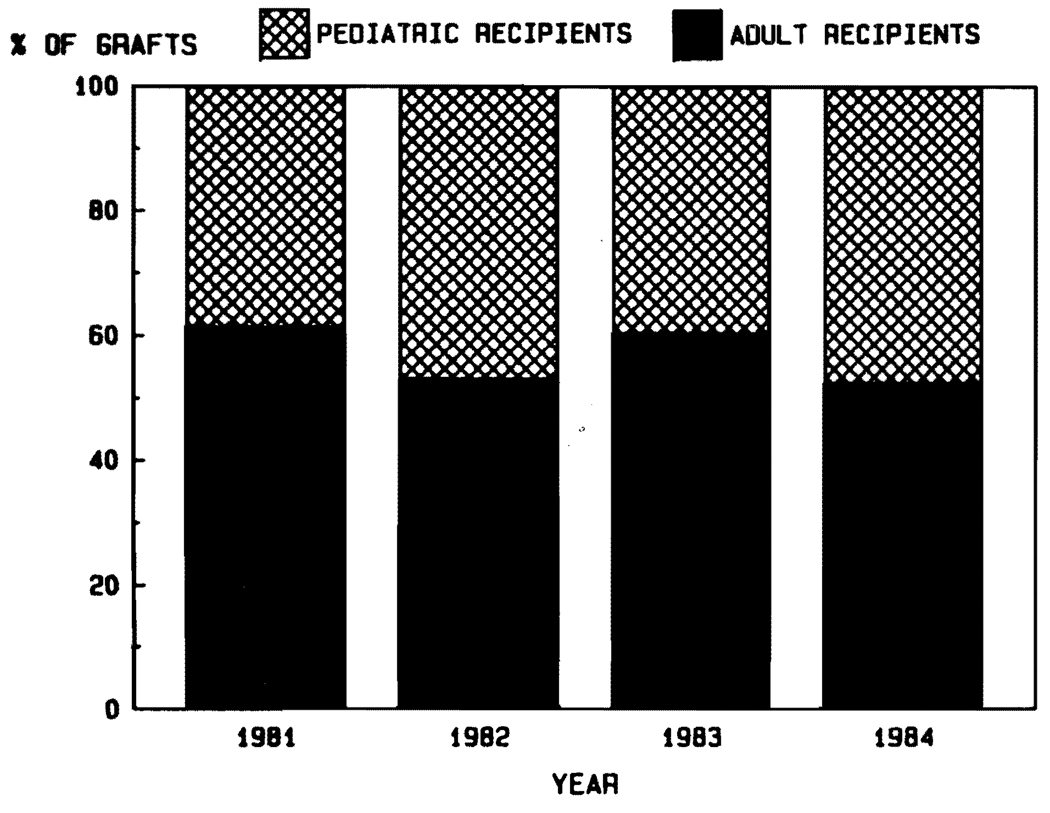
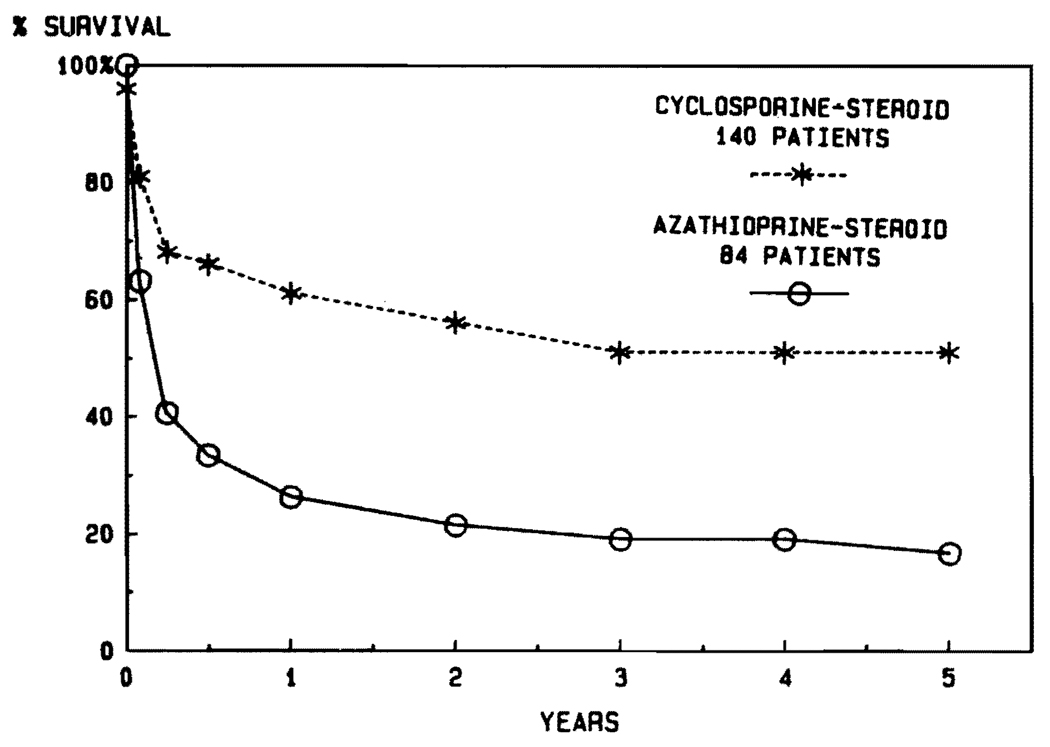
Aside from the fact that the disease profiles leading to transplantation are different in children and adults, another justification for stratification into adult and pediatric categories is the influence of age on survival. It was noted in the days of conventional immunosuppression that the results were better in pediatric recipients (Fig. 6). The disparity in pediatric versus adult cases has been even more striking during the cyclosporine era (Fig. 7). The actuarial 5-year survival in adults is projected at about 50%, compared with more than 70% for the pediatric recipients (Fig. 7). In view of the importance of the age factor, it will be necessary for groups reporting results to stipulate age distribution in their series. In our own experience using conventional immunosuppression from 1963 to 1979, half of the recipients were infants, children, and teenagers. In the subsequent years using cyclosporine, the pediatric component has never been that high (Fig. 8).
FIG. 6.
Results with adult versus pediatric liver transplantation under conventional immunosuppression between 1963 and early 1980.
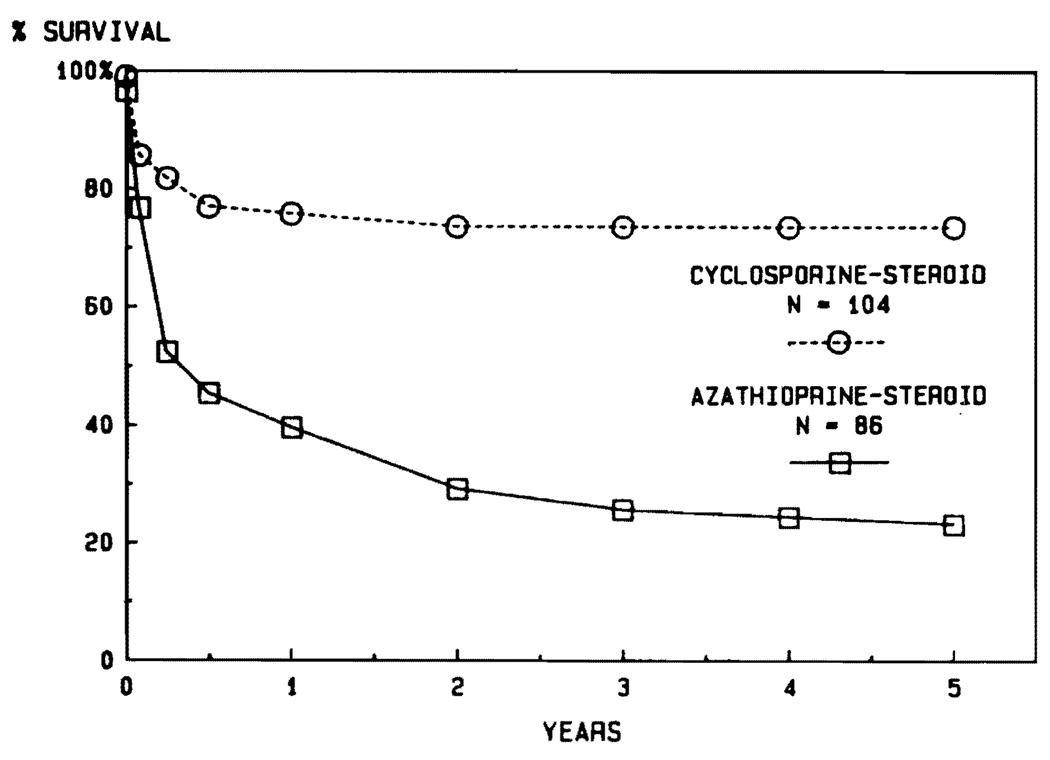
FIG. 7.
Comparison of results in adult and pediatric recipients during the cyclosporine era of 1980 to 1984.
FIG. 8.
Percentages of liver transplantations in pediatric versus adult recipients at the University of Pittsburgh from 1981 through most of 1984.
With the appropriate age stratification, meaningful comparisons become possible between what was achievable in the precyclosporine era versus now. In adults, the projected 5-year survival after liver transplantation, while still unsatisfactory, is nearly three times better than it was previously (Fig. 9). In children, the divergence of results using conventional immunosuppression compared with the present time is even more striking (Fig. 10).
FIG. 9.
Survival of adult liver recipients in the precyclosporine versus the cyclosporine eras.
FIG. 10. Survival of pediatric patients in the precyclosporine versus the cyclosporine eras.
Notice the remarkably high survival of children treated with cyclosporine-steroids during the first postoperative year as well as the fact that subsequent losses were extremely uncommon.
Influence of Diseases on Prognosis
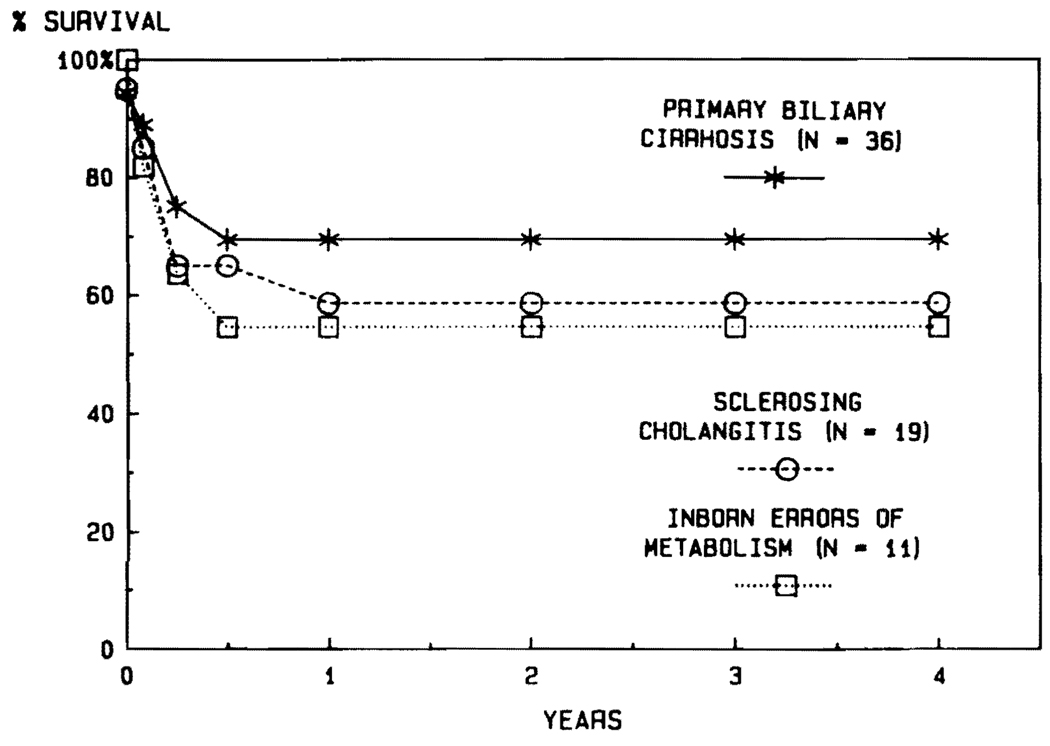
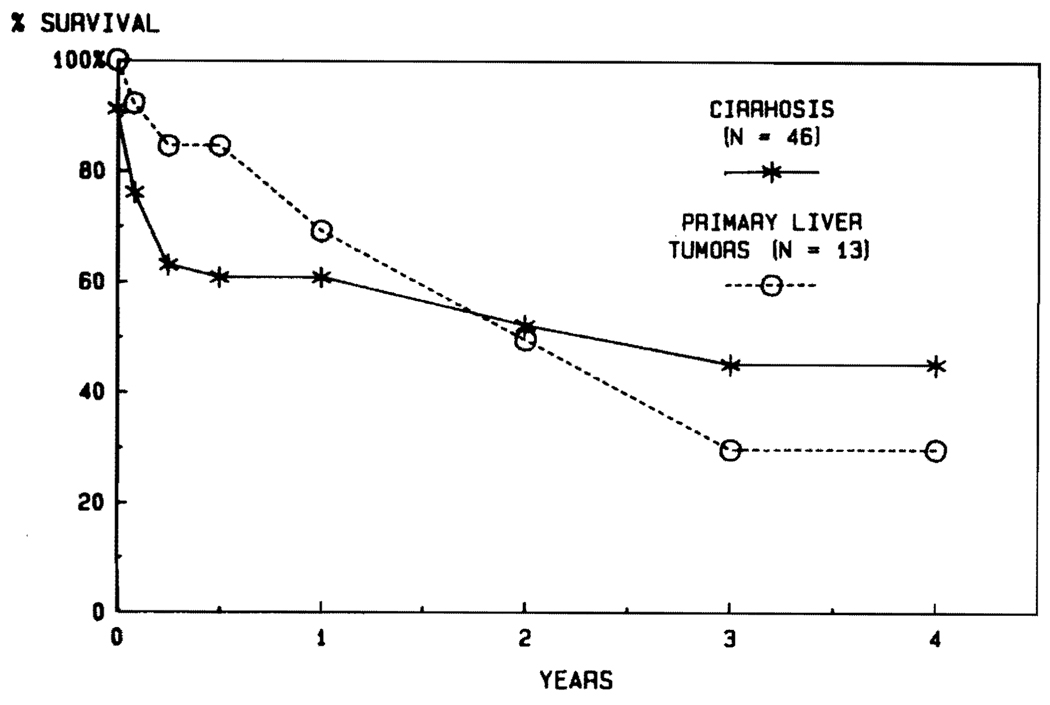
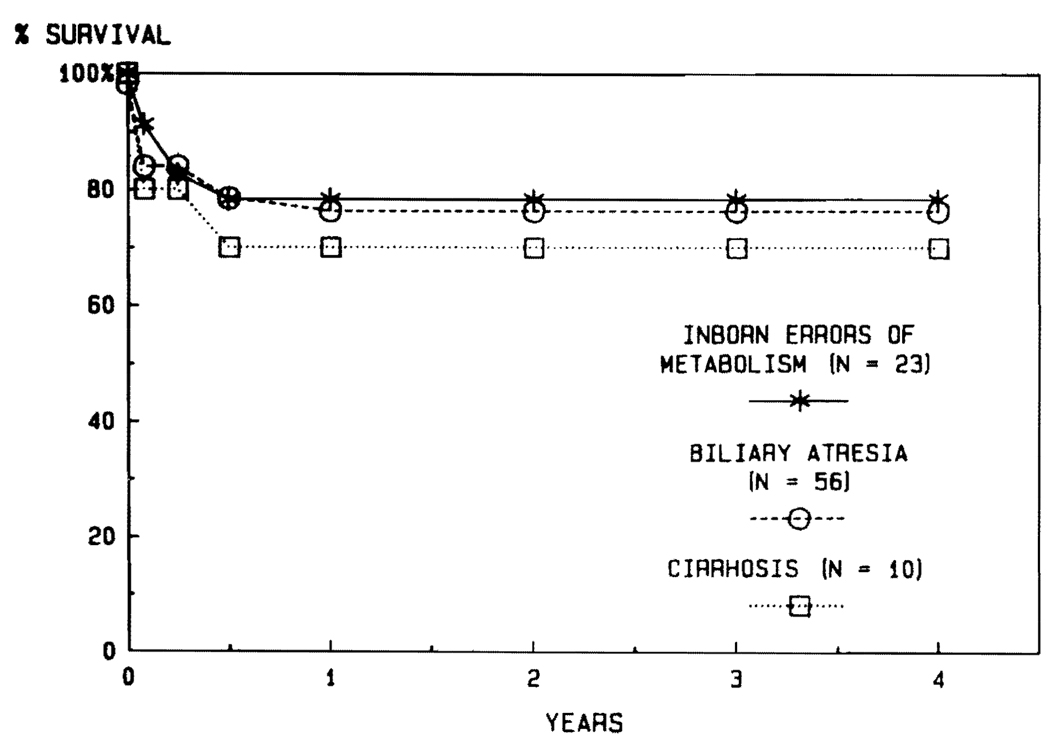
There are no diseases for which transplantation has been carried out in the past that can be automatically precluded from future trials. Usually, the nature of the original disease has not profoundly influenced the outcome after transplantation. For example, the results in adults have been similar with such diverse diseases as primary biliary cirrhosis, sclerosing cholangitis, and inborn errors of metabolism (Fig. 11). Nevertheless, there may be some high-risk diseases. So far, the results with postnecrotic cirrhosis and with primary hepatic tumors have been inferior (Fig. 12). With cirrhosis, the principal explanations have been the technical difficulties of the operation caused by the pathologic process, the generally poor condition of the patients, and almost universal recapitulation of their original chronic active hepatitis in B virus carriers.
FIG. 11.
The lack of influence of the underlying disease in adults treated for primary biliary cirrhosis, sclerosing cholangitis, and inborn errors of metabolism.
FIG. 12. Life survival curves of patients with cirrhosis and primary hepatic malignancy.
Note the very high survival of patients with malignant disease during the first half year (856), but with a steady decline thereafter, which was due primarily to the development of metastases.
In patients whose reason for liver replacement was a primary hepatic malignancy that could not be removed by conventional subtotal hepatic resection, the early mortality has been quite low, with more than 80% of the recipients being alive at 6 months. The steady decline thereafter (Fig. 12) has been caused by recurrent tumor, which can be expected in 80% or more of patients who live long enough for metastases to be detected. The only acceptable results thus far have been in patients with the slowgrowing and nonaggressive fibrolamellar hepatomas, which recently have been recognized to be a favorable variant within the larger hepatoma category.45 No patient has ever been cured of a duct cell carcinoma by liver transplantation. This has been unexpected, since the small duct cell carcinomas at the confluence of the right and left hepatic ducts (Klatskin tumors) were once thought to be an almost ideal indication for liver replacement.
In children, the results have been about the same in all of the main disease categories (Fig. 13). It was thought once that the technical problems in frequently reoperated children with biliary atresia would result in an increased mortality. Almost all such infants and children have had portoenterostomies and many have had multiple later surgical interventions in and around the hepatic hilum. Although transplantation is technically much more difficult under such circumstances, there has been no demonstrable penalty in terms either of early or late survival (Fig. 13).
FIG. 13.
Lack of influence of underlying disease on the survival of children undergoing liver transplantation.
ROLE OF RETRANSPLANTATION
Before the cyclosporine era, retransplantation in the event of failure of the first liver was almost never successful.25 The effectiveness of retransplantation has improved greatly since the introduction of cyclosporine, with an expected 1-year survival of almost 50%, as documented elsewhere in this issue of Seminars. The success rate for patients whose grafts have been in place for some time and failed slowly because of rejection has been especially high. The worst results have been in patients with immediate and serious technical complications and those whose grafts have undergone a rapid and uncontrolled rejection in the first week or two.
The role of retransplantation in the future has been somewhat clouded by the enormous economic ramifications of early technical or other complications serious enough to warrant replacement of the graft. In his article in this issue of Seminars, Luebs has provided data about these fiscal implications at the Children’s Hospital of Pittsburgh.
Both in adults and in children, but particularly the latter, technical complications have played an important role in necessitating attempts at retransplantation.46 The lesson has been clear that if a perfect operation is not performed the first time for any reason, the cost will be prodigous and will have to be borne by the patient or more commonly the health insurance carrier. In future years, it will become important to try to identify those patients for whom retransplantation offers little or no chance of survival so that expensive and ineffective attempts can be avoided with some degree of accuracy.
DIAGNOSIS OF POSTOPERATIVE LIVER DYSFUNCTION
In the early days of liver transplantation, the development of hepatic dysfunction postoperatively was quite naturally attributed to rejection. This diagnosis was usually correct, but not always. Frequent alternative explanations were complications of biliary tract reconstruction; infection with hepatitis B virus, cytomegalovirus, herpes, or adenovirus; and drug toxicity. The ability accurately to differentiate these causes from rejection has resulted from the great advances in hepatology, infectious disease, radiology, and pathology of the last two decades. With the combination of a hepatitis antigen/antibody screen, noninvasive radiologic imaging techniques, cholangiography, angiography on occasion, and needle biopsy of the liver, treatment can be tailored for each individual patient in a far more effective way.
SUMMARY
During the last 5 years, liver transplantation has become a service as opposed to an experimental operation. The most important factor in making this possible has been the introduction of cyclosporine-steroid therapy. At the same time, liver transplantation has been made more practical by improvements in diagnosing and managing other causes of postoperative hepatic dysfunction. Tissue typing and matching have played no role in improving the results of liver transplantation. With the demonstration that preformed antibody states are irrelevant, even avoidance of positive cross-matches caused by cytotoxic antibodies and observance of ABO blood group barriers have become unnecessary if the recipient’s needs are great. With the exceptions of malignancy and cirrhosis, the nature of the underlying hepatic disease has not profoundly influenced the results. Retransplantation has played an important role in improving survival, although the costs of retransplantation have been extremely high.
Acknowledgment
Supported by Research Grants from the Veterans Administration and Project Grant No. AM-29961 from the National Institutes of Health, Bethesda, Maryland.
REFERENCES
- 1.Starzl TE, Kaupp HA, Brock DR, et al. Reconstructive problems in canine liver homotransplantation with special reference to the postoperative role of hepatic venous flow. Surg Gynecol Obstet. 1960;111:733–743. [PMC free article] [PubMed] [Google Scholar]
- 2.Moore FD, Wheeler HB, Demissianos HV, et al. Experimental whole-organ transplantation of the liver and of the spleen. Ann Surg. 1960;152:374387. [PMC free article] [PubMed] [Google Scholar]
- 3.Starzl TE, Marchioro TL, von Kaulla K, et al. Homotransplantation of the liver in humans. Surg Gynecol Obstet. 1963;117:659–676. [PMC free article] [PubMed] [Google Scholar]
- 4.Starzl TE, Marchioro TL, Porter KA, et al. Factors determining short- and long-term survival after orthotopic liver homotransplantation in the dog. Surgery. 1965;58:131–155. [PMC free article] [PubMed] [Google Scholar]
- 5.Starzl TE. Experience in Hepatic Transplantation. Philadelphia: W.B. Saunders; 1969. (with the assistance of Putnam CW) [Google Scholar]
- 6.Starzl TE, Marchioro TL, Porter KA, et al. The use of heterologous antilymphoid agents in canine renal and liver homotransplantation, and in human renal homotransplantation. Surg Gynecol Obstet. 1967;124:301–318. [PMC free article] [PubMed] [Google Scholar]
- 7.Starzl TE, Groth CG, Brettschneider L, et al. Orthotopic homotransplantation of the human liver. Ann Surg. 1968;168:392–415. doi: 10.1097/00000658-196809000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murray JE, Merrill JP, Dammin GJ, et al. Kidney transplantation in modified recipients. Ann Surg. 1962;156:337–355. doi: 10.1097/00000658-196209000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Starzl TE, Marchioro TL, Waddell WR. The reversal of rejection in human renal homografts with subsequent development of homograft tolerance. Surg Gynecol Obstet. 1963;117:385–395. [PMC free article] [PubMed] [Google Scholar]
- 10.Hume DM, Magee JH, Kauffman HM, Jr, et al. Renal homotransplantation in man in modified recipients. Ann Surg. 1963;158:608–644. doi: 10.1097/00000658-196310000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woodruff MFA, Robson JS, Nolan B, et al. Homotransplantation of kidney in patients treated by preoperative local irradiation and postoperative administration of an antimetabolite (Imuran) Lancet. 1963;2:675–682. doi: 10.1016/s0140-6736(63)90465-3. [DOI] [PubMed] [Google Scholar]
- 12.Murray JE, Merrill JP, Harrison JH, et al. Prolonged survival of human kidney homografts by immunosuppressive drug therapy. N Engl J Med. 1963;268:1315–1323. doi: 10.1056/NEJM196306132682401. [DOI] [PubMed] [Google Scholar]
- 13.Starzl TE. Experience in Renal Transplantation. Philadelphia: W.B. Saunders; 1964. [Google Scholar]
- 14.Opelz G, Michey MR, Terasaki PI. HLA matching and cadaver kidney transplant survival in North America: Influence of center variation and presensitization. Transplantation. 1977;23:490–497. doi: 10.1097/00007890-197706000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Starzl TE, Porter KA, Iwasaki Y, et al. In: The use of antilymphocyte globulin in human renal homotransplantation. Wolstenholme GEW, O’Connor M, editors. London, J and A Churchill: Antilymphocytic Serum; 1967. pp. 4–34. [Google Scholar]
- 16.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 17.Cosimi AB, Burton RC, Colvin RB, et al. Treatment of acute renal allograft rejection with OKT3 monoclonal antibody. Transplantation. 1981;36:535–539. doi: 10.1097/00007890-198112000-00018. [DOI] [PubMed] [Google Scholar]
- 18.Norman DJ, Barry JM, Henell K, et al. Reversal of acute allograft rejection with monoclonal antibody. Transplant Proc. 1985;17:39–41. [Google Scholar]
- 19.Goldstein G, Schindler J, Sheahan M, et al. Orthoclone OKT3 treatment of acute renal allograft rejection. Transplant Proc. 1985;17:129–131. [PubMed] [Google Scholar]
- 20.Starzl TE, Putnam CW, Halgrimson CG, et al. Cyclophosphamide and whole organ transplantation in humans. Surg Gynecol Obstet. 1971;133:981–991. [PMC free article] [PubMed] [Google Scholar]
- 21.Franksson C. Survival of homografts of skin in rats depleted of lymphocytes by chronic drainage from the thoracic duct. Lancet. 1964;1:1331–1332. doi: 10.1016/s0140-6736(64)91451-5. [DOI] [PubMed] [Google Scholar]
- 22.Starzl TE, Weil R, III, Koep LJ, et al. Thoracic duct drainage before and after cadaveric kidney transplantation. Surg Gynecol Obstet. 1979;149:815–821. [PMC free article] [PubMed] [Google Scholar]
- 23.Strober S, Slavin S, Fuks Z, et al. Transplantation tolerance after total lymphoid irradiation. Transplant Proc. 1979;11:1032–1038. [PubMed] [Google Scholar]
- 24.Najarian JS, Ferguson RM, Sutherland DER, et al. Fractionated total lymphoid irradiation (TLI) as preparative immunosuppression in high risk renal transplantation. Ann Surg. 1982;196:442–452. doi: 10.1097/00000658-198210000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Starzl TE, Iwatsuki S, Van Thiel DH, et al. Evolution of liver transplantation. Hepatology. 1982;2:614–636. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borel JF, Feurer C, Gubler HU, Stahelin H. Biological effects of cyclosporin A: A new antilymphocytic agent. Agents Actions. 1976;6:468–475. doi: 10.1007/BF01973261. [DOI] [PubMed] [Google Scholar]
- 27.Calne RY, Rolles K, White DJG, et al. Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreases, and 2 livers. Lancet. 1979;2:1033–1036. doi: 10.1016/s0140-6736(79)92440-1. [DOI] [PubMed] [Google Scholar]
- 28.Hanto DW, Gajl-Peczalska KJ, Frizzera G, et al. Epstein-Barr virus (EBV) induced polyclonal and monoclonal B-cell lymphoproliferative disease occurring after renal transplantation. Ann Surg. 1983;198:356–369. doi: 10.1097/00000658-198309000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Starzl TE, Weil R, III, Iwatsuki S, et al. The use of cyclosporin A and prednisone in cadaver kidney transplantation. Surg Gynecol Obstet. 1980;151:17–26. [PMC free article] [PubMed] [Google Scholar]
- 30.Starzl TE, Nalesnik MA, Porter KA, et al. Reversibility of lymphomas and lymphoproliferative lesions developing under cyclosporine-steroid therapy. Lancet. 1984;1:583–587. doi: 10.1016/s0140-6736(84)90994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Penn I, Hammond W, Brettschneider L, Starzl TE. Malignant lymphomas in transplantation patients. Transplant Proc. 1969;1:106–112. [PMC free article] [PubMed] [Google Scholar]
- 32.Starzl TE, Penn I, Putnam CW, et al. Iatrogenic alterations of immunologic surveillance in man and their influence on malignancy. Transplant Rev. 1971;7:112–145. doi: 10.1111/j.1600-065x.1971.tb00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Penn I. The price of immunotherapy. Curr Probl Surg. 1981;18:681–751. doi: 10.1016/s0011-3840(81)80011-1. [DOI] [PubMed] [Google Scholar]
- 34.Editorial: Lymphoma in organ transplant recipients. Lancet. 1984;1:601–603. [PubMed] [Google Scholar]
- 35.Klintmalm GBG, Iwatsuki S, Starzl TE. Nephrotoxicity of cyclosporin-A in liver and kidney transplant patients. Lancet. 1981;1:470–471. doi: 10.1016/s0140-6736(81)91851-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Starzl TE, Hakala TR, Rosenthal JT, et al. Variable convalescence and therapy after cadaveric renal transplantation under cyclosporin A and steroids. Surg Gynecol Obstet. 1982;154:819–825. [PMC free article] [PubMed] [Google Scholar]
- 37.Slapak M, Geoghegan T, Digard N, et al. The use of low-dose cyclosporine in combination with azathioprine and steroids in renal transplantation. Transplant Proc. 1985;17:1222–1226. [PubMed] [Google Scholar]
- 38.Illner W-D, Land W, Habersetzer R, et al. Cyclosporine in combination with azathioprine and steroids in cadaveric renal transplantation. Transplant Proc. 1985;17:1181–1184. [Google Scholar]
- 39.Starzl TE, Klintmalm GBG, Weil R, III, et al. Cyclosporin-A and steroid therapy in 66 cadaver kidney recipients. Surg Gynecol Obstet. 1981;153:486–494. [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenthal JT, Hakala TR, Iwatsuki S, et al. Cadaveric renal transplantation under cyclosporine-steroid therapy. Surg Gynecol Obstet. 1983;157:309–315. [PMC free article] [PubMed] [Google Scholar]
- 41.Starzl TE, Klintmalm GBG, Porter KA, et al. Liver transplantation with use of cyclosporin-A and prednisone. N Engl J Med. 1981;305:266–269. doi: 10.1056/NEJM198107303050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Starzl TE, Iwatsuki S, Shaw BW, Jr, Gordon RD. Orthotopic liver transplantation in 1984. Transplant Proc. 1985;17:250–258. [Google Scholar]
- 43.Starzl TE, Putnam CW, Ishikawa M, et al. Current policies in hepatic transplantation: Candidacy of patients with alcoholic liver disease or preformed antidonor antibodies and a reappraisal of biliary duct reconstruction. NY Acad Sci. 1975;252:145–158. doi: 10.1111/j.1749-6632.1975.tb19151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iwatsuki S, Iwaki Y, Kano T, et al. Successful liver transplantation from cross match positive donors. Transplant Proc. 1981;13:286–288. [PMC free article] [PubMed] [Google Scholar]
- 45.Starzl TE, Iwatsuki S, Shaw BW, Jr, et al. Treatment of fibrolamellar hepatoma with partial hepatectomy or with totl hepatectomy and liver transplantation. Surg Gynecol Obstet. 1986 January;162 [PMC free article] [PubMed] [Google Scholar]
- 46.Shaw BW, Jr, Gordon RD, Iwatsuki S, Starzl TE. Hepatic retransplantation. Transplant Proc. 1985;17:264–271. [PMC free article] [PubMed] [Google Scholar]