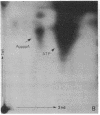
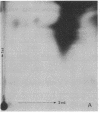
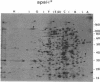
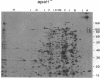
Abstract
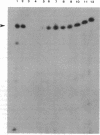
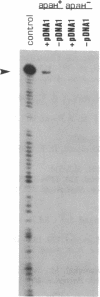
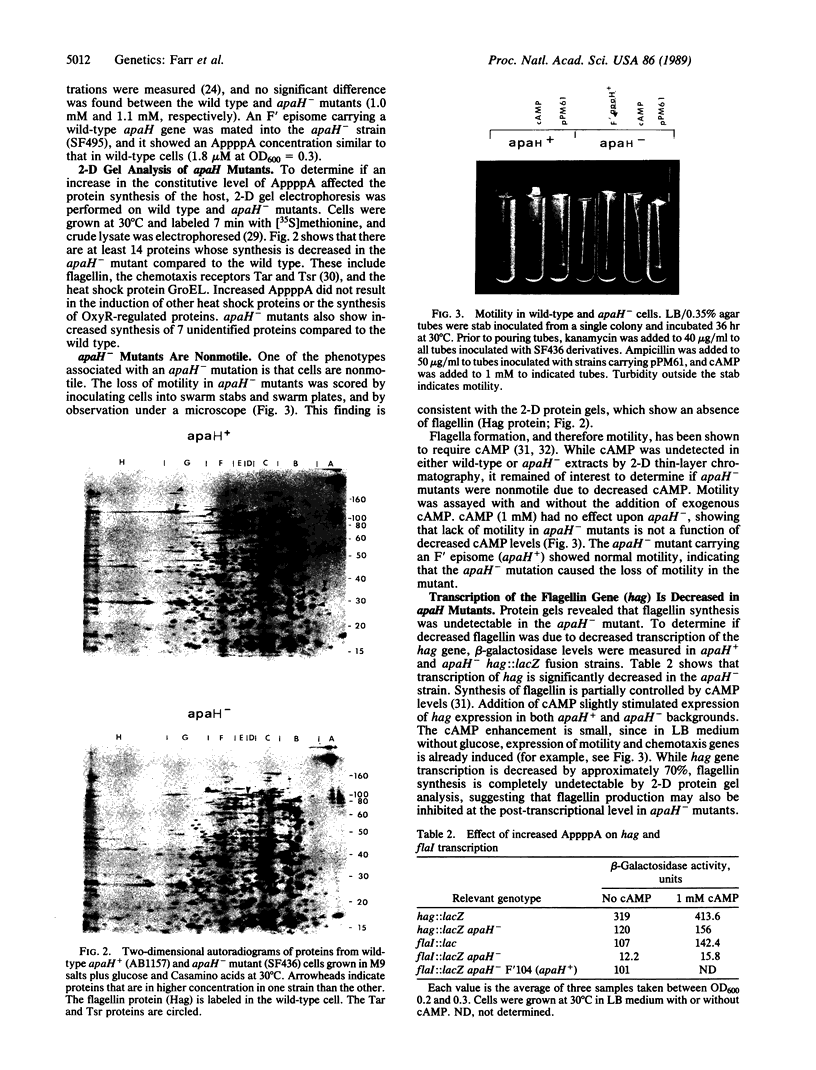
apaH- mutants lack the hydrolase responsible for degradation of AppppN dinucleotides in Escherichia coli and show a greater than or equal to 16-fold increase in AppppA under nonstress conditions. These mutants lack detectable activity of sigma F, a factor required for transcription of motility and chemotaxis genes. Expression of the flbB/flaI operon, thought to encode sigma F, is decreased in apaH- mutants, and there appears to be a general decrease in expression of genes regulated by cAMP-binding protein and cAMP as well.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper M. D., Ames B. N. Transport of antibiotics and metabolite analogs by systems under cyclic AMP control: positive selection of Salmonella typhimurium cya and crp mutants. J Bacteriol. 1978 Jan;133(1):149–157. doi: 10.1128/jb.133.1.149-157.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnosti D. N., Chamberlin M. J. Secondary sigma factor controls transcription of flagellar and chemotaxis genes in Escherichia coli. Proc Natl Acad Sci U S A. 1989 Feb;86(3):830–834. doi: 10.1073/pnas.86.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnara A. S., Finch L. R. Quantitative extraction and estimation of intracellular nucleoside triphosphates of Escherichia coli. Anal Biochem. 1972 Jan;45(1):24–34. doi: 10.1016/0003-2697(72)90004-8. [DOI] [PubMed] [Google Scholar]
- Baker J. C., Jacobson M. K. Determination of diadenosine 5',5''',-P1,P4-tetraphosphate levels in cultured mammalian cells. Anal Biochem. 1984 Sep;141(2):451–460. doi: 10.1016/0003-2697(84)90070-8. [DOI] [PubMed] [Google Scholar]
- Bartlett D. H., Frantz B. B., Matsumura P. Flagellar transcriptional activators FlbB and FlaI: gene sequences and 5' consensus sequences of operons under FlbB and FlaI control. J Bacteriol. 1988 Apr;170(4):1575–1581. doi: 10.1128/jb.170.4.1575-1581.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchin-Roland S., Blanquet S., Schmitter J. M., Fayat G. The gene for Escherichia coli diadenosine tetraphosphatase is located immediately clockwise to folA and forms an operon with ksgA. Mol Gen Genet. 1986 Dec;205(3):515–522. doi: 10.1007/BF00338091. [DOI] [PubMed] [Google Scholar]
- Bochner B. R., Ames B. N. Complete analysis of cellular nucleotides by two-dimensional thin layer chromatography. J Biol Chem. 1982 Aug 25;257(16):9759–9769. [PubMed] [Google Scholar]
- Bochner B. R., Lee P. C., Wilson S. W., Cutler C. W., Ames B. N. AppppA and related adenylylated nucleotides are synthesized as a consequence of oxidation stress. Cell. 1984 May;37(1):225–232. doi: 10.1016/0092-8674(84)90318-0. [DOI] [PubMed] [Google Scholar]
- Bochner B. R., Zylicz M., Georgopoulos C. Escherichia coli DnaK protein possesses a 5'-nucleotidase activity that is inhibited by AppppA. J Bacteriol. 1986 Nov;168(2):931–935. doi: 10.1128/jb.168.2.931-935.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier J., Sanchez R. Lysyl-tRNA synthetase from Escherichia coli K12. Chromatographic heterogeneity and the lysU-gene product. Biochem J. 1987 Nov 15;248(1):43–51. doi: 10.1042/bj2480043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr S. B., Touati D., Kogoma T. Effects of oxygen stress on membrane functions in Escherichia coli: role of HPI catalase. J Bacteriol. 1988 Apr;170(4):1837–1842. doi: 10.1128/jb.170.4.1837-1842.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant J. A. Stringent control in E. coli. Annu Rev Genet. 1979;13:393–415. doi: 10.1146/annurev.ge.13.120179.002141. [DOI] [PubMed] [Google Scholar]
- Garges S., Adhya S. Sites of allosteric shift in the structure of the cyclic AMP receptor protein. Cell. 1985 Jul;41(3):745–751. doi: 10.1016/s0092-8674(85)80055-6. [DOI] [PubMed] [Google Scholar]
- Goerlich O., Foeckler R., Holler E. Mechanism of synthesis of adenosine(5')tetraphospho(5')adenosine (AppppA) by aminoacyl-tRNA synthetases. Eur J Biochem. 1982 Aug;126(1):135–142. doi: 10.1111/j.1432-1033.1982.tb06757.x. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Helmann J. D., Chamberlin M. J. DNA sequence analysis suggests that expression of flagellar and chemotaxis genes in Escherichia coli and Salmonella typhimurium is controlled by an alternative sigma factor. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6422–6424. doi: 10.1073/pnas.84.18.6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horii Z., Clark A. J. Genetic analysis of the recF pathway to genetic recombination in Escherichia coli K12: isolation and characterization of mutants. J Mol Biol. 1973 Oct 25;80(2):327–344. doi: 10.1016/0022-2836(73)90176-9. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Theriot L. Mutants of Escherichia coli K-12 defective in DNA repair and in genetic recombination. Genetics. 1966 Jun;53(6):1137–1150. doi: 10.1093/genetics/53.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komeda Y. Transcriptional control of flagellar genes in Escherichia coli K-12. J Bacteriol. 1986 Dec;168(3):1315–1318. doi: 10.1128/jb.168.3.1315-1318.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer G. F., Baker J. C., Ames B. N. Near-UV stress in Salmonella typhimurium: 4-thiouridine in tRNA, ppGpp, and ApppGpp as components of an adaptive response. J Bacteriol. 1988 May;170(5):2344–2351. doi: 10.1128/jb.170.5.2344-2351.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenney K., Shimatake H., Court D., Schmeissner U., Brady C., Rosenberg M. A system to study promoter and terminator signals recognized by Escherichia coli RNA polymerase. Gene Amplif Anal. 1981;2:383–415. [PubMed] [Google Scholar]
- Mechulam Y., Fromant M., Mellot P., Plateau P., Blanchin-Roland S., Fayat G., Blanquet S. Molecular cloning of the Escherichia coli gene for diadenosine 5',5'''-P1,P4-tetraphosphate pyrophosphohydrolase. J Bacteriol. 1985 Oct;164(1):63–69. doi: 10.1128/jb.164.1.63-69.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Plateau P., Fromant M., Blanquet S. Heat shock and hydrogen peroxide responses of Escherichia coli are not changed by dinucleoside tetraphosphate hydrolase overproduction. J Bacteriol. 1987 Aug;169(8):3817–3820. doi: 10.1128/jb.169.8.3817-3820.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primakoff P., Artz S. W. Positive control of lac operon expression in vitro by guanosine 5'-diphosphate 3'-diphosphate. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1726–1730. doi: 10.1073/pnas.76.4.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M., Simon M. Characterization of Escherichia coli flagellar mutants that are insensitive to catabolite repression. J Bacteriol. 1974 Dec;120(3):1196–1203. doi: 10.1128/jb.120.3.1196-1203.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens J. C., Artz S. W., Ames B. N. Guanosine 5'-diphosphate 3'-diphosphate (ppGpp): positive effector for histidine operon transcription and general signal for amino-acid deficiency. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4389–4393. doi: 10.1073/pnas.72.11.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullmann A., Joseph E., Danchin A. Cyclic AMP as a modulator of polarity in polycistronic transcriptional units. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3194–3197. doi: 10.1073/pnas.76.7.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsumi R., Tanabe H., Nakamoto Y., Kawamukai M., Sakai H., Himeno M., Komano T., Hirota Y. Inhibitory effect of adenosine 3',5'-phosphate on cell division of Escherichia coli K-12 mutant derivatives. J Bacteriol. 1981 Sep;147(3):1105–1109. doi: 10.1128/jb.147.3.1105-1109.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanBogelen R. A., Kelley P. M., Neidhardt F. C. Differential induction of heat shock, SOS, and oxidation stress regulons and accumulation of nucleotides in Escherichia coli. J Bacteriol. 1987 Jan;169(1):26–32. doi: 10.1128/jb.169.1.26-32.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E. A., Mowry K. L., Clegg D. O., Koshland D. E., Jr Tandem duplication and multiple functions of a receptor gene in bacterial chemotaxis. J Biol Chem. 1982 May 10;257(9):4673–4676. [PubMed] [Google Scholar]
- Way J. C., Davis M. A., Morisato D., Roberts D. E., Kleckner N. New Tn10 derivatives for transposon mutagenesis and for construction of lacZ operon fusions by transposition. Gene. 1984 Dec;32(3):369–379. doi: 10.1016/0378-1119(84)90012-x. [DOI] [PubMed] [Google Scholar]
- Winans S. C., Elledge S. J., Krueger J. H., Walker G. C. Site-directed insertion and deletion mutagenesis with cloned fragments in Escherichia coli. J Bacteriol. 1985 Mar;161(3):1219–1221. doi: 10.1128/jb.161.3.1219-1221.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T., Gots J. S. Requirement of adenosine 3', 5'-cyclic phosphate for flagella formation in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1970 Aug;103(2):513–516. doi: 10.1128/jb.103.2.513-516.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamecnik P. C., Stephenson M. L., Janeway C. M., Randerath K. Enzymatic synthesis of diadenosine tetraphosphate and diadenosine triphosphate with a purified lysyl-sRNA synthetase. Biochem Biophys Res Commun. 1966 Jul 6;24(1):91–97. doi: 10.1016/0006-291x(66)90415-3. [DOI] [PubMed] [Google Scholar]