Abstract
BACKGROUND:
Autoimmune hepatitis is characterized by hepatocellular inflammation often progressing to cirrhosis. Standard treatment consists of corticosteroids and azathioprine. For the 20% of patients with refractory disease or those who are intolerant to medication, there is no standardized treatment.
OBJECTIVE:
To evaluate mycophenolate mofetil (MMF) as an alternative therapy for autoimmune hepatitis.
METHODS:
The present retrospective study identified all patients with autoimmune hepatitis who were treated with MMF over a 10-year period at the Henry Ford Hospital (Michigan, USA). These patients were evaluated for tolerance and response.
RESULTS:
Of the 90 patients participating in the study, 48% had a complete response, 32% experienced relapses and 21% were refractory. MMF was initiated in 21 patients – 12 (57%) for refractory disease and nine (43%) for medication intolerance. Of the 12 patients converted for refractory disease, all showed biochemical improvement but none had a complete response. Of the patients converted due to intolerance, 88% maintained complete remission. For all patients converted to MMF, there was a mean decrease in steroid dose from 18.9 mg/day to 7.8 mg/day (P=0.01).
CONCLUSIONS:
In patients with autoimmune hepatitis who were intolerant to conventional therapy, MMF was well tolerated, with 88% of patients maintained in remission. MMF did not induce remission in those refractory to conventional therapy; however, it resulted in a significant decrease in steroid use. Prospective studies are needed to better assess the role of MMF as an alternative therapy.
Keywords: Autoimmune liver disease, Difficult to treat, Mycophenolate
Abstract
HISTORIQUE :
L’hépatite auto-immune se caractérise par une inflammation hépatocellulaire qui évolue souvent en cirrhose. Le traitement standard se compose de corticoïdes et d’azathioprine. Pour les 20 % de patients ayant une maladie réfractaire ou qui sont intolérants aux médicaments, il n’existe pas de traitement standardisé.
OBJECTIF :
Évaluer le mofétil de mycophénolate (MMF) comme traitement contre l’hépatite auto-immune.
MÉTHODOLOGIE :
Dans le cadre de la présente étude prospective, les chercheurs ont retracé tous les patients atteints d’hépatite auto-immune traités par MMF depuis dix ans au Henry Ford Hospital (Michigan, États-Unis). Ils ont évalué la tolérance et la réponse de ces patients.
RÉSULTATS :
Sur les 90 patients qui ont participé à l’étude, 48 % avaient une réponse complète au traitement, 32 % ont subi des récidives et 21 % étaient réfractaires. Le MMF a été amorcé chez 21 patients, soit 12 (57 %) en raison d’une maladie réfractaire et neuf (43 %), en raison d’une intolérance aux médicaments. Les 12 patients qui ont changé de traitement en raison d’une maladie réfractaire ont tous démontré une amélioration biochimique, mais aucun n’a présenté une réponse complète, tandis que 88 % des patients qui ont changé de traitement en raison d’une intolérance ont maintenu une rémission complète. Chez tous les patients qui sont passés au MMF, on a constaté une diminution moyenne de la dose de stéroïdes, qui a chuté de 18,9 mg/jour à 7,8 mg/jour (P=0,01).
CONCLUSIONS :
Chez les patients atteints d’une hépatite auto-immune intolérants au traitement classique, le MMF était bien toléré, 88 % des patients demeurant en rémission. Le MMF ne suscitait pas de rémission chez les patients réfractaires au traitement classique, mais favorisait une diminution importante de l’utilisation des stéroïdes. Des études prospectives s’imposent pour mieux évaluer le rôle du MMF comme solution thérapeutique.
Autoimmune hepatitis (AIH) is a necroinflammatory disease of the liver of unclear etiology, characterized by progressive hepatocellular inflammation, hypergammaglobulinemia and serum autoantibodies (1). Patients with sustained high transaminase levels, defined as greater than 10 times the upper limit of normal, and elevated gammaglobulin levels have a three-year mortality rate of 50% (1). Cirrhosis is found at presentation in 25% of patients. These patients have a five-year mortality rate close to 60% (1).
Treatment is indicated for patients with sustained elevation of transaminase levels, or the presence of bridging necrosis or multiacinar necrosis on histological examination (1). Standard treatment consists of corticosteroids alone or in combination with azathioprine (AZA) (2).
Remission is achieved in more than 80% of cases, and long-term treatment with AZA with or without steroids is needed to prevent relapses and maintain remission. Relapsing AIH may be seen in up to 70% of patients within three years of treatment discontinuation (1). Close to 20% of patients develop an adverse outcome despite adequate therapy (1). The adverse outcomes are broadly classified as treatment failure, incomplete response and intolerance of side effects (drug toxicity) (1).
Unfortunately, side effects are associated with long-term treatment with steroids and AZA. Eighty per cent of patients undergoing chronic therapy with steroids, defined as more than two years of treatment, develop obesity and hirsutism (1). Serious side effects such as diabetes, hypertension, osteoporosis and vertebral fracture may develop after prolonged treatment with prednisone doses of greater than 10 mg/day (1). Complications from AZA including cytopenias, cholestatic liver failure, nausea and emesis develop in 10% of patients (1,2).
Alternative therapies are warranted for individuals who are refractory or intolerant to standard therapy. Currently, there is no standardized treatment for these individuals. Various immunosuppressive agents have been used with variable success including cyclosporine, tacrolimus, budesonide (2) and mycophenolate mofetil (MMF).
MMF inhibits inosine monophosphate dehydrogenase, which restricts lymphocyte-specific DNA synthesis (1). It has been successfully used following heart, kidney and liver transplant without reported hepatotoxicity (3). A few case reports and limited retrospective studies have advocated the use of MMF for AIH (4). While some studies (5) showed that MMF was effective both in induction and maintaining remission, others (4) showed the opposite result. The aim of our study was to describe our experience with MMF in the treatment of 21 patients who were refractory or intolerant to conventional therapy.
METHODS
Patients
The present retrospective study of all patients with a diagnosis of AIH at the Henry Ford Hospital (Michigan, USA) between 1995 and 2004 was reviewed and approved by the hospital’s institutional review board. The diagnosis of AIH was made by one of four academic hepatologists at the Henry Ford Hospital, and was based on a constellation of clinical, serological, biochemical and histological findings. Patients with common viral hepatitides, hereditary hemochromatosis, Wilson’s disease, alpha-1 antitrypsin deficiency, primary biliary cirrhosis, primary sclerosing cholangitis, alcohol and nonalcoholic fatty liver disease were excluded. Liver biopsy results were not available for every patient; the international AIH criteria could not be universally applied.
Conventional treatment consisted of steroid therapy with or without AZA for at least six months. Prednisone was initiated at a dose of 40 mg/day and was tapered by 10 mg every two weeks if possible. AZA was started at 50 mg/day and was increased to 150 mg/day for patients with a suboptimal biochemical response.
Refractory disease was defined as persistent elevation of transaminase levels, defined as two or more times the upper limit of normal, despite adequate treatment with conventional therapy for at least six months. Partial responders were defined as those with transaminase levels of between one and two times the upper limit of normal despite treatment for six months. Relapsing disease was defined as the elevation of transaminase levels to two or more times the upper limit of normal within six months of discontinuation or tapering of treatment. Intolerance was defined as adverse events preventing adequate steroid or AZA dosing to induce or maintain remission.
MMF was used for patients with disease refractory to treatment or who were intolerant. Patients with partial response remained on standard treatment. The starting dose of MMF at the Henry Ford Hospital was 500 mg twice a day. It was increased to 2 g/day as determined by the primary hepatologist. The variables of interest were demographic features and laboratory results including baseline alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels, at the end of the conventional therapy period, and at six months and one year after initiating MMF. The primary end points were response to MMF and the ability to wean off prednisone based on biochemical response. Response to MMF was defined as complete remission, refractory disease despite MMF, partial response to MMF, relapse of AIH and drug toxicity.
Tabulation of clinical variables and subsequent analysis was performed using SPSS (SPSS Inc, USA). The Pearson’s χ2 test was used to compare frequency of treatment response and failure. A paired sample t test was used to compare mean amino-transferase levels and steroid doses, with P<0.05 considered to be statistically significant.
RESULTS
Between 1995 and 2004, 90 patients with AIH were identified at the Henry Ford Hospital. After an average of 42 months of treatment and follow-up, 42 patients (47%) experienced complete clinical and laboratory resolution of their disease, 29 (32%) had relapsing disease and 19 (21%) had a partial or no response to prednisone and/or AZA. Of the 48 patients who did not exhibit a complete response, MMF was initiated in 21, as shown in Table 1.
TABLE 1.
Distribution of patients receiving mycophenolate mofetil (MMF) based on response to conventional therapy and the reason for initiating MMF
| Reason for initiating MMF |
Response to conventional therapy |
||
|---|---|---|---|
| Complete (n=42) | Relapse (n=29) | None/partial (n=19) | |
| Refractory to therapy (group 1) | 0 | 6 | 6 |
| Medication intolerance (group 2) | 6 | 1 | 2 |
Data presented as n
All patients were undergoing steroid treatment for at least six months, and 20 of 21 were on AZA for at least six months before initiation of MMF (median 15 months). The median dose of AZA before the initiation of MMF was 100 mg/day (range 50 mg/day to 150 mg/day).
The reason for MMF conversion was failure of conventional treatment (group 1) in 57%, and intolerance to steroids or AZA (group 2) in 43%. The main demographic features of each group are summarized in Table 2. There were no significant differences between the demographic and laboratory features of the two groups. Baseline histological features were available for 15 patients, which are shown in Table 3. Twenty per cent of patients had bridging fibrosis or cirrhosis at baseline. Sufficient information to calculate AIH score was available for five patients, all of whom had a score greater than 15, meeting the diagnostic criteria for definite AIH (6).
TABLE 2.
Demographic and laboratory features of the two groups of patients treated with mycophenolate mofetil before initiating therapy
| Total |
Conventional therapy |
P | ||
|---|---|---|---|---|
| Failure | Intolerance | |||
| Age, years | 50 (25–80) | 46.3 (25–61) | 55.7 (35–80) | 0.12 |
| Female, % | 81 | 75 | 89 | 0.73 |
| Weight, kg | 79 (47–117) | 82 (47–117) | 75 (61–91) | 0.92 |
| Alkaline phosphatase, U/L (normal <140 U/L) | 232 (82–1522) | 156 (91–260) | 346 (82–1522) | 0.30 |
| Aspartate aminotransferase, U/L (normal <35 U/L) | 801 (64–3967) | 880 (64–3967) | 695 (95–3099) | 0.41 |
| Alanine aminotransferase (normal <40 U/L) | 762 (72–3218) | 746 (72–3218) | 782 (94–1659) | 0.79 |
| Immunoglobulin G, g/L (normal range 7 g/L–16 g/L) | 22.1 | 18.8 | 26.7 | 0.34 |
| White blood cell count, ×109/L | 7.7 (2.2–11.2) | 7.6 (3.6–11.2) | 7.8 (2.2–10.9) | 0.90 |
Data presented as mean (range) unless indicated otherwise
TABLE 3.
Available baseline histological features of 15 patients started on mycophenolate mofetil
| Histology | n (%) |
|---|---|
| Interphase hepatitis | 4 (26) |
| Lymphoplasmocytic infiltrate | 5 (33) |
| Biliary changes | 1 (7) |
| Interphase hepatitis + lymphoplasmocytic infiltrate | 5 (33) |
MMF was initiated at a median starting dose of 1 g/day and was adjusted based on laboratory and clinical response. At the end of one year, the median dose of MMF was 1.5 g/day (range 0.5 g/day to 2.0 g/day).
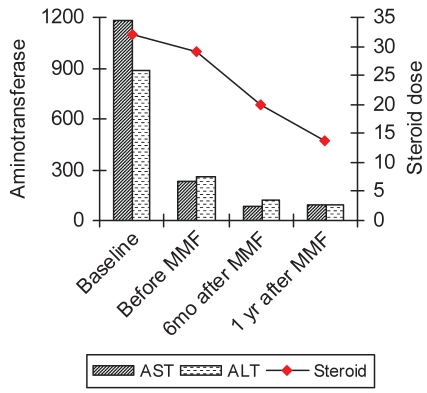
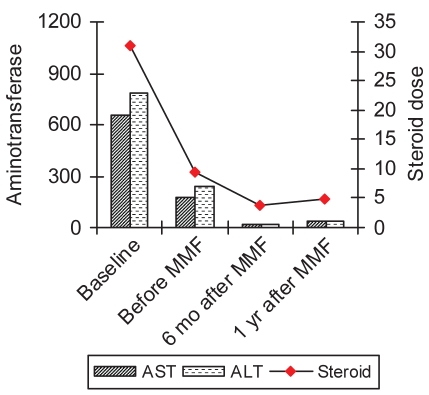
In both groups switched to MMF, the mean aminotransferase levels were lower at the six-month and one-year cross section, although it did not reduce the frequency of relapses in the interim or after one year or more (Figures 1 and 2). For all patients converted to MMF, there was a mean decrease in steroid use from 18.9 mg/day to 7.8 mg/day (P=0.01).
Figure 1).
Transaminase levels (U/L) and steroid dose (mg/day of prednisone), before treatment and during the course of treatment in patients refractory to standard therapy. ALT Alanine aminotransferase; AST Aspartate aminotransferase; MMF Mycophenolate mofetil; mo Months; yr Year
Figure 2).
Transaminase levels (U/L) and steroid dose (mg/day of prednisone) before treatment and during the course of treatment in patients intolerant to standard therapy. ALT Alanine aminotransferase; AST Aspartate aminotransferase; MMF Mycophenolate mofetil; mo Months; yr Year
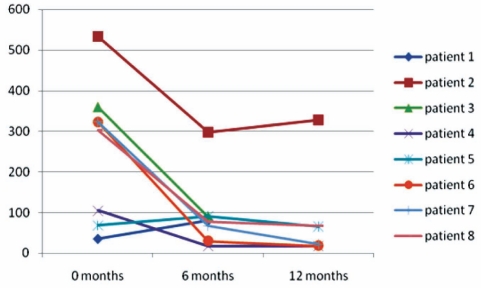
Twelve of 21 patients were converted to MMF because of refractory disease (group 1), eight of whom had a follow-up of greater than six months. The other four patients did not return for follow-up. The mean ALT levels significantly decreased from 256 U/L to 93 U/L at six months (P=0.012), and to 86 U/L at one year (P=0.012). However, none of the patients in this group were considered to have a sustained biochemical resolution of disease secondary to multiple relapses in the interim (Figure 3). Although the mean steroid (prednisone) requirement decreased from 29 mg/day to 17.5 mg/day, and 13.7 mg/day at the six-month and one-year interval, respectively, none of the patients could be weaned off steroids completely. Of the remaining eight patients in this group, six were still on MMF at the end of one year. One patient developed gastrointestinal side effects and one was considered to be a nonresponder. In the latter, MMF was stopped at six months and cyclosporine was initiated.
Figure 3).
Change in alanine aminotransferase levels (U/L) after implementation of mycophenolate mofetil in the ‘refractory group’
Of the nine patients who were converted to MMF for intolerance to conventional treatment (group 2), eight (88%) maintained complete remission following MMF conversion (P=0.002). In this group, the mean ALT levels improved from 244 U/L to 44 U/L (P=0.26), and 37 U/L (P=0.25) at six months and one year, respectively. The prednisone requirement decreased from a mean of 9.5 mg/day to 3.8 mg/day at six months (P=0.02), and 2.2 mg/day at one year (P=0.01). At the end of one year, five of nine patients were totally weaned off steroids.
Overall, MMF treatment was well tolerated, with 88% (15 of 17) of patients remaining on treatment at the end of follow-up. MMF was discontinued in one patient after seven months due to gastrointestinal symptoms that included nausea, vomiting and diarrhea. MMF was discontinued in another patient after eight months secondary to significant worsening of transaminase levels. There was no evidence of bone marrow suppression or leukopenia associated with MMF. The mean white blood cell count slightly decreased from 7.7×109/L to 6.8×109/L without clinical manifestations.
DISCUSSION
Nearly 20% of patients with AIH will fail or be intolerant to conventional medical therapy. Treatment of these patients is often challenging because no standardized therapy exists.
Many studies have reported the effect of other immunomodulators on the course of AIH for these patients. These drugs include mycophenolate, cyclosporine, cyclophosphamide, budesonide, methotrexate and tacrolimus (7,8). Cyclosporine is the most extensively studied and is the only medication that has been evaluated in an open-label trial, with good success for both steroid-naive and steroid-refractory patients (9,10).
MMF is an ester prodrug designed to increase the bioavailability of the active metabolite mycophenolic acid (5). It affects the de novo purine synthesis pathway in lymphocytes. It ultimately results in decreased DNA synthesis and, consequently, decreased proliferation of T lymphocytes. Its toxicity profile is similar to that of AZA (5).
Most of the available data regarding MMF are from the transplant setting. MMF has been superior to AZA in preventing acute and chronic allograft rejection in liver transplant recipients (11). Furthermore, it is well tolerated by patients, and the risk for opportunistic infections is not significantly greater (11). Similar results have been observed from studies on kidney transplant recipients treated with MMF versus AZA (12). The side effect profile, with the exception of diarrhea, is similar to AZA. MMF did not cause nephrotoxicity, hepatotoxicity or significant myelotoxicity when compared with AZA (12).
There are case reports and four case series (4,5,13–15), all single-centre experiences, investigating the role of MMF on AIH as a salvage therapy. The results of theses studies were based on laboratory resolution with or without histological changes. A study of seven patients (15) showed resolution of transaminitis in 70% of patients; at seven months, there was histological improvement in all patients and decreased steroid requirements. In another case series of five patients (4), all showed biochemical remission with MMF. In a multicentre study conducted in Canada (5), of 11 patients who were treated with MMF for refractory disease, 65% demonstrated a complete response. The prednisone dose was reduced by an average of 15.3 mg/day over a six-month period.
In contrast, a retrospective study by Cjaza and Carpenter (13) compared the effect of MMF with high-dose steroids in refractory patients. Compared with steroids, MMF was not able to induce laboratory or histological remission.
All of these studies are limited by their small population size and were not able to divide patients into subgroups (eg, intolerant and refractory) similar to the current study.
To our knowledge, our study is the largest to examine the treatment of AIH with MMF. We have the additional advantage of being able to analyze the difference in MMF treatment for those patients who failed standard treatment versus those who were intolerant to either steroids or AZA.
The results of our study showed a complete response and remission rate of 48% across all AIH patients over a four-year period. Most investigations of AIH report success rates of as high as 70% to 90% over five years (2,7,9,13,16). The reason for the higher relapse rates and resistant disease in our patient population is unclear. We postulate that the greater number of these difficult-to-treat patients was seen because our study was performed in a tertiary care centre. Accordingly, we see more patients referred with challenging disease. Our centre appears to use MMF more liberally, with 25% of our patients receiving this drug. One should consider these facts when interpreting the MMF results.
The majority of patients – regardless of their response to the initial therapeutic regimen – experienced improvement or stable suppression of transaminase activity. However, the treatment of AIH with MMF was not uniformly effective, and clear differences between the two groups were seen.
In patients with AIH who were intolerant to conventional therapy, MMF was well tolerated. Nearly 90% of the patients who were switched to MMF remained in clinical and laboratory remission after one year. Overall, these patients required lower doses of steroids, with only a few patients requiring chronic steroid therapy.
Conversely, MMF was not as effective in patients who were refractory to conventional therapy. MMF was unable to induce remission in this group. This is not surprising because failure to respond is indicative of aggressive liver disease. Nevertheless, MMF facilitated a significant reduction in steroid use for this group as well. Furthermore, these patients still exhibited a marked improvement in biochemical markers in response to MMF; however, due to the small number of patients in this group, the changes were not statistically significant. We suggest that MMF be used as a salvage therapy for these patients
Overall, both groups tolerated MMF well. We did not observe any significant side effects or opportunistic infections with MMF. However, our follow-up was limited to one year. One patient developed significant gastrointestinal symptoms requiring discontinuation of the medication.
While recommending MMF for salvage therapy in patients with refractory disease appears to be reasonable in spite of other studies, advocating MMF as a first-line therapy should be done with caution. A greater than 10-fold cost difference exists between conventional and novel therapy regimens (17). This should be considered in future studies as a balancing measure.
A limitation of the present study was the participation of four hepatologists who functioned without a standardized protocol for the patients who failed conventional therapy with steroids and AZA. Differing doses of MMF were used as well as different tapering schedules for steroids.
We must also acknowledge that we defined response solely according to biochemical and clinical response. As mentioned earlier, not every patient underwent a liver biopsy to help confirm the diagnosis of AIH. Because histological improvement lags behind clinical and laboratory improvement by three to eight months (18), the lack of longer follow-up with repeat biopsy is one of the shortcomings of the present study.
SUMMARY
We believe that MMF should strongly be considered as an alternative therapy for patients with an initial good response to conventional therapy who develop side effects from AZA, and also as a salvage measure in patients refractory to conventional therapy with the intent of lowering steroid requirements. Larger, prospective studies are needed to better assess the role of MMF as a first- and second-line agent for the treatment of AIH.
REFERENCES
- 1.Czaja AJ. Autoimmune liver disease. In: Walton RE, Boyer TB, editors. Hepatology: A Textbook of Liver Disease. 4th edn. Vol. 2. Philadelphia: Saunders; 2002. pp. 1163–94. [Google Scholar]
- 2.Czaja AJ. Autoimmune liver disease. Curr Opin Gastroenterol. 2006;22:234–40. doi: 10.1097/01.mog.0000218959.48064.7f. [DOI] [PubMed] [Google Scholar]
- 3.Oo YH, Neuberger J. Use of mycophenolate in the treatment of autoimmune hepatitis. Liver Int. 2005;25:687–91. doi: 10.1111/j.1478-3231.2005.01123.x. [DOI] [PubMed] [Google Scholar]
- 4.Devlin SM, Swain MG, Urbanski SJ, Burak KW. Mycophenolate mofetil for the treatment of autoimmune hepatitis in patients refractory to standard therapy. Can J Gastroenterol. 2004;18:321–6. doi: 10.1155/2004/504591. [DOI] [PubMed] [Google Scholar]
- 5.Chatur N, Ramji A, Bain VG, et al. Transplant immunosuppressive agents in non-transplant chronic autoimmune hepatitis: The Canadian Association for the Study of the Liver (CASL) experience with mycophenolate mofetil and tacrolimus. Liver Int. 2005;25:723–7. doi: 10.1111/j.1478-3231.2005.01107.x. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez PA, Berg FB, Bianchi L, et al. International Autoimmune Hepatitis Group report Review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929–38. doi: 10.1016/s0168-8278(99)80297-9. [DOI] [PubMed] [Google Scholar]
- 7.Gordon FD, Simpson M. Use of immunomodulary agents is difficult in treating autoimmune hepatitis patients. J Clin Gastroenterol. 2004;38:729–30. doi: 10.1097/01.mcg.0000139177.33502.47. [DOI] [PubMed] [Google Scholar]
- 8.Aqel BA, Machicao V, Rosser B, Satyanarayana R, Harnois DM, Dickson RC. Efficacy of tacrolimus in the treatment of steroid refractory autoimmune hepatitis. J Clin Gastroenterol. 2004;38:805–9. doi: 10.1097/01.mcg.0000139050.67178.be. [DOI] [PubMed] [Google Scholar]
- 9.Fernandes NF, Redeker AG, Vierling JM, Villamil FG, Fong TL. Cyclosporine therapy in patients with steroid resistant autoimmune hepatitis. Am J Gastroenterol. 1999;94:241–8. doi: 10.1111/j.1572-0241.1999.00807.x. [DOI] [PubMed] [Google Scholar]
- 10.Malekzadeh R, Nasseri-Moghaddam S, Kaviani MJ, Taheri H, Kamalian N, Sotoudeh M. Cyclosporine A is a promising alternative to corticosteroids in autoimmune hepatitis. Dig Dis Sci. 2001;46:1321–7. doi: 10.1023/a:1010683817344. [DOI] [PubMed] [Google Scholar]
- 11.Wiesner R, Rabkin J, Klintmalm G, et al. A randomized double-blind comparative study of mycophenolate mofetil and azathioprine in combination with cyclosporine and corticosteroids in primary liver transplant recipients. Liver Transpl. 2001;7:442–50. doi: 10.1053/jlts.2001.23356. [DOI] [PubMed] [Google Scholar]
- 12.Sollinger HW. Mycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients. U.S. Renal Transplant Mycophenolate Mofetil Study Group. Transplantation. 1995;60:225–32. doi: 10.1097/00007890-199508000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Czaja AJ, Carpenter HA. Empiric therapy of autoimmune hepatitis with mycophenolate mofetil: Comparison with conventional treatment for refractory disease. J Clin Gastroenterol. 2005;39:819–25. doi: 10.1097/01.mcg.0000177260.72692.e8. [DOI] [PubMed] [Google Scholar]
- 14.Brunt EM, Di Bisceglie AM. Histologic changes after the use of mycophenolate mofetil in autoimmune hepatitis. Human Pathol. 2004;35:509–12. doi: 10.1016/j.humpath.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 15.Richardson PD, James PD, Ryder SD. Mycophenolate mofetil for maintenance of remission in autoimmune hepatitis in patients resistant to or intolerant of azathioprine. J Hepatol. 2000;33:371–5. doi: 10.1016/s0168-8278(00)80271-8. [DOI] [PubMed] [Google Scholar]
- 16.van den Berg AP. Autoimmune hepatitis: Pathogenesis, diagnosis and treatment. Scand J Gastroenterol Suppl. 1998;225:66–9. doi: 10.1080/003655298750027254. [DOI] [PubMed] [Google Scholar]
- 17.Heneghan MA, Al-Chalabi T, McFarlane IG. Cost-effectiveness of pharmacotherapy for autoimmune hepatitis. Expert Opin Pharmacother. 2006;7:145–56. doi: 10.1517/14656566.7.2.145. [DOI] [PubMed] [Google Scholar]
- 18.Czaja AJ, Bianchi FB, Carpenter HA, et al. Treatment challenges and investigational opportunities in autoimmune hepatitis. Hepatology. 2005;41:207–15. doi: 10.1002/hep.20539. [DOI] [PubMed] [Google Scholar]