Abstract
BACKGROUND:
Hepatitis C virus (HCV) genotype 4 is a common infection in Egypt and is the leading cause of liver disease.
OBJECTIVE:
To study the efficacy and safety of a novel 20 kD pegylated interferon alpha-2a derived from Hansenula polymorpha in combination with ribavirin for the treatment of Egyptian patients with genotype 4 chronic hepatitis C (CHC).
METHODS:
One hundred seven patients with genotype 4 CHC were involved in the present study. Liver biopsy was performed in all patients. All patients received a fixed weekly dose of 160 μg of a novel pegylated interferon in combination with ribavirin in standard and adjusted doses. Serum HCV RNA levels were assessed by a real-time sensitive polymerase chain reaction assay at four, 12, 48 and 72 weeks after the start of therapy. Patients demonstrating an early virological response (EVR) completed a 48-week course of treatment.
RESULTS:
The overall sustained virological response (SVR) was 60.7%. The SVR in patients with a rapid virological response was significantly higher (91.7%) than in patients with complete EVR (67.74%) (P=0.033) and partial EVR (56.14%) (P=0.003). SVR was also significantly higher in patients with a low degree of liver fibrosis according to Metavir score (F1 and F2) (67.57%) compared with those with a high degree of liver fibrosis (F3 and F4) (45.45%) (P=0.017). The baseline viral load had no impact on SVR in the present series nor were any serious adverse events reported.
CONCLUSION:
The novel pegylated interferon alpha-2a assessed in the present study was effective for the treatment of patients with genotype 4 CHC, and was safe and well tolerated.
Keywords: Chronic hepatitis C, Genotype 4, Hansenula polymorpha, Pegylated interferon
Abstract
HISTORIQUE :
Le virus de l’hépatite C (VHC) de génotype 4 est une infection courante en Égypte et constitue la principale cause de maladie hépatique.
OBJECTIF :
Étudier l’efficacité et l’innocuité d’un nouvel interféron alpha-2a pégylé 20 kD dérivé de l’Hansenula polymorpha associé à de la ribavirine pour traiter des patients égyptiens atteints d’hépatite C chronique (HCC) de génotype 4.
MÉTHODOLOGIE :
Cent sept patients atteints d’HCC de génotype 4 ont participé à la présente étude. Ils ont tous subi une biopsie hépatique et reçu une dose hebdomadaire fixe de 160 μg d’un nouvel interféron pégylé en association avec de la ribavirine en doses standards et rajustées. Les taux d’ARN du VHC sérique ont été évalués au moyen du titrage d’une réaction en chaîne de la polymérase sensible en temps réel quatre, 12, 48 et 72 semaines après le début du traitement. Les patients démontrant une réponse virologique précoce (RVP) ont reçu un traitement de 48 semaines.
RÉSULTATS :
La réponse virologique soutenue (RVS) globale était de 60,7 %. La RVS chez les patients ayant une réponse virologique rapide était considérablement plus importante (91,7 %) que chez les patients ayant une RVP complète (67,74 %) (P=0,033) et partielle (56,14 %) (P=0,003). La RVS était également considérablement plus importante chez des patients ayant une fibrose hépatique légère selon l’indice de Metavir (F1 et F2) (67,57 %) que chez ceux ayant une fibrose hépatique marquée (F3 et F4) (45,45 %) (P=0,017). La charge virale de départ n’avait pas de répercussions sur la RVS dans la présente série, et aucun événement indésirable grave n’a été déclaré.
CONCLUSION :
Le nouvel interféron alpha-2 pégylé évalué dans la présente étude était efficace, sécuritaire et bien toléré pour le traitement des patients atteints d’HCC de génotype 4.
The prevalence of chronic hepatitis C (CHC) in Egypt is extremely high, affecting 15% to 20% of the population. Hepatitis C virus (HCV) is the leading cause of liver disease in Egypt and is one of the country’s major health problems. Genotype 4 is the predominant genotype of HCV in Egyptian patients (up to 91%) (1). Genotype is one of the most important pretreatment factors that affect response to therapy (2). Genotype 4 is the least studied HCV genotype, and is prevalent in developing countries in Africa and the Middle East. This particular genotype was considered difficult to treat with the combination of conventional interferon and ribavirin (3–6). However, after the introduction of pegylated interferon, subsequent studies reported favourable response to treatment with pegylated interferon alpha (α)-2a and α-2b in combination with ribavirin (7–11), with the exception of a single report (12).
In the past decade, only two pegylated interferon products have been available: pegylated interferon α-2a (Pegasys, Hoffmann-LaRoche, Switzerland) and pegylated interferon α-2b (PegIntron, Schering-Plough, USA). Interferon therapy will likely remain the backbone of treatment for CHC despite the large number of oral anti-HCV agents under development. Attempts to introduce novel interferons with the aim of increasing therapeutic efficacy, reducing adverse events and/or reducing the cost of therapy are important. Many novel interferons, such as consensus interferon and albinterferon, were developed and studied for their efficacy in the treatment of CHC in the past few years. Albinterferon α-2b was reported to be effective for the treatment of CHC genotypes 2 and 3 (13) and genotype 1 (14), with the advantage of a better dosing schedule but at the expense of cost. In 2007, a novel interferon was introduced in Egypt and was approved for the treatment of CHC. Reiferon Retard (Rhein MinaPharm, Egypt) is a linear 20 kD pegylated interferon α-2a derived from Hansenula polymorpha. The cost of this novel interferon is markedly less than that of the existing peginterferons. To date, only a single study (15) investigating the efficacy and safety profile of this novel pegylated interferon for the treatment of Egyptian patients with genotype 4 CHC is available.
The H polymorpha expression system has been known for its superior characteristics for decades. Due to an increasing number of products and protein candidates derived from this expression system, it has been gaining greater popularity in recent years. H polymorpha represents a stable, robust and safe expression system (GRAS 1 organism), which boasts one of the highest productivities ever described for a recombinant protein (13.5 g/L of recombinant phytase). In addition, production processes based on H polymorpha technology are very cost effective. The cost effectiveness is strongly related to very short fermentation times and to a significantly reduced number of downstream steps, resulting in a higher purity, with no forms of oxidized interferon being detected (16). The aim of the present study was to assess the efficacy and safety profile of this novel 20 kD pegylated interferon α-2a derived from H polymorpha (Reiferon Retard) in combination with ribavirin for the treatment of Egyptian patients with genotype 4 CHC.
METHODS
Patients
One hundred seven patients with CHC were included in the present study during the period from March 2007 to December 2009. All patients provided written informed consent before enrollment in conformance with the Declaration of Helsinki of 1979. The study protocol was in accordance with national protocols for the treatment of CHC approved by the ministry of health in Egypt. All of the pharmaceutical products used were approved by the local health authorities. Inclusion criteria comprised the following: treatment-naive patients with no history of previous antiviral treatment for CHC, 18 years of age and older, the presence of detectable serum HCV RNA (genotype 4) using a real-time sensitive polymerase chain reaction (PCR) assay, clinical and laboratory evidence of compensated liver disease (absence of ascites, encephalopathy or esophageal varices, serum bilirubin level of less than 15 mg/L, serum albumin level of greater than 35 g/L and an international normalized ratio of less than 1.5), acceptable hematological values (hemoglobin level greater than 120 g/L, neutrophil level greater than 1500/mm3 and platelet count of greater than 90,000/mm3), serum creatinine level of less than 1.5 mg/dL and a body mass index of less than 30 kg/m2. Exclusion criteria were as follows: the presence of hepatitis B surface antigen or serum antihepatitis B core antigen antibodies, major depressive illness, solid organ transplant, schistosomiasis or concomitant clinically significant disease (uncontrolled diabetes mellitus, significant ischemic heart disease, severe hypertension, autoimmune disorders and thyroid disease).
Patient evaluation and liver histology
All patients were clinically assessed by full history and thorough clinical examination. Laboratory tests included the following: complete blood count, alanine aminotransferase (ALT), aspartate aminotransferase, bilirubin, international normalized ratio, serum albumin, creatinine, fasting and postprandial blood glucose, circulating schistosomal antigen and thyroid-stimulating hormone. Abdominal ultrasound examination was performed in all patients. Serum HCV RNA levels were assessed using a real-time sensitive PCR assay (Cobas Amplicor HCV monitor, version 2.0, Roche Diagnostics; lower limit of quantitation = 50 IU/mL). HCV genotyping was performed by restriction fragment length polymorphism analysis. A baseline viral load of 600,000 IU/mL or greater was defined as high viral load (HVL), while a level ranging from the lower detection limit of the assay (50 IU/mL) to less than 600,000 IU/mL was defined as low viral load (LVL).
Liver biopsy was performed in all patients using a Menghini needle (Hepafix 1.4, B Braun Melsungen AG, Germany). Biopsy specimens were assessed by an experienced pathologist who was blinded to the clinical and laboratory data. Metavir score was used for the assessment of necroinflammation grade (A0 to A3) and the degree of fibrosis (F0 to F4). Upper gastrointestinal endoscopy was performed in patients with F3 and F4 fibrosis grade to exclude the presence of esophageal or gastric varices.
Study design and assessment of response
All patients received a fixed weekly dose of 160 μg of a novel 20 kD linear pegylated interferon α-2a (Reiferon Retard) derived from H polymorpha in combination with ribavirin in standard and adjusted doses. Response to treatment was assessed by measuring follow-up serum HCV RNA levels at four, 12, 48 and 72 weeks after the start of treatment, and defined as the following:
Rapid virological response (RVR): undetectable serum HCV RNA after four weeks of treatment.
Early virological response (EVR): 12 weeks after initiation of treatment, undetectable HCV RNA in serum was considered to be a complete EVR (cEVR), while a baseline decrease in serum HCV RNA of 2 log units or greater was considered to be a partial EVR (pEVR).
End of treatment response (ETR): undetectable serum HCV RNA at the end of treatment (after 48 weeks).
Sustained virological response (SVR): undetectable serum HCV RNA after 24 weeks from the end of treatment (ie, 72 weeks from the start of treatment).
Treatment was discontinued if no EVR – either complete or partial – was achieved; these patients were defined as nonresponders. Early responders continued treatment for a total of 48 weeks. Patients who achieved RVR were considered to be part of the rapid responder subset, while patients achieving EVR – either complete or partial – without RVR were considered to be part of the early responder subset.
Assessment of safety
Throughout the entire treatment period, regular patient monitoring for adverse events was performed at the clinical and laboratory level. Temporary discontinuation of peginterferon was considered if neutrophil counts fell to below 500/mm3. Dose reduction of peginterferon by 50% was initiated if the neutrophil count was less than 750/mm3 and/or platelet counts were less than 60,000/mm3. Adjustment of ribavirin dose was initiated when hemoglobin levels fell to below 100 g/L. Dose reduction for both drugs was continued until improvement of the adverse event, after which the original dose was subsequently reinstituted. Discontinuation of therapy was considered if adverse events persisted or worsened for more than four weeks without improvement after dose reduction was initiated.
Statistical analysis
Numerical patient values are presented as mean ± SD. Patients’ characteristics were compared according to SVR rate using the χ2 test for categorical variables.
RESULTS
The present study included 107 patients: 60 men and 47 women with a mean age of 46.08±8.38 years (range 21 to 61 years). The major demographic and clinical characteristics of the patients are summarized in Table 1. Serum ALT levels were not significantly associated with the grade of necroinflammation at liver biopsy (χ2=3.296; P=0.51) (Table 2). Despite the fact that most patients with grade A3 necroinflammation demonstrated elevated ALT levels compared with patients with grade A1, the difference was not statistically significant (P=0.069).
TABLE 1.
Patients’ baseline demographic and laboratory data (n=107)
| Sex, male/female, n (%) | 60 (56.07)/47(43.93) |
| Age, years (mean ± SD) | 46.08±8.38 |
| Body mass index, kg/m2 (mean ± SD) | 28.03±1.327 |
| Alanine aminotransferase, IU/dL (mean ± SD) | 53.093±24.762 |
| Aspartate aminotransferase, IU/dL (mean ± SD) | 48.384±31.731 |
| Hepatitis C virus RNA, IU/mL (mean ± SD) | 53,5579.9±50,8857.3 |
| Metavir necroinflammation score, n (%) | |
| A0 | 0 (0) |
| A1 | 18 (16.82) |
| A2 | 67 (62.62) |
| A3 | 22 (20.56) |
| Metavir fibrosis score, n (%) | |
| F0 | 0 (0) |
| F1 | 26 (24.3) |
| F2 | 48 (44.86) |
| F3 | 24 (22.43) |
| F4 | 9 (8.41) |
TABLE 2.
Relationship between serum alanine aminotransferase (ALT) levels and necroinflammation
| Necroinflammation score |
ALT level |
|
|---|---|---|
| Normal | Elevated | |
| A1 (n=18) | 10 (55.56) | 8 (44.44) |
| A2 (n=67) | 27 (40.3) | 40 (59.7) |
| A3 (n=22) | 6 (27.27) | 16 (72.73) |
Data presented as n (%)
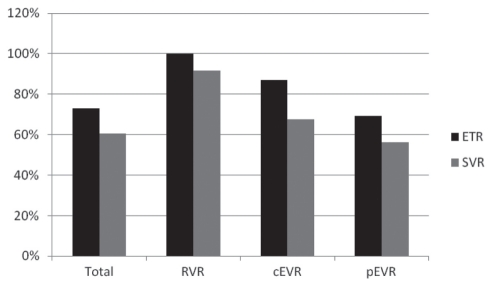
Ninety-four patients (87.85%) from the present series achieved either pEVR or cEVR, and completed the 48-week treatment course. The response to combination therapy is summarized in Table 3. Overall, ETR was 72.9%, SVR was 60.7% and the relapse rate was 16.67%. Patients who achieved an RVR comprised 22.4% of the study population. All of these patients achieved cEVR and ETR. The percentage of patients who achieved cEVR and pEVR without an RVR were 28.97% and 36.45%, respectively. The highest SVR rate occurred in patients with RVR (91.7%), and this rate was significantly higher than in patients with cEVR (67.74%; χ2=4.539; P=0.033) and patients with pEVR (56.14%; χ2=8.768; P=0.003). The difference in the SVR rate between patients with cEVR and pEVR was not statistically significant (χ2=0.936; P=0.333). Differences in relapse rates were not statistically significant among patient subsets regarding rate of virus elimination, despite being somewhat higher in patients with cEVR and pEVR than in patients with RVR. Response to treatment is summarized in Figure 1.
TABLE 3.
Response to combination therapy in all patients
| ETR | SVR | Relapse | ||
|---|---|---|---|---|
| Total | 107 (100) | 78 (72.9) | 65 (60.7) | 13 (16.67) |
| RVR | 24 (22.43) | 24 (100) | 22 (91.7) | 2 (8.33) |
| cEVR | 31 (28.97) | 27 (87.1) | 21 (67.74)* | 6 (22.22) |
| pEVR | 39 (36.45) | 27 (69.23)*** | 22 (56.14)** | 5 (18.52) |
Data presented as n (%).
P=0.033 compared with rapid virological responders (RVR);
P=0.003 compared with RVR;
P=0.00025 compared with RVR. cEVR Complete early virological responders; ETR End of treatment response; pEVR Partial early virological responders; SVR Sustained virological response
Figure 1).
Treatment response in all patients. ETR End-of-treatment response; cEVR Complete early virological response; pEVR Partial early virological response; RVR Rapid virological response; SVR Sustained virological response
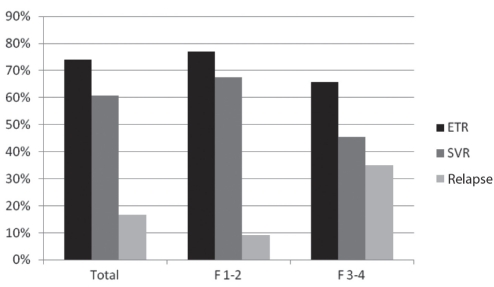
The degree of liver fibrosis was significantly associated with ETR, SVR and relapse rate. Patients with Metavir F1 and F2 degree of liver fibrosis achieved higher ETR rates (74.3%) than patients with an F3 or F4 degree of liver fibrosis (65.7%; χ2=4.956; P=0.026). SVR was also higher in patients with F1 and F2 (67.57%) compared with patients with F3 and F4 degree of fibrosis (45.45%; χ2=5.665; P=0.017) (Table 4, Figure 2). The same difference was also identified in relapse rate (F1 and F2, 9.1%; F3 and F4, 34.8%; χ2=7.708; P=0.005).
TABLE 4.
Response to combination therapy according to degree of liver fibrosis
| Fibrosis score | Total, n | ETR | SVR | Relapse |
|---|---|---|---|---|
| F1–F2 | 74 | 55 (74.3)* | 50 (67.57)** | 5 (9.1)*** |
| F3–F4 | 33 | 23 (65.7) | 15 (45.45) | 8 (34.8) |
Data presented as n (%) unless indicated otherwise.
P=0.026;
P=0.017;
P=0.005. ETR End-of-treatment response; SVR Sustained virological response
Figure 2).
Comparison of response to treatment and degree of hepatic fibrosis based on Metavir score (F1 to F4). ETR End-of-treatment response; SVR Sustained virological response
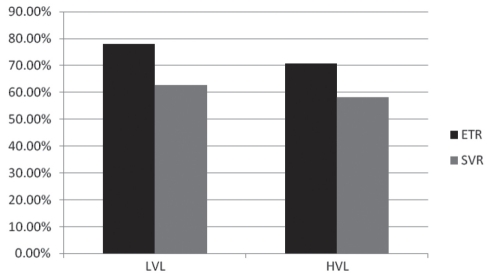
ETR in patients with LVL (74.58%) was slightly higher than in those with HVL (70.83%), but this difference was not statistically significant (χ2=0.188; P=0.665). The difference between SVR in patients with LVL (62.71%) and patients with HVL (58.33%) was also not significant (χ2=0.213, P=0.645 [Table 5, Figure 3]). Relapse rates in these two subsets of patients were also not statistically significant (15.91% in LVL; 17.65% in HVL; χ2=0.0417; P=0.838).
TABLE 5.
Response to combination therapy according to baseline virological load
| Total | ETR | SVR | Relapse | |
|---|---|---|---|---|
| LVL | 59 (55.14) | 44 (74.58) | 37 (62.71) | 7 (15.91) |
| HVL | 48 (44.86) | 34 (70.83) | 28 (58.33) | 6 (17.65) |
Data presented as n (%). High virological load (HVL; serum hepatitis C virus [HCV] RNA level of greater than 600,000 IU/mL) and low virological load (LVL; serum HCV RNA level of 50 IU/mL to less than 600,000 IU/mL) were both determined using a sensitive real-time polymerase chain reaction assay (Cobas Amplicor HCV monitor, version 2.0, Roche Diagnostics, USA, lower limit of quantitation = 50 IU/mL); ETR End-of-treatment response; SVR Sustained virological response
Figure 3).
Comparison of treatment response between the patient group with a baseline low virological load (LVL; serum hepatitis C virus [HCV] RNA level of 50 IU/mL to less than 600,000 IU/mL) and the group with a baseline high virological load (HVL; serum HCV RNA level of greater than 600,000 IU/mL) using a sensitive real-time polymerase chain reaction assay (Cobas Amplicor HCV monitor, version 2.0, Roche Diagnostics, USA, lower limit of quantitation = 50 IU/mL). ETR End-of-treatment response; SVR Sustained virological response
Patient characteristics including age, sex, body mass index, baseline serum ALT levels, fasting blood glucose and thyroid-stimulating hormone were not significantly associated with response to treatment.
Adverse events of treatment are summarized in Table 6. Hematological abnormalities were the most common of these events. However, none of these events required the discontinuation of treatment in any patients included in the present study. Temporary dose reduction of peginterferon by 50% (one to two doses) was indicated in three patients (two for neutropenia and one for thrombocytopenia). Dose adjustment of ribavirin according to hemoglobin level was indicated in 19 patients. The minimum dose of ribavirin used after reduction was 800 mg/day. Erythropoietin- or granulocyte-stimulating factors were not used. Citalopram (10 mg/day orally), a serotonin reuptake inhibitor, was used successfully in six patients to control depressive symptoms. Other minor adverse events included pruritus – which was controlled by antihistaminic agents in most cases – cough and fatigue. No treatment-related mortality has since occurred.
TABLE 6.
Major adverse events of treatment
| Neutropenia (<1000/mL) | 19 (17.76) |
| Thrombocytopenia (<75,000/mL) | 13 (12.15) |
| Hemolytic anemia (related to ribavirin) | 19 (17.76) |
| Fatigue | 14 (13.08) |
| Pruritus | 7 (6.54) |
| Depression | 6 (5.61) |
| Cough | 5 (4.67) |
Data presented as n (%)
DISCUSSION
Response to treatment of genotype 4 CHC was a matter of debate. Earlier reports have shown that these patients respond poorly to conventional interferon and ribavirin combination therapy (3–6). However, this view was changed after the introduction of pegylated interferon. In a meta-analysis (7), the rate of SVR in patients with genotype 4 CHC using peginterferon and ribavirin combination therapy was 55%. Studies involving Egyptian patients with genotype 4 CHC reported SVR rates of 56% to 67.9% after treatment with the existing two peginterferon α-2a and α-2b agents (8–11), with the exception of a single report (12) that demonstrated an SVR rate of 33.3% after treatment with peginterferon α-2b. In a single study (15), the efficacy of a novel 20 kDa linear pegylated interferon α-2a (Reiferon Retard) was evaluated in Egyptian patients with genotype 4 CHC. This study reported an SVR of 56% following 48 weeks of combination therapy. Recently, a comparative study (17) of the two forms of peginterferon α-2a and α-2b reported a similar efficacy of both in the treatment of CHC patients infected with genotype 1, noting that the chemical difference of pegylation had no effect on the therapeutic efficacy of the drug. The overall SVR rate in our series was 60.7%, which is consistent with previous results in both international and Egyptian studies of patients with genotype 4 CHC.
The probability of achieving an SVR in the present study was tested against several factors including the rate of viral clearance from circulation, the degree of liver fibrosis and baseline viral load. The highest rate of SVR was achieved in patients who achieved an RVR and cleared viral RNA from circulation within four weeks of treatment. The concept of the effect of viral kinetics on the likelihood of response to treatment was recently introduced to the scope of studies regarding CHC treatment with combination peginterferon and ribavirin. An early report (18) demonstrated that RVR was a strong predictive factor of SVR regardless of genotype or treatment regimen. The RVR rate was estimated to be 15% to 20% for genotype 1 and 66% for genotypes 2 and 3 (18,19). In a retrospective analysis (18), the predictive value of RVR in patients with genotype 1 treated with peginterferon alfa-2a in achieving SVR was 91%. In another study (9), the RVR rate was 22% in Egyptian patients with genotype 4 CHC treated with peginterferon α-2b. SVR rates in patients who achieved RVR were 88% following 48 weeks of treatment and 86% following 24 weeks of treatment (9). Furthermore, the predictive value of RVR in achieving SVR in patients infected with genotype 4 HCV and treated with peginterferon α-2a was reported to be 98% (10). In our series, the RVR rate was 22.43% and, of these, 91.7% achieved SVR – results that are comparable with those reported for the existing peginterferons (α-2a and α-2b) in the context of viral clearance rate. The SVR rate in our series was significantly higher in patients with RVR than in patients with EVR alone. However, the difference between SVR rates in patients with cEVR and pEVR was not significant.
The degree of liver fibrosis was widely studied as a pretreatment predictor of response to therapy in patients with CHC. Generally, the higher the degree of fibrosis, the lower the likelihood of response (20–22). This could be explained on the basis of insufficient delivery of peginterferon due to reduced peripheral portal flow (23) or decreased expression of hepatic interferon receptors with the progression of fibrosis (24). In our study, the rate of SVR was significantly higher in patients with Metavir F1 and F2 liver fibrosis than in patients with F3 and F4; this finding was related to both the lower rate of ETR and higher rates of relapse in patients with a higher degree of fibrosis.
There was no impact of baseline virological load on the rate of SVR in the present study despite that this factor was recognized to be one of the pretreatment predictors of response to therapy by many authors. The real-time PCR assay used in our study is sensitive, and we do not believe that its detection range could be a factor in this contradiction. However, our findings are consistent with two previously published studies using peginterferon α-2a (8) and α-2b (12) in Egyptian patients with genotype 4 CHC. These two studies, together with our study, shared a rather low mean baseline serum HCV RNA level, which may explain this contradiction. The adverse events that occurred in our patients were not sufficiently serious to discontinue treatment, which suggests good drug tolerability. Most of these events were mild to moderate and responded to the dose adjustment required. These findings are consistent with previous reports (25–27) regarding the safety of a combination peginterferon and ribavirin regimen in patients with CHC.
Serum ALT level was not a good predictor of the degree of hepatic necroinflammation in our series. This finding undermines the reliability of solely using this particular biochemical test in the decision-making process regarding treatment initiation. Therefore, liver biopsy remains the gold standard test in this respect. The need for noninvasive tests for the assessment of necroinflammation and degree of fibrosis remains.
CONCLUSION
A novel 20 kD pegylated interferon α-2a (Reiferon retard) derived from H polymorpha is an effective agent for the treatment of genotype 4 CHC, with a reasonable SVR rate (60.7%), and is well tolerated and safe.
Footnotes
CONFLICTS OF INTEREST: The authors have no conflicts of interest to declare.
REFERENCES
- 1.Ray SC, Arthur RR, Carella A, Bukh J, Thomas D. Genetic epidemiology of hepatitis C virus throughout Egypt. J Infect Dis. 2000;182:698–707. doi: 10.1086/315786. [DOI] [PubMed] [Google Scholar]
- 2.Zeuzem S. Heterogeneous virologic response rates to interferon based therapy in patients with chronic hepatitis C: Who responds less well? Ann Intern Med. 2004;40:370–81. doi: 10.7326/0003-4819-140-5-200403020-00033. [DOI] [PubMed] [Google Scholar]
- 3.El-Zayadi A, Simmonds P, Dabbous H, Prescott L, Selim O, Ahdy A. Response to interferon-alpha of Egyptian patients infected with hepatitis C virus genotype 4. J Viral Hepat. 1996;3:261–4. doi: 10.1111/j.1365-2893.1996.tb00052.x. [DOI] [PubMed] [Google Scholar]
- 4.El-Zayadi A, Selim O, Haddad S, et al. Combination treatment of interferon alpha-2b and ribavirin in comparison to interferon monotherapy in treatment of chronic hepatitis C genotype 4 patients. Ital J Gastroenterol Hepatol. 1999;31:472–5. [PubMed] [Google Scholar]
- 5.Kamal SM, Madwar MA, Peters T, Fawzy R, Rasenack J. Interferon therapy in patients with chronic hepatitis C and schistosomiasis. J Hepatol. 2000;32:172–4. doi: 10.1016/s0168-8278(00)80207-x. [DOI] [PubMed] [Google Scholar]
- 6.Koshy A, Madda JP, Marcellin P, Martinot M. Treatment of hepatitis C virus genotype 4-related cirrhosis: Ribavirin and interferon combination compared with interferon alone. J Clin Gastroenterol. 2002;35:82–5. doi: 10.1097/00004836-200207000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Khuroo MS, Dahab ST. Meta-analysis: A randomized trial of peginterferon plus ribavirin for the initial treatment of chronic hepatitis C genotype 4. Aliment Pharmacol Ther. 2004;20:931–8. doi: 10.1111/j.1365-2036.2004.02208.x. [DOI] [PubMed] [Google Scholar]
- 8.El Makhzangy H, Esmat G, Said M, et al. Response to pegylated interferon alfa-2a and ribavirin in chronic hepatitis C genotype 4. J Med Virol. 2009;81:1576–83. doi: 10.1002/jmv.21570. [DOI] [PubMed] [Google Scholar]
- 9.Kamal SM, El Kamary SS, Shardell MD, et al. Pegylated interferon alpha-2b plus ribavirin in patients with genotype 4 chronic hepatitis C: The role of rapid and early virologic response. Hepatology. 2007;46:1732–40. doi: 10.1002/hep.21917. [DOI] [PubMed] [Google Scholar]
- 10.Derbala MF, El Dweik NZ, Al Kaabi SR, et al. Viral kinetics of HCV genotype-4 during pegylated interferon alpha 2a: Ribavirin therapy. J Vir Hepatol. 2008;15:591–9. doi: 10.1111/j.1365-2893.2008.00988.x. [DOI] [PubMed] [Google Scholar]
- 11.Derbala M, Rizk N, Al-Kaabi, et al. Adiponectin changes in HCV-genotype 4: Relation to liver histology and response to treatment. J Vir Hepatit. 2009;16:689–6. doi: 10.1111/j.1365-2893.2009.01096.x. [DOI] [PubMed] [Google Scholar]
- 12.Derbala M, Amer A, Bener A, Lopez AC, Omar M, El Ghannam M. Pegylated interferon-alpha 2b-ribavirin combination in Egyptian patients with genotype 4 chronic hepatitis. J Viral Hepatitis. 2005;12:380–5. doi: 10.1111/j.1365-2893.2005.00604.x. [DOI] [PubMed] [Google Scholar]
- 13.Bain VG, Kaita KD, Marotta P, et al. Safety and antiviral activity of albinterferon alfa-2b dosed every four weeks in genotype 2/3 chronic hepatitis C patients. Clin Gastroenterol Hepatol. 2008;6:701–6. doi: 10.1016/j.cgh.2008.02.056. [DOI] [PubMed] [Google Scholar]
- 14.Zeuzem S, Yoshida EM, Benhamou Y, et al. Albinterferon alfa-2b dosed every two or four weeks in interferon-naïve patients with genotype 1 chronic hepatitis C. Hepatology. 2008;48:407–17. doi: 10.1002/hep.22403. [DOI] [PubMed] [Google Scholar]
- 15.Esmat G, Abdel-Fattah S. Evaluation of a novel pegylated interferon alpha-2a (Reiferon Retard®) in Egyptian patients with chronic hepatitis C-genotype 4. Dig Liv Dis. 2009;(Suppl 3):17–9. [Google Scholar]
- 16.Gellissen G. Hansenula polymorpha – Biology and Applications. Weinheim: Wiley-VCH; 2002. p. 352. [Google Scholar]
- 17.McHutchison JG, Lawitz EJ, Shiffman ML, et al. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med. 2009;6:580–93. doi: 10.1056/NEJMoa0808010. 361; [DOI] [PubMed] [Google Scholar]
- 18.Ferenci P, Fried MW, Shiffman ML, et al. Predicting sustained virological responses in chronic hepatitis C patients treated with peginterferon alfa-2a (40 KD)/ribavirin. J Hepatol. 2005;43:425–33. doi: 10.1016/j.jhep.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Shiffman ML, Suter F, Bacon BR, et al. Peginterferon alfa-2a and ribavirin for 16 or 24 weeks in HCV genotype 2 or 3. N Engl J Med. 2007;357:124–34. doi: 10.1056/NEJMoa066403. [DOI] [PubMed] [Google Scholar]
- 20.Davis GL, Lau JY. Factors predictive of a beneficial response to therapy of hepatitis C. Hepatology. 1997;26(Suppl 1):122S–127S. doi: 10.1002/hep.510260721. [DOI] [PubMed] [Google Scholar]
- 21.Poynard T, Marcellin P, Lee SS, et al. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT) Lancet. 1998;352:1426–32. doi: 10.1016/s0140-6736(98)07124-4. [DOI] [PubMed] [Google Scholar]
- 22.Karino Y, Toyota J, Sugawara M, et al. Hepatitis C virus genotypes and hepatic fibrosis regulate 24-h decline of serum hepatitis C virus RNA during interferon therapy in patients with chronic hepatitis C. J Gastroenterol Hepatol. 2003;18:404–10. doi: 10.1046/j.1440-1746.2003.03009.x. [DOI] [PubMed] [Google Scholar]
- 23.Koda M, Murawaki Y, Kawasaki H, Ikawa S. Portal blood velocity and portal blood flow in patients with chronic viral hepatitis: Relation to histological liver fibrosis. Hepatogastroenterology. 1996;43:199–202. [PubMed] [Google Scholar]
- 24.Lee CM, Kee KM, Hung CH, et al. Hepatic interferon receptor mRNA expression: Clinical relevance and its relationship with effectiveness of interferon plus ribavirin therapy in patients with genotype 1b hepatitis C virus infection. Antivir Ther. 2006;11:17–23. [PubMed] [Google Scholar]
- 25.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomised trial. Lancet. 2001;358:958–65. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 26.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–82. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 27.Hadziyannis SJ, Sette H, Morgan TR, Jr, et al. PEGASYS International Study Group Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: A randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346–55. doi: 10.7326/0003-4819-140-5-200403020-00010. [DOI] [PubMed] [Google Scholar]