Abstract
Mycobacterium avium paratuberculosis (MAP) is an obligate intracellular organism that has frequently been associated with Crohn’s disease (CD). Because CD is a chronic inflammatory condition, many researchers have speculated that an infectious agent must be the cause of CD. MAP has often been proposed to be one such agent; however, despite considerable research, the evidence remains inconclusive. Higher levels of MAP have been found in the tissues and blood of CD patients than in controls, forming the foundation for much of the research into the role of MAP in CD and the primary argument in support of a causative role for MAP in CD. MAP is a slow-growing and fastidious organism that is difficult to grow in culture and, therefore, challenging to detect in patients. As a result, there has been variability in the results of studies attempting to detect the presence of MAP in CD patients, and considerable controversy over whether this organism has a causative role in the etiology of CD. Two main hypotheses exist with respect to the role of MAP in CD. The first is that MAP is a principal cause of CD, while the second is that MAP is more prevalent because of the immune dysfunction seen in CD but does not play a causative role. Clinicians are often faced with questions regarding the role of this organism and the need to treat it. The present article attempts to provide an overview of the controversy including the nature of the mycobacterium, the difficulty in detecting it, the use of antimycobacterial agents to treat it and the effect of immunosuppressive agents – all from a clinician’s perspective. Although the role of MAP in CD remains controversial and an area of considerable research, it is currently only of academic interest because there is no clinically useful test to identify the presence of the organism, and no evidence to support the use of antibiotics to eradicate it for the treatment of CD.
Keywords: Crohn’s disease, Infliximab, Mycobacterium avium paratuberculosis
Abstract
La Mycobacterium avium paratuberculosis (MAP) est un organisme intracellulaire obligatoire souvent associée à la maladie de Crohn (MC). Puisque la MC est une maladie inflammatoire chronique, de nombreux chercheurs spéculent qu’un agent infectieux doit en être responsable. On a souvent postulé que la MAP puisse être l’un de ces agents. Cependant, malgré des recherches considérables, les données probantes demeurent non concluantes. On a découvert des taux plus élevés de MAP dans les tissus et le sang de patients atteints de la MC que dans ceux des sujets témoins, ce qui a jeté les bases d’une grande partie de la recherche sur le rôle de la MAP dans la MC et qui constitue le principal argument à l’appui du rôle étiologique de la MAP dans la MC. La MAP est un organisme exigeant à la croissance lente qu’il est difficile de cultiver. Il est donc difficile à déceler chez les patients. C’est pourquoi on constate une certaine variabilité dans les résultats des études visant à déceler la présence de MAP chez les personnes atteintes de la MC et qu’une importante controverse perdure sur son rôle causal éventuel dans l’étiologie de la MC. Il existe deux grandes hypothèses quant au rôle de la MAP dans la MC. La première, c’est que la MAP constitue l’une des causes principales de la MC, et la deuxième, c’est qu’elle est plus prévalente en raison de la dysfonction immune observée en cas de MC, mais qu’elle n’y joue pas un rôle causal. Les cliniciens font souvent face à des questions au sujet du rôle de cet organisme et du besoin de le traiter. Le présent article vise à donner un aperçu de la controverse, y compris la nature de la mycobactérie, la difficulté à la déceler, le recours à des agents antimycobactériens pour la traiter et l’effet de ses agents immunosuppresseurs, le tout selon le point de vue d’un clinicien. Même si le rôle de la MAP dans la MC demeure controversé et qu’il suscite de nombreuses recherches, il demeure limité à un intérêt théorique puisqu’il n’existe aucun test utile en clinique pour déterminer la présence de cet organisme et aucune donnée probante pour étayer que des antibiotiques pourront l’éradiquer dans le cadre du traitement de la MC.
Crohn’s disease (CD) is a chronic transmural inflammatory systemic condition that has the potential to involve any part of the human intestinal tract. The highest incidence and prevalence of CD has been reported in Northern Europe, the United Kingdom and North America, where rates appear to be stabilizing (1). The etiology of the disease remains unknown; however, CD is generally believed to involve genetic, environmental and immune factors in association with an inappropriate inflammatory response (2). Mycobacterium avium subspecies paratuberculosis (MAP) is an obligate intracellular mycobacteria that has been proposed to be a possible environmental cause of CD.
The debate over the evidence supporting the role of MAP has been growing since the initial culture of this bacteria from the intestinal tissues of several patients with CD by Chiodini et al (3) in 1984. MAP is best known as the causative agent of Johne’s disease – an inflammatory bowel disease of cattle – which is similar both clinically and pathologically to CD (4). Both diseases have similar symptoms including weight loss and chronic diarrhea. They are both chronic inflammatory illnesses characterized by the formation of granulomata and inflammatory changes in the small bowel and colon. The similarity between the two disease states supports the hypothesis that MAP is the etiological agent of CD; however, to date, the evidence has been inconclusive.
Newer means of MAP culture and detection, genetic susceptibility studies and attempts at treating MAP in CD have added more evidence to both sides of the debate; however, most of these data are only of academic interest to the practising physician and of little clinical benefit to the patient. In the present article, we attempt to review some of the newer evidence both for and against the role of MAP as the etiological agent of CD. We focus on the areas of specific clinical relevance to the practising physician.
MAP
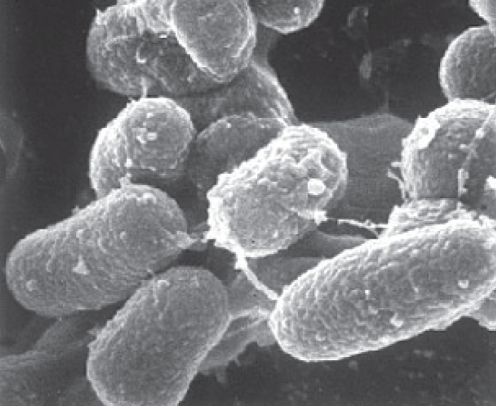
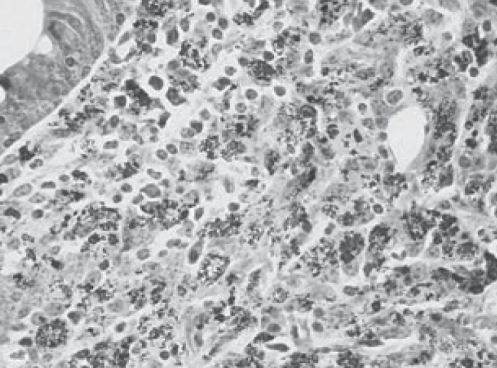
MAP has a thick, waxy cell wall composed of 60% lipid (Figure 1). This cell wall makes the organism acid fast, hydrophobic and resistant to chemical (chlorine) and heat (pasteurization) destruction (Figure 2). The thick wall also restricts the uptake of nutrients, making the organism very slow to grow and, as a result, very difficult to culture (5). MAP is a subspecies member of the M avium complex; however, it has many phenotypic differences from the other M avium subspecies (6). The M avium complex typically causes disease only in an immunocompromised host; however, MAP has been shown to be pathogenic in immunocompetent ruminants and primates (7). This would imply that, if MAP could infect immunocompetent patients and cause CD, it should be found in abundance in the blood or tissues of those patients; however, this has not been the case (8). MAP is distinguished from the other mycobacterium species by its extremely slow growth, its inability to produce mycobactin and the presence of the insertion sequence (IS) 900 (5). It has been postulated that the difficulty in isolating the bacteria from humans relates to the low bacillary burden or dormancy of the mycobacteria (9). The inherent difficulties in culturing and detecting this agent are at the cornerstone of the debate as to whether MAP is a host-associated pathogen or simply ubiquitous in the environment.
Figure 1).
Scanning electron micrograph showing the thick, waxy coat of Mycobacterium avium paratuberculosis. Courtesy of Johne’s Testing Center, School of Veterinary Medicine, University of Wisconsin (Wisconsin, USA)
Figure 2).
Ziehl-Neelsen stain from the terminal ileum of a cow showing the acid-fast nature of Mycobacterium avium paratuberculosis. Courtesy of Johne’s Testing Center, School of Veterinary Medicine, University of Wisconsin (Wisconsin, USA)
Proposed pathogenesis
There are several proposed mechanisms of MAP involvement in the pathogenesis of CD. Most theories are based on the idea that MAP (an environmental pathogen) is ingested by a susceptible host (genetic susceptibility), and penetrates the intestinal wall via a leaky epithelium. The mycobacteria induces disease by stimulating a dysregulated inflammatory response and is perpetuated by a maladaptive immune response. MAP is proposed to enter the human host through a contaminated retail milk or water supply. Once inside the host, it is taken up by intestinal macrophages where MAP has been shown to exist in a spheroplast form (ie, lacking a cell wall) (10). In animals, MAP can exist in this latent or nonpathogenic state for years. Once inside the macrophage, the mycobacteria causes the cell to produce a number of inflammatory mediators including tumour necrosis factor-alpha (TNF-α), interleukin (IL)-1 and IL-10 (11), which are all involved in the initiation and maintenance of inflammation encountered in CD. These inflammatory mediators stimulate naive T cells to preferentially differentiate into T-helper 1 cells which, in turn, stimulate macrophages to secrete the large amounts of TNF-α, IL-1 and IL-6 seen in CD (1). These inflammatory cytokines and activated natural killer cells mediate the tissue damage seen in CD. A recent study (12) showed that peripheral blood mononuclear cells (PBMCs) isolated from patients with CD can be induced to produce more T cells and secrete higher levels of TNF-α and IL-10 by coincubating the cells with MAP. The authors suggested that previous exposures and immune engagement with MAP is more common in patients with CD.
Detection of MAP
MAP was initially cultured directly from the pathology specimens of two patients with CD (3). This finding formed the basis for the subsequent search for evidence linking MAP to CD. Other attempts to directly culture MAP from human tissues were inconsistent, largely because of the slow growth and fastidious nature of this organism. Unlike Mycobacterium tuberculosis, MAP lacks a cell wall that can be positively Ziehl-Neelsen stained. Improvement in culture media and the discovery of the MAP-specific DNA insertion sequence IS900 has enabled the detection of MAP from the blood and tissue of infected individuals. The IS900 element is highly specific for the MAP organism (13), and its detection by polymerase chain reaction (PCR) methods is considered to be the reference standard for distinguishing MAP from other mycobacteria (14). With the assistance of PCR to detect IS900, MAP has been successfully cultured and identified directly from human intestinal tissue, where it was found more frequently in CD patients than in controls (13). MAP has also been successfully cultured from PBMCs of patients with CD (15).
Children at an early stage of CD diagnosis have also been shown to have higher levels of MAP in mucosal biopsies and PBMCs than in noninflammatory bowel disease (IBD) patients (16). In this study, the authors collected mucosal biopsies and/or PBMC specimens from 142 children – 62 with CD, 26 with ulcerative colitis (UC) and 54 with non-IBD. Using the IS900 DNA sequence and PCR, they found that 39% of patients with CD and 16% of those in the non-IBD group tested positive for MAP. MAP-positive DNA was detected in 16% of the CD patients, 8% of the UC patients and none (0%) of the non-IBD patients. A small subset of patients had mucosal biopsies cultured for viable MAP, with 40% of the CD patients having positive cultures; none of the UC or non-IBD patients were culture positive.
In-situ hybridization (ISH) has been used to detect MAP DNA obtained from human tissues, but with variable results. A recent meta-analysis (9) of MAP detection methods found the results of PCR assays to be more reliable than those using in-situ hybridization techniques. PCR detection of the IS900 DNA sequence has limited utility in clinical practice for a variety of reasons. Most of the PCR tests are performed on tissue samples which, unlike peripheral blood, require surgery or endoscopy to obtain. There have been variable results with the use of PCR, which may be due to the varying sensitivities of the assay used, the low numbers of bacteria present and an inability to distinguish viable MAP from MAP DNA (9). Furthermore, PCR is expensive, labour intensive and not widely available to the practising clinician, making it impractical as a screening test for CD patients.
Serological testing for antibodies to p35 and p36 (MAP-specific antigens) was reported to be higher in CD patients than controls (17); however, these antigens are also present in M avium species avium, therefore, diluting the specificity of these tests. Another case-control study using an ELISA-based technique with MAP-specific antibodies found no difference in the serum antibody levels of patients with CD, UC and controls (18).
A newer method has recently been tested using protein tyrosine phosphatase A, which is produced by MAP when it infects macrophages. This protein appears to be involved in blocking signal transduction of the host, thereby preventing macrophages from killing the bacteria (19). Using an ELISA, antibodies directed against these proteins, which are specific to MAP, have been reliably found in the serum of infected patients (20). This may prove to be a more reliable method of detecting MAP with good clinical utility because it can be easily performed on serological specimens.
THE EVIDENCE (TABLE 1)
TABLE 1.
Evidence supporting and not supporting Mycobacterium avium subspecies paratuberculosis (MAP) as the etiological agent in Crohn’s disease (CD)
|
The evidence supporting MAP as a cause of CD |
|
|
The evidence not supporting MAP as a cause of CD |
|
Johne’s disease
Proponents of a causative role for MAP in CD acknowledge the similarities between Johne’s disease and CD. However, despite similarities to Johne’s disease, CD is not the same entity. The fibrosis, fistula, fissures and pseudopolyps commonly encountered in CD are typically absent in Johne’s disease (21). Furthermore, humans who are exposed to animals infected with MAP do not show a higher prevalence of CD, as evidenced by the fact that farmers whose cattle had Johne’s disease have not been shown to have a higher prevalence of CD (22). MAP has been found in milk and water supplies, and is capable of surviving commercial pasteurization methods (23,24). However, evidence supporting the consumption of foods containing MAP as a cause of CD is lacking (25).
Presence of MAP
Multiple studies (15,16,26,27) have detected MAP in the tissues and blood of CD patients with a greater frequency than those without CD. A recent meta-analysis (9) of the nucleic acid-based detection methods for MAP in CD, which mainly involves the use of PCR to detect the IS900 sequence, showed that the majority of studies have found higher levels of MAP in CD patients. However, MAP has also been isolated from individuals without CD, albeit in smaller numbers (13,28). The study by Kirkwood et al (16) demonstrated no association between MAP detected in tissue and PBMC samples. The authors proposed several mechanisms for this lack of association. It may have been due to the collection of specimens at different stages of disease, because gut biopsy samples did not contain foci of infection, or that MAP exists as either a local infection confined to the gut mucosa or as a systemic infection with limited tissue invasion. Finding MAP in healthy controls and UC patients suggests that MAP infection is not sufficient to cause CD, and that other factors are necessary to induce disease. MAP has also been cultured from the breast milk of two mothers with CD, while not found in five lactating controls (29), suggesting that, in CD, MAP is a systemic infection able to enter the breast milk. Alternatively, as mentioned previously, MAP may have systemic and localized forms of infection that do not necessarily occur simultaneously. Despite finding MAP in breast milk, there is no evidence to support the vertical transmission of MAP and CD to offspring (30).
Immune dysfunction
The NOD2/CARD15 gene has previously been shown to be a susceptibility gene for the development of CD (11,31). Patients harbouring these mutations have a defective innate response to bacterial infection; thus, it has been proposed that the ineffective clearance of intracellular MAP is the link between MAP and CD (32). Further evidence to support this notion comes from the fact that PBMCs obtained from CD patients with mutations in NOD2 display defective recognition of MAP antigens (33). Furthermore, cows possessing single nucleotide polymorphisms in the CARD15 gene were shown to have greater susceptibility to MAP infection (34).
The persistence of viable MAP within macrophages may be a mechanism by which MAP could thrive under conditions of immunosuppressive treatment. In fact, the opposite has been reported for immunosuppressive therapy with corticosteroids, which have been associated with decreased levels of MAP DNA in the intestinal tissues of CD patients (14). It is generally accepted that MAP is a paucibacillary infection and an obligate intracellular, cell wall-deficient organism similar to other mycobacterial species. M tuberculosis proliferates with anti-TNF-α antibodies or corticosteroid treatment. Mycobacterium intracellulare flourishes as CD4 T cell counts fall with acquired immunosuppression; however, similar results have not been found in MAP infection (30,35). It is difficult to reconcile the idea that MAP would be pathogenic because of a defective innate host immune response, yet decreased levels of MAP were detected under conditions of immunosuppression with corticosteroids in which similar mycobacteria flourish. One possible explanation is that MAP does not actually cause CD but is able to grow in the presence of the immune dysfunction and inflammation encountered in CD. MAP-specific high TNF-α secretion has been found in CD but not in normal or IBD control patients, suggesting that there is a defect in the handling of MAP in CD (32). The answer may lie in the effect that anti-TNF-α antibodies have on MAP levels in CD patients. To date, there have been no published studies regarding the effects of anti-TNF-α antibodies on MAP levels.
Antibiotics
Several studies have investigated treating CD with antituberculous drugs. A meta-analysis (36) of the randomized controlled trials of antituberculous therapy for maintenance of remission in CD provided mixed results. There were two trials that showed a statistically significant benefit from using antituberculous therapy when steroids were used to induce remission and the antituberculous drugs were used as maintenance therapy. The pooled OR for these trials was 3.37 (95% CI 1.38 to 8.24), with an absolute risk reduction of 29% and a number needed to treat of 3. However, in the three trials that did not use steroids to induce remission, there was no benefit from therapy. The pooled OR for the maintenance of remission was 0.70 (95% CI 0.39 to 1.25), with a number needed to treat of 15. The authors concluded that antituberculous therapies may be beneficial when they are used following a course of antituberculous drugs and corticosteroids to induce remission (36).
Selby et al (37), attempted to answer this question directly. Their trial of antibiotics with activity against MAP provided some of the strongest evidence against the role of MAP as the etiological agent of CD. In this trial, 213 adult patients with active CD were randomly assigned to receive either clarithromycin, rifabutin and clofazimine, or placebo medications for 16 weeks. All of the patients were also given oral prednisolone 40 mg/day on a predetermined tapering dose to zero over 16 weeks. Those who were in remission at the end of the 16-week period continued their study medications for a total of two years, with a further one-year follow-up after discontinuation of medication. At week 16, there were significantly more subjects in remission in the antibiotic arm (66%) than in the placebo arm (50%) (P=0.02). Of the 122 patients entering the maintenance phase at 16 weeks, there was no significant difference between the antibiotic treatment and control groups throughout the remainder of the treatment phase or the one-year follow-up with respect to relapses of disease. The authors concluded that their study did not support a significant ongoing pathogenic role for MAP in the majority of patients with CD. One potential problem with this trial was that the presence of MAP was not determined before, during or after therapy; therefore, efficacy of the therapy at eradicating MAP could not be determined.
CAUSATION
When investigating the etiology of CD, one must consider the burden of proof that would be required to establish causation. In the 1880s, Koch (38) originally postulated four requirements for establishing causation in disease (Table 2). Ironically, Koch’s postulates were formulated in reference to establishing M tuberculosis as the etiological agent in tuberculosis. However, with the discovery of cholera and viral pathogens, it became clear that Koch’s postulates did not apply in all situations. The accepted definition for establishing causation continued to evolve over time and, by 1965, Hill’s criteria (39) formed a new standard (Table 3). Although more universally applicable, Hill still considered these rules as only a guideline or ‘viewpoints’ for establishing causation. MAP as a cause of CD can be a ‘necessary’ cause (MAP must be present for CD to exist), a ‘sufficient’ cause (MAP in the absence of other factors is capable of causing CD) or a ‘component’ cause (neither necessary nor sufficient, but does contribute to disease). Most of the research in the area of MAP in CD has attempted to identify MAP as a sufficient cause and has focused on trying to prove Koch’s postulates. That is, identifying the pathogen – MAP – isolating it from individuals with the disease, culturing it and giving it to individuals without the disease to induce disease. In fact, there is one report (3) of MAP being isolated and cultured from a patient with CD and given to a young goat, subsequently inducing Johne’s disease. Whether considering Koch’s postulates, Hill’s criteria or other methods of validating causal inference, it remains clear that there is no consensus on the role of MAP in CD. However, consideration of causal criteria is useful in that it helps to highlight the multicausal nature of a complex disease such as CD (40).
TABLE 2.
Koch’s postulates
|
From reference 38
TABLE 3.
Hill’s criteria
|
There appears to be two main possibilities for the role of MAP in CD. The first is that MAP is, in fact, a major ‘component’ cause of CD. The second is that MAP’s presence in patients with CD is not contributing to the etiology of CD but is a consequence of the immune dysregulation and inflammation already present. From a clinical standpoint, the evidence teaches us about the possible irrelevance of this distinction. We know that there is currently no effective anti-MAP treatment that significantly improves symptoms in CD. We are unlikely to be able to eradicate MAP from the environment and our food supply and, even if we could, there is no convincing evidence that doing so would impact the prevalence of CD. Furthermore, there is no proven safe, reliable and inexpensive method of detecting MAP that could be used in the diagnosis and, ultimately, the treatment of patients.
Perhaps it is more useful to consider MAP as being ‘associated’ with or a risk factor for developing CD. Other risk factors would include genetic factors such as the NOD2/CARD15 gene variations, and environmental factors such as smoking and nonsteroidal anti-inflammatory drug use. Such a risk factor model would enable us to tailor treatment toward the identified risk factors of each individual patient. As an analogy, consider the Framingham data (41), which have been used to identify multiple risk factors as predictors for the development of coronary artery disease. Management for coronary disease is aimed at all of the identified risk factors for coronary disease within an individual patient rather than focussing on one main etiology. With respect to CD, this means directing treatment toward all of the contributing causes of CD, which may, in the future, include MAP as well as immune dysfunction.
The highest priority for further defining MAP as a risk factor or cause of CD should be the development of a reliable, readily accessible and, ideally, inexpensive method of detection. Such a test would not only help to better define the role of MAP in CD but also change the way we manage this complex disease. A recent trial (20) used one such test to compare the antibodies against the MAP-specific protein – protein tyrosine phosphatase A – in patients with CD and control subjects. The results revealed a statistically significant difference between the antibody levels in the CD group versus the control group. If MAP infection is ‘associated’ with (rather than causing) CD, MAP may prove to be a marker of inflammation or disease activity. In this situation, a clinically useful test to detect MAP will be critical to move MAP from the research setting into the clinic.
CONCLUSION
CD is a complex disease of unknown etiology, with genetic, environmental and immunological factors all likely being contributors. While many studies have found evidence of higher levels of MAP in CD patients than healthy individuals, there is no conclusive evidence supporting MAP as a cause of CD. The two main hypotheses for the role of MAP in CD are that MAP is a significant cause of CD or, alternatively, that MAP is more prevalent in CD because of the immune dysfunction found in individuals with CD. For the clinician, there is no effective method to detect MAP, nor is there any evidence to support the use of anti-MAP therapies to treat patients with CD. However, hopefully, in the near future, there will be a more reliable test to detect MAP that will better define its role in the pathogenesis of CD and aid in the managenment of this disease.
REFERENCES
- 1.Baumgart DC, Carding SR. Inflammatory bowel disease: Cause and immunobiology. Lancet. 2007;369:1627–40. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- 2.Podolsky DK. Medical progress. N Engl J Med. 2002;347 doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 3.Chiodini RJ, Van Kruiningen HJ, Thayer WR, Merkal RS, Coutu JA. Possible role of mycobacteria in inflammatory bowel disease. Dig Dis Sci. 1984;29:1073–9. doi: 10.1007/BF01317078. [DOI] [PubMed] [Google Scholar]
- 4.McKenna SL, Keefe GP, Tiwari A, VanLeeuwen J, Barkema HW. Johne’s disease in Canada part II: Disease impacts, risk factors, and control programs for dairy producers. Can Vet J. 2006;47:1089–99. [PMC free article] [PubMed] [Google Scholar]
- 5.Rowe MT, Grant IR. Mycobacterium avium ssp. paratuberculosis and its potential survival tactics. Letters in Appl Microbiol. 2006;42:305–11. doi: 10.1111/j.1472-765X.2006.01873.x. [DOI] [PubMed] [Google Scholar]
- 6.Behr MA, Kapur V. The evidence for Mycobacterium paratuberculosis in Crohn’s disease. Curr Opin Gastroenterol. 2008;24:17–21. doi: 10.1097/MOG.0b013e3282f1dcc4. [DOI] [PubMed] [Google Scholar]
- 7.Mendoza JL, Lana R, Díaz-Rubio M. Mycobacterium avium subspecies paratuberculosis and its relationship with Crohn’s disease. World J Gastroenterol. 2009;15:417–22. doi: 10.3748/wjg.15.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pierce ES. Where are all the Mycobacterium avium subspecies paratuberculosis in patients with Crohn’s disease? PLoS Pathog. 2009;5:e1000234. doi: 10.1371/journal.ppat.1000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abubakar I, Myhill D, Aliyu SH, Hunter PR. Detection of Mycobacterium avium subspecies paratuberculosis from patients with Crohn’s disease using nucleic acid-based techniques: A systematic review and meta-analysis. Inflamm Bowel Dis. 2008;14:401–10. doi: 10.1002/ibd.20276. [DOI] [PubMed] [Google Scholar]
- 10.Chiodini R, Van Kruiningen H, Thayer W, Coutu J. Spheroplastic phase of mycobacteria isolated from patients with Crohn’s disease. J Clin Microbiol. 1986;24:357–63. doi: 10.1128/jcm.24.3.357-363.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogura Y, Bonen DK, Inohara N, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603–6. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 12.Sibartie S, Scully P, Keohane J, et al. Mycobacterium avium subsp. paratuberculosis (MAP) as a modifying factor in Crohn’s disease. Inflamm Bowel Dis. 2009 doi: 10.1002/ibd.21052. (In press). [DOI] [PubMed] [Google Scholar]
- 13.Bull TJ, McMinn EJ, Sidi-Boumedine K, et al. Detection and verification of Mycobacterium avium subsp. paratuberculosis in fresh ileocolonic mucosal biopsy specimens from individuals with and without Crohn’s disease. J Clin Microbiol. 2003;41:2915–23. doi: 10.1128/JCM.41.7.2915-2923.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Autschbach F, Eisold S, Hinz U, et al. High prevalence of Mycobacterium avium subspecies paratuberculosis IS900 DNA in gut tissues from individuals with Crohn’s disease. Gut. 2005;54:944–9. doi: 10.1136/gut.2004.045526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naser SA, Ghobrial G, Romero C, Valentine JF. Culture of Mycobacterium avium subspecies paratuberculosis from the blood of patients with Crohn’s disease. Lancet. 2004;364:1039–44. doi: 10.1016/S0140-6736(04)17058-X. [DOI] [PubMed] [Google Scholar]
- 16.Kirkwood CD, Wagner J, Boniface K, et al. Mycobacterium avium subspecies paratuberculosis in children with early-onset Crohn’s disease. Inflamm Bowel Dis. 2009:1643–55. doi: 10.1002/ibd.20967. [DOI] [PubMed] [Google Scholar]
- 17.Naser SA, Hulten K, Shafran I, Graham DY, El-Zaatari FA. Specific seroreactivity of Crohn’s disease patients against p35 and p36 antigens of M. avium subsp. paratuberculosis. Vet Microbiol. 2000;77:497–504. doi: 10.1016/s0378-1135(00)00334-5. [DOI] [PubMed] [Google Scholar]
- 18.Bernstein CN, Blanchard JF, Rawsthorne P, Collins MT. Population-based case control study of seroprevalence of Mycobacterium paratuberculosis in patients with Crohn’s disease and ulcerative colitis. J Clin Microbiol. 2004;42:1129–35. doi: 10.1128/JCM.42.3.1129-1135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bach H, Sun J, Hmama Z, Av-Gay Y. Mycobacterium avium subsp. paratuberculosis PtpA is an endogenous tyrosine phosphatase secreted during infection. Infect Immun. 2006;74:6540–6. doi: 10.1128/IAI.01106-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ko HH, Bach H, Raizman EA, Enns RA, Bressler B. Presence of serum antibodies against secreted proteins from Mycobacterium avium spp paratuberculosis in patients with Crohn’s disease. American Gastroenterological Society Digestive Disease Week; Chicago, Illinois. June 30 to May 4, 2009. [Google Scholar]
- 21.Chacon O, Bermudez LE, Barletta RG. Johne’s disease, inflammatory bowel disease, and Mycobacterium paratuberculosis. Annu Rev Microbiol. 2004;58:329–63. doi: 10.1146/annurev.micro.58.030603.123726. [DOI] [PubMed] [Google Scholar]
- 22.Jones PH, Farver TB, Beaman B, Cetinkaya B, Morgan KL. Crohn’s disease in people exposed to clinical cases of bovine paratuberculosis. Epidemiol Infect. 2006;134:49–56. doi: 10.1017/S0950268805004681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grant IR, Hitchings EI, McCartney A, Ferguson F, Rowe MT. Effect of commercial-scale high-temperature, short-time pasteurization on the viability of Mycobacterium paratuberculosis in naturally infected cows’ milk. Appl Environ Microbiol. 2002;68:602–7. doi: 10.1128/AEM.68.2.602-607.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Millar D, Ford J, Sanderson J, et al. IS900 PCR to detect Mycobacterium paratuberculosis in retail supplies of whole pasteurized cows’ milk in England and Wales. Appl Environ Microbiol. 1996;62:3446–52. doi: 10.1128/aem.62.9.3446-3452.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abubakar I, Myhill DJ, Hart AR, et al. A case-control study of drinking water and dairy products in Crohn’s disease – further investigation of the possible role of Mycobacterium avium paratuberculosis. Am J Epidemiol. 2007;165:776–83. doi: 10.1093/aje/kwk067. [DOI] [PubMed] [Google Scholar]
- 26.Sanderson JD, Moss MT, Tizard ML, Hermon-Taylor J. Mycobacterium paratuberculosis DNA in Crohn’s disease tissue. Gut. 1992;33:890–6. doi: 10.1136/gut.33.7.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hulten K, El-Zimaity HM, Karttunen TJ, et al. Detection of Mycobacterium avium subspecies paratuberculosis in Crohn’s diseased tissues by in situ hybridization. Am J Gastroenterol. 2001;96:1529–35. doi: 10.1111/j.1572-0241.2001.03751.x. [DOI] [PubMed] [Google Scholar]
- 28.Sechi LA, Gazouli M, Ikonomopoulos J, et al. Mycobacterium avium subsp. paratuberculosis, genetic susceptibility to Crohn’s disease, and Sardinians: The way ahead. J Clin Microbiol. 2005;43:5275–7. doi: 10.1128/JCM.43.10.5275-5277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naser SA, Schwartz D, Shafran I. Isolation of Mycobacterium avium subsp paratuberculosis from breast milk of Crohn’s disease patients. Am J Gastroenterol. 2000;95:1094–5. doi: 10.1111/j.1572-0241.2000.01954.x. [DOI] [PubMed] [Google Scholar]
- 30.Sartor RB. Does Mycobacterium avium subspecies paratuberculosis cause Crohn’s disease? Gut. 2005;54:896–8. doi: 10.1136/gut.2004.055889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goyette P, Labbe C, Trinh TT, Xavier RJ. Molecular pathogenesis of inflammatory bowel disease: Genotypes, phenotypes and personalized medicine. Ann Med. 2007;39:177–99. doi: 10.1080/07853890701197615. [DOI] [PubMed] [Google Scholar]
- 32.Clancy R, Zen R, Turton J, Pang G, Wettstein A. Molecular evidence for Mycobacterium avium subspecies paratuberculosis (MAP) in Crohn’s disease correlates with enhanced TNF-a secretion. Dig Liver Dis. 2007;39:445–51. doi: 10.1016/j.dld.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Ferwerda G, Kullberg BJ, de Jong DJ, et al. Mycobacterium paratuberculosis is recognized by toll-like receptors and NOD2. J Leukoc Biol. 2007;82:1011–8. doi: 10.1189/jlb.0307147. [DOI] [PubMed] [Google Scholar]
- 34.Pindeo PJ, Buergelt CD, Donovan GA, et al. Association between CARD15/NOD2 gene polymorphisms and paratuberculosis infection in cattle. Vet Microbiol. 2009;134:346–52. doi: 10.1016/j.vetmic.2008.09.052. [DOI] [PubMed] [Google Scholar]
- 35.Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor-a-neutralizing agent. N Engl J Med. 2001;345:1098–104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 36.Borgaonkar M, MacIntosh D, Fardy J, Simms L. Anti-tuberculous therapy for maintenance of remission in Crohn‘s disease. Cochrane reviews. 2000;(2):CD000299. doi: 10.1002/14651858.CD000299. [DOI] [PubMed] [Google Scholar]
- 37.Selby W, Pavli P, Crotty B, et al. Two-year combination antibiotic therapy with clarithromycin, rifabutin, and clofazimine for Crohn’s disease. Gastroenterology. 2007;132:2313–9. doi: 10.1053/j.gastro.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 38.Koch R. “2 die aetiologie der tuberkulose”. Mitt Kaiser Gesundh. 1884;2:1–88. [Google Scholar]
- 39.Page GP, George V, Go RC, Page PZ, Allison DB. “Are we there yet?”: Deciding when one has demonstrated specific genetic causation in complex diseases and quantitative traits. Am J Human Genet. 2003;73:711–9. doi: 10.1086/378900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rothman KJ, Greenland S. Causation and causal inference in epidemiology. Am J Public Health. 2005;95(Suppl 1):144–50. doi: 10.2105/AJPH.2004.059204. [DOI] [PubMed] [Google Scholar]
- 41.Wilson PWF, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 42.Hill AB. The environment and disease: Association or causation? Proc R Soc Med. 1965;58:295. [PMC free article] [PubMed] [Google Scholar]
- 43.Bentley RW, Keenan JI, Gearry RB, Kennedy MA, Barclay ML, Roberts RL. Incidence of Mycobacterium avium subspecies paratuberculosis in a population-based cohort of patients with Crohn’s disease and control subjects. Am J Gastroenterol. 2008;103:1168–72. doi: 10.1111/j.1572-0241.2007.01742.x. [DOI] [PubMed] [Google Scholar]
- 44.Olsen I, Wiker HG, Johnson E, Langeggen H, Reitan LJ. Elevated antibody responses in patients with Crohn’s disease against a 14-kDa secreted protein purified from Mycobacterium avium subsp. paratuberculosis. Scand J Immunol. 2001;53:198–203. doi: 10.1046/j.1365-3083.2001.00857.x. [DOI] [PubMed] [Google Scholar]
- 45.Olsen I, Wiker HG, Johnson E, Langeggen H, Reitan LJ. Elevated antibody responses in patients with Crohn’s disease against MPP14, a 14 kDa secreted protein purified from Mycobacterium avium subsp. paratuberculosis. Acta Vet Scand. 2003;44:287. [PubMed] [Google Scholar]