Abstract
This study investigated the effects of dual endothelin (ET) receptor blockade in rat models of liver ischemia and reperfusion injury (IRI). Three models of IRI were used: (1) in vivo total hepatic warm ischemia with portal shunting for 60 minutes with control (saline) and treatment groups (15 mg/kg tezosentan intravenously prior to reperfusion), (2) ex vivo hepatic perfusion after 24 hours of cold storage in University of Wisconsin solution with control and treatment groups (10 mg/kg tezosentan in the perfusate), and (3) syngeneic liver transplantation (LT) after 24 hours of cold storage in University of Wisconsin solution with control and treatment groups (10 mg/kg tezosentan intravenously prior to reperfusion). Tezosentan treatment significantly improved serum transaminase and histology after IRI in all 3 models. This correlated with reduced vascular resistance, improved bile production, and an improved oxygen extraction ratio. Treatment led to a reduction in neutrophil infiltration and interleukin-1 beta and macrophage inflammatory protein 2 production. A reduction in endothelial cell injury as measured by purine nucleoside phosphorylase was seen. Survival after LT was significantly increased with tezosentan treatment (90% versus 50%). In conclusion, this is the first investigation to examine dual receptor ET blockade in 3 models of hepatic IRI and the first to use the parenterally administered agent tezosentan. The results demonstrate that in both warm and cold IRI tezosentan administration improves sinusoidal hemodynamics and is associated with improved tissue oxygenation and reduced endothelial cell damage. In addition, reduced tissue inflammation, injury, and leukocyte chemotactic signaling were seen. These results provide compelling data for the further investigation of the use of tezosentan in hepatic IRI.
Hepatic dysfunction following ischemia and reperfusion injury (IRI) represents an inevitable consequence of liver surgery and liver transplantation (LT). IRI can manifest as a transient elevation of liver enzymes or may result in liver failure. The growing disparity between the number of suitable donors and the number of candidates awaiting LT has prompted renewed efforts to use extended criteria livers, which can be associated with poor graft function due, in part, to IRI. Novel strategies to prevent IRI are needed to ensure the safe use of such organs and increase the potential donor organ pool.
The precise mechanism of hepatic IRI remains elusive despite extensive investigations. The initial events elicited during this process are characterized by oxidative injury and sinusoidal flow disturbances.1 Then, secondary inflammation occurs through the infiltration of neutrophils, which is mediated through Kupffer cell–derived chemotactic signals.1 The final common pathway of liver IRI involves a continuum of interrelated processes, all of which culminate in varying degrees of hepatocellular dysfunction/death.
Endothelin (ET) is a potent vasoactive peptide that is synthesized and released from endothelial cells (EC).2 Three subtypes of ET have been isolated (ET-1, ET-2, and ET-3), and 2 ET receptors have been identified (ETA and ETB).3 ET-1 exerts the strongest vasoconstrictive properties and displays the highest affinity for ETA receptor.3 There is evidence that ET-1 is an important mediator in the microcirculatory disturbances following hepatic IRI. Following ischemia, elevated ET-1 levels have been reported in the suprahepatic vena cava4 and portal vein,5 indicating that ET-1 is produced in the sinusoidal and mesenteric circulations during periods of hepatic ischemia. The pathogenic role of ET-1 in hepatic IRI may be related to its vasoconstrictive properties resulting in microcirculatory derangements and hypoxic conditions and thereby leading to hepatocellular damage.4,5 ET receptors are abundant on the hepatic stellate cell (ie, Ito cell), which has been identified as a primary target for ET-mediated sinusoidal constriction.6 Additionally, in vitro data have demonstrated the association of ET with hepatic Kupffer cells, which stimulates the synthesis and release of platelet activating factor.7 A potent phospholipid mediator, platelet activating factor has an important role in platelet aggregation and inflammation during hepatic IRI.8 Unfortunately, the exact role of ET in hepatic IRI remains unclear.
Tezosentan, designed for parenteral use, is a novel compound with high water solubility that competitively antagonizes the specific binding of ET-1 and ET-3 on cells and tissues carrying ETA and ETB receptors, with inhibitory constants in the nanomolar range. Tezosentan exhibits an elimination half-life of 2 hours in rats9 and 3.3 to 3.9 hours in humans,10 and this makes it particularly attractive for use in the peritransplant period. Tezosentan has never been investigated in models of liver IRI but has demonstrated efficacy in experimental models of renal IRI.11
We investigated, using experimental models of warm and cold ischemia, the impact of tezosentan on the microhemodynamic, biochemical, and histological manifestations of hepatic IRI.
MATERIALS AND METHODS
Animals
Inbred male Sprague-Dawley rats weighing between 250 and 300 g (Harlan Sprague Dawley, Indianapolis, IN) were used for all experiments. Animals were cared for according to the guidelines of the University of California–Los Angeles Institutional Animal Research Committee and the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health. Rats were allowed water ad libitum and access to standard rat chow until 12 hours before experiments.
Tezosentan
Tezosentan [Veletri, Ro 61-0612; 5-isopropyl-pyridine-2-sulfonic acid 6-(2-hydroxy-ethoxy)-5-(2-methoxy-phenoxy)-2-(2-1H-tetrazol-5-yl-pyridin-4-yl)-pyrimidin-4-ylamide; molecular weight = 649.6 Da] was supplied by Actelion Pharmaceuticals (Allschwil, Switzerland); it was dissolved in saline at 1 mg/mL and administered intravenously with previously established dosing strategies.9
In Vivo Warm Ischemia and Reperfusion Model
The first hepatic IRI model was total hepatic ischemia (THI) with placement of a portojugular shunt (PJS) to prevent portal and mesenteric venous congestion. Rats underwent midline laparotomy and cannulation of a cecal branch of the mesenteric vein with a 20-gauge angiocatheter (Becton Dickinson Infusion Therapy Systems, Sandy, UT). The external jugular vein was cannulated with an 18-gauge angiocatheter. The catheters were then connected with silastic tubing (1.01-mm internal diameter; Dow Corning Co., Midland, MI). A 3-way connector (Cole-Parmer Instrument Co., Chicago, IL) was used for the administration of normal saline (20 mL/kg) and to assess the patency and flow of the shunt. After an intravenous sodium heparin (100 U/kg) injection, the portal vein, hepatic artery, and common bile duct were occluded with an atraumatic clamp for 60 minutes. After removal of the clamp, the cecal vein was decannulated and ligated. The external jugular catheter was maintained for serial blood draws at 5 minutes, 2 hours, 6 hours, and 24 hours following reperfusion. Blood samples were collected for serum aspartate aminotransferase (AST) measurement with an autoanalyzer from ANTECH Diagnostics (Irvine, CA). Serum levels of purine nucleoside phosphorylase (PNP) were measured with a spectrophotometer at 24 hours post-reperfusion as an index of ischemic damage to the EC. The rats were sacrificed at 24 hours post-reperfusion, at which time a portion of liver tissue was snap-frozen; the remaining tissue samples were fixed in 10% buffered formalin. The following 2 groups were examined with this model: a control group [THI/PJS with administration of normal saline (n = 6)] and a treatment group [THI/PJS with intravenous tezosentan (15 mg/kg) 5 minutes prior to reperfusion (n = 6)].
Ex Vivo Cold Ischemia and Reperfusion Model
The second hepatic IRI model was the ex vivo isolated rat liver perfusion apparatus (IRLPA) as previously described.12 Briefly, after midline laparotomy incision, donor rats were heparinized with cannulation of the portal vein, common bile duct, and inferior vena cava. The liver was flushed via the portal vein with University of Wisconsin solution (10 mL; Viaspan, Dupont Pharma, Wilmington, DE), procured, and stored for 24 hours at 4°C. Livers were then perfused ex vivo for 2 hours on IRLPA. The perfusate consisted of whole rat blood diluted with Kreb’s bicarbonate solution to a hematocrit of 15%, the temperature was maintained at 37°C, the pH was buffered at 7.4, and the portal flow was adjusted to keep the portal pressure constant at 13 cm of water. Portal blood flow was recorded at 30-minute intervals and is expressed as milliliters per minute per gram of wet liver tissue. Blood samples were collected at 30-minute intervals for AST measurement, and total bile production (μL/g of wet liver) was measured. Blood samples were obtained from portal venous inflow and hepatic venous outflow for blood gas analysis at 30-minute intervals. The hepatic oxygen extraction ratio (ERO2) was calculated by the division of oxygen consumption (VO2) by oxygen transport (TO2), which is the difference in the arterial and venous oxygen content (CxO2) divided by the arterial oxygen content (CaO2):
CxO2 was calculated according to the following equation:
where [Hb] is the hemoglobin concentration, SO2 is the saturation of oxygen, and pO2 is the pressure of oxygen. At the conclusion of the experiment, a portion of the liver was snap-frozen, and the remainder of the tissue was fixed in 10% buffered formalin. Two groups were studied in this model: a control group [no treatment (n = 4)] and a treatment group [tezosentan (10 mg/kg) diluted in a perfusate solution (n = 4)].
Syngeneic LT Model
The last IRI model used was rat LT as previously described.13 Donor livers were procured and stored in University of Wisconsin solution at 4°C for 24 hours. LT without hepatic artery reconstruction was performed according to the cuff technique,14 with the anhepatic phase lasting less than 20 minutes. Treatment rats were given tezosentan (15 mg/kg intravenously) just prior to portal vein anastomosis. Control animals received no treatment. One group of rats was sacrificed at 24 hours with blood and liver tissue collected for analysis (n = 4 rats per group). A second group of rats was sacrificed on postoperative day 7 to assess differences in survival (n = 10 rats per group). All animals underwent necropsy, with all deaths attributed to liver failure because of the lack of any other significant surgical or organ pathology.
PNP Assay
Serum levels of PNP, an enzyme localized in the cytoplasm of sinusoidal EC,15 were assayed with a spectrophotometer at 25°C. Changes in absorbance at 293 nM were detected during 5-minute incubation with phosphate-dependent conversion of inosine to uric acid in the presence of sufficient xanthine oxidase.16 The reaction mixture consisted of 1.35 mL of a 100 mM potassium phosphate buffer (pH 7.4), 0.05 mL of a 7.5 mM inosine solution, 0.05 mL of 10 U/mL xanthine oxidase, and 0.05 mL of heparinized serum as a sample. For a blank control, 0.05 mL of phosphate buffer was added instead of the sample. The PNP concentration (U/mL) was calculated as follows: [(ΔA/minute test – change in absorbance (ΔA)/minute blank)/12]/0.05 mL, where 12 represents the millimolar extinction coefficient of uric acid at 293 nm. One unit of PNP was defined as the quantity of enzyme causing the phosphorolysis of 1.0 μmol of inosine to hypoxanthine and ribose 1-phosphate per minute at pH 7.4 at 25°C. Potassium phosphate, inosine, and xanthine oxidase were purchased from Sigma Chemical Co. (St. Louis, MO).
Reverse-Transcription Polymerase Chain Analysis Cytokine Analysis
Total RNA was extracted from liver tissue samples frozen at −70°C, and the RNA concentration was determined with a spectrophotometer. A 5-μg sample of RNA was reverse-transcribed into complementary DNA with oligo (dT) primers. Reverse-transcriptase polymerase chain reaction was performed by different cycle numbers at the annealing temperature for each primer pair: 60 cycles at 60°C [interleukin-1 beta (IL-1β)], 35 cycles at 60°C [macrophage inflammatory protein 2 (MIP-2)], and 35 cycles at 63°C (β-actin). To compare the relative level of each cytokine in different samples, all samples were normalized against the respective β-actin template complementary DNA ratio. The following cytokines/ chemokines were analyzed: IL-1β and MIP-2.
Tissue Myeloperoxidase (MPO) Assay
MPO was measured as a marker for tissue neutrophil content17 as previously described.12 Frozen tissue was thawed and homogenized in a mixture of 4 mL of 0.5% hexadecyltrimethylammonium bromide (Sigma) and a 50 mM potassium phosphate buffer solution buffered to a pH of 5.0. Each sample was then centrifuged at 13,000 rpm for 15 minutes at 4°C, and the supernatants were combined with hydrogen peroxide–sodium acetate and tetramethylbenzidine solutions (Sigma). The change in spectrophotomorphic absorbance was measured at 655 nm. One unit of MPO activity was defined as the quantity of enzyme degrading 1 mmol of peroxide per minute at 25°C per gram of tissue.
Histological Analysis
Formalin-stored tissue specimens were embedded in paraffin and cut into 4-mm sections. Representative cross sections of the liver were examined by routine hematoxylin and eosin staining. Samples were analyzed by a single pathologist (C.L.) in a blinded manner.
Statistical Analysis
All data are expressed as the mean ± standard deviation. Differences between experimental groups were analyzed in the ex vivo IRLPA with a paired 2-tailed Student t test. The survival difference in the LT model was analyzed with a chi-square log-rank test. Statistical comparisons in the in vivo THI/PJS were analyzed with 1-way analysis of variance with the Tukey-Fisher least-significant difference criterion to establish significant differences between groups. A P value less than 0.05 was considered statistically significant.
RESULTS
In Vivo Warm Ischemia and Reperfusion Model
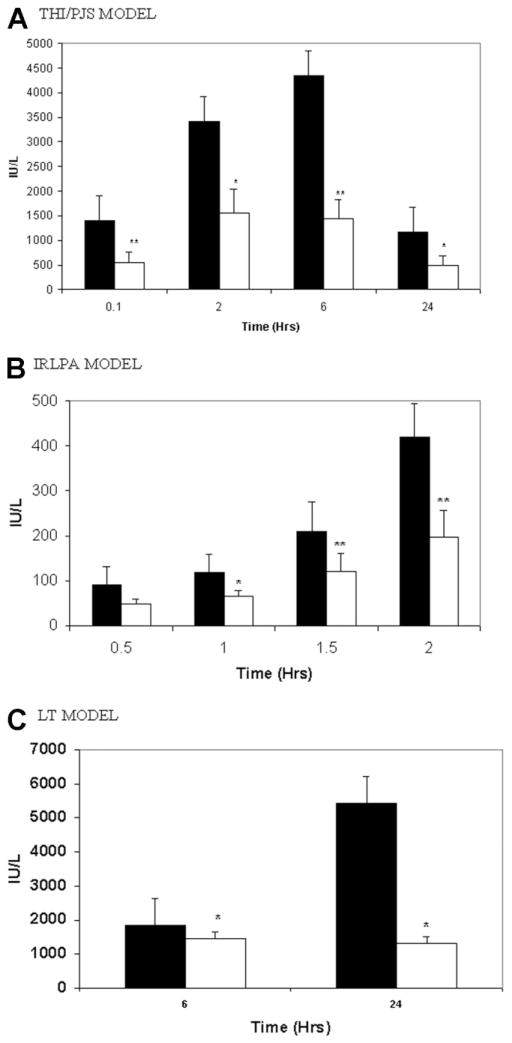
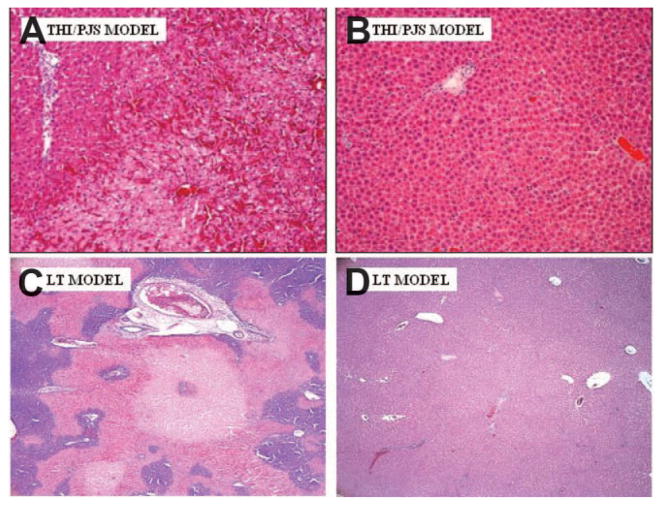
Figure 1A illustrates AST after 60 minutes of warm THI/PJS and 24 hours of reperfusion. Tezosentan administration significantly attenuated AST levels at all study time points in comparison with the control. Additionally, liver histological evaluation at 24 hours demonstrated a significant reduction in hepatocellular necrosis in the treated livers (Fig. 2B) in comparison with the control group (Fig. 2A). Serum PNP levels were measured as a marker for sinusoidal EC injury. Untreated rats showed a significant elevation of serum PNP 24 hours after IRI (control: 0.44 ± 0.07 U/mL); tezosentan treatment led to a significant reduction in PNP levels (tezosentan: 0.24 ± 0.05 U/mL; P = 0.029 versus controls). Reverse-transcriptase polymerase chain reaction analysis of IL-1β and MIP-2 demonstrated that tezosentan administration significantly inhibited the production of both IL-1β (0.71 ± 0.27 IL-1β/β-actin for tezosentan versus 1.13 ± 0.22 IL-1β/β-actin for control; P = 0.004 versus controls) and MIP-2 (0.50 ± 0.13 MIP-2/β-actin for tezosentan versus 0.78 ± 0.17 MIP-2/β-actin for control; P = 0.009 versus controls) following 24 hours of reperfusion.
Figure 1.
Serum AST reduction after tezosentan treatment in (A) THI/PJS, (B) IRLPA, and (C) LT. (A) Control (black bar) and tezosentan-treated (white bar) rats underwent THI/PJS. Each bar represents the mean ± standard deviation (*P < 0.05, **P < 0.02; n = 6 per group). (B) The AST level is shown after reperfusion on IRLPA with sampling performed at 30, 60, 90, and 120 minutes. Control animals are shown with a black bar, and tezosentan-treated animals are shown with a white bar. Each bar represents the mean ± standard deviation (*P < 0.05, **P < 0.02; n = 4 per group). (C) Serum AST levels after LT are shown at the analysis time points of 6 and 24 hours. Again, control animals are shown with a black bar, and tezosentan-treated animals are shown with a white bar. Each bar represents the mean ± standard deviation (*P < 0.05; n = 4 per group). Abbreviations: AST, aspartate aminotransferase; IRLPA, isolated rat liver perfusion apparatus; LT, liver transplantation; PJS, portojugular shunt; THI, total hepatic ischemia.
Figure 2.
Liver histopathology is improved after treatment with tezosentan. Representative photomicrographs of the liver after (A,B) THI/PJS and (C,D) LT are shown. (A,C) Control samples are on the left, whereas (B,D) tezosentan-treated samples are on the right (40× magnification, hematoxylineosin stain). Abbreviations: LT, liver transplantation; PJS, portojugular shunt; THI, total hepatic ischemia.
ISOLATED RAT LIVER PERFUSION APPARATUS
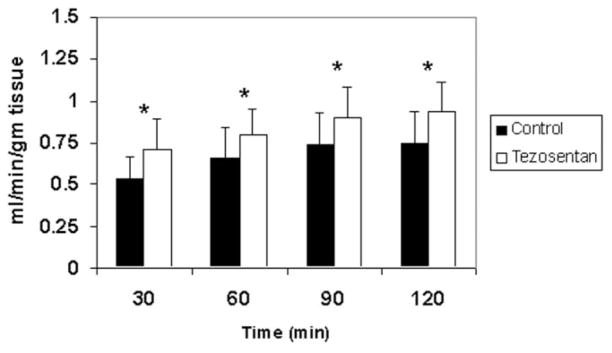
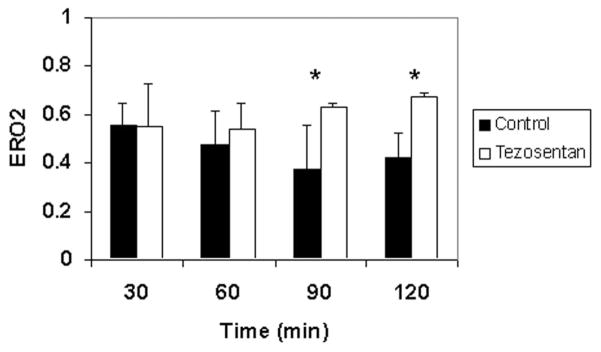
Next, with IRLPA, the effect of warm ischemia and ex vivo reperfusion was assessed. Tezosentan administration significantly diminished AST release into the perfusate at 60, 90, and 120 minutes following reperfusion in comparison with controls (Fig. 1B). Cumulative bile production was markedly improved in livers treated with tezosentan (20.6 ± 6.7 μL/g of liver tissue) versus untreated control livers (9.5 ± 4.4 μL/g of liver tissue; P = 0.0018). With the portal inflow pressure kept at a constant 13 cm of water, portal blood flow (adjusted for liver weight) was directly measured as an index of sinusoidal resistance. As shown in Fig. 3, administration of tezosentan significantly improved portal blood flow at each time point studied (P < 0.05 versus control group), and this indicated decreased sinusoidal resistance. ERO2 was assessed at 30-minute intervals during the use of IRLPA (Fig. 4). ERO2 was initially equivalent in both control and tezosentan groups at 30 minutes, but by 60 minutes, although ERO2 deteriorated steadily thereafter in the control group, tezosentan showed a steady improvement over time, with differences reaching statistical significance at 90 and 120 minutes. Furthermore, markedly elevated MPO activity was seen in controls (1.97 ± 0.21 U/g of tissue) versus tezosentan treatment (1.46 ± 0.09 U/g of tissue; P = 0.0015 versus controls).
Figure 3.
Portal blood flow is improved after treatment with tezosentan. With the isolated rat liver perfusion apparatus, portal blood flow at 30-minute time intervals was measured during 120 minutes of ex vivo reperfusion. Control results are shown as black bars, and tezosentan results are shown as white bars. Flow on the y axis is expressed as milliliters per minute per gram of liver tissue. Each bar represents the mean ± standard deviation (*P < 0.05; n = 4 per group).
Figure 4.
ERO2 steadily improves during the use of the isolated rat liver perfusion apparatus after tezosentan treatment. ERO2, as calculated with hemoglobin and blood gas measurements, provides a global assessment of hepatic oxygen consumption and viability. ERO2 was measured at 30-minute intervals during the use of the isolated rat liver perfusion apparatus, with the control results shown as black bars and the tezosentan results shown as white bars. Each bar represents the mean ± standard deviation (*P < 0.02; n = 4 per group). Abbreviation: ERO2, oxygen extraction ratio.
Syngeneic LT Model
The LT model was used as it combines both cold and warm ischemia followed by reperfusion and expands in vivo analysis beyond IRLPA. Tezosentan treatment decreased hepatocyte injury, as evidenced by a significant (P < 0.05) reduction in AST levels at both study time points (Fig. 1C). This was associated with markedly improved liver histopathology. The tezosentan group showed minimal necrosis at 24 hours (Fig. 2D), whereas control tissues showed extensive pan lobular coagulative necrosis (Fig. 2C). Proinflammatory IL-1β and chemotactic MIP-2 mRNA levels were also significantly reduced in the tezosentan group (0.81 ± 0.15 IL-1β/β-actin for tezosentan versus 2.58 ± 0.23 IL-1β/β-actin for control; P < 0.0001 versus controls) and MIP-2 (0.60 ± 0.11 MIP-2/β-actin for tezosentan versus 1.91 ± 0.19 MIP-2/β-actin for control; P < 0.0001 versus controls). Tezosentan treatment reduced neutrophil infiltration as evidenced by a marked reduction in MPO activity (1.63 ± 0.19 U/g of tissue for tezosentan versus 4.65 ± 0.20 U/g of tissue for control; P < 0.0001 versus controls). Lastly, untreated control rats had a 50% 7-day survival following LT, whereas tezosentan-treated recipients had a 90% 7-day survival (Fig. 5; P = 0.033). All deaths occurred within 24 hours of LT and were consistent with hepatic dysfunction at necropsy.
Figure 5.
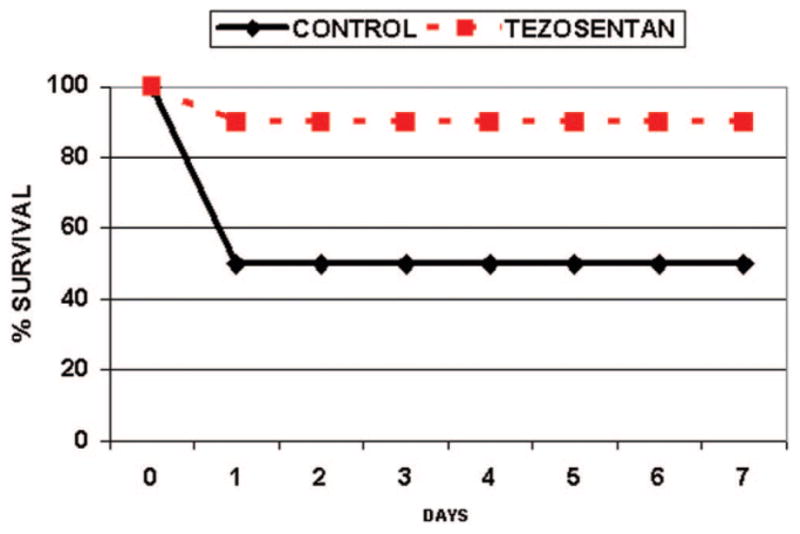
Survival after liver transplantation is improved with tezosentan treatment. Survival 7 days after liver transplantation was assessed in control and tezosentan-treated rats (n = 10 animals per group). The control is shown as the solid line with diamonds, and tezosentan is shown as the dashed line with squares. Results are expressed as the actual survival percentage versus the day (P = 0.033).
DISCUSSION
ET has been implicated in various pathophysiological conditions involving hepatic vasoconstriction and hypoxia.4 Hypoxic conditions are capable of inducing ET expression in cultured EC.18 In the liver, the Ito cell expresses the highest concentration of the ETA type receptor and is responsible for ET-induced sinusoidal vasoconstriction.6 Recent studies of hepatic IRI by Zhang et al.19 and Witzigmann et al.20 have implicated the ETA type receptor as the predominant mediator. The role of the Kupffer cell and EC, which possess predominantly ETB type receptors, remains controversial. A few studies have shown that stimulation of ETB receptors using a receptor-specific agonist can create increased portal pressure, but this response may not be directly mediated by ETB and instead may be exerted through the production of other vasomediators.21 Because of these controversies, we feel that the most beneficial approach to ET receptor blockade appears to be dual receptor antagonism.
Clozel et al.9 characterized tezosentan as a potent ET-1 antagonist with a high affinity for both ETA and ETB receptors. Tezosentan was synthesized as a non-peptide derivative of bosentan (Ro 47-0203) to achieve improved water solubility for intravenous administration while maintaining high potency for both receptors.9 It has undergone extensive pharmacological profiling in both animal models and humans, including studies relevant to intravenous administration in the face of hepatic and renal impairment.9,10,22,23 All of these data make this agent an attractive drug worthy of studies on IRI.
Experimentally, the efficacy of tezosentan has been demonstrated only in a renal IRI model.11 No studies have been performed to date with this agent in hepatic IRI. Therefore, we undertook this investigation to (1) study the efficacy of tezosentan in hepatic IRI, (2) determine if this agent did indeed warrant further preclinical investigations into hepatic IRI, and (3) robustly test this agent using substantial liver injuries in both warm and cold ischemia models.
Bosentan is an older generation nonpeptide, dual receptor antagonist that has commonly been used in many published studies.24 Speigel et al.25 noted reduced serum AST/alanine aminotransferase and improved tissue oxygenation in a bosentan-treated rat model of warm total hepatic IRI. The same group26 used increasing doses of bosentan in a warm total hepatic IRI model to show a dose response efficacy. Koeppel et al.27 used bosentan treatment and intravital microscopy in a rat lobar warm IRI model to show improved microvascular perfusion with decreased leukocyte-endothelial interactions. Ricciardi et al.28 administered bosentan in a porcine hepatic cold IRI followed by ex vivo perfusion model and determined that portal perfusion and resistance were improved, oxygen consumption and bile production were enhanced, and response to bile acid challenge was augmented. Significantly, however, indices of hepatocellular damage were unchanged in comparison with untreated controls. Although bosentan does demonstrate efficacy, the agent does not appear to have clinical potential for an acute injury such as IRI, thus rendering these studies purely experimental. Peptide ET antagonists have also been investigated,29 although the use of this class of ET receptor antagonists is felt to be of limited clinical utility.24
In our hands, all 3 IRI models used in this study represent a severe and lethal hepatic injury as shown by control animal survival. Prior investigations25–29 using other agents employed less severe degrees of hepatic injury either by limiting the ischemia time or by applying the ischemia in a lobar fashion. Furthermore, this model demonstrated that neutrophil tissue infiltration as measured by MPO is reduced after treatment with a dual ET receptor antagonist. Others have noted fewer neutrophil-EC interactions using in vivo microscopy,26 but none have expanded this to show that these reduced interactions actually lead to a reduction in tissue infiltration. We feel that this is important as the neutrophil is considered a major mediator of hepatic IRI.17
The use of an ex vivo IRLPA in these experiments affords a significant advantage in that it allows the direct measurement of macrovascular hemodynamics, tissue oxygenation, and bile production. Tezosentan treatment led to marked improvements in oxygen extraction, a parameter shown to strongly correlate with hepatocellular dysfunction.30 Although other studies have measured tissue oxygenation,25 these investigations differ fundamentally as measurements were obtained with topical probes, thereby reflecting oxygenation in only a small and superficial region. We feel that the use of the ERO2 ratio used herein represents a more accurate approach as it provides a more global assay of hepatic oxygen consumption. We are aware of only 1 investigation that has attempted to directly measure hepatic oxygen extraction after treatment with bosentan.28
The macrovascular hemodynamic data obtained with the ex vivo IRLPA are another important result of this type of therapy. One of the major mechanisms of ET receptor antagonism is the amelioration of the ET-dependent vasoconstriction. Using intravital microscopy, other studies have demonstrated that the use of ET receptor antagonists leads to microvascular flow improvement, including sinusoidal dilatation and reduced intravascular cell adhesion.26,27 However, these studies examined only a few microvascular beds in the superficial regions of the liver and extrapolated these data to represent microvascular flow in the organ as a whole. Using the ex vivo IRLPA, we can directly and accurately measure portal venous flow and thus calculate a whole organ vascular resistance that reflects both macrovascular and microvascular beds. Our data demonstrate marked improvements in portal venous flow and resistance and complement the data reported previously.26,27 Only 1 other study has attempted to directly measure portal venous flow in an ex vivo model using bosentan.28
Bile production during reperfusion represents an important and significant indicator of hepatic function.31 The use of the ex vivo IRLPA allows direct measurement of bile production, and tezosentan-treated livers showed a marked increase in cumulative bile production. Ricciardi et al.28 also examined bile production after bosentan administration and reported similar findings.
The third model used in this study examines the effects of cold and warm IRI through LT and is important as it tests the ability of the liver to recover from the injury thus facilitating animal survival. Hepatocyte function and architecture were preserved, and neutrophil infiltration was markedly minimized as evidenced by the reduction in MPO levels in treated animals. Inflammatory cytokine levels were also significantly decreased in the tezosentan-treated group. Furthermore, survival results indicate that tezosentan administration was indeed associated with a markedly improved outcome.
Although much of hepatic IRI research has focused on biochemical and histological parameters of parenchymal cell damage, the direct injury sustained by the EC has received relatively little attention. The EC has been previously described as the least tolerant of non-parenchymal hepatic cells to IRI, as it represents the initial target of oxidative and inflammatory-mediated damage.1 PNP is a cytoplasmic enzyme that catalyzes the conversion of inosine to hypoxanthine. Measurement of serum PNP levels is a sensitive marker of EC oxidative injury and may predict irreversible hepatic IRI.15 Using a model of THI/PJS, we observed a significant suppression of serum PNP levels with tezosentan administration. Although the mechanism behind this effect is unknown, a direct EC protective effect by the agent and an indirect effect mediated through improved perfusion and oxygenation are both plausible.
Finally, we investigated the cytokines IL-1β and MIP-2 because IL-1β has been implicated in stimulating oxygen free radical production32 and MIP-2 is known to act as a neutrophil chemotactic signal.33 The results indicated that tezosentan treatment was associated with a reduction in the production of mRNA for IL-1β and MIP-2 following hepatic IRI. Although the elaboration of these chemokines is thought to arise from the hepatic macrophage,33 other cell types may contribute to this response, including circulating monocytes and neutrophils.1,17 An interesting observation is that the treatment-induced reduction in the inflammatory cytokines in our model correlates with a reduction in neutrophil infiltration during ex vivo hepatic reperfusion; this suggests a link between ET-mediated cytokine production and neutrophil recruitment. Another possible explanation for this observed effect may be related to a direct effect of ET on neutrophil adhesion to EC as ET-1 has been shown to stimulate neutrophil adhesion to cultured EC by affecting the expression of adhesion molecules in cardiac IRI models.34 An untested hypothesis is that ET receptor blockade may result in reduced expression of adhesion molecules and therefore reduced tissue infiltration by leukocytes.
In summary, using 3 different models of both warm and cold severe hepatic IRI, we have shown that administration of a dual ET receptor antagonist, tezosentan, is associated with marked improvements in outcomes. On the basis of the known physiology of ET, we feel that this dual receptor antagonist acts to directly improve global hepatic blood flow, as demonstrated by the data herein. We theorize that this may represent a critical step in the complex cascade of events leading to hepatic IRI as we also observed improvements in other indices of tissue injury such as hepatocyte necrosis/injury and EC injury. However, these improvements may not necessarily result directly from the drug itself but may result from other cellular protective mechanisms that arise secondarily which were not examined in the present investigation. As with many studies, several new avenues of investigation can be initiated from these results in an attempt to better understand the complex mechanisms surrounding the use of dual ET receptor antagonism in IRI.
In conclusion, the dual ET receptor antagonist tezosentan, when administered in rodent models of hepatic IRI, is associated with improved outcomes. While acknowledging that further preclinical mechanistic investigation is warranted, we feel that tezosentan may provide an important and relevant strategy in protecting early liver function after various ischemic insults, including transplantation. However, it must be emphasized that many of the ET receptor antagonist effects seen in rodent models may not be directly translated into the human situation.
Acknowledgments
This study was funded by the Dumont Research Fund and the JoAnn Barr Foundation.
Abbreviations
- AST
aspartate aminotransferase
- CaO2
arterial oxygen content
- CxO2
arteriovenous oxygen difference
- EC
endothelial cell(s)
- ERO2
oxygen extraction ratio
- ET
endothelin
- [Hb]
hemoglobin concentration
- IL-1β
interleukin-1 beta
- IRI
ischemia and reperfusion injury
- IRLPA
isolated rat liver perfusion apparatus
- LT
liver transplantation
- MIP-2
macrophage inflammatory protein 2
- MPO
myeloperoxidase
- PJS
portojugular shunt
- PNP
purine nucleoside phosphorylase
- pO2
pressure of oxygen
- SO2
saturation of oxygen
- THI
total hepatic ischemia
- TO2
oxygen transport
- VO2
oxygen consumption
References
- 1.Farmer DG, Amersi F, Kupiec-Weglinski JW, Busuttil RW. Current status of ischemia and reperfusion injury in the liver. Transplant Rev. 2000;14:106–126. [Google Scholar]
- 2.Yanagisawa M, Kurihara H, Kimura S, Goto K, Masaki T. A novel peptide vasoconstrictor, endothelin, is produced by vascular endothelium and modulates smooth muscle Ca2+ channels. J Hypertens Suppl. 1988;6:S188–S191. doi: 10.1097/00004872-198812040-00056. [DOI] [PubMed] [Google Scholar]
- 3.Sakurai T, Yanagisawa M, Takuwa Y, Miyazaki H, Kimura S, Goto K, Masaki T. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990;348:732–735. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- 4.Goto M, Takei Y, Kawano S, Nagano K, Tsuji S, Masuda E, et al. Endothelin-1 is involved in the pathogenesis of ischemia/reperfusion liver injury by hepatic microcirculatory disturbances. Hepatology. 1994;19:675–681. doi: 10.1002/hep.1840190319. [DOI] [PubMed] [Google Scholar]
- 5.Mitsuoka H, Suzuki S, Sakaguchi T, Baba S, Miwa M, Konno H, Nakamura S. Contribution of endothelin-1 to microcirculatory impairment in total hepatic ischemia and reperfusion injury. Transplantation. 1999;67:514–520. doi: 10.1097/00007890-199902270-00004. [DOI] [PubMed] [Google Scholar]
- 6.Zhang JX, Bauer M, Clemens MG. Vessel- and target cell-specific actions of endothelin-1 and endothelin-3 in rat liver. Am J Physiol. 1995;269(pt 1):G269–G277. doi: 10.1152/ajpgi.1995.269.2.G269. [DOI] [PubMed] [Google Scholar]
- 7.Mustafa SB, Gandhi CR, Harvey SA, Olson MS. Endothelin stimulates platelet-activating factor synthesis by cultured rat Kupffer cells. Hepatology. 1995;21:545–553. [PubMed] [Google Scholar]
- 8.Zhou W, McCollum MO, Levine BA, Olson MS. Inflammation and platelet-activating factor production during hepatic ischemia/reperfusion. Hepatology. 1992;16:1236–1240. [PubMed] [Google Scholar]
- 9.Clozel M, Ramuz H, Clozel JP, Breu V, Hess P, Loffler BM, et al. Pharmacology of tezosentan, new endothelin receptor antagonist designed for parenteral use. J Pharmacol Exp Ther. 1999;290:840–846. [PubMed] [Google Scholar]
- 10.Dingemanse J, Gunawardena KA, van Giersbergen PL. Comparison of the pharmacokinetics, pharmacodynamics and tolerability of tezosentan between Caucasian and Japanese subjects. Br J Clin Pharmacol. 2006;61:405–413. doi: 10.1111/j.1365-2125.2006.02586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilhelm SM, Stowe NT, Robinson AV, Schulak JA. The use of the endothelin receptor antagonist, tezosentan, before or after renal ischemia protects renal function. Transplantation. 2001;71:211–216. doi: 10.1097/00007890-200101270-00007. [DOI] [PubMed] [Google Scholar]
- 12.Dulkanchainun TS, Goss JA, Imagawa DK, Shaw GD, Anselmo DM, Kaldas F, et al. Reduction of hepatic ischemia/reperfusion injury by a soluble P-selectin glycoprotein ligand-1. Ann Surg. 1998;227:832–840. doi: 10.1097/00000658-199806000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amersi F, Buelow R, Kato H, Ke B, Coito AJ, Shen XD, et al. Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury. J Clin Invest. 1999;104:1631–1639. doi: 10.1172/JCI7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamada N, Calne RY. Orthotopic liver transplantation in the rat. Technique using cuff for portal vein anastomosis and biliary drainage. Transplantation. 1979;28:47–50. [PubMed] [Google Scholar]
- 15.Rao PN, Walsh TR, Makowka L, Rubin RS, Weber T, Snyder JT, Starzl TE. Purine nucleoside phosphorylase: a new marker for free oxygen radical injury to the endothelial cell. Hepatology. 1990;11:193–198. doi: 10.1002/hep.1840110206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffee PA, May R, Robertson BD. Purine nucleoside phosphorylase from Salmonella typhimurium and rat liver. Methods Enzymol. 1978;51:517–524. doi: 10.1016/s0076-6879(78)51072-0. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation. 1993;55:1265–1272. doi: 10.1097/00007890-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Kourembanas S, Marsden PA, McQuillan LP, Faller DV. Hypoxia induces endothelin gene expression and secretion in cultured human endothelium. J Clin Invest. 1991;88:1054–1057. doi: 10.1172/JCI115367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang XY, Francis RJ, Sun CK, Wheatley AM. Endothelin receptor A blockade ameliorates hypothermic ischemia-reperfusion-related microhemodynamic disturbances during liver transplantation in the rat. J Surg Res. 2002;102:63–70. doi: 10.1006/jsre.2001.6246. [DOI] [PubMed] [Google Scholar]
- 20.Witzigmann H, Ludwig S, Escher E, Armann B, Gabel G, Teupser D, et al. Administration of a selective endothelina receptor antagonist (BSF 208075) improves hepatic warm ischemia/reperfusion injury in pigs. Transplant Proc. 2002;34:2387–2388. doi: 10.1016/s0041-1345(02)03289-x. [DOI] [PubMed] [Google Scholar]
- 21.Bauer M, Bauer I, Sonin NV, Kresge N, Baveja R, Yokoyama Y, et al. Functional significance of endothelin B receptors in mediating sinusoidal and extrasinusoidal effects of endothelins in the intact rat liver. Hepatology. 2000;31:937–947. doi: 10.1053/he.2000.5922. [DOI] [PubMed] [Google Scholar]
- 22.Dingemanse J, van Giersbergen PL. Influence of mild liver impairment on the pharmacokinetics of tezosentan, a drug excreted unchanged into bile. Br J Clin Pharmacol. 2004;57:344–348. doi: 10.1046/j.1365-2125.2003.01987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Giersbergen PL, Dingemanse J. Effect of severe renal impairment on the pharmacokinetics and tolerability of the parenteral endothelin antagonist tezosentan. Int J Clin Pharmacol Ther. 2003;41:261–266. doi: 10.5414/cpp41261. [DOI] [PubMed] [Google Scholar]
- 24.Roux S, Breu V, Ertel SI, Clozel M. Endothelin antagonism with bosentan: a review of potential applications. J Mol Med. 1999;77:364–376. doi: 10.1007/s001090050363. [DOI] [PubMed] [Google Scholar]
- 25.Spiegel HU, Uhlmann D, Scommotau S, Giersch B, Sulkowski U. Effect of the endothelin receptor antagonist bosentan on postischemic oxygen supply of the liver. J Invest Surg. 1996;9:439–445. doi: 10.3109/08941939609025861. [DOI] [PubMed] [Google Scholar]
- 26.Uhlmann D, Uhlmann S, Palmes D, Spiegel HU. Endothelin receptor blockade as a therapeutic strategy in ameliorating postischemic damage to the liver microcirculation. J Cardiovasc Pharmacol. 2000;36(suppl 1):S351–S353. doi: 10.1097/00005344-200036051-00102. [DOI] [PubMed] [Google Scholar]
- 27.Koeppel TA, Kraus T, Thies JC, Gebhard MM, Otto G, Post S. Effects of mixed ETA and ETB-receptor antagonist (Ro-47-0203) on hepatic microcirculation after warm ischemia. Dig Dis Sci. 1997;42:1316–1321. doi: 10.1023/a:1018830929913. [DOI] [PubMed] [Google Scholar]
- 28.Ricciardi R, Schaffer BK, Shah SA, Quarfordt SH, Banner BF, Wheeler SM, et al. Bosentan, an endothelin antagonist, augments hepatic graft function by reducing graft circulatory impairment following ischemia/reperfusion injury. J Gastrointest Surg. 2001;5:322–329. doi: 10.1016/s1091-255x(01)80055-x. [DOI] [PubMed] [Google Scholar]
- 29.Kitayama Y, Yamanaka N, Kawamura E, Kuroda N, Okamoto E. Hepatoprotective effect of the endothelin receptor antagonist TAK-044 against ischemia-reperfusion injury in the canine liver. Hepatology. 1997;25:938–942. doi: 10.1002/hep.510250425. [DOI] [PubMed] [Google Scholar]
- 30.Goto M, Kawano S, Yoshihara H, Takei Y, Hijioka T, Fukui H, et al. Hepatic tissue oxygenation as a predictive indicator of ischemia-reperfusion liver injury. Hepatology. 1992;15:432–437. doi: 10.1002/hep.1840150313. [DOI] [PubMed] [Google Scholar]
- 31.Karwinski W, Husoy AM, Farstad M, Soreide O. Sixty minutes of normothermic ischemia in the rat liver: correlation between adenine nucleotides and bile excretion. J Surg Res. 1989;46:99–103. doi: 10.1016/0022-4804(89)90210-2. [DOI] [PubMed] [Google Scholar]
- 32.Shirasugi N, Wakabayashi G, Shimazu M, Shito M, Kawachi S, Kitajima M. Interleukin-1 receptor blockade attenuates oxygen-derived free radical production and micro-circulatory disturbances in ischemia/reperfusion injury in the liver. Transplant Proc. 1997;29:371–373. doi: 10.1016/s0041-1345(96)00124-8. [DOI] [PubMed] [Google Scholar]
- 33.Lentsch AB, Yoshidome H, Cheadle WG, Miller FN, Edwards MJ. Chemokine involvement in hepatic ischemia/reperfusion injury in mice: roles for macrophage inflammatory protein-2 and Kupffer cells. Hepatology. 1998;27:507–512. doi: 10.1002/hep.510270226. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Farre A, Riesco A, Espinosa G, Digiuni E, Cernadas MR, Alvarez V, et al. Effect of endothelin-1 on neutrophil adhesion to endothelial cells and perfused heart. Circulation. 1993;88:1166–1171. doi: 10.1161/01.cir.88.3.1166. [DOI] [PubMed] [Google Scholar]