Abstract
Previous studies have shown that herpes simplex virus type 1 (HSV-1) replication is inhibited by the cyclin-dependent kinase (cdk) inhibitor roscovitine. One roscovitine-sensitive cdk that functions in neurons is cdk5, which is activated in part by its binding partner, p35. Because HSV establishes latent infections in sensory neurons, we sought to determine the role p35 plays in HSV-1 replication in vivo. For these studies, wild-type (wt) and p35-/- mice were infected with HSV-1 using the mouse ocular model of HSV latency and reactivation. The current results indicate that p35 is an important determinant of viral replication in vivo.
Keywords: cdk5, HSV-1, HSV-1 latency and reactivation, p35, p35 knockout mice
Introduction
Herpes simplex virus type 1 (HSV-1) is a prevalent human pathogen that causes cold sores and is the leading cause of infectious blindness in western industrialized countries (Fields et al, 2007). Upon infection at a peripheral site, HSV-1 undergoes lytic replication and ultimately establishes a latent infection in sensory neurons, which can reactivate from latency upon physical or emotional stress. The molecular mechanisms that lead to the establishment of latency and promote reactivation are largely unknown.
Cyclin-dependent kinases (cdks) are cellular factors involved in the cell cycle and are critical determinants as to whether an HSV infection will be lytic or remain latent. To date at least 13 cdks have been described (Malumbres and Barbacid, 2009). Several cdk inhibitors block progression at the G1/S and G2/M boundaries of the cell cycle. Notably, two cdk inhibitors, roscovitine (rosco) and olomoucine (olo), also inhibit HSV-1 replication by blocking viral transcription, viral DNA replication, and reactivation (Schang et al, 1998, 1999, 2000, 2002). One roscovitine-sensitive cdk of interest is cdk5, a serine/threonine kinase that regulates neuronal positioning during brain development (Ohshima et al, 1996) and modulates intracellular transport in neurons (Smith and Tsai, 2002).
Cdk5 activity is, in large part, regulated by p35 or its cleaved form, p25; both proteins act as specific activators of cdk5 (Lew et al, 1994; Tsai et al, 1994). p35 has been reported to localize primarily in the cytoplasm and/or cell membrane of neurons (Nikolic et al, 1996, 1998; Ohshima et al, 1996). Coexpression of cdk5 and p35 leads cdk5 to localize to p35-containing cellular compartments, suggesting that the subcellular location of cdk5 is influenced by p35, putting cdk5 in close proximity with several of its substrates. p35 knockout mice display a reverse packing order of cortical neurons during brain development, leading to cortical lamination defects and a low level of sporadic adult seizures and lethality (Chae et al, 1997). Cdk5 activity is also regulated by a related isoform of p35, known as p39 (Tang and Wang, 1996). It has been shown that cdk5 activity is important for survival, as mice lacking both p35 and p39 show a high degree of embryonic lethality (Ko et al, 2001). p35-/- mice are viable; thus, it is not known whether there exists a specific subset of cdk5 that is uniquely activated by p35 or if the observable phenotypes in p35-/- mice are simply a result of an overall lower level of cdk5 activity. Because cdk5 is important in several neuronal processes and is a roscovitine-sensitive cdk, we hypothesized that a lack of p35 would result in diminished viral replication. To initially test this hypothesis, we infected wild-type (wt) and p35-/- mice ocularly with HSV-1 and monitored viral replication and reactivation in vivo.
Results
To determine whether p35 contributes to viral shedding in the eyes, 6- to 8-week-old p35-/- (Chae et al, 1997)) and wt (C57BL/6) mice were infected with wt HSV-1 (strain KOS) at 1 × 105 plaque-forming units (PFU) per eye, and eye swabs were taken on days 1, 3, 5, and 7 post infection (p.i.). As shown in Figure 1, the HSV-1 titers were reduced in p35-/- mice on days 3 and 5 p.i. 12-fold and 9-fold, respectively, relative to C57BL/6 mice (P = .0095 on day 3 and P = .0005 on day 5, unpaired t test). On days 1 and 7 p.i., viral titers were similar or marginally affected between the two mouse strains. Viral shedding was at or near the limit of detection on day 7 p.i., as has been previously reported for wt mice (Halford et al, 2004). We observed that ocular titers for both strains of mice did not exceed input inoculum levels. Two published reports, however, indicate that de novo viral replication was being measured in our experiments, as opposed to viral clearance (Leib et al, 1989; Strelow and Leib, 1995). Our results indicate that p35 is necessary for efficient viral shedding in the eyes.
Figure 1.
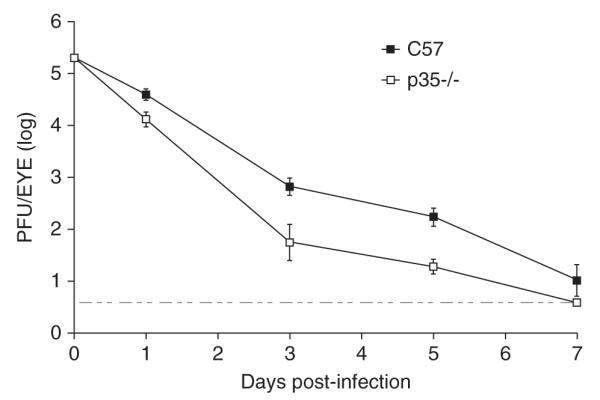
HSV-1 ocular shedding in p35-/- mice. C57BL/6 and p35-/- mice were ocularly infected with 1 × 105 PFU/eye, and eye swabs were taken on days 1, 3, 5, and 7 post infection (p.i.). Viral titers of eye swabs (n = 8 per time point) were determined by standard plaque assays. Results displayed are logarithmic means, with error bars showing the standard error of the means (SEMs). The horizontal stippled line represents the lower limit of detection. Results from one experiment are shown; similar results were observed in a repeat experiment.
We then examined acute viral replication in neurons of the trigeminal ganglia (TG) of ocularly infected mice on days 1, 3, 5, and 7 p.i. Results from these experiments (Figure 2) showed that viral growth was decreased 16-fold by day 3 p.i. and 17-fold by day 5 p.i. when p35-/- TG titers were compared to those of C57BL/6 mice (P = .0003 and P = .0002, respectively, unpaired t test). Viral replication was similar between the two groups on days 1 and 7 p.i. TG titers in wt mice on day 7 approached the limit of detection, which has been reported by others (Jung et al, 2004).
Figure 2.
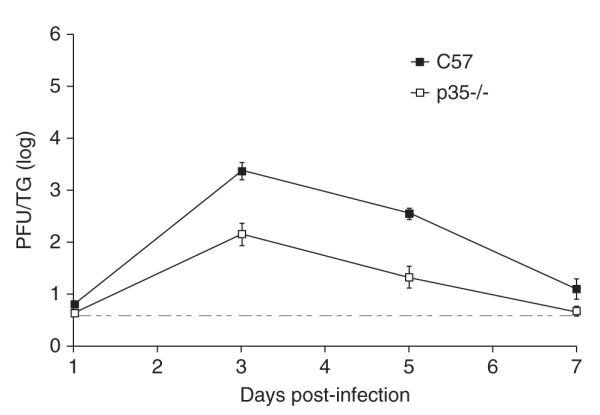
Acute viral replication in murine TG. TG from C57BL/6 and p35-/- infected mice were removed days 1, 3, 5, and 7 p.i., and TG homogenates were titered by standard plaque assays (n = 8 per time point). The results displayed are logarithmic means, with the error bars representing the SEMs. The horizontal stippled line represents the lower limit of detection. Results from one experiment are shown; similar results were observed in a repeat experiment.
To assess the capability of HSV-1 to establish latency in the two different mouse strains, viral DNA loads were determined by real-time polymerase chain reaction (PCR) from latently infected TG 30 days p.i. As shown in Figure 3, there was an 8.3-fold decrease in latent HSV-1 genomes present in p35-/- TG compared to wt TG (P = .001, unpaired t test).
Figure 3.
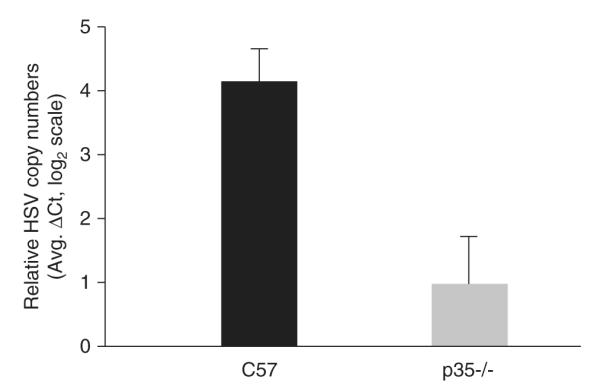
Latent viral DNA loads in wt and p35-/- mice. TG were removed from C57BL/6 and p35-/- latently infected mice at 30 days p.i., and DNA was isolated from each TG. The relative establishment of HSV-1 latency for each mouse strain (n = 14 per group) was determined by real-time PCR using primers that amplified the HSV-1 UL50 gene. UL50 gene levels were normalized to a DNA loading control, the mouse adipsin gene. Data shown are logarithmic (base 2) means. Error bars represent SEMs. Mockinfected samples were below the limit of detection for the UL50 gene (data not shown).
To determine whether this decrease in establishing a latent infection affected viral reactivation, explant cultures were done with latently infected wt and p35-/- TG 30 days p.i. and cocultured on Vero cells (Link and Schaffer, 2007). In these assays, HSV-1 reactivated from 100% of wt mice TG by day 5, whereas only 25% of p35-/- day mice TG reactivated by 5 (Figure 4), which was statistically significant (P = .0013, Fisher’s exact test). Prior to heat shock treatment, only 33% of p35-/- the TG had reactivated; efficiency of reactivation increased to 58% in the p35-/- strain by day 15 post explant.
Figure 4.
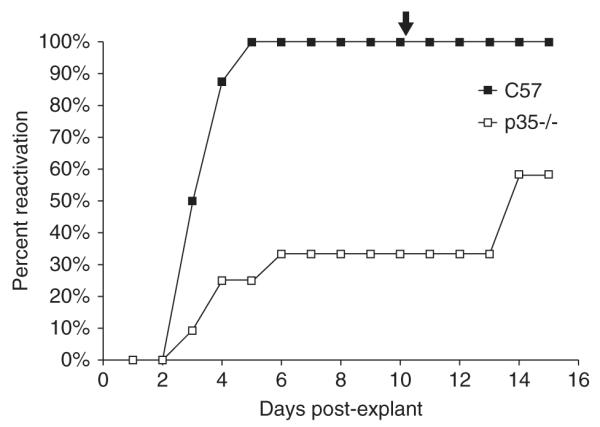
HSV-1 reactivation in latently infected TG. On days 30 p.i., TG were removed from C57BL/6 and p35-/- latently infected mice and explanted on Vero cells. Medium from each sample was monitored daily for infectious virus over a period of 15 days (n = 8 for C57BL/6, n = 12 for p35-/-). The arrow at the top of the figure (day 10 post explant) indicates that samples were heat shocked at 43°C for 3 h to induce further reactivation. The results from one of two experiments are shown. The percent reactivation is the cumulative amount of reactivation for each set of samples analyzed.
Discussion
The results of this study indicate that p35 is required for efficient HSV-1 replication in mice and suggest that HSV-1 utilizes cdk5 activity to promote high levels of viral growth. Consistent with our results, one report showed that cdk5 and p35 are coexpressed in adult mouse corneal epithelium (Gao et al, 2002). Cdk5 has been reported to play roles in the adhesion, migration, and differentiation of lens and corneal epithelial cells (reviewed in Rosales and Lee, 2006). Clearly, the absence of p35 significantly diminished acute ocular replication. Thus, HSV-1 appears to take advantage of the non-neuronal functions of cdk5 and p35 to facilitate efficient shedding in the eyes of mice.
Acute viral replication was also reduced in the TG of p35-/- mice. It is known that viral replication in the eyes can influence productive replication in the TG (Leib et al, 1989). However, at least one published report supports the hypothesis that the reduction in TG titers we observed in p35-/- mice cannot be solely attributable to reductions in HSV-1 ocular shedding. This study showed that maximal ocular and TG titers for wt HSV-1 are approximately the same in vivo, although TG titers peak 2 days later than the ocular titers (Thompson et al, 2009). Given that there was a minor decrease (<3-fold) in the peak of p35-/- ocular titers on day 1 p.i., this decrease alone cannot account for the significant reduction (16-fold) in the peak of p35-/- TG titers on day 3 p.i. Taken together, these results strongly suggest that p35 plays a role in enhancing lytic infection in sensory neurons. This decrease in acute replication in p35-/- neurons appears to have reduced the ability of HSV-1 to establish an efficient latent infection (an 8.3-fold decrease), which consequently can impact reactivation. The reductions in acute TG replication and viral latency in p35-/- mice are consistent with the model that the level of viral DNA replication in TG neurons during acute infection determines how efficiently HSV-1 can establish a latent infection (Coen et al, 1989; Katz et al, 1990; Kosz-Vnenchak et al, 1990; Sawtell et al, 2006). Although not within the confines of this initial report, future experiments will be needed to establish equivalent latent infections in both mouse strains in order to determine whether p35 is required for efficient reactivation. A role for p35 and cdk5 in reactivation is very plausible given that one study showed the cdk inhibitor rosco blocked HSV-1 antigen expression and explant-induced reactivation in neurons (Schang et al, 2002). Whether p35 has one or more functions independent of cdk5 in the mouse ocular model of HSV latency remains to be determined.
The molecular mechanisms by which p35 and cdk5 facilitate HSV-1 productive infection have not been determined. Given cdk5’s multiple activities, several possibilities exist, which are not necessarily mutually exclusive. A recent publication has indicated that cdk5 appears to protect neurons from cell death (Zhang et al, 2010). Consequently, p35 and cdk5 may prevent virally induced apoptosis in a subset of acutely and/or latently infected neurons. Because cdk5 plays a role in trafficking (Smith and Tsai, 2002), a decrease in cdk5 activity due to a lack of p35 could impair the transport of viral nucleocapsids or proteins via microtubule-associated motors, especially in neurons. In addition to transport, cdk5 and p35 have been reported to translocate into the nuclei of neurons (Ino and Chiba, 1996; Qu et al, 2002), phosphorylate, and alter the activity of cellular transcription factors such as MEF2, p53, and Stat3 (Fu et al, 2004; Gong et al, 2003; Lee et al, 2007), which may activate viral gene expression. Moreover, roscovitine-sensitive cdks have been shown to alter the posttranslational modification states of several HSV-1 regulatory proteins (e.g., ICP0, ICP4, ICP22) (Advani et al, 2001; Schang et al, 1999), and in the case of ICP0 and ICP4, these modifications have been linked to their transcriptional activities (Davido et al, 2002; Schang et al, 1999). Whether an indirect or direct effect, the lack of p35 could diminish the transcription of HSV-1 genes, impairing viral replication. Additional studies will address these potential mechanisms.
Methods
Infection of mice
Animals were handled according to the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996). wt (C57BL/6; The Jackson Laboratories and Charles River Laboratories) and p35-/- mice (a gift from Dr. L.H. Tsai, Harvard Medical School [Chae et al, 1997]) were anesthetized, and their corneas scarified and bilaterally infected with wt HSV-1 (strain KOS) at 1 × 105 plaque-forming units (PFU) per eye in 3 μl as previously described (Balliet and Schaffer, 2006).
Acute ocular and trigeminal ganglia titers
Eye swabs were taken on days 1, 3, 5, and 7 post infection (p.i.) as previously published (Balliet and Schaffer, 2006), using a cotton-tipped applicator to collect tear film from each eye. Each trigeminal ganglion from an infected mouse was harvested on days 1, 3, 5, and 7 p.i., and frozen and homogenized as described (Balliet and Schaffer, 2006). Viral titers for samples were determined by standard plaque assays on Vero cells (Schaffer et al, 1973).
Latent viral DNA loads
DNA was isolated by phenol chloroform extraction from TG (Halford and Schaffer, 2000) of latently infected mice 30 days p.i., and real-time PCR was performed by amplifying two specific genes: relative HSV-1 genome loads were determined using primers specific for the UL50 gene (sequences courtesy of Dr. Lynda Morrison, St. Louis University) and normalized against the mouse adipsin gene (Strand and Leib, 2004).
Ex vivo reactivation
Reactivation assays were carried out as described by Link and Schaffer (Link and Schaffer, 2007). Briefly, each explanted trigeminal ganglion was cut into eight pieces and placed into a well of a 24-well plate seeded with 3 × 104 Vero cells in 1.5 ml of Dulbecco’s modified Eagle’s medium (DMEM) containing 5% fetal bovine serum. Every 24 h, 150 μl of medium was removed from each sample and monitored for cytopathic effect on a Vero cell mono-layer. On day 10 post explantation, cells were heat shocked at 43°C for 3 h as an additional stimulus for reactivation. Sampling was continued until day 15 post explantation.
Acknowledgments
This research was supported in part by an NFGRF grant (2302-072) from the University of Kansas Center for Research. The authors would like to thank Joshua Hilliard for technical assistance and members of the Davido lab for helpful suggestions related to this project.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Advani SJ, Hagglund R, Weichselbaum RR, Roizman B. Posttranslational processing of infected cell proteins 0 and 4 of herpes simplex virus 1 is sequential and reflects the subcellular compartment in which the proteins localize. J Virol. 2001;75:7904–7912. doi: 10.1128/JVI.75.17.7904-7912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balliet JW, Schaffer PA. Point mutations in herpes simplex virus type 1 oriL, but not in oriS, reduce pathogenesis during acute infection of mice and impair reactivation from latency. J Virol. 2006;80:440–450. doi: 10.1128/JVI.80.1.440-450.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae T, Kwon YT, Bronson R, Dikkes P, Li E, Tsai LH. Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures, and adult lethality. Neuron. 1997;18:29–42. doi: 10.1016/s0896-6273(01)80044-1. [DOI] [PubMed] [Google Scholar]
- Coen DM, Kosz-Vnenchak M, Jacobson JG, Leib DA, Bogard CL, Schaffer PA, Tyler KL, Knipe DM. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc Natl Acad Sci U S A. 1989;86:4736–4740. doi: 10.1073/pnas.86.12.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davido DJ, Leib DA, Schaffer PA. The cyclindependent kinase inhibitor roscovitine inhibits the transactivating activity and alters the posttranslational modification of herpes simplex virus type 1 ICP0. J Virol. 2002;76:1077–1088. doi: 10.1128/JVI.76.3.1077-1088.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields BN, Knipe DM, Howley PM. Fields Virology. 5th ed Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia: 2007. [Google Scholar]
- Fu AK, Fu WY, Ng AK, Chien WW, Ng YP, Wang JH, Ip NY. Cyclin-dependent kinase 5 phosphorylates signal transducer and activator of transcription 3 and regulates its transcriptional activity. Proc Natl Acad Sci U S A. 2004;101:6728–6733. doi: 10.1073/pnas.0307606100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Negash S, Guo HT, Ledee D, Wang HS, Zelenka P. CDK5 regulates cell adhesion and migration in corneal epithelial cells. Mol Cancer Res. 2002;1:12–24. [PubMed] [Google Scholar]
- Gong X, Tang X, Wiedmann M, Wang X, Peng J, Zheng D, Blair LA, Marshall J, Mao Z. Cdk5-mediated inhibition of the protective effects of transcription factor MEF2 in neurotoxicity-induced apoptosis. Neuron. 2003;38:33–46. doi: 10.1016/s0896-6273(03)00191-0. [DOI] [PubMed] [Google Scholar]
- Halford WP, Balliet JW, Gebhardt BM. Re-evaluating natural resistance to herpes simplex virus type 1. J Virol. 2004;78:10086–10095. doi: 10.1128/JVI.78.18.10086-10095.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford WP, Schaffer PA. Optimized viral dose and transient immunosuppression enable herpes simplex virus ICP0-null mutants to establish wild-type levels of latency in vivo. J Virol. 2000;74:5957–5967. doi: 10.1128/jvi.74.13.5957-5967.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ino H, Chiba T. Intracellular localization of cyclin-dependent kinase 5 (CDK5) in mouse neuron: CDK5 is located in both nucleus and cytoplasm. Brain Res. 1996;732:179–185. doi: 10.1016/0006-8993(96)00523-9. [DOI] [PubMed] [Google Scholar]
- Jung HW, Jung CR, Choi BK, Vinay DS, Hill JM, Gebhardt BM, Kwon BS. Herpesvirus infection of ICAM-1-deficient mice. Curr Eye Res. 2004;29:201–208. doi: 10.1080/02713680490504650. [DOI] [PubMed] [Google Scholar]
- Katz JP, Bodin ET, Coen DM. Quantitative polymerase chain reaction analysis of herpes simplex virus DNA in ganglia of mice infected with replication-incompetent mutants. J Virol. 1990;64:4288–4295. doi: 10.1128/jvi.64.9.4288-4295.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J, Humbert S, Bronson RT, Takahashi S, Kulkarni AB, Li E, Tsai LH. p35 and p39 are essential for cyclin-dependent kinase 5 function during neurodevelopment. J Neurosci. 2001;21:6758–6771. doi: 10.1523/JNEUROSCI.21-17-06758.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosz-Vnenchak M, Coen DM, Knipe DM. Restricted expression of herpes simplex virus lytic genes during establishment of latent infection by thymidine kinase-negative mutant viruses. J Virol. 1990;64:5396–5402. doi: 10.1128/jvi.64.11.5396-5402.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Kim HS, Lee SJ, Kim KT. Stabilization and activation of p53 induced by Cdk5 contributes to neuronal cell death. J Cell Sci. 2007;120:2259–2271. doi: 10.1242/jcs.03468. [DOI] [PubMed] [Google Scholar]
- Leib DA, Coen DM, Bogard CL, Hicks KA, Yager DR, Knipe DM, Tyler KL, Schaffer PA. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J Virol. 1989;63:759–768. doi: 10.1128/jvi.63.2.759-768.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew J, Huang QQ, Qi Z, Winkfein RJ, Aebersold R, Hunt T, Wang JH. A brain-specific activator of cyclin-dependent kinase 5. Nature. 1994;371:423–426. doi: 10.1038/371423a0. [DOI] [PubMed] [Google Scholar]
- Link MA, Schaffer PA. Herpes simplex virus type 1 C-terminal variants of the origin binding protein (OBP), OBPC-1 and OBPC-2, cooperatively regulate viral DNA levels in vitro, and OBPC-2 affects mortality in mice. J Virol. 2007;81:10699–10711. doi: 10.1128/JVI.01213-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- National Research Council . Guide for the care and use of laboratory animals. National Academy Press; Washington, DC: 1996. [Google Scholar]
- Nikolic M, Chou MM, Lu W, Mayer BJ, Tsai LH. The p35/Cdk5 kinase is a neuron-specific Rac effector that inhibits Pak1 activity. Nature. 1998;395:194–198. doi: 10.1038/26034. [DOI] [PubMed] [Google Scholar]
- Nikolic M, Dudek H, Kwon YT, Ramos YF, Tsai LH. The cdk5/p35 kinase is essential for neurite outgrowth during neuronal differentiation. Genes Dev. 1996;10:816–825. doi: 10.1101/gad.10.7.816. [DOI] [PubMed] [Google Scholar]
- Ohshima T, Ward JM, Huh CG, Longenecker G, Veeranna Pant HC, Brady RO, Martin LJ, Kulkarni AB. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci U S A. 1996;93:11173–11178. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu D, Li Q, Lim HY, Cheung NS, Li R, Wang JH, Qi RZ. The protein SET binds the neuronal Cdk5 activator p35nck5a and modulates Cdk5/p35nck5a activity. J Biol Chem. 2002;277:7324–7332. doi: 10.1074/jbc.M107270200. [DOI] [PubMed] [Google Scholar]
- Rosales JL, Lee KY. Extraneuronal roles of cyclin-dependent kinase 5. Bioessays. 2006;28:1023–1034. doi: 10.1002/bies.20473. [DOI] [PubMed] [Google Scholar]
- Sawtell NM, Thompson RL, Haas RL. Herpes simplex virus DNA synthesis is not a decisive regulatory event in the initiation of lytic viral protein expression in neurons in vivo during primary infection or reactivation from latency. J Virol. 2006;80:38–50. doi: 10.1128/JVI.80.1.38-50.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer PA, Aron GM, Biswal N, Benyesh-Melnick M. Temperature-sensitive mutants of herpes simplex virus type 1: isolation, complementation and partial characterization. Virology. 1973;52:57–71. doi: 10.1016/0042-6822(73)90398-x. [DOI] [PubMed] [Google Scholar]
- Schang LM, Bantly A, Schaffer PA. Explant-induced reactivation of herpes simplex virus occurs in neurons expressing nuclear cdk2 and cdk4. J Virol. 2002;76:7724–7735. doi: 10.1128/JVI.76.15.7724-7735.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schang LM, Phillips J, Schaffer PA. Requirement for cellular cyclin-dependent kinases in herpes simplex virus replication and transcription. J Virol. 1998;72:5626–5637. doi: 10.1128/jvi.72.7.5626-5637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schang LM, Rosenberg A, Schaffer PA. Transcription of herpes simplex virus immediate-early and early genes is inhibited by roscovitine, an inhibitor specific for cellular cyclin-dependent kinases. J Virol. 1999;73:2161–2172. doi: 10.1128/jvi.73.3.2161-2172.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schang LM, Rosenberg A, Schaffer PA. Roscovitine, a specific inhibitor of cellular cyclin-dependent kinases, inhibits herpes simplex virus DNA synthesis in the presence of viral early proteins. J Virol. 2000;74:2107–2120. doi: 10.1128/jvi.74.5.2107-2120.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DS, Tsai LH. Cdk5 behind the wheel: a role in trafficking and transport? Trends Cell Biol. 2002;12:28–36. doi: 10.1016/s0962-8924(01)02181-x. [DOI] [PubMed] [Google Scholar]
- Strand SS, Leib DA. Role of the VP16-binding domain of vhs in viral growth, host shutoff activity, and pathogenesis. J Virol. 2004;78:13562–13572. doi: 10.1128/JVI.78.24.13562-13572.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strelow LI, Leib DA. Role of the virion host shutoff (vhs) of herpes simplex virus type 1 in latency and pathogenesis. J Virol. 1995;69:6779–6786. doi: 10.1128/jvi.69.11.6779-6786.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Wang JH. Cyclin-dependent kinase 5 (Cdk5) and neuron-specific Cdk5 activators. Prog Cell Cycle Res. 1996;2:205–216. doi: 10.1007/978-1-4615-5873-6_20. [DOI] [PubMed] [Google Scholar]
- Thompson RL, Preston CM, Sawtell NM. De novo synthesis of VP16 coordinates the exit from HSV latency in vivo. PLoS Pathog. 2009;5:e1000352. doi: 10.1371/journal.ppat.1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai LH, Delalle I, Caviness VS, Jr, Chae T, Harlow E. p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature. 1994;371:419–423. doi: 10.1038/371419a0. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li H, Herrup K. Cdk5 nuclear localization is p27-dependent in nerve cells: implications for cell cycle suppression and caspase-3 activation. J Biol Chem. 2010;285:14052–14061. doi: 10.1074/jbc.M109.068262. [DOI] [PMC free article] [PubMed] [Google Scholar]


