Abstract
Working memory has traditionally been viewed as independent of the hippocampus and related medial temporal lobe structures. Yet memory-impaired patients with medial temporal lobe damage are sometimes impaired at remembering relational information (e.g., an object and its location) across delays as short as a few seconds. This observation has raised the possibility that medial temporal lobe structures are sometimes critical for maintaining relational information, regardless of whether the task depends on working or long-term memory. An alternative possibility is that these structures are critical for maintaining relational information only when the task exceeds working memory capacity and depends instead on long-term memory. To test these ideas, we drew on a method used previously in a classic study of digit span in patient HM that distinguished immediate memory from long-term memory. In two experiments, we assessed the ability of four patients with medial temporal lobe lesions to maintain varying numbers of object–location associations across a 1 s retention interval. In both experiments, the patients exhibited a similar pattern of performance. They performed similarly to controls when only a small number of object–location associations needed to be maintained, and they exhibited an abrupt discontinuity in performance with larger set sizes. This pattern of results supports the idea that maintenance of relational information in working memory is intact after damage to the hippocampus and related medial temporal lobe structures and that damage to these structures impairs performance only when the task depends on long-term memory.
Introduction
The distinction between immediate and long-term memory has been fundamental to understanding how the brain organizes its memory functions (Atkinson and Shiffrin, 1968; Milner, 1972; Squire, 2009). Early studies of memory-impaired patients with medial temporal lobe (MTL) damage found immediate and working memory to be intact, despite markedly impaired performance on tasks of long-term memory (Drachman and Arbit, 1966; Wickelgren, 1968; Baddeley and Warrington, 1970). Thus, working memory [the capacity to maintain temporarily a limited amount of information in mind (Baddeley and Hitch, 1974)] has been thought to be independent of MTL structures, whereas these same structures are essential for the formation of long-term memory.
This view has been challenged by recent reports that patients with MTL lesions are impaired on some tasks even when the retention interval is as short as a few seconds (Hannula et al., 2006; Nichols et al., 2006; Olson et al., 2006a,b; Hartley et al., 2007; Ezzyat and Olson, 2008; Finke et al., 2008). These striking impairments have been interpreted as reflecting impaired working memory (sometimes termed short-term memory), especially when what has to be remembered involves relations between items (e.g., object–location associations) (Hannula et al., 2006; Olson et al., 2006b; Finke et al., 2008). Yet, as discussed by several of the authors just cited, it can be difficult to know when task performance depends on working memory and when the capacity of working memory has been exceeded such that performance depends on long-term memory.
The findings in an earlier study of digit span in memory-impaired patients (Drachman and Arbit, 1966) suggest a method for making this distinction. In that study, participants heard digit strings of increasing length. Each string was repeated until it was reported back correctly. Then, a new string of digits was presented that contained one digit more than the preceding string. Controls made their first errors with strings of eight digits and were eventually able to repeat strings as long as 20 digits. By contrast, patients with MTL damage exhibited a sharp discontinuity in performance as the string length increased. For example, patient HM repeated six digits correctly, but then failed at seven digits, even after 25 repetitions of the same string. It was suggested that the patients performed normally at short string lengths because they could rely on their intact immediate memory, but they exhibited an abrupt decline in performance at the point at which immediate memory capacity was exceeded such that performance now depended on long-term memory.
In two experiments, we used the method of Drachman and Arbit (1966) to examine memory for object–location associations after brief delays in patients with bilateral MTL damage. If working memory is intact in these patients, they should perform as well as controls at small set sizes but exhibit an abrupt discontinuity in performance at larger set sizes. Alternatively, if MTL damage impairs working memory on some tasks, then the patients should be impaired in these tasks even at small set sizes, and even when controls perform perfectly in these conditions.
Materials and Methods
Experiment 1
Participants.
Four memory-impaired patients participated (Table 1). Three patients had bilateral lesions thought to be limited to the hippocampus (CA fields, dentate gyrus, and subicular complex). KE became amnesic in 2004 after an episode of ischemia associated with kidney failure and toxic shock syndrome. LJ (the only female) became amnesic in 1988 during a 6 month period with no known precipitating event. Her memory impairment has been stable since that time. GW became amnesic in 2001 following a drug overdose and associated respiratory failure. One patient (GP) had severe memory impairment resulting from viral encephalitis, together with intact perceptual and intellectual functions (Bayley et al., 2006; Shrager et al., 2006). He has demonstrated virtually no new learning since the onset of his amnesia, and during repeated testing over many weeks he did not recognize that he had been tested before (Bayley et al., 2005a).
Table 1.
Characteristics of memory-impaired patients
| Patient | Gender | Age (years) | Education (years) | IQ (WAIS-III) | WMS-R |
||||
|---|---|---|---|---|---|---|---|---|---|
| Attention | Verbal | Visual | General | Delay | |||||
| KE | M | 67 | 13.5 | 108 | 114 | 64 | 84 | 72 | 55 |
| LJ | F | 71 | 12 | 101 | 105 | 83 | 60 | 69 | <50 |
| GW | M | 49 | 12 | 108 | 105 | 67 | 86 | 70 | <50 |
| GP | M | 61 | 16 | 98 | 102 | 79 | 62 | 66 | 50 |
The Wechsler Adult Intelligence Scale-III (WAIS-III) and the Wechsler Memory-Scale Revised (WMS-R) yield mean scores of 100 in the normal population, with a SD of 15. The WMS-R does not provide numerical scores for individuals who score <50. M, Male; F, female; IQ, intelligence quotient.
Estimates of medial temporal lobe damage were based on quantitative analysis of magnetic resonance images of the patients compared with data for 19 (for KE, GW, and GP) or 11 (for LJ) controls (Gold and Squire, 2005). Nine coronal magnetic resonance images from each patient, together with detailed descriptions of the lesions, appear in supplemental Figure 1 (available at www.jneurosci.org as supplemental material). KE, LJ, and GW had an average bilateral reduction in hippocampal volume of 49, 46, and 48%, respectively (all values >3 SDs from the control mean). On the basis of two patients (LM and WH) with similar bilateral volume loss in the hippocampus for whom detailed postmortem neurohistological information was obtained (Rempel-Clower et al., 1996), this degree of volume loss likely reflects nearly complete loss of hippocampal neurons. The volume of the parahippocampal gyrus, by contrast, is reduced by 17, −8, and 12%, respectively (all values within 2 SDs of the control mean). GP had average bilateral reductions in hippocampal volume of 96%. The volume of the parahippocampal gyrus (temporopolar, perirhinal, entorhinal, and parahippocampal cortices) is reduced by 93%.
Additional measurements, based on four controls for each patient, were performed for the frontal, lateral temporal, parietal, and occipital lobes; insular cortex; and fusiform gyrus (Bayley et al., 2005b). For all patients, the volumes of each of these regions were within 16% of the control volumes, and none of the patients has volume reductions >2 SDs of the control mean.
Nine controls also participated (eight males; mean age, 62.2 ± 3.2 years; mean education, 14.8 years).
Materials and procedure.
The procedure was based on modifications of earlier studies of object–location memory using arrays of toys as memoranda (Smith and Milner, 1981, 1989; Crane and Milner, 2005). The stimuli consisted of 60 small, nameable objects and their exact duplicates (Fig. 1A), plus two additional objects that were used for practice. On average, the objects measured 6.9 cm long, 4.0 cm wide, and 2.8 cm high.
Figure 1.
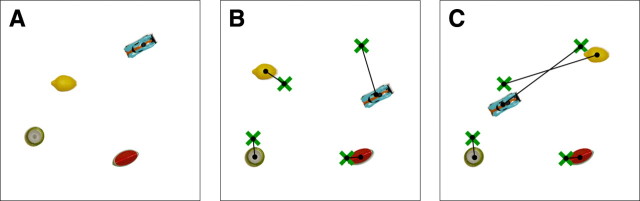
Experiment 1. A, At study, participants named the objects in an array (from one to five) and tried to memorize their exact locations. Participants then immediately moved to an adjacent table where they attempted to place the objects in their original arrangement (retention interval, ∼1 s). B, A typical test trial in which a participant has attempted to place each object in its original location. The green crosses illustrate each object's original location, and the line links each cross to the location in which the object was placed at test. In this example, the displacement error involves placing one or more of the objects in an incorrect location that no object had occupied originally. C, A test trial illustrating another kind of displacement error where each object is placed near a location that had been occupied originally, but the locations of two objects are interchanged.
Participants completed four test blocks, each consisting of a trial involving 1, 2, 3, 4, or 5 objects. Within each block, the first trial used one object, and then the set size was sequentially increased. Unique objects were used for each trial. At each stage, the same objects were used for all participants (e.g., a toy car was always used for a set size of one in the first block).
Before each trial, the experimenter arranged the objects in a pseudorandom pattern on a 60 × 60 cm white tabletop (Fig. 1). Care was taken that the objects were well distributed and that they were not arranged in an easily identifiable pattern such as a square or a straight line. In addition, no object was closer than 7 cm from the edge of the table or closer than 7 cm from any other object. Participants were instructed that they would be shown an array of objects and that they should point to each object, name each object, and study their exact locations. They were also told how much time was available for study (5 s per object). Participants then saw the array for the first time. Immediately after study, participants moved to an adjacent 60 × 60 cm white table where duplicate objects had been placed in the middle of the tabletop (∼1 s retention interval). Participants were reminded that their task was to place the objects in their original locations. It was emphasized that participants should be as accurate as possible in placing the objects. There was no time limit. Measurement of each object's displacement from its original location was calculated from photographs of the test array taken after each trial (see Scoring, below).
Before testing, participants completed practice with two objects. To emphasize the importance of accuracy, the array of two objects was presented again as needed, until both objects were placed within 5 cm of their original location.
Scoring.
Before each study array was presented, the position of each object was marked on a piece of translucent Plexiglas overlaying the array. Then, after participants finished arranging the objects on each trial, the marked Plexiglas was placed over the array, and a photograph was taken and subsequently imported to Matlab. For scoring, the distance between each object's location at test and that same object's location at study (as marked on the Plexiglas) was measured from each photograph using the Matlab ruler tool (Fig. 1B,C). An average of these displacement scores for all the objects in the array was then calculated to yield a mean displacement score for each set size across all four blocks of the experiment.
Experiment 2
Participants.
These were the same as in experiment 1. Experiments 1 and 2 were administered at least 6.5 weeks apart (mean = 12.5 weeks).
Materials and procedure.
The stimuli consisted of 56 small, nameable objects and their exact duplicates, plus two additional objects that were used for practice. Fifty-four of these objects were also used in experiment 1.
The general procedure was the same as in experiment 1 (i.e., objects were arranged in an array in a pseudorandom pattern, unique objects were used for each trial, testing moved sequentially from small to larger set sizes, and the intratrial interval was ∼1 s). At study, participants saw 1–7 objects. At test, they again tried to place each object as it was originally. In this experiment, participants continued with study and test trials at the same set size (with the same objects and object locations) until they succeeded in reaching a criterion or failed 10 times in succession (see Scoring, below).
Controls were tested once with set sizes 1–7. Patients were tested twice with set sizes 1–7. The two test sessions were scheduled at least 1 week apart. Unique objects were used for each of the two tests.
Scoring.
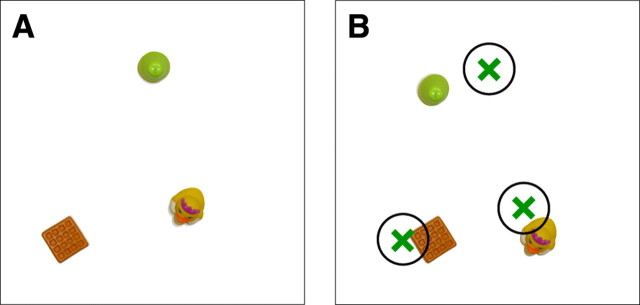
As in experiment 1, the position of each object at study was marked on Plexiglas. Then, after participants finished arranging the objects on each trial, the Plexiglas was placed over the array. The experimenter then placed cardboard circles (5 cm radius) on the marked locations. To reach criterion, each object in the array needed to be in contact with its corresponding circle (i.e., the edge of each object needed to be placed within 5 cm of where it was originally centered) (Fig. 2). In this way, it could be quickly determined whether criterion had been reached on any given trial. The score was the number of trials needed to reach criterion per set size (scores averaged over two test sessions for each patient). A score of 11 was given when participants failed to reach criterion after 10 attempts. For some patients, the test proved so taxing that testing had to be discontinued before the largest set size was presented. In these cases, the patient average is based on the available patient data.
Figure 2.
Experiment 2. A, At study, participants named the objects in an array (from one to seven) and tried to memorize their exact locations. Participants then immediately moved to an adjacent table where they attempted to place the objects in their original arrangement (retention interval, ∼1 s). B, A typical test trial in which a participant has attempted to place each object in its original location. The green crosses illustrate each object's original location. In the trial illustrated, the participant did not reach criterion because one of the objects was placed outside the circle (5 cm radius) that defined the object's original location.
Results
Experiment 1
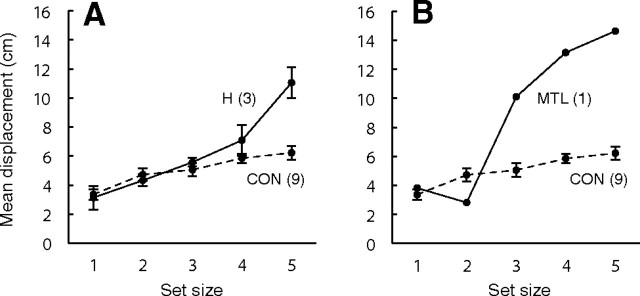
The patients with hippocampal lesions (n = 3) and patient GP, with large medial temporal lobe lesions, exhibited a similar pattern of performance. They performed as well as controls at small set sizes but were markedly impaired at larger set sizes. The performance of patients with hippocampal lesions (measured as mean object displacement for each set size) was intact for set sizes 1 through 3, began to decline at set size 4, and declined sharply at set size 5 (Fig. 3A). Thus, the patients were able to maintain in memory a small number of object–location associations as well as controls, but they made substantial errors when asked to remember five object–location associations (mean displacement, 11.2 vs 6.2 cm; t(10) = 4.9, p < 0.001). The MTL patient GP performed normally at set sizes 1 and 2 but his performance declined sharply at larger set sizes (Fig. 3B). The pattern of results was the same when performance was measured as mean maximum displacement as a function of set size (i.e., the mean of the largest displacements in each of the four trials at a given set size).
Figure 3.
Experiment 1. Participants saw one to five objects in an array and then, on an adjacent table, attempted to place them in their original locations. The data show the mean distance that the objects were displaced from their original locations as a function of set size. A, Patients with circumscribed hippocampal damage (H). B, Patient GP with large medial temporal lobe lesions (MTL); CON, controls. Error bars indicate SEM.
The displacement errors made by the patients were of two kinds. Most of the errors involved placing one or more of the objects in an incorrect location that no object had occupied originally (Fig. 1B). The other kind of error occurred when each object was placed near a location that had been occupied originally, but the locations of two (or more) objects were interchanged (Fig. 1C). All of the hippocampal patients and the medial temporal lobe patient GP exhibited this second kind of error (GW, one of four trials at set size 5; LJ, one of four trials at set sizes 4 and 5; KE, one of four trials at set sizes 4 and 5; GP, two of four trials at set size of 4 and one of four trials at set size 5). One control also exhibited this second kind of error at set size 4, another control exhibited this error at a set size of 5, and a third control exhibited this error at both set sizes 4 and 5. Thus, neither patients nor controls exhibited this second kind of error until the array consisted of at least four objects.
We also asked whether some displacement errors occurred because participants correctly maintained the spatial relationships among the objects in the array but displaced the entire array by some amount (e.g., all objects placed seven cm below their original locations). One control exhibited this kind of error (displacing the entire array) at set 2. No patient exhibited this error.
Experiment 2
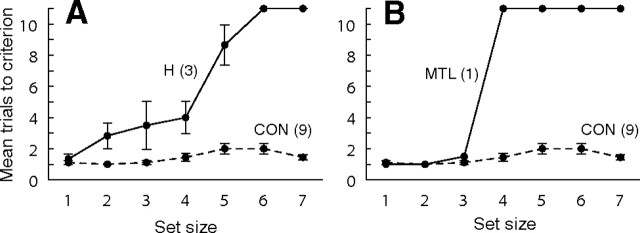
As in experiment 1, the patients with hippocampal lesions and patient GP exhibited a similar pattern of performance. They performed well at small set sizes but then declined abruptly at larger set sizes. The performance of the hippocampal patients (measured as mean number of trials needed to reach criterion at each set size) declined a little beginning at a set size of 2 and then declined sharply beginning at set size 5 (Fig. 4A). The MTL patient (GP) performed as well as controls for set sizes 1, 2, and 3 but was unable to reach criterion for any set sizes larger than 3 (Fig. 4B). By contrast, no control ever needed more than four trials to reach criterion, even with large set sizes. The pattern of results was similar when the displacement measure from experiment 1 was used to assess performance (using the first trials at each set size).
Figure 4.
Experiment 2. Participants saw one to seven objects in an array and then, on an adjacent table, attempted to place them in their original locations. The data show the number of trials needed to reach criterion as a function of set size [all objects in contact with a circle (5 cm radius) around the original location]. A score of 11 was assigned if criterion was not reached within 10 trials. A, Patients with circumscribed hippocampal damage (H). B, Patient GP with large medial temporal lobe lesions (MTL); CON, controls. Error bars indicate SEM.
By both measures (the number of trials needed to reach criterion and the mean displacement on the first trial), the performance of the patients with hippocampal lesions was variable at set sizes 2, 3, and 4 across the two test sessions. Accordingly, we examined individual performance of the hippocampal patients in each of their two test sessions (Fig. 5). These data indicate that each patient demonstrated, in at least one test session, an ability to perform as well as controls at set sizes 1, 2, 3, and 4 (GW, session 2; LJ, session 1; KE, session 2). In addition, in every test session, a sharp discontinuity appeared between the learning score obtained at small set sizes and the learning score obtained at large set sizes. Indeed, in every session, each patient reached a set size at which they failed to reach criterion within 10 trials and, at set sizes 6 and 7, none of the patients reached criterion. Note that the test proved so taxing for patient LJ (in both sessions) and for patient KE (in one session) that testing was discontinued before the largest set size was presented.
Figure 5.
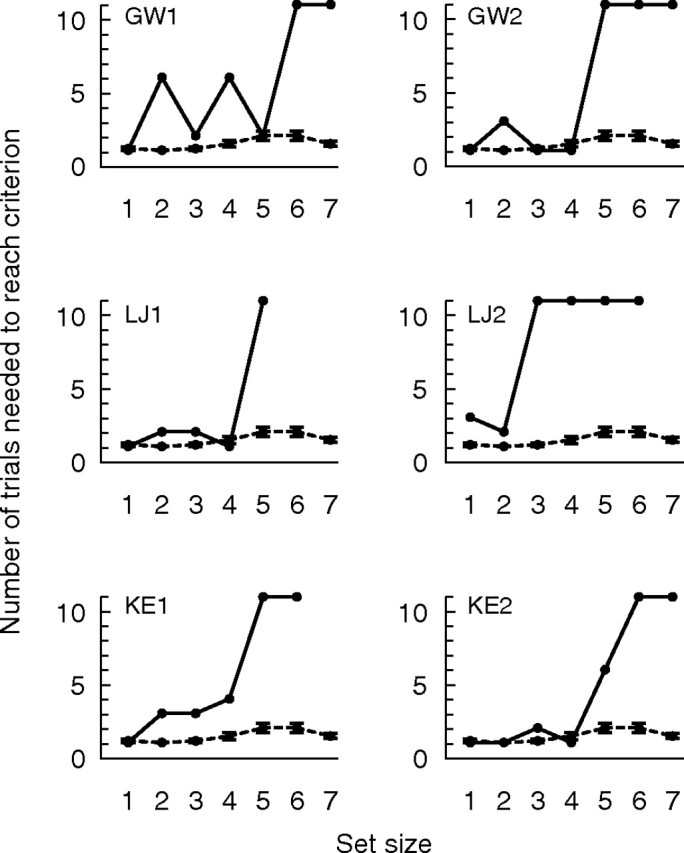
Experiment 2. Individual patient data from Figure 4 is shown. Patients (solid lines) were tested twice and controls (dashed lines) were tested once. Each panel shows an individual hippocampal patient's performance on a single test session along with control performance.
Nearly all the errors were of the same two kinds as in experiment 1 (i.e., one or more objects were placed in a location that no object had occupied originally or the locations of two or more objects were interchanged). In addition, in two cases (both at a set size of 2), the patients correctly maintained the spatial relationships between the two objects but displaced the entire array by a small amount (GW in four of six trials, session 1; KE in one of three trials, session 1). Note that one control also exhibited this kind of error (displacing the entire array) at set 2 in experiment 1. At small set sizes (1 through 4), where the hippocampal patients sometimes did not perform as well as controls (Fig. 5), the errors that resulted in a failure to reach criterion were typically small (displacing a single object a little outside the allowed boundary). By contrast, at set sizes 5 through 7, many of the objects in the array were typically misplaced, and the displacements tended to be large. Also, at the larger set sizes (5 through 7), all patients exhibited the second kind of error (i.e., the location of two or more objects were interchanged). This type of error was uncommon in smaller set sizes (four instances at set size 4 and one instance at set size 3). Interestingly, one control also exhibited this kind of error (interchanging objects) at set sizes 4 and 5, and another control exhibited this error at set size 6. Thus, with the exception of one trial in one test session (GW at set size 3), both patients and controls first exhibited this kind of error at set size 4. Note that in experiment 1 this kind of error also first appeared, for both patients and controls, at set size 4.
Discussion
We investigated the role of the hippocampus and related medial temporal lobe structures in maintenance of relational information across a short retention interval. It has been suggested that medial temporal lobe structures are sometimes critical for maintaining relational information, regardless of whether the task depends on working memory or long-term memory. An alternative possibility is that these structures are critical for maintaining relational information only when the task exceeds working memory capacity and depends instead on long-term memory. To test these ideas, we assessed in two experiments the performance of four patients with medial temporal lobe damage on a task that required participants to maintain a number of object–location associations across a 1 s retention interval. In both experiments, the patients exhibited a similar pattern of performance. They performed similarly to controls when only a small number of object–location associations needed to be maintained. Furthermore, they exhibited an abrupt decline in performance when more object locations needed to be remembered.
Our findings are reminiscent of the classic observations of patient HM (Drachman and Arbit, 1966). HM could repeat back strings of one to six digits without error but then failed at seven digits even after 25 repetitions of the same digit string. The marked discontinuity in HM's performance as he moved from six to seven digits was interpreted to mean that his immediate memory capacity was exceeded when seven digits were presented and that performance now depended on long-term memory. The abrupt discontinuity in performance that we observed suggests a similar interpretation.
Other studies of visual working memory have identified a capacity limit smaller than was found with digits and similar to what we have found here. Typically, only three to four simple visual objects can be maintained (Cowan, 2001; Fukuda et al., 2010). Our data suggest a similar capacity limit on the number of object–location associations that can be maintained. Working memory capacity for visual material may be more limited than for material presented verbally and may have distinct neural substrates as well (Baddeley, 2003). Nonetheless, both kinds of working memory are capacity-limited and require active maintenance.
In our task, memory for the objects themselves could potentially be maintained by both visual and verbal strategies. Memory for the spatial location of those objects presumably required maintenance by a visual strategy. First, object location memory in this task is impaired by right but not left temporal lobectomy (Smith and Milner, 1981; Crane and Milner, 2005). Second, in posttest interviews, participants reported that they tried to retain “a snapshot” of the array.
It had been suggested previously that medial temporal lobe structures (particularly the hippocampus) are critical for maintaining relational information in some tasks, even when the task depends on working memory and retention delays as short as 3 s (Hannula et al., 2006; Olson et al., 2006b). In Hannula et al. (2006), participants decided whether an object in a scene had changed location compared with its location in a scene presented earlier. Study trials were interleaved with probe trials that appeared either immediately after the corresponding study trial, five trials later, or nine trials later (lags of 1, 5, or 9). The patients were impaired at remembering the locations of objects even at a lag of 1 when no stimuli intervened between study and test. In Olson et al. (2006b), participants studied three objects, each presented one at a time in one cell of a 3 × 3 grid. After a delay of 1 or 8 s, an object was presented in one of the nine cells, and the participant decided whether it had been presented in that same location during study or whether it had been presented in a different location (i.e., a test of object–location associations). The patients were impaired even at a delay of 1 s.
In Hannula et al. (2006), it is possible that the task depended on long-term memory rather than working memory, even at a lag of 1. Specifically, the test format required participants to maintain a number of scenes in mind throughout testing, because participants did not know whether the next trial would probe a scene presented one, five, or nine scenes earlier. That is, even at a lag of 1, a substantial memory load was required to perform well. In Olson et al. (2006b), patients were impaired in only one of two experiments that tested memory for three object–location associations after 1 s. In our earlier study with this same procedure (Shrager et al., 2008), patients performed as well as controls at remembering up to six object–location associations after 1 s (for further discussion of the different findings in the two reports, see Shrager et al., 2008).
Patient GP, who had large medial temporal lesions, exhibited the most striking demonstration of intact performance at small set sizes (Figs. 3B, 4B), together with an abrupt decline in performance at larger set sizes. For example, in experiment 1, GP exhibited intact performance at set sizes 1 and 2, but his performance declined abruptly at set size 3. In experiment 2, GP reached criterion as quickly as controls for 1, 2, and 3 object–location associations. When the set size was increased by only one more object (set size 4), GP failed to reach criterion even after 10 attempts with the same array of objects (Fig. 4B). This pattern of performance is strikingly similar to the pattern of performance exhibited by patient HM on the digit task (Drachman and Arbit, 1966).
The three patients with circumscribed hippocampal lesions exhibited a pattern of performance similar to patient GP and similar to the patients in the earlier study on digit span (Drachman and Arbit, 1966). In experiment 1, the patients exhibited intact performance at set sizes 1–3. Their performance began to decline at set size 4, and declined sharply at set size 5 (Fig. 3A). In experiment 2, the patients exhibited a modest impairment at set sizes 1–4 and an abrupt decline in performance at set size 5 (Fig. 4A).
Although in experiment 2 the patients with hippocampal lesions did exhibit, on average, a modest impairment at small set sizes (2, 3, and 4), all the patients were able to perform as well as controls at these same set sizes in at least one of the two test sessions (Fig. 5). In addition, at small set sizes, patient GP performed as well as controls on both test sessions of experiment 2. GP was also consistently the best motivated and most attentive of all the patients. Moreover, the modest impairment apparent in the average score for the hippocampal patients (Fig. 4A) was influenced particularly by the first test session for patient GW (Fig. 5, upper left), and GW tended to be less careful than the others.
In summary, we explored memory for relational information (object–location associations) after a brief delay in patients with medial temporal lobe damage. Patients performed similarly to controls when only a small number of object locations needed to be maintained in memory. All patients then exhibited an abrupt decline in performance at larger set sizes. In addition, both patients and controls first made a particular type of error (interchanging the location of objects) at larger set sizes. This pattern of results supports the idea that maintenance of relational information in working memory is intact after damage to the hippocampus and related medial temporal lobe structures and that damage to these structures impairs performance only when the task depends on long-term memory.
Footnotes
This work was supported by the Medical Research of the Department of Veterans Affairs, National Institute of Mental Health Grant MH24600, and the Metropolitan Life Foundation. We thank Jennifer Frascino, Lisa Graves, Anna van der Horst, Ashley Knutson, David Roemer, and Christine Smith for assistance.
References
- Atkinson RC, Shiffrin RM. Human memory: a proposed system and its control processes. In: Spence KW, Spence JT, editors. The psychology of learning and motivation: advances in research and theory. Vol 2. New York: Academic; 1968. [Google Scholar]
- Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci. 2003;4:829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Hitch GJ. Working memory. In: Bower GH, editor. The psychology of learning and motivation: advances in research and theory. New York: Academic; 1974. pp. 47–89. [Google Scholar]
- Baddeley AD, Warrington EK. Amnesia and the distinction between long- and short-term memory. J Verbal Learn Verbal Behav. 1970;9:176–189. [Google Scholar]
- Bayley PG, Frascino JC, Squire LR. Robust habit learning in the absence of awareness and independent of the medial temporal lobe. Nature. 2005a;436:550–553. doi: 10.1038/nature03857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley PG, Gold JJ, Hopkins RO, Squire LR. The neuroanatomy of remote memory. Neuron. 2005b;46:799–810. doi: 10.1016/j.neuron.2005.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley PJ, Hopkins RO, Squire LR. The fate of old memories after medial temporal lobe damage. J Neurosci. 2006;26:13311–13317. doi: 10.1523/JNEUROSCI.4262-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: a reconsideration of mental storage capacity. Behav Brain Sci. 2001;24:87–114. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- Crane J, Milner B. What went where?: Impaired object-location learning in patients with right hippocampal lesions. Hippocampus. 2005;15:216–231. doi: 10.1002/hipo.20043. [DOI] [PubMed] [Google Scholar]
- Drachman DA, Arbit J. Memory and the hippocampal complex. II. Is memory a multiple process? Arch Neurol. 1966;15:52–61. doi: 10.1001/archneur.1966.00470130056005. [DOI] [PubMed] [Google Scholar]
- Ezzyat Y, Olson IR. The medial temporal lobe and visual working memory: comparisons across tasks, delays, and visual similarity. Cogn Affect Behav Neurosci. 2008;8:32–40. doi: 10.3758/cabn.8.1.32. [DOI] [PubMed] [Google Scholar]
- Finke C, Braun M, Ostendorf F, Lehmann TN, Hoffmann KT, Kopp U, Ploner CJ. The human hippocampal formation mediates short-term memory of colour-location associations. Neuropsychologia. 2008;46:614–623. doi: 10.1016/j.neuropsychologia.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Awh E, Vogel EK. Discrete capacity limits in visual working memory. Curr Opin Neurobiol. 2010;20:177–182. doi: 10.1016/j.conb.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JJ, Squire LR. Quantifying medial temporal lobe damage in memory-impaired patients. Hippocampus. 2005;15:79–85. doi: 10.1002/hipo.20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Tranel D, Cohen NJ. The long and the short of it: relational memory impairments in amnesia, even at short lags. J Neurosci. 2006;26:8352–8359. doi: 10.1523/JNEUROSCI.5222-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley T, Bird CM, Chan D, Cipolotti L, Husain M, Vargha-Khadem F, Burgess N. The hippocampus is required for short-term topographical memory in humans. Hippocampus. 2007;17:34–48. doi: 10.1002/hipo.20240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, Vogel EK. The capacity of visual working memory for features and conjunctions. Nature. 1997;390:279–281. doi: 10.1038/36846. [DOI] [PubMed] [Google Scholar]
- Milner B. Disorders of learning and memory after temporal lobe lesions in man. Clin Neurosurg. 1972;19:421–446. doi: 10.1093/neurosurgery/19.cn_suppl_1.421. [DOI] [PubMed] [Google Scholar]
- Nichols EA, Kao YC, Verfaellie M, Gabrieli JD. Working memory and long-term memory for faces: evidence from fMRI and global amnesia for involvement of the medial temporal lobes. Hippocampus. 2006;16:604–616. doi: 10.1002/hipo.20190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Moore KS, Stark M, Chatterjee A. Visual working memory is impaired when the medial temporal lobe is damaged. J Cogn Neurosci. 2006a;18:1087–1097. doi: 10.1162/jocn.2006.18.7.1087. [DOI] [PubMed] [Google Scholar]
- Olson IR, Page K, Moore KS, Chatterjee A, Verfaellie M. Working memory for conjunctions relies on the medial temporal lobe. J Neurosci. 2006b;26:4596–4601. doi: 10.1523/JNEUROSCI.1923-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempel-Clower NL, Zola SM, Squire LR, Amaral DG. Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. J Neurosci. 1996;16:5233–5255. doi: 10.1523/JNEUROSCI.16-16-05233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrager Y, Gold JJ, Hopkins RO, Squire LR. Intact visual perception in memory-impaired patients with medial temporal lobe lesions. J Neurosci. 2006;26:2235–2240. doi: 10.1523/JNEUROSCI.4792-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrager Y, Levy DA, Hopkins RO, Squire LR. Working memory and the organization of brain systems. J Neurosci. 2008;28:4818–4822. doi: 10.1523/JNEUROSCI.0710-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Milner B. The role of the right hippocampus in the recall of spatial location. Neuropsychologia. 1981;19:781–793. doi: 10.1016/0028-3932(81)90090-7. [DOI] [PubMed] [Google Scholar]
- Smith ML, Milner B. Right hippocampal impairment in the recall of spatial location: encoding deficit or rapid forgetting? Neuropsychologia. 1989;27:71–81. doi: 10.1016/0028-3932(89)90091-2. [DOI] [PubMed] [Google Scholar]
- Squire LR. The legacy of patient H.M. for neuroscience. Neuron. 2009;61:6–9. doi: 10.1016/j.neuron.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickelgren WA. Sparing of short-term memory in an amnesic patient: implications for strength theory of memory. Neuropsychologia. 1968;6:235–244. [Google Scholar]