Summary
Recent advances in single-cell assays have focused attention on the fact that even members of a genetically identical group of cells or organisms in identical environments can exhibit variability in drug sensitivity, cellular response, and phenotype. Underlying much of this variability is stochasticity in gene expression, which can produce unique proteomes even in genetically identical cells. Here we discuss the consequences of non-genetic cell-to-cell variability in the cellular response to drugs and its potential impact for treatment of human disease.
Introduction
It has long been recognized that differences from one cell to the next can arise through variation in the extracellular environment (e.g. morphogen gradients during embryogenesis) or from genomic alteration (e.g. genetic heterogeneity within a tumor). Only recently has it become clear that intracellular biochemical fluctuations can also have profound effects on phenotype. These fluctuations cause genetically identical cells to vary significantly in their responsiveness to stimuli and drugs even in a uniform environment [1,2**,3**,4,5*,6,7**,8*]. Such cell-to-cell differences arise from stochasticity in the biochemical reactions. Gene expression is one of the best understood of these stochastic processes (see Box 1), but cytoskeletal rearrangement, protein localization, post-translational modification and formation of protein complexes are almost certainly also involved in generating variability. The net result is the creation of cell populations with the same genome but unique proteomes.
Box 1. Stochastic gene expression.
An early and influential experiment analyzed the sources of noise in gene expression by tagging two copies of the same promoter with two different fluorescent proteins in E. coli [46]. The authors found that both “extrinsic noise” (affecting both gene copies equally, but varying from cell to cell) and “intrinsic noise” (caused by the inherent stochasticity in transcription and translation, and random between gene copies) contributed to cell-to-cell variability. This intrinsic noise arises in bacteria because proteins are produced in translational bursts from mRNA transcripts [47] and because mRNA molecules themselves are produced in transcriptional bursts [13]. In mammalian cells, however, transcription plays a more dominant role. Work in Chinese hamster ovary cells showed that transcriptional bursts are infrequent but long-lasting, caused by random transitions between inactive and active states of genes. These bursts produce high variation from cell to cell in mRNA levels and consequently in protein number [48*].
Network architecture has significant impact on noise propagation in complex signaling systems. Noise in upstream regulators can propagate to downstream genes, thus adding to the intrinsic noise in the gene’s output [49]. Negative feedback can reduce the effects of noise by compensating for deviations from a set point [50], while positive feedback can have the opposite effect, amplifying small fluctuations. Cooperativity helps to maintain a state, buffering it against small fluctuations, and providing a long-term protein-level memory [51].
Transient heritability is an interesting property of phenotypic variability arising from cell-to-cell differences at the proteome level [5*,9*,10**]. While genetic differences are stably passed on to progeny and epigenetic modifications are heritable for 10–105 cell divisions [11], the state of the proteome is heritable on a much shorter timescale (one to a few divisions) [5*,9*,10**]. The proteomes of recently born sister cells are similar, owing to binomial partitioning of abundant cellular components during cell division [12,13]. The phenotypes of sister cells are therefore more similar than those of two cells chosen at random, although stochastic fluctuations cause sister cells to decorrelate rapidly [5*,8*,10**].
The timescales and mechanisms underlying stochastic, epigenetic, and genetic variation are distinct, but the three types of variation interact (Figure 1a). Most obviously, genetic and epigenetic factors determine the mean levels or activities of proteins, and stochastic processes determine dispersion around the mean. However, the proteome also influences epigenetic regulation of gene expression [14,15] and it has been suggested that the expression levels of certain proteins influence the probability that mutations will be fixed [16].
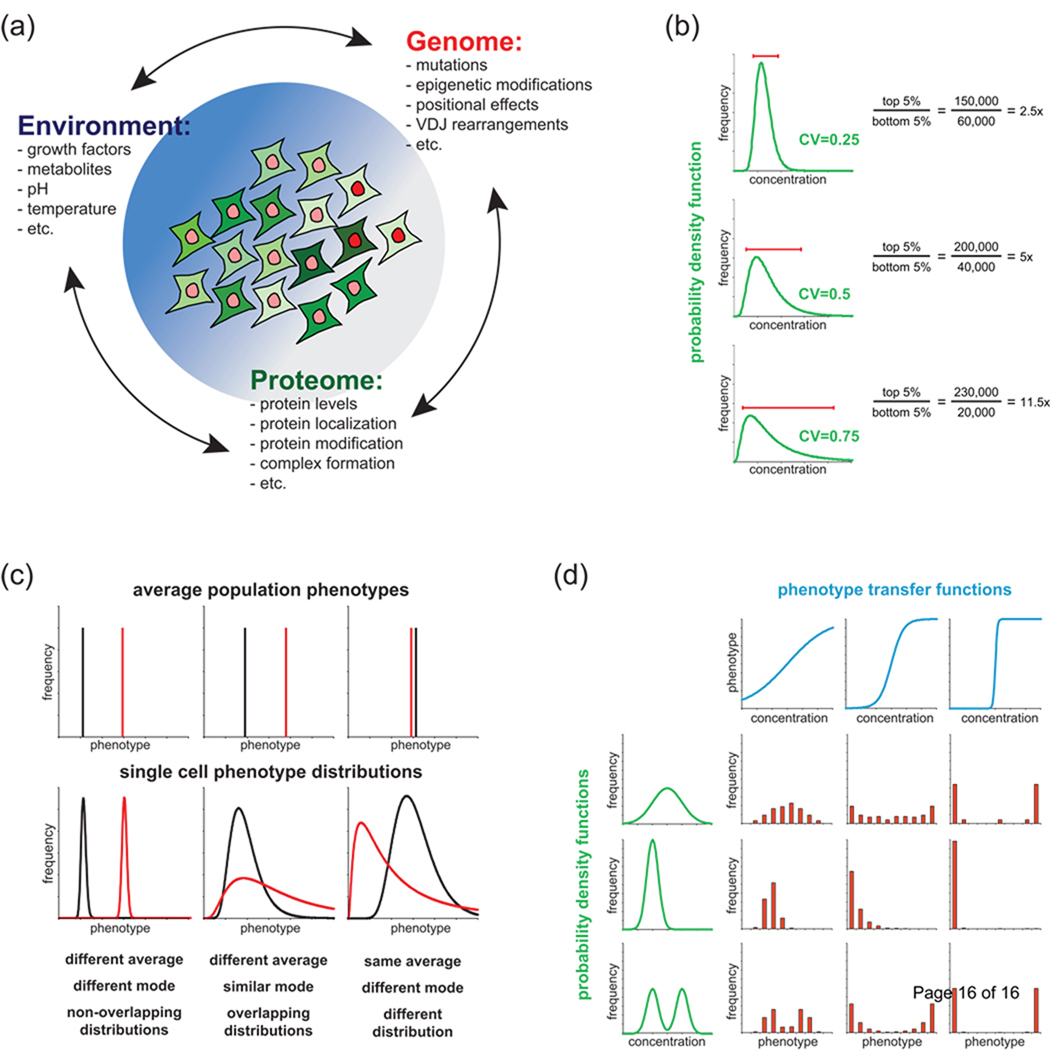
Figure 1. Cell-to-cell variability of the proteome gives rise to phenotypic heterogeneity.
(a) Interaction among factors that determine average cellular phenotype and variance around the average. Combinations of environmental (blue), genomic (red) and proteomic (green) variation can cause heterogeneity in an initially homogenous population. (b) Probability density functions of three lognormal distributions with different coefficients of variation (CV; standard deviation divided by the mean). Red bars show the increasing disparity in protein levels between cells in the bottom and the top 5th percentiles. Protein numbers for the top and bottom 5th percentile and their ratio are shown for each distribution, assuming a mean of 100,000 proteins per cell. (c) Three hypothetical cases illustrating how the mean phenotype of a population (top row) can arise from different underlying phenotypic distributions (bottom row) at the single cell level. (d) Illustration of the effects of various transfer functions linking protein levels to phenotypes. The histograms (red) represent the distribution of phenotypes in a population generated by applying the univariate transfer functions (blue) to the protein level probability density functions (green).
The distribution of protein abundance and resulting variability in phenotype
When the effects of cell size are taken into account, the concentration distribution for many proteins across a clonal population of mammalian cells is well-fit by a log-normal distribution [2**,5*], with a coefficient of variation (CV, equal to the standard deviation divided by the mean) of ~0.1 to ~0.6 [2**,5*,10**]. Log-normal distributions are long-tailed so that even a tight distribution (CV=0.25) contains cells in the 95th percentile that have 2.5 times the abundance of a particular protein than cells in 5th percentile, and this spread increases dramatically with increasing CV (Figure 1b). Some distributions are even bimodal, with potentially dramatic effects on cell fate [17*]. It is not known why distributions differ [2**,10**,18–20] but regulatory processes may be involved [19,20]. In mammalian cells, the relative levels of most proteins do not appear to be highly correlated [10**], although correlation is observed in the case of cell cycle regulated genes [18]. Based on current observations, uncorrelated, long-tailed distributions in protein abundance should be considered the default state; deviations from this probably reflect the operation of active processes that affect variability (feedback control on transcription, for example).
We are accustomed to thinking about phenotypes as discrete states in which a single genotype maps to a distinct phenotype. However, the existence of a unique proteome in each cell means that a single genotype can give rise to a range of phenotypes, with varying degrees of overlap (Figure 1c). Whether the phenotypic range is wide or narrow cannot easily be predicted a priori. For example, in the case of receptor-mediated apoptosis, the time between ligand exposure and the commitment to death is very variable from one cell to the next but the time between commitment to apoptosis and completion of caspase-mediated cell killing is not [5*,21–23]. Is phenotypic variation from one cell to the next simply the inevitable consequence of building pathways from imprecise components (proteins that vary in abundance, for example) or might it actually have adaptive advantage? It has been proposed that intrinsic variability in otherwise identical lymphocytes is necessary for correct operation of the adaptive immune response [24].
In general, the connection between the levels of a set of interacting proteins and a phenotype depends on the precise biochemistry of the regulatory process. This is true for the mean levels of a protein (determined genetically) and for fluctuations around the mean. We can approximate the relationship between a protein’s level and a cellular response by a transfer function. The phenotype distribution across a population thus depends on both the heterogeneous protein distribution and the complexity of the transfer function (Figure 1d). In some cases, the function is linear, at least over a limited range, but nonlinear, non-monotonic relationships and thresholds are likely more common [1,25]. Moreover, while some phenotypes are influenced primarily by a single protein [7**,9*], most are controlled by multiple factors [2**,5*,26,27], so intuiting cellular phenotype from unique proteomes is not trivial. Therefore, understanding phenotype at a quantitative level requires methods for measuring variation (see below) and development of modeling frameworks that are able to account both for variation in the proteome and the complexity of transfer functions that determine phenotype (see Box 2).
Box 2. Modeling cell-to-cell variability.
The amount and detail of information available about a biochemical process and the goals of the analysis determine how cell-to-cell variability should be modeled. The simplest models are conceptual or diagrammatic and attempt to account for the mean values of key variables (e.g. rates of physiological processes) and their dispersion about the mean [3**]. More rigorous are methods based on stochastic differential equations (SDEs) or the Langevin approach in which the biochemical reactions are cast as deterministic differential equations with an added noise term [49]. Finally, the chemical master equation, which describes the probability that individual molecules will collide and react in a given time interval, can be approximated numerically using the Stochastic Simulation Algorithm (SSA) or Gillespie Algorithm [52].
If the goal is to study the stochastic process in detail, then use of SDEs or the SSA is necessary, but it is also possible to separate the causes and consequences of stochasticity. For example, if we want to understand cell-to-cell variability in phenotype arising from differences in the levels of relatively abundant proteins, then we can combine deterministic models with distributions of initial protein concentrations and arrive at reasonable and useful approximations. The processes that generate the distribution in parameter values are stochastic, but given a measured or assumed distribution at the outset of the simulation, we can then use deterministic differential equations. This greatly facilitates parameter estimation, simulation, and model interpretation. Using such a hybrid approach merely awaits the development of a practical implementation of the modeling methodology.
Measuring cell-to-cell variation
Linking protein expression levels to phenotype is most commonly performed using fixed and live-cell microscopy. Only a limited number of cellular events are visible using bright field or phase-contrast illumination (e.g. changes in cell size, morphology, motility, cell division) and fluorescent biosensors are needed to assay specific biochemical processes. Cell permeable dyes, such as JC-1 used to measure changes in mitochondrial membrane potential during apoptosis, can simply be added to cell growth media, while genetically encoded reporters have to be actively inserted into cells. When expressed in cells, activity-sensitive fluorescent biosensors can report on protein-protein association [28], protein conformation [29], protein cleavage [1], and protein modifications [30]. The ectopic expression of fluorescently tagged proteins has also been used to monitor changes in protein subcellular localization [31], protein expression and degradation [8*], and changes in cell cycle phase [32]. One challenge with these reporters is that expressing them in cells can perturb the phenomena under study. To minimize this, fluorescent reporters can be fused to genes at their normal genomic loci, so that native promoters are retained [10**,19,20,33]. Libraries of such cell lines have been used to correlate protein levels and localization in drug-treated cells with cell fate [7**].
Immunofluorescence-based assays such as flow cytometry [6,34] and high-throughput immunofluorescence microscopy [35,36] have the advantage that no reporters are required, and all assays can be carried out in unaltered cells, often allowing for the transition of an established assay into new cell lines or even primary tissue samples. However, these assays do not provide information on temporal dynamics and are limited by the availability of specific antibodies. Ultimately, we would like to obtain data on the complete proteomes of single cells, but this is not feasible using current technology. High-dimensional flow cytometry [37,38], improved microdissection [39] and high-sensitivity mass spectrometry [40] may make systematic characterization of proteomes at the single-cell level possible. Until then, the obvious approach is to measure protein distributions by flow cytometry and phenotypes by live-cell microscopy.
Potential implications of cell-to-cell variability in pharmacology and human disease
How might emerging understanding of non-genetic cell-to-cell variability impact pharmacology and the treatment of human disease? A significant problem in cancer is fractional killing or incomplete growth inhibition of tumor cells [41]. Multiple explanations of fractional killing have been proposed including variation in the access of tumor cells to a drug, genetic heterogeneity, drug insensitivity during certain cell cycle phases [42,43] and, most recently the presence of drug-resistant cancer stem cells [44]. However, natural fluctuations in the proteome and resulting dispersion in drug responsiveness of cells [3**,4,5*,7**] is likely to be an important but poorly appreciated factor. For example, protein expression outliers in key stem cell markers such as Nanog and Sca-1 have been shown to influence pluripotency and can remix to repopulate the full distribution of expression levels [9*,17*]. Similarly, drug-resistant cancer stem cells, rather than being a static genetically or epigenetically distinct subfraction, may represent population outliers resulting from proteomic heterogeneity.
If proteomic fluctuations indeed play a critical role in drug sensitivity, new approaches to maximizing therapeutic efficacy may be required. Fractional killing is often combated by dosing patients repeatedly with the idea that cells that are resistant to the first round of therapy will be killed in the second round, perhaps because they are in a different cell cycle phase [42,43]. However, if fluctuations in the proteome play a significant role in fractional killing, then repeat treatment may not work because outliers in the dose-response profile will survive to repopulate the full distribution [5*,10**,45]. Nevertheless, it may be possible to identify compounds that alter the distribution of proteome states so as to reduce the fraction of non-responders. Combination therapy is standard in cancer care, but targeting the dispersion of phenotypic responses has not, to our knowledge, been examined as a strategy. Preliminary data suggests it might work however, since heterogeneity in the responses of cells to drugs such as a death ligand can be altered by changing the expression levels of apoptotic regulators [5*]. The way forward would appear to involve understanding which proteins contribute most to variability in response to a particular drug and then attempting to target these proteins.
In many cases, the phenotypic heterogeneity within a population is not simply a binary choice between death and survival. For example, cells treated with microtubule poisons such as nocodazole can respond with as many as seven distinct phenotypes [3**]. Thus, following exposure to a drug, even a relatively homogeneous population of cells can become significantly more diverse. This induced phenotypic heterogeneity is likely to be even more complicated when multiple drugs are employed, especially if one drug causes permanent genomic changes. As a consequence, it is exceedingly difficult to predict the optimal treatment regimen with the large number of chemotherapeutics employed at this time.
Conclusions
The complexity of cellular response to drugs suggests that it will be valuable to study pre-clinical pharmacology using sophisticated single-cell measurement of both mean behaviors and variation about the mean in proteome and phenotype. While the phenomena of stochastic gene expression, proteome fluctuations and cell-to-cell variability in phenotypes have clearly been established in the context of single-celled organisms and cell lines, the full impact on pharmacology and the treatment of human disease is currently unknown. These concepts may actually be clinically applicable in the short term, as relevant single cell data is already being collected in pharmacological screens, histopathology samples, and flow cytometry analysis of blood. Advances in mathematical modeling methods are also required to account for stochastic biochemical processes, dispersion in model parameters, and the impact on phenotype. Nonetheless, there is every reason to believe that non-genetic variability in the proteome will be recognized, alongside genetic changes and local environment, as an important factor in the development and treatment of human disease.
Acknowledgements
This work was supported by National Institute of Health (NIH grants) GM68762 and CA112967.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albeck JG, Burke JM, Aldridge BB, Zhang M, Lauffenburger DA, Sorger PK. Quantitative analysis of pathways controlling extrinsic apoptosis in single cells. Mol Cell. 2008;30:11–25. doi: 10.1016/j.molcel.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Feinerman O, Veiga J, Dorfman JR, Germain RN, Altan-Bonnet G. Variability and robustness in T cell activation from regulated heterogeneity in protein levels. Science. 2008;321:1081–1084. doi: 10.1126/science.1158013.. ** This study linked variability in the activation of a clonal population of T cells to endogenous variability in the levels of two proteins, CD8 and SHP-1. The authors measured co-variation in the levels of these two proteins and using modeling, showed that this co-variation reduced the diversity of T cell response.
- 3. Gascoigne KE, Taylor SS. Cancer cells display profound intra- and interline variation following prolonged exposure to antimitotic drugs. Cancer Cell. 2008;14:111–122. doi: 10.1016/j.ccr.2008.07.002.. ** This paper characterized the response of 15 cell lines to three different classes of antimitotic drugs and found significant inter- as well as intra-line variation, with cells within any given cell line exhibiting multiple distinct phenotypes in response to treatment.
- 4.Orth JD, Tang Y, Shi J, Loy CT, Amendt C, Wilm C, Zenke FT, Mitchison TJ. Quantitative live imaging of cancer and normal cells treated with Kinesin-5 inhibitors indicates significant differences in phenotypic responses and cell fate. Mol Cancer Ther. 2008;7:3480–3489. doi: 10.1158/1535-7163.MCT-08-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Spencer SL, Gaudet S, Albeck JG, Burke JM, Sorger PK. Non-genetic origins of cell-to-cell variability in TRAIL-induced apoptosis. Nature. 2009;459:428–432. doi: 10.1038/nature08012.. * This paper examined why, in a clonal population, some cells die after a death-inducing stimulus, while others survive indefinitely. Using time-lapse imaging, flow cytometry, and mathematical modeling, the authors showed that stochastic differences in the levels of proteins are the primary causes of cell-to-cell variability in the timing and probability of death.
- 6.Irish JM, Hovland R, Krutzik PO, Perez OD, Bruserud O, Gjertsen BT, Nolan GP. Single cell profiling of potentiated phospho-protein networks in cancer cells. Cell. 2004;118:217–228. doi: 10.1016/j.cell.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 7. Cohen AA, Geva-Zatorsky N, Eden E, Frenkel-Morgenstern M, Issaeva I, Sigal A, Milo R, Cohen-Saidon C, Liron Y, Kam Z, et al. Dynamic proteomics of individual cancer cells in response to a drug. Science. 2008;322:1511–1516. doi: 10.1126/science.1160165.. ** This paper used time-lapse imaging of a library of proteins tagged with yellow fluorescent protein at their endogenous locus to monitor drug responses at a single cell level. The authors identified two proteins whose levels rise in cells that survive treatment with the cancer drug camptothecin and decrease in cells that died, thereby correlating variability arising subsequent to drug treatment with cell fate.
- 8. Geva-Zatorsky N, Rosenfeld N, Itzkovitz S, Milo R, Sigal A, Dekel E, Yarnitzky T, Liron Y, Polak P, Lahav G, et al. Oscillations and variability in the p53 system. Mol Syst Biol. 2006;2 doi: 10.1038/msb4100068. 2006 0033. * This study described the dynamic changes of p53 and Mdm2 expression in genetically identical cells. In response to gamma irradiation, a high degree of cell-to-cell variability was observed, with some cells showing persistent oscillations. While these oscillations were random in amplitude, the oscillating behavior of recently divided sister cells was found to be correlated.
- 9. Chang HH, Hemberg M, Barahona M, Ingber DE, Huang S. Transcriptome-wide noise controls lineage choice in mammalian progenitor cells. Nature. 2008;453:544–547. doi: 10.1038/nature06965.. * Chang et al. found highly variable expression levels of the stem-cell marker Sca-1 in a clonal population of multipotent haematopoietic cells. Expression levels correlated with lineage choice and extreme outliers with very high or very low expression were able to repopulate the original protein distribution.
- 10. Sigal A, Milo R, Cohen A, Geva-Zatorsky N, Klein Y, Liron Y, Rosenfeld N, Danon T, Perzov N, Alon U. Variability and memory of protein levels in human cells. Nature. 2006;444:643–646. doi: 10.1038/nature05316.. ** This pioneering study measured fluctuations over time in the levels of 20 proteins tagged with yellow fluorescent protein at their endogenous locus in human cells. This method allowed dynamic observation of protein level heterogeneity and examined whether cells that have higher than average protein levels eventually become lower. This mixing occurred for all proteins, and the half-life for mixing time was found to range between 0.8 and 2.5 cell generations.
- 11.Rando OJ, Verstrepen KJ. Timescales of genetic and epigenetic inheritance. Cell. 2007;128:655–668. doi: 10.1016/j.cell.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 12.Rosenfeld N, Young JW, Alon U, Swain PS, Elowitz MB. Gene regulation at the single-cell level. Science. 2005;307:1962–1965. doi: 10.1126/science.1106914. [DOI] [PubMed] [Google Scholar]
- 13.Golding I, Paulsson J, Zawilski SM, Cox EC. Real-time kinetics of gene activity in individual bacteria. Cell. 2005;123:1025–1036. doi: 10.1016/j.cell.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson S, Pillus L. Modifying chromatin and concepts of cancer. Curr Opin Genet Dev. 1999;9:175–184. doi: 10.1016/S0959-437X(99)80027-6. [DOI] [PubMed] [Google Scholar]
- 15.Kouzarides T. Histone acetylases and deacetylases in cell proliferation. Curr Opin Genet Dev. 1999;9:40–48. doi: 10.1016/s0959-437x(99)80006-9. [DOI] [PubMed] [Google Scholar]
- 16.Cowen LE, Lindquist S. Hsp90 potentiates the rapid evolution of new traits: drug resistance in diverse fungi. Science. 2005;309:2185–2189. doi: 10.1126/science.1118370. [DOI] [PubMed] [Google Scholar]
- 17. Kalmar T, Lim C, Hayward P, Munoz-Descalzo S, Nichols J, Garcia-Ojalvo J, Martinez Arias A. Regulated fluctuations in nanog expression mediate cell fate decisions in embryonic stem cells. PLoS Biol. 2009;7:e1000149. doi: 10.1371/journal.pbio.1000149.. * By monitoring the expression levels of the transcription factor Nanog, the authors found two distinguishable states in isogenic embryonic stem cells. The high Nanog state is stable while the low Nanog state is unstable and promotes differentiation. Mathematical modeling suggested that transcriptional noise underlies this behavior.
- 18.Sigal A, Milo R, Cohen A, Geva-Zatorsky N, Klein Y, Alaluf I, Swerdlin N, Perzov N, Danon T, Liron Y, et al. Dynamic proteomics in individual human cells uncovers widespread cell-cycle dependence of nuclear proteins. Nat Methods. 2006;3:525–531. doi: 10.1038/nmeth892. [DOI] [PubMed] [Google Scholar]
- 19.Newman JR, Ghaemmaghami S, Ihmels J, Breslow DK, Noble M, DeRisi JL, Weissman JS. Single-cell proteomic analysis of S. cerevisiae reveals the architecture of biological noise. Nature. 2006;441:840–846. doi: 10.1038/nature04785. [DOI] [PubMed] [Google Scholar]
- 20.Bar-Even A, Paulsson J, Maheshri N, Carmi M, O'Shea E, Pilpel Y, Barkai N. Noise in protein expression scales with natural protein abundance. Nat Genet. 2006;38:636–643. doi: 10.1038/ng1807. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein JC, Kluck RM, Green DR. A single cell analysis of apoptosis. Ordering the apoptotic phenotype. Ann N Y Acad Sci. 2000;926:132–141. doi: 10.1111/j.1749-6632.2000.tb05607.x. [DOI] [PubMed] [Google Scholar]
- 22.Tyas L, Brophy VA, Pope A, Rivett AJ, Tavare JM. Rapid caspase-3 activation during apoptosis revealed using fluorescence-resonance energy transfer. EMBO Rep. 2000;1:266–270. doi: 10.1093/embo-reports/kvd050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albeck JG, Burke JM, Spencer SL, Lauffenburger DA, Sorger PK. Modeling a snap-action, variable-delay switch controlling extrinsic cell death. PLoS Biol. 2008;6:2831–2852. doi: 10.1371/journal.pbio.0060299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawkins ED, Turner ML, Dowling MR, van Gend C, Hodgkin PD. A model of immune regulation as a consequence of randomized lymphocyte division and death times. Proc Natl Acad Sci U S A. 2007;104:5032–5037. doi: 10.1073/pnas.0700026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu RC, Pesce CG, Colman-Lerner A, Lok L, Pincus D, Serra E, Holl M, Benjamin K, Gordon A, Brent R. Negative feedback that improves information transmission in yeast signalling. Nature. 2008;456:755–761. doi: 10.1038/nature07513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu F, Fan D, Qi J, Zhu H, Zhou Y, Yang C, Zhu Z, Xiong D. Co-expression of cytokeratin 8 and breast cancer resistant protein indicates a multifactorial drug-resistant phenotype in human breast cancer cell line. Life Sci. 2008;83:496–501. doi: 10.1016/j.lfs.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 27.Violini S, D'Ascenzo S, Bagnoli M, Millimaggi D, Miotti S, Canevari S, Pavan A, Dolo V. Induction of a multifactorial resistance phenotype by high paclitaxel selective pressure in a human ovarian carcinoma cell line. J Exp Clin Cancer Res. 2004;23:83–91. [PubMed] [Google Scholar]
- 28.Overton MC, Blumer KJ. G-protein-coupled receptors function as oligomers in vivo. Curr Biol. 2000;10:341–344. doi: 10.1016/s0960-9822(00)00386-9. [DOI] [PubMed] [Google Scholar]
- 29.Goley ED, Rodenbusch SE, Martin AC, Welch MD. Critical conformational changes in the Arp2/3 complex are induced by nucleotide and nucleation promoting factor. Mol Cell. 2004;16:269–279. doi: 10.1016/j.molcel.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 30.Harvey CD, Ehrhardt AG, Cellurale C, Zhong H, Yasuda R, Davis RJ, Svoboda K. A genetically encoded fluorescent sensor of ERK activity. Proc Natl Acad Sci U S A. 2008;105:19264–19269. doi: 10.1073/pnas.0804598105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson DE, Ihekwaba AE, Elliott M, Johnson JR, Gibney CA, Foreman BE, Nelson G, See V, Horton CA, Spiller DG, et al. Oscillations in NF-kappaB signaling control the dynamics of gene expression. Science. 2004;306:704–708. doi: 10.1126/science.1099962. [DOI] [PubMed] [Google Scholar]
- 32.Sakaue-Sawano A, Kurokawa H, Morimura T, Hanyu A, Hama H, Osawa H, Kashiwagi S, Fukami K, Miyata T, Miyoshi H, et al. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell. 2008;132:487–498. doi: 10.1016/j.cell.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 33.Sigal A, Danon T, Cohen A, Milo R, Geva-Zatorsky N, Lustig G, Liron Y, Alon U, Perzov N. Generation of a fluorescently labeled endogenous protein library in living human cells. Nat Protoc. 2007;2:1515–1527. doi: 10.1038/nprot.2007.197. [DOI] [PubMed] [Google Scholar]
- 34.Krutzik PO, Crane JM, Clutter MR, Nolan GP. High-content single-cell drug screening with phosphospecific flow cytometry. Nat Chem Biol. 2008;4:132–142. doi: 10.1038/nchembio.2007.59. [DOI] [PubMed] [Google Scholar]
- 35.Perlman ZE, Slack MD, Feng Y, Mitchison TJ, Wu LF, Altschuler SJ. Multidimensional drug profiling by automated microscopy. Science. 2004;306:1194–1198. doi: 10.1126/science.1100709. [DOI] [PubMed] [Google Scholar]
- 36.Loo LH, Wu LF, Altschuler SJ. Image-based multivariate profiling of drug responses from single cells. Nat Methods. 2007;4:445–453. doi: 10.1038/nmeth1032. [DOI] [PubMed] [Google Scholar]
- 37.Bandura DR, Baranov VI, Ornatsky OI, Antonov A, Kinach R, Lou X, Pavlov S, Vorobiev S, Dick JE, Tanner SD. Mass Cytometry: Technique for Real Time Single Cell Multitarget Immunoassay Based on Inductively Coupled Plasma Time-of-Flight Mass Spectrometry. Anal Chem. 2009 doi: 10.1021/ac901049w. [DOI] [PubMed] [Google Scholar]
- 38.Sachs K, Perez O, Pe'er D, Lauffenburger DA, Nolan GP. Causal protein-signaling networks derived from multiparameter single-cell data. Science. 2005;308:523–529. doi: 10.1126/science.1105809. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez AS, Espina BH, Espina V, Liotta LA. Automated laser capture microdissection for tissue proteomics. Methods Mol Biol. 2008;441:71–90. doi: 10.1007/978-1-60327-047-2_5. [DOI] [PubMed] [Google Scholar]
- 40.Rubakhin SS, Churchill JD, Greenough WT, Sweedler JV. Profiling signaling peptides in single mammalian cells using mass spectrometry. Anal Chem. 2006;78:7267–7272. doi: 10.1021/ac0607010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berenbaum MC. In vivo determination of the fractional kill of human tumor cells by chemotherapeutic agents. Cancer Chemother Rep. 1972;56:563–571. [PubMed] [Google Scholar]
- 42.Chabner B, Longo DL. Cancer Chemotherapy and Biotherapy: Principles and Practice. edn 4th. Philadelphia: Lippincott Willians & Wilkins; 2006. [Google Scholar]
- 43.Skeel RT. Handbook of Cancer Chemotherapy. edn 6th. Lippincott Williams & Wilkins; 2003. [Google Scholar]
- 44.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 45.Brock A, Chang H, Huang S. Non-genetic heterogeneity - a mutation-independent driving force for the somatic evolution of tumours. Nat Rev Genet. 2009 doi: 10.1038/nrg2556. [DOI] [PubMed] [Google Scholar]
- 46.Elowitz MB, Levine AJ, Siggia ED, Swain PS. Stochastic gene expression in a single cell. Science. 2002;297:1183–1186. doi: 10.1126/science.1070919. [DOI] [PubMed] [Google Scholar]
- 47.Ozbudak EM, Thattai M, Kurtser I, Grossman AD, van Oudenaarden A. Regulation of noise in the expression of a single gene. Nat Genet. 2002;31:69–73. doi: 10.1038/ng869. [DOI] [PubMed] [Google Scholar]
- 48. Raj A, Peskin CS, Tranchina D, Vargas DY, Tyagi S. Stochastic mRNA synthesis in mammalian cells. PLoS Biol. 2006;4:e309. doi: 10.1371/journal.pbio.0040309.. * Raj and co-workers used a modified fluorescence in situ hybridization method to visualize individual mRNA molecules in mammalian cells. They observed extensive stochastic variation in mRNA levels in isogenic cells, owing to random switching between active and inactive states of the reporter gene.
- 49.Pedraza JM, van Oudenaarden A. Noise propagation in gene networks. Science. 2005;307:1965–1969. doi: 10.1126/science.1109090. [DOI] [PubMed] [Google Scholar]
- 50.Dublanche Y, Michalodimitrakis K, Kummerer N, Foglierini M, Serrano L. Noise in transcription negative feedback loops: simulation and experimental analysis. Mol Syst Biol. 2006;2:41. doi: 10.1038/msb4100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suel GM, Garcia-Ojalvo J, Liberman LM, Elowitz MB. An excitable gene regulatory circuit induces transient cellular differentiation. Nature. 2006;440:545–550. doi: 10.1038/nature04588. [DOI] [PubMed] [Google Scholar]
- 52.Gillespie DT. Exact Stochastic Simulation of Coupled Chemical Reactions. The Journal of Physical Chemistry. 1977;81:2340–2361. [Google Scholar]