Abstract
A major pathway for chronic ethanol-induced liver injury is ethanol-induced oxidant stress. Several pathways contribute to mechanisms by which ethanol induces oxidant stress. While some studies support a role for cytochrome P450 2E1 (CYP2E1), others do not. Most previous studies were conducted in the intragastric infusion model of ethanol administration. There is a need to develop oral models of significant liver injury and to evaluate the possible role of CYP2E1 in ethanol actions in such models. We evaluated chronic ethanol-induced liver injury, steatosis and oxidant stress in wild type (WT) mice, CYP2E1 knockout (KO) mice and in humanized CYP2E1 knockin (KI) mice, where the human 2E1 was added back to mice deficient in the mouse 2E1. WT mice and the CYP2E1 KO and KI mice (both provided by Dr F. Gonzalez, NCI) were fed a high fat Lieber-DeCarli ethanol liquid diet for 3 weeks; pair-fed controls received dextrose. Ethanol produced fatty liver and oxidant stress in WT mice but liver injury (transaminases, histopathology) was minimal. Ethanol-induced steatosis and oxidant stress was blunted in the KO mice (no liver injury) but restored in the KI mice. Signicant liver injury was produced in the ethanol-fed KI mice with elevated transaminases, necrosis, and increased levels of collagen type 1 and smooth muscle actin. This liver injury in the KI mice was associated with elevated oxidant stress and elevated levels of the human CYP2E1 compared to levels of the mouse 2E1 in WT mice. Activation of JNK and decreased levels of Bcl-2 and Bcl-XL were observed in the ethanol-fed KI mice compared to the other groups. Fatty liver in the WT and the KI mice was associated with lower levels of PPAR alpha and acyl CoA oxidase. No such changes were found in the ethanol-fed KO mice. These results show that CYP2E1 plays a major role in ethanol-induced fatty liver and oxidant stress. It is the absence of CYP2E1 in the KO mice responsible for the blunting of steatosis and oxidant stress since restoring the CYP2E1 restores the fatty liver and oxidant stress. Moreover, it is the human CYP2E1 which restores these effects of ethanol which suggests that results on fatty liver and oxidant stress from rodent models of ethanol intake and mouse CYP2E1 can be extrapolated to human models of ethanol intake and to human CYP2E1.
Keywords: Chronic Alcohol, CYP2E1, Fatty Liver, Oxidative Stress, Hepatotoxcity
Introduction
The mechanisms by which alcohol causes cell injury are still not clear. A major pathway that is a focus of considerable research is the role of lipid peroxidation and oxidative stress in alcohol toxicity [1–4]. Many pathways have been suggested to play a key role in how alcohol induces oxidative stress including redox state changes, mitochondrial damage, ethanol-induced increase in endotoxin levels and TNFα production, mobilization of iron, ethanol modulation of antioxidant defense systems, and ethanol induction of CYP2E1[1–4].
While some studies support a role for CYP2E1 in the hepatotoxic actions of ethanol [5–9], others do not [10–12].There is concern over the specificity of some of the inhibitors utilized in these studies, e.g. gadolinium chloride may affect levels of cytochrome P450 enzymes such as CYP2E1 beyond inactivation of Kuppfer cells [13,14] or diallyl sulfide may increase levels of antioxidants such as heme-oxygenase-1 besides inhibiting CYP2E1 [15]. Bradford et al [16] reported that CYP2E1 but not NADPH oxidase was required for ethanol-induced oxidative DNA damage in rodent liver and may play a key role in ethanol-associated hepatocarcinogenesis, whereas NADPH oxidase but not CYP2E1 played the major role in ethanol-induced hepatotoxicity. Why CYP2E1 may be important for one mode of liver injury, DNA damage, but not necrosis and pathological changes is not clear. Additional study is needed in assessing the role of CYP2E1 in the actions of ethanol.
Almost all the studies evaluating a possible role for CYP2E1 in ethanol hepatotoxicity have employed the intragastric infusion model of liver injury, since significant injury occurs in this model [5–8,10–12,16,17]. Most oral models of ethanol administration e.g. the typical Lieber-DeCarli model do not show the presence of significant liver injury beyond steatosis or small elevations in transaminase levels. There is clearly a need for oral models of ethanol treatment which result in the development of significant liver injury. There is also a need to evaluate the role of CYP2E1 in the actions of ethanol in oral models of ethanol administration. In the Lieber-DeCarli model, fatty liver and oxidative stress develops. These events are associated with induction of CYP2E1. However, direct evidence that CYP2E1 plays a role in the ethanol-induced fatty liver or oxidative stress in this oral model of ethanol administration is needed. Morgan and collaborators reported that in a transgenic mouse overexpressing CYP2E1, ethanol administration produced liver injury [18,19}. We recently reported that CYP2E1 plays a role in experimental fatty liver in an oral, Lieber-DeCarli ethanol feeding model [20]. Fatty liver was observed in wild type mice but not in CYP2E1 knockout mice fed ethanol chronically. The goal of the current report was to extend this previous study to humanized CYP2E1 knockin mice. Because of concerns of extrapolating results with mouse enzymes to human enzymes and to overcome species differences in P450 expression, and specificity between rodents and humans, transgenic humanized mouse models expressing major P450 enzymes were developed by Gonzalez and colleagues [21]. The human P450 transgene was introduced onto the corresponding null mouse background [22] to create a humanized P450 mouse in the absence of the corresponding mouse P450 [23]. Therefore, functional activities and differences between human and mouse CYPs can be directly compared with this model. In the humanized CYP2E1 knockin mouse [23], some differences were observed compared to the wild type mice expressing mouse CYP2E1 such as rates of p-nitrophenol (but not chlorzoxazone) oxidation and sensitivity to toxicity by acetaminophen. A major goal of the current report was to validate that the decreased fatty liver produced by chronic ethanol feeding in CYP2E1 knockout mice and the decreased ethanol-induced oxidative stress was indeed due to the deficiency in CYP2E1 by evaluating whether such actions by ethanol are restored when CYP2E1 is restored in the knockout mice. Another important goal was to study whether human CYP2E1 could effectively mimic mouse CYP2E1 in contributing to ethanol-induced fatty liver, oxidant stress and liver injury and thus allow assessment of the human CYP2E1 to modulate actions of chronic ethanol administration in a whole animal model system.
Materials and Methods
Animals and Ethanol Treatment
SV/129-background CYP2E1-knockout [22] and humanized CYP2E1 transgenic mice [23] were kindly provided by Dr. Frank J. Gonzalez (Laboratory of Metabolism, National Cancer Institute, Bethesda, MD), and breeding colonies established at Mount Sinai. Female SV129 wild type mice were purchased from Charles River Laboratory. All mice were housed in temperature-controlled animal facilities with 12-hour light/12-hour dark cycles and were permitted consumption of tap water and Purina standard chow ad libitum until being fed the liquid diets. The mice received humane care, and experiments were carried out according to the criteria outlined in the Guide for the Care and Use of Laboratory Animals and with approval of the Mount Sinai Animal Care and Use Committee.
Female WT, KO, and KI mice were initially fed the control liquid dextrose diet (Bio-Serv, Frenchtown, NJ) for 3 days to acclimate them to the liquid diet. Afterward, the mice were fed either the liquid ethanol diet (Bio-Serv, Frenchtown, NJ) or the control liquid dextrose diet, as described by Lieber and DeCarli [24] for 3 weeks. The content of ethanol was gradually increased every 3–4 days from 10% (1.77% [vol/vol]) of total calories to 20% (3.54% [vol/vol]), 25% (4.42% [vol/vol]), 30% (5.31% [vol/vol]), and finally 35% of total calories (6.2% [vol/vol]). The control mice were pair-fed the control dextrose diet on an isoenergetic basis. The ethanol-fed mice had access to their rations ad libitum, and the conditions of wild-type, knockout, and humanized transgenic mice were comparable. The amount of food consumed by CYP2E1-knockout mice, the knockin mice and the wild-type mice was approximately the same.
No mice died in any group after 3 weeks of feeding with the control or ethanol-containing diet. Before being sacrificed, the mice were fasted overnight and body weight was measured. Blood was collected and serum was separated. Whole liver was removed and liver weight measured; the liver was rapidly excised into fragments and washed with cold saline, and one aliquot of tissue was placed in 10% formalin solution for paraffin blocking. Another piece was placed in Tissue-Tek O.C.T Compound, and then was frozen and cut into 10-micron frozen sections for oil red O staining. and for DHE fluorescence assays. The remaining aliquots were stored at −80°C for further assays. Liver homogenates were prepared in ice-cold 0.15 M KCl. All samples were stored at −80°C in aliquots.
Liver Histology and Immunohistochemistry
Liver sections were stained with H&E for pathological evaluation. Steatosis was quantified as the percentage of cells containing fatty droplets. Necroinflammation was quantified as the number of clusters of 5 or more inflammatory cells per square millimeter. Five 200× fields per liver were examined (one 200× field area = 0.95 mm2). Histopathology was performed on a fee for service basis by the Mount Sinai Pathology Core. The pathologist (S.C.W) was unaware of the treatment groups when evaluating the slides.
Immunohistochemical staining for CYP2E1, 3-nitrotyrosine (3-NT) protein adducts, 4-HNE protein adducts, collagen α type 1 and smooth muscle actin proteins was performed by using anti-CYP2E1 antibody (a gift from Dr. Jerome Lasker, Hackensack Biomedical Research Institute, Hackensack, NJ), anti-3-NT adducts IgG (Upstate, Lake Placid, NY), anti-4-HNE adducts IgG (Calbiochem, LaJolla CA), anti-collagen α1, and anti-α-smooth muscle actin (SMA, Millipore) IgGs followed by a Broad Spectrum (AEC) Histostain Plus Kit (Invitrogen). DHE fluorescence was assayed by incubating frozen tissue sections of liver with 40 uM DHE (Molecular Probes, Eugene OR) plus 1 mM NADPH applied to the tissue surface for 30 min in a light-protected humid chamber. After rinsing, fluoresecence of the liver section was detected by a fluoresecence microscope. Control sections were incubated with DHE but without NADPH.
Biochemical Assays
Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST) and ethanol were assayed using Infinity kits (Thermo Electron, Melbourne, Australia) and an ethanol assay kit (Biovision, Mountain View, CA), respectively as previously described [20]. Liver tumor necrosis factor α (TNFα) was assayed using a TNFα ELISA kit (Biosource, Camarillo, CA). Liver triglyceride (TG) was determined using an Infinity kit (Thermo Electron, Melbourne, Australia). Hepatic levels of GSH and TBARs were determined as previously described [20,25]. CYP2E1 activity was measured by the rate of oxidation of p-nitrophenol to p-nitrocatechol by isolated hepatic microsomes [26]. Immunoblots for β-actin, CYP2E1, peroxisome proliferator-activated receptor α (PPARα), acyl CoA oxidase (AOX), stearoyl CoA desaturase-1 (SCD-1), Bcl-2, Bcl XL, pJNK, JNK and antioxidant enzymes were carried out as previously described [20,25]. The antibody against acyl-CoA oxidase was a gift from Professor Paul Van Veldhoven, (K.U. Leuven, Belgium). Polyclonal antibody against SCD was from Cell Signaling Technology, Inc (Danvers MA). Antibodies against PPARα, Bcl 2, Bcl-XL, pJNK, JNK, catalase, GPx-4, thioredoxin, SOD-1 and SOD-2 were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).Blots were quantified using the Image J (version 1.37v) software program from NIH. Ratios were expressed as protein/actin or pJNK/JNK.
Statistics
Results are expressed as means ± SEMs. Statistical evaluation was carried out by one-way ANOVA followed by the Student-Newman-Keuls post hoc test.
Results
Levels of CYP2E1 and TNFα after Chronic Ethanol Feeding
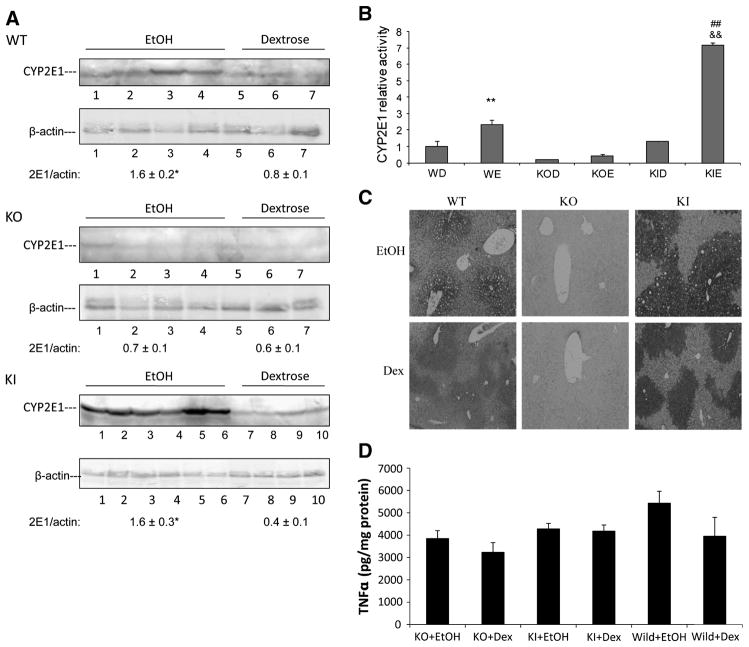
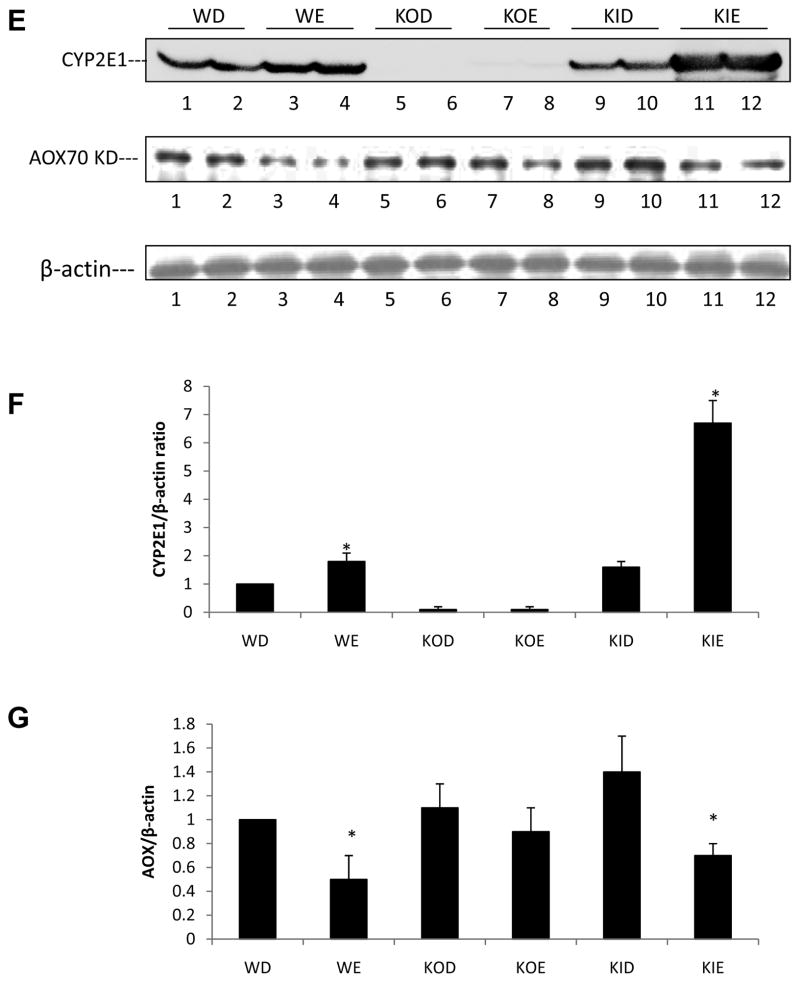
Female wild type SV129 mice, CYP2E1 knockout mice (KO) and CYP2E1 knockin mice (KI) in which the human CYP2E1 was added to replace the knocked out mouse CYP2E1 were fed ethanol for three weeks. Pair-fed controls received isocaloric dextrose. All mice were killed after an overnight fast. Serum ethanol levels were similar between the 3 groups of ethanol-fed mice at sacrifice. Ethanol elevated mouse CYP2E1 levels (Fig 1A) and CYP2E1 catalytic activity (oxidation of p-nitrophenol) (Fig 1B) about two-fold in wild type mice. Levels of CYP2E1 and oxidation of PNP were either very low or not detectable in the dextrose or ethanol-fed KO mice (Fig. 1A, 1B). Levels and activity of human CYP2E1 were elevated in the ethanol-fed KI mice to an even greater extent than that found for mouse CYP2E1 in the ethanol-fed WT mice (Fig. 1A,1B). Immunohistochemistry showed that chronic ethanol feeding elevated CYP2E1 in WT mice and more strikingly in KI mice in the pericentral zone of the liver acinus. No CYP2E1 was observed in the ethanol- or dextrose-fed KO mice (Fig. 1C).. Hepatic levels of TNFα were not significantly different between the ethanol and dextrose fed mice and between the three genotypes (Fig. 1D).
Figure 1. CYP2E1 activities and expression in WT, CYP2E1 knockout and humanized CYP2E1 knockin mice fed dextrose or ethanol diets.
A. Western blotting analysis for CYP2E1 expression. A typical immunoblot is shown and CYP2E1/actin ratios from 3–6 pairs of mice in each group are indicated. * P <0.05 compared with dextrose group.
B. Microsomal PNP activities. ** P<0.01, compared with WT dextrose (WD) group; ## P<0.01, compared with KI dextrose (KID) group; && P<0.01, compared with WT ethanol (WE) group. KOD, CYP2E1 knockout mice fed dextrose diet; KOE, CYP2E1 knockout mice fed ethanol diet; KIE, humanized CYP2E1 knockin mice fed ethanol diet. (n= 3–6 pairs of mice in each group).
C. Immunohistochemistry staining to determine CYP2E1 in the liver.
D. TNFα levels in liver lysates were analyzed by an ELISA method. (n= 3–6 pairs of mice in each group).
Ethanol-induced Hepatotoxicity
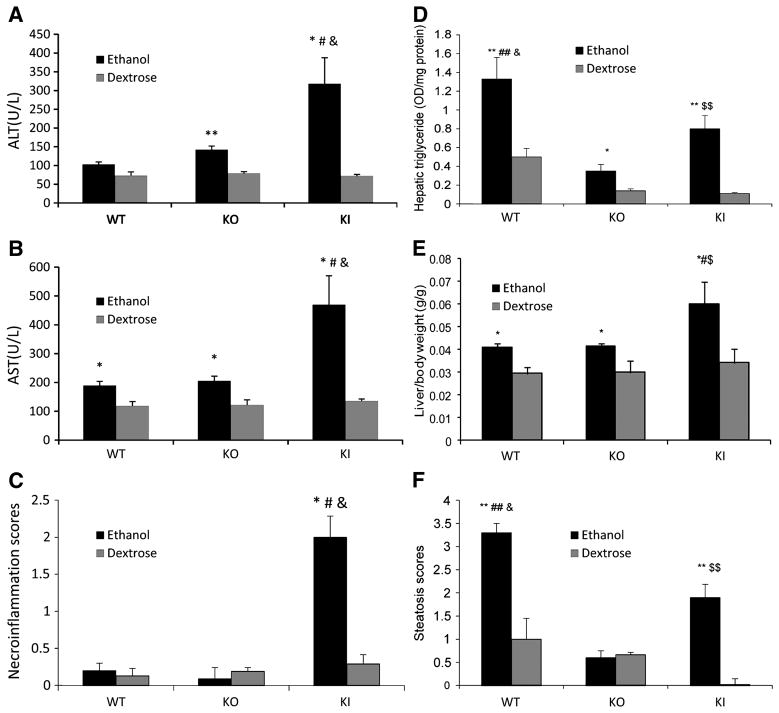
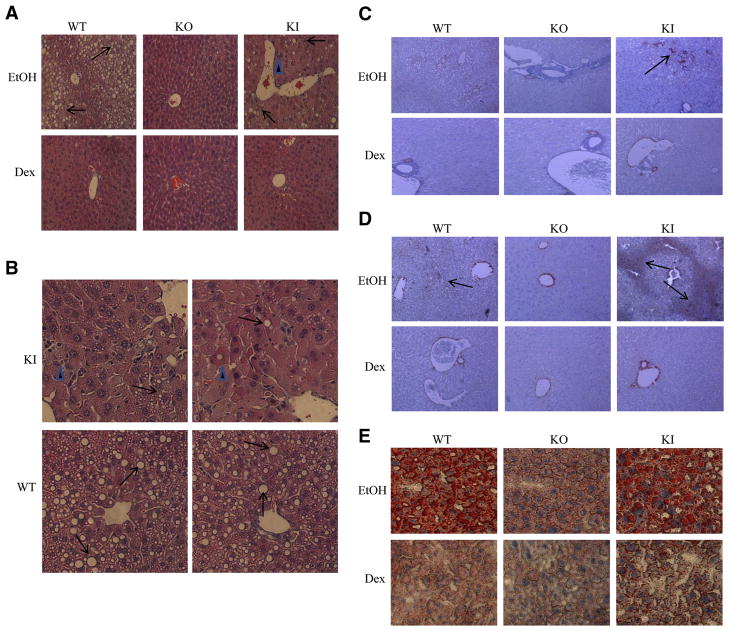
Serum ALT and AST levels were elevated less than two-fold by ethanol in the WT mice and a similar increase was found with the KO mice (Fig. 2A,2B). Serum ALT and AST levels were increased about four- five-fold by ethanol in the KI mice, an increase greater than the increase by ethanol in the WT or KO mice. Histopathology observation revealed the expected presence of steatosis in the ethanol-fed WT mice but little or no indication for necrosis (Fig. 3A,3B). Steatosis was much lower and necrosis was not observed in the ethanol-fed KO mice. However, both necrosis and steatosis were found with the ethanol-fed KI mice; areas with cell swelling, focus necrosis and inflammatory infiltration were observed (Fig. 3A,3B). Necroinflammation scores were elevated only for the ethanol-fed KI mice (Fig. 2C). No changes in the activity of caspases 3 or 9 nor TUNEL staining were found in the ethanol-fed KI mice compared to dextrose controls (data not shown) suggesting necrotic but not apoptotic liver injury was occurring under these conditions.
Figure 2. Ethanol-induced hepatotoxicity in WT, CYP2E1 knockout and humanized CYP2E1 knockin mice. Results are from 3 to 6 pairs of mice in each group.
A. Serum ALT. B. Serum AST. * P<0.05 and ** P<0.01, compared with dextrose group; # P<0.05, compared with WT ethanol group; & P<0.05, compared with KO ethanol group.
C. Necroinflammation scores from H&E staining. * P<0.05, compared with dextrose group; # P<0.05, compared with WT ethanol group; & P<0.05, compared with KO ethanol group.
D.Hepatic triglycerides. *P<0.05 and **P<0.01 compared with dextrose group; ## P< 0.01 compared with ethanol KO group; & P< 0.05 compared with KI ethanol group; $$ P < 0.01 compared with KO ethanol group.
E. Liver to body wt ratio. * P< 0.05 compared with dextrose group; # P< 0.05 compared with WT ethanol group; $ P< 0.05 compared with KO ethanol group.
F. Steatosis scores from H&E staining. ** P< 0.01 compared with dextrose group; ## P< 0.01 compared with KO ethanol group; & P< 0.05 compared with KI ethanol group; $$ P <0.01 compared with KO ethanol group.
Figure 3. Ethanol-induced steatosis and hepatotoxicity.
A.. H&E staining (low power). Arrows show lipid droplets, arrow heads show necroinflammation.
B. H&E staining (high power). Arrows show single cell death, arrow heads show inflammation. Results from 2 WT and 2 KI mice are shown.
C. Immunohistochemistry staining for α-smooth muscle actin. Arrows show positive staining.
D. Immunohistochemistry staining for collagen αI. Arrows show positive staining.
E. Oil Red O staining for lipid.
Ethanol Induced Fatty Liver
As mentioned above, ethanol induced steatosis in WT and KI mice but to a much lesser extent in KO mice. Further evaluation showed elevated oil red staining in WT and KI mice to a greater extent than in KO mice (Fig. 3E). The oil red staining, similar to the histopathology suggested a more elevated steatosis in the WT mice as compared to the KI mice. Hepatic triglycerides were elevated by ethanol about two-fold in the KO mice, about seven-fold in the KI mice and about three-fold in the WT mice (Fig. 2D). Triglyceride levels were higher in both the dextrose-fed and ethanol-fed WT mice than the corresponding KO and KI mice, likely accounting for the “only” three fold increase by ethanol in WT mice compared to the 7-fold increase in KI mice. The steatosis score confirmed that ethanol produced fatty liver in the WT and KI mice, but not the KO mice (Fig. 2F). Interestingly, while the steatosis score was highest in the ethanol-fed WT mice, the necroinflammatory score was highest in the ethanol-fed KI mice. Both scores were lowest in the ethanol KO mice.
The liver to body wt ratio was elevated by about 35% by ethanol in WT mice (Fig. 2E). A similar increase by ethanol was found with the KO mice, despite only a modest increase in triglycerides as compared to the WT mice. A near two-fold increase in the liver to body wt ratio was found with the ethanol-fed KI mice even though triglycerides were elevated by ethanol in these mice to a lesser extent than the WT mice. This suggests that other macromolecules besides fat contribute to elevated liver/body wt ratio in the ethanol-fed KO and KI mice.
Ethanol-Induced Fibrosis
In general, ethanol-induced liver injury is modest in the typical Lieber-DeCarli diet, and fibrosis is not typically observed. This is confirmed by the lack of an increase in immunohistochemical detection of smooth muscle actin (SMA), a marker of hepatic stellate cell activation in liver from ethanol-fed WT mice, as well as the KO mice (Fig. 3C). However, some staining for SMA was observed in livers from the ethanol-fed KI mice (Fig. 3C). Immunohistochemical staining for collagen revealed thick bands of collagen throughout the liver of the ethanol-fed KI mice while in the WT and the KO mice, no positive staining for collagen in the liver itself was observed except for blood vessels (Fig 3D).
Ethanol-Induced Oxidant Stress
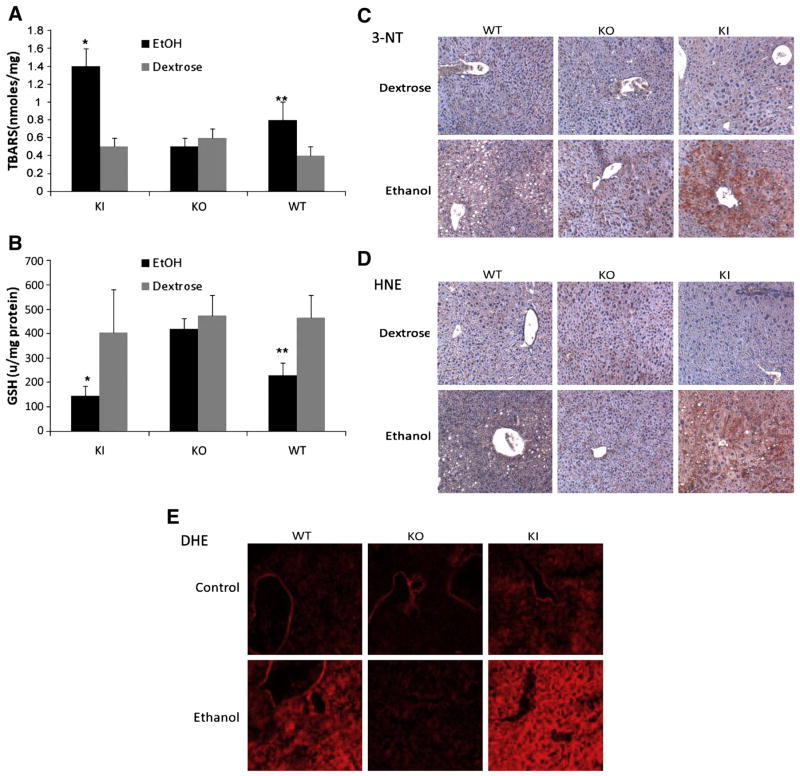
There are many possible pathways and mechanisms by which chronic ethanol feeding can elevate oxidant stress, and induction of CYP2E1 is one of these [27]. Lipid peroxidation, as assayed by formation of thiobarbituric acid-reactive substances (TBARs) in the liver, was increased 2-fold by ethanol in WT mice and 3-fold in KI mice (Fig. 4A). No increase by ethanol was found in livers from the KO mice. GSH levels were lowered about 50% by ethanol in livers from WT mice and about 70% in livers from the KI mice (Fig. 4B).. Ethanol had no effect on GSH levels in livers from KO mice. Formation of 3-nitrotyrosine protein adducts is one assay for detection of peroxynitrite formation [28]. Low levels of 3-NT staining were observed in livers from the ethanol-fed WT and KO mice, but a more intense staining was observed in livers from the ethanol-fed KI mice (Fig. 4C). 4- HNE protein adducts as detected by immunohistochemistry were elevated in WT mice and to a greater extent in KI mice as compared to dextrose controls(4D). Ethanol did not elevate 4-HNE adducts in the KO mice. In situ detection of ROS using the superoxide sensitive probe DHE showed that ethanol elevated DHE fluorescence in WT and to a greater extent in KI mice but not in the KO mice (Fig 4E). Thus, levels of oxidant stress induced by ethanol appeared to follow a similar pattern of induction of CYP2E1 by ethanol, being highest in the CYP2E1 KI mice, intermediate in the WT mice, and lowest in the CYP2E1 KO mice.
Figure 4. Ethanol-induced oxidative stress.
Chronic ethanol induced oxidative stress was determined by measuring levels of TBARS (A), or GSH (B) in liver lysates. *, **, P<0.05 compared with their dextrose control groups respectively (n=3–6). C. Immunohistochemistry staining for 3-NT protein adducts in mouse liver. D. Immunohistochemistry staining for 4-HNE protein adducts. E. ROS detection by DHE fluorescence.
Levels of some antioxidant enzymes were assayed by immunoblot analysis. Ethanol had little or no effect on the levels of catalase, thioredoxin (Trx), SOD-1 or SOD-2 in any group (data not shown). Ethanol lowered the levels of GPx-4 55%, 58% and 39% in the WT, KO and KI mice, respectively as compared to their appropriate dextrose-fed control (data not shown). This decline in GPx-4 levels by ethanol, unlike the 5 parameters of oxidant stress discussed above (increase in TBARs, decrease in GSH, increase in 3-NT and 4-HNE staining, increase in DHE fluorescence) appears to be independent of CYP2E1 since a comparable decline was found in CYP2E1 KO mice as with the WT mice.
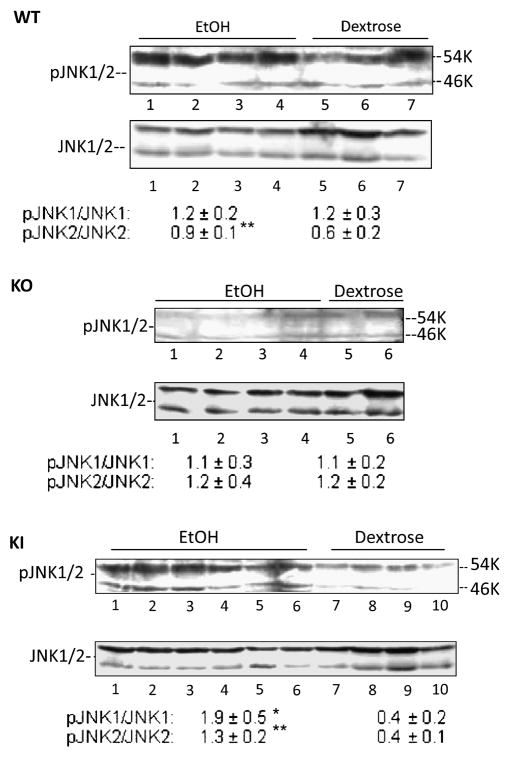
Activation of JNK
Activation of JNK has been shown to be important in many models of hepatotoxicity [29–32], including CYP2E1 potentiation of LPS or TNF liver injury [33,34]. There are few studies evaluating the role of JNK, if any, in ethanol-induced hepatotoxicity and steatosis. Fig. 5 shows that JNK2 (54KDa) was elevated 50% (0.6 to 0.9 pJNK2/JNK2 ratio) in wild type mice chronically fed ethanol as compared to pair-fed controls. This modest activation of JNK2 did not occur in the CYP2E1 knockout mice fed ethanol. However activation of JNK2 was restored, and even magnified (3-fold) in the CYP2E1 knockin mice fed ethanol (Fig. 5). JNK1 (46 KDa), which was not activated in the ethanol-fed wild type or CYP2E1 knockout mice, was activated 5-fold in the ethanol fed CYP2E1 knockin mice (Fig. 5). We did not observe any activation of p38MAPK by ethanol in any group (data not shown).
Figure 5. The activation of JNK MAP kinase.
The phosphorylation of JNK1 or JNK2 was determined by immunoblot analysis of liver lysates. The activity was determined by the ratios of pJNK/JNK which are listed under the blots and are from 3–6 pairs of mice. *, **, P<0.05 compared with dextrose fed groups respectively.
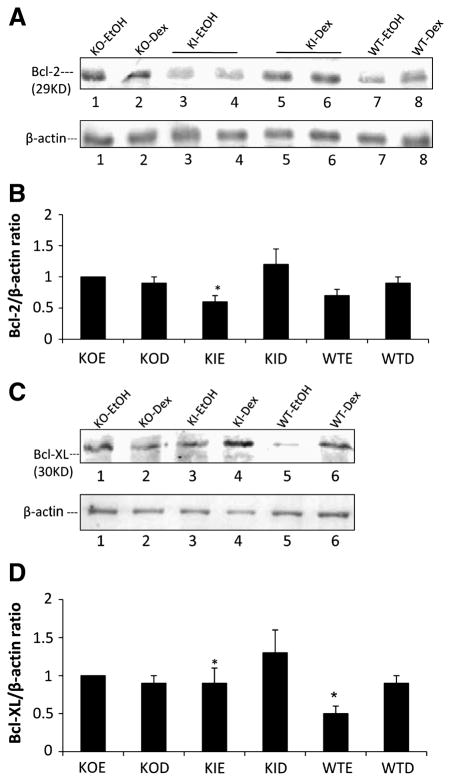
Levels of Bcl-2 and Bcl-XL
Bcl-2 and Bcl-XL are antiapoptotic members of the Bcl-2 family and can protect against certain models of cell/tissue injury, including alcohol toxicity [35,36]. Levels of Bcl-2 were lowered by about 50% in the ethanol-fed KI mice (Fig 6A,6B). Levels of Bcl-XL were significantly lowered about 40% by ethanol in livers of WT mice and by 30 % in the KI mice (Fig. 6B,6C). Ethanol had no effect on the levels of Bcl-2 and Bcl-XL in the KO mice.
Figure 6. Levels of Bcl-2 and Bcl-XL.
Levels of Bcl-2 (A) and Bcl-XL (C) were determined by immunoblots. Ratios with β-actin are shown in the bar graphs (B, D) and are from 3–6 pairs of mice. *, P<0.05 compared with dextrose fed groups.
Levels of Lipogenic and Lipolytic Factors
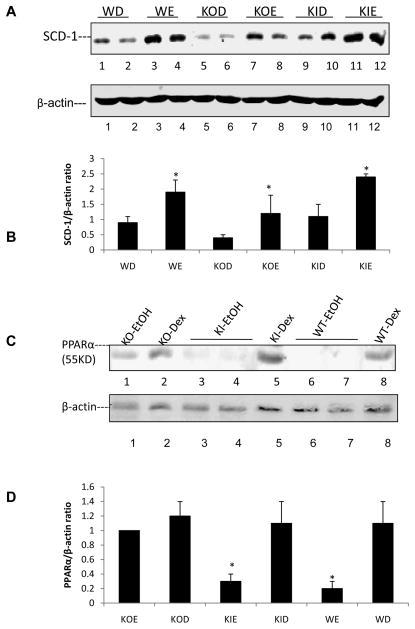
The master regulator of lipogenesis SREBP, was shown to be elevated by ethanol in some models e.g. low fat intake with a subsequent increase in lipogenic enzymes such as acetylCoA carboxylase [37–41]. A master regulator of lipolysis, PPARα, was shown to be decreased by ethanol in those models, with a subsequent decrease in lipolytic enzymes such as acyl CoA oxidase [37–41]. Such changes play a key role in the overall mechanism by which ethanol induces a fatty liver. We did not observe any changes in levels of SREBP or ACC by ethanol in any of the groups of mice (data not shown). Levels of SCD-1 were increased by ethanol in all the groups of mice, although the levels were lower in both the dextrose and ethanol fed KO mice (Fig. 7A). Levels of PPARα were decreased by ethanol in both the WT and the KI mice, but ethanol had no effect on PPARα levels in the KO mice (Fig. 7B). A downstream target of PPARα, peroxisomal acyl CoA oxidase, responded in a similar manner, being decreased by ethanol in WT and KI mice but no effect was found with KO mice (Fig. 7C). Previously [20], we found that levels of PPARα mRNA but not protein were decreased by ethanol in WT mice while levels of AOX mRNA and protein were decreased. In the current study, levels of AOX as well as PPARα protein were decreased by ethanol in the WT mice. The only difference between the two studies was the time of ethanol feeding, three (current study) versus four weeks [20] so it is not clear why the effect of ethanol on PPARα protein but not AOX protein levels differ between the 2 studies.
Figure 7. Effects of chronic ethanol on liver lipogenic and lipolytic proteins.
Immunoblots were carried out to determine the levels of SCD-1 (A,), PPARα (C) and CYP2E1 plus AOX (E) proteins. *, **, P<0.05 compared with their dextrose groups respectively. The bar graphs (B, D, F, G) show the protein/actin ratios from 3–6 pairs of mice
Discussion
Fatty liver is a uniform and early response of the liver to ethanol consumption. Previously, fatty liver was considered benign, however, it is now known that fatty liver can increase sensitivity to hepatotoxins such as lipopolysaccharide (LPS) and high levels of fatty acids promote lipotoxicity [42–44]. Hence, there is a need to understand the mechanisms responsible for fatty liver production by ethanol. Early hypotheses for mechanisms responsible for fatty liver production by ethanol included redox state changes (elevated NADH) when ethanol was metabolized by alcohol dehydrogenase, elevated formation of acetyl CoA from ethanol/acetaldehyde oxidation and impairment of β-oxidation of fatty acids [37]. Recent studies by Crabb, You and colleagues have identified new mechanisms which regulate the synthesis and the oxidation of fatty acids as being central to how ethanol produces fatty liver. Ethanol has been shown to elevate levels of SREBP-1c, a master transcriptional regulator of lipogenic enzymes, but to lower levels of PPARα, a master transcriptional regulator of lipolytic enzymes. Decreased activation of AMPK by ethanol plays a role in these effects [38–41]. What remains unclear is how ethanol is upregulating or activating lipogenic enzymes while downregulating lipolytic enzymes. Possible mechanisms may involve acetaldehyde, homocysteine-induced ER stress, and oxidant stress [38–41, 45,46].
Is there a role for CYP2E1 in ethanol-induced fatty liver? In the IG infusion model, Kono et al [11] reported that after feeding ethanol for 4 weeks, there was no difference in ethanol-induced steatosis between CYP2E1 knockout mice and wild type mice. Similarly, in this model, Wan et al [47] reported that ethanol infusion for 21 days promoted fat accumulation in CYP2E1 knockout mice but, surprisingly, not in the wild type mice. In contrast, the CYP2E1 inhibitor chlormethiazole (CMZ) was reported to blunt ethanol-induced fatty liver in the IG model [9].
We previously showed that ethanol-induced fatty liver using the high fat Lieber-DeCarli model was blunted in CYP2E1 KO mice as compared to WT mice [20]. These results were confirmed in the current report as evident by histopathology, oil-red staining and biochemical assay of hepatic triglyceride levels in livers from WT mice fed ethanol compared to CYP2E1 KO mice fed ethanol. We believe two important new points arise from results of Fig. 2 and 3. First, it is the absence of CYP2E1 in the KO mice and not secondary effects associated with the knockout that plays a major role in the blunting of the ethanol-induced fatty liver in the KO mice since restoring the presence of CYP2E1 in these mice restores the fatty liver. Second, human CYP2E1 was the CYP2E1 which restores the ethanol-induced steatosis, which indicates that human CYP2E1 can potentiate development of fatty liver in an oral model of chronic ethanol administration. This suggests that results on fatty liver extrapolated from rodent models of ethanol intake can be extrapolated to human models of ethanol intake with respect to effects of CYP2E1.
Associated with the fatty liver induced by ethanol was an increase in oxidative/nitrosative stress as reflected by the increase in TBARS, 3-NT and 4-HNE staining, and DHE fluorescence and the decrease in GSH levels in the WT mice fed ethanol. CYP2E1 is suggested be a major contributor to ethanol-induced oxidant stress. Results in Fig. 4 show that ethanol caused oxidative stress in WT mice, but the ethanol-induced oxidative stress was decreased in CYP2E1 KO mice. It is the absence of CYP2E1 in the CYP2E1 KO mice that plays a major role in blunting of the ethanol-induced oxidant stress since restoring CYP2E1 restores oxidative/nitrosative stress. As discussed above for fatty liver, human CYP2E1 was the CYP2E1 which restores the oxidative/nitrosative stress indicating that human CYP2E1 can potentiate development of oxidative/nitrosative stress in an oral model of chronic ethanol administration, and that results on oxidative/nitrosative stress extrapolated from rodent models of ethanol intake can be extrapolated to human models of ethanol intake.
It appears that ethanol-induced steatosis may be related to oxidative stress as shown by Diluzio [48] who reported that antioxidants can prevent the ethanol-induced fatty liver, and studies showing that overexpression of the copper-zinc superoxide dismutase-1 can partially reduce the ethanol steatosis [49]. CYP2E1-generated ROS contributes to the ethanol-induced oxidant stress which may explain its role in the ethanol-induced steatosis. However, mechanisms for ethanol-induced steatosis are likely to be more complex than increases in CYP2E1/cytokines/oxidant stress e.g ethanol did not induce fatty liver in C1q knockout mice despite elevating CYP2E1, TNFα, IL-6 and oxidant stress and the authors suggested a role for the classical complement pathway in contributing to the pathogenesis of ethanol-induced liver injury [50]. Blockade of PPARα function by chronic ethanol administration plays a key role in the mechanism of ethanol-induced steatosis [51] as PPARα knockout mice fed ethanol develop extensive fatty liver and liver injury [52] whereas PPARα activating ligands such as clofibrate [53] or WY14643 [51] decrease the ethanol fatty liver. Levels of PPARα, and a downstream target of PPARα, AOX, were decreased more than 50% by ethanol administration to WT mice and CYP2E1 KI mice, but no effect was found with the CYP2E1 KO mice. This suggests the ethanol-induced decline in PPARα may be due, at least in part to CYP2E1, or more likely, CYP2E1-derived ROS. These results differ from Wan et al [47] who using the intragastric infusion model of ethanol administration, found that ethanol decreased PPARα levels and induced fatty liver to a greater extent in CYP2E1 KO mice than WT mice. We believe these differences may relate, in part, to differences in endotoxemia and TNFα levels in the intragastric versus oral models of ethanol administration, i.e. ROS may be produced in CYP2E1 KO mice fed ethanol intragastrically largely via TNFα generated by endotoxemia. In our oral model of ethanol administration, TNFα levels were not elevated (Fig. 1D), leading us to speculate that CYP2E1, not TNFα, is a major mechanism for ethanol-induced oxidant stress followed by steatosis.
The decrease in PPAR-α produced by ethanol in WT and KI mice may contribute to the development of fatty liver by decreasing fatty acid oxidation. This remains to be evaluated. With respect to fatty acid synthesis, we could not detect increases in SREBP, acetyl CoA carboxylase or fatty acyl synthase levels in the ethanol-fed WT or KI mice. However, as found by other [54,55] levels of stearoyl CoA desaturase-1 were increased by ethanol in the WT mice as well as the KO and the KI mice. SCD-1 has been shown to promote hepatic steatosis as SCD-1 deficiency prevents liver steatosis in various mouse models [56,57]. Oleate, the product of the SCD-1 catalyzed desaturation of stearic acid normalized the decreased hepatic lipogenesis of the SCD -1 KO mice, and it has been suggested that endogenous oleate synthesis catalyzed by SCD-1 acts as a metabolic switch which influences the balance of energy (fat) storage versus energy (fat) oxidation [57]. SCD-1 deficiency increases the phosphorylation state of AMPK and hepatic AMPK activity [58], hence, elevating SCD-1 would inactivate AMPK, thereby promoting lipogenesis. However, we did not find an increase in the pAMPK/AMPK ratio in the ethanol-fed WT or KI mice (data not shown). In addition, ethanol increased levels of SCD-1 in the CYP2E1 KO mice in which no increase in steatosis was observed suggesting that the increase in SCD-1 is not a major explanation for the CYP2E1-stimulation of steatosis.
Significant liver injury generally does not occur in most oral models of ethanol intake, and there is a need to develop such models for mechanistic and preventive/therapeutic designs. A modified low carbohydrate ethanol liquid diet caused mild liver injury in rats [59,60]. A two-fold elevation in ALT levels and mild liver injury occurred in female rats fed fish oil plus ethanol orally [61]. Although CYP2E1 was elevated in these models, whether the elevated CYP2E1 played a role in the liver injury was not directly evaluated. ALT, AST and necroinflammatory scores were higher in ethanol-fed CYP2E1 KI mice compared to WT and CYP2E1 KO mice. Increases in smooth muscle actin and collagen type 1 protein were observed in the ethanol-fed KI mice which may be suggestive of an initiation of fibrosis. Perhaps longer periods of ethanol feeding in the KI mice may provide a valuable model of ethanol-induced fibrosis and stellate cell activation. CYP2E1 levels and activity were about two-fold higher in the CYP2E1 KI mice compared to the wild type mice after the ethanol feeding suggesting the elevated liver injury may be due to the elevated content of CYP2E1 although the possibility that the human CYP2E1 is more reactive than the mouse CYP2E1 in promoting this injury remains to be evaluated. The elevated liver injury is associated with an elevated oxidative/nitrosative stress in the ethanol-fed CYP2E1 KI mice and we speculate that the latter plays a key role in the former. This will be evaluated in future studies examining the effects of antioxidants such as N-acetylcysteine, vitamins E and C and inhibitors of the inducible nitric oxide synthase e.g.1400W. The elevated liver injury is also associated with an increase in activation of the JNK mitogen activated protein kinase. Activation of JNK has been shown to be important in many models of hepatotoxicity [30–33], including CYP2E1 potentiation of LPS or TNF-α liver injury [34,35]. Activated JNK can phosphorylate and thereby inactivate Bcl-2 and Bcl-XL [62]. Levels of these two anti-apoptotic proteins were lowered by chronic ethanol feeding in CYP2E1 KI mice in association with activation of JNK, but not in the CYP2E1 KO mice in which JNK was not activated by the ethanol feeding. Despite this decline in levels of Bcl-2 and Bcl-XL, the ethanol injury was necrotic not apoptotic. While chronic ethanol-induced liver injury was usually considered to be necrotic [4,5,8], ethanol-induced apoptosis has also been observed [36,50]. Most likely whether liver injury induced by ethanol is necrotic or apoptotic or necroapoptotic may reflect amount of and time of ethanol feeding, mode of ethanol administration, whether significant endotoxemia occurs, the severity of mitochondrial injury and ATP depletion, the mouse strain and other factors such as pro and anti inflammatory cytokine elevation, fat content, type of fat. We have not yet assayed levels of proapoptotic members of the Bcl-2 family or ATP levels which may regulate a possible switch between apoptosis and necrosis in the CYP2E1 KI mice, nor conducted time courses of ethanol feeding to assess if an early apoptosis switches to necrosis. Future experiments will evaluate the ability of an inhibitor of JNK activation such as SP600125 to prevent the chronic ethanol-induced liver injury in the CYP2E1 KI mice. SP600125 was effective in preventing the CYP2E1 potentiation of LPS or TNF-α liver injury [33]. We speculate the elevated oxidative/nitrosative stress in ethanol-fed KI mice may play a role in the activation of JNK e.g. the upstream MAPKKK ASK-1 is activated when its inhibitor thioredoxin-1 is oxidized by ROS, and dissociates off ASK-1, which then allows ASK-1 to activate downstream MAPKKs and subsequently JNK [63,64]. ROS also inactivate MAPK phosphatases which dephosphorylate activated MAPK such as JNK [65]. The activation status of JNK will be assessed in the above proposed experiments evaluating the effects of antioxidants on the ethanol-induced liver injury in KI mice. Although other models of hepatotoxicity produced by overexpression of CYP2E1 have been reported e.g. a transgenic model of CYP2E1 overexpression [18,19], or administration of an adenovirus expressing CYP2E1 to mice [66], the advantage of the CYP2E1 KI model is that the actions of the human CYP2E1 can be studied in the total absence of the mouse CYP2E1 thus allowing assessment of the role of human CYP2E1 in ethanol-induced oxidative stress and liver injury. Another point to consider is that the ethanol-induced liver toxicity observed in the intragastric infusion model of ethanol administration is typically associated with endotoxemia and activation of Kupffer cells with the subsequent production of TNF-α [67–70]. Alcohol-induced liver injury in this model is deceased when gram negative bacteria are deleted from the gut by treatment with lactobacillus or antibiotics [71] or when anti-TNF-α antibodies are administered [72] or when TNF-α receptor 1 knockout mice were treated with ethanol [73]. We did not observe any elevation of TNF-α in livers of any of the ethanol-treated mice (Fig. 1), therefore the liver injury in the ethanol-fed KI mice is not associated with an elevation of TNF-α. Whether the injury is TNF-α-independent or requires basal levels of TNF-α will require further studies, e.g. use of clodronate liposomes to deplete Kupffer cells [74]or anti- TNF-α antibodies [72].
Acknowledgments
These studies were supported by USPHS Grants 1RO1 AA 017425 and 018790 from The National Institute on Alcohol Abuse and Alcoholism
ABBREVIATIONS
- AOX
acyl CoA oxidase
- CYP2E1
cytochrome P4502E1
- DHE
dihydroethidine
- 4-HNE
4-hydroxynonenal
- GSH
reduced glutathione
- IG
intragastric
- KO
knockout
- KI
knockin
- 3-NT
3-nitrotyrosine
- PPARα
peroxisome proliferator receptor α
- ROS
reactive oxygen species
- SCD-1
stearoyl CoA desaturase -1
- SREBP
sterol regulatory element-binding protein
- TBARs
thiobarbituric acid-reactive components
- TNFα
tumor necrosis factor α
- WT
wild type
References
- 1.Nordman R, Riviere C, Rouach H. Implication of free radical mechanisms in ethanol-induced cellular injury. Free Rad Biol Med. 1992;12:219–240. doi: 10.1016/0891-5849(92)90030-k. [DOI] [PubMed] [Google Scholar]
- 2.Bondy SC. Ethanol toxicity and oxidative stress. Toxicol Lett. 1992;63:231–242. doi: 10.1016/0378-4274(92)90086-y. [DOI] [PubMed] [Google Scholar]
- 3.Cederbaum AI. Introduction serial review: alcohol, oxidative stress and cell injury. Free Rad Biol Med. 2001;31:1524–1526. doi: 10.1016/s0891-5849(01)00741-9. [DOI] [PubMed] [Google Scholar]
- 4.Arteel GE. Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology. 2003;124:778–790. doi: 10.1053/gast.2003.50087. [DOI] [PubMed] [Google Scholar]
- 5.French SW, Morimoto M, Reitz RC, Koop D, Klopfenstein B, Estes, et al. Lipid peroxidation, CYP2E1 and arachidonic acid metabolism in alcoholic liver disease in rats. J Nutr. 1997;127:907S–911S. doi: 10.1093/jn/127.5.907S. [DOI] [PubMed] [Google Scholar]
- 6.Castillo T, Koop DR, Kamimura S, Triadafilopoulos G, Tsukamoto H. Role of cytochrome P4502E1 in ethanol, carbon tetrachloride and iron-dependent microsomal lipid peroxidation. Hepatology. 1992;16:992–996. doi: 10.1002/hep.1840160423. [DOI] [PubMed] [Google Scholar]
- 7.Morimoto M, Zern A, Hagbjork AL, Ingelman-Sundberg M, French SW. Fish oil, alcohol and liver pathology: role of cytochrome P450 2E1. Proceedings Society of Experimental Biology and Medicine. 1994;207:197–205. doi: 10.3181/00379727-207-43807. [DOI] [PubMed] [Google Scholar]
- 8.Nanji AA, Zhao S, Sadrzadeh SMH, Dannenberg AJ, Tahan SR, Waxman DJ. Markedly enhanced cytochrome P4502E1 induction and lipid peroxidation is associated with severe liver injury in fish oil-ethanol-fed rats. Alcoholism Clin Exp Res. 1994;18:1280–1285. doi: 10.1111/j.1530-0277.1994.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 9.Gouillon Z, Lucas D, Li J, Hagbjork AL, French BA, Fu P, et al. Inhibition of ethanol-induced liver disease in the intragastric feeding rat model by chlormethiazole. Proc Soc Exp Biol Med. 2000;224:302–308. doi: 10.1046/j.1525-1373.2000.22435.x. [DOI] [PubMed] [Google Scholar]
- 10.Koop DR, Klopfenstei B, Iimuro Y, Thurman RG. Gadolinium chloride blocks alcohol-dependent liver toxicity in rats treated chronically with intragastric alcohol despite the induction of CYP2E1. Mol Pharmacol. 1997;51:944–950. doi: 10.1124/mol.51.6.944. [DOI] [PubMed] [Google Scholar]
- 11.Kono H, Bradford BU, Yin M, Sulik KK, Koop D, Thurman RG, et al. CYP2E1 is not involved in early alcohol-induced liver injury. Am J Physiol. 1999;277:G1259–G1267. doi: 10.1152/ajpgi.1999.277.6.G1259. [DOI] [PubMed] [Google Scholar]
- 12.Isayama F, Froh M, Bradford BU, Mckim SE, Kadiiska MB, Arteel GE, et al. The CYP inhibitor 1-aminobenzotriazole does not prevent oxidative stress associated with alcohol-induced liver injury in rats and mice. Free Rad Biol Med. 2003;35:1568–1581. doi: 10.1016/j.freeradbiomed.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Badger DA, Kuester RK, Sauer JM, Sipes LG. Gadolinium chloride reduces Cytochrome p450: relevance to chemical induced hepatotoxicity. Toxicology. 1997;121:143–153. doi: 10.1016/s0300-483x(97)00065-6. [DOI] [PubMed] [Google Scholar]
- 14.Jarvelainen HA, Fang C, Ingelman-Sundberg M, Lukkan TA, Sippel H, Lindros KO. Kupffer cell inactivation alleviates ethanol-induced steatosis and CYP2E1 induction but not inflammatory responses in rat liver. J Hepatol. 2000;32:900–910. doi: 10.1016/s0168-8278(00)80094-x. [DOI] [PubMed] [Google Scholar]
- 15.Gong P, Hu B, Cederbaum AI. Dially sulfide induces heme oxygenase-1 through MAPK pathway. Arch Biochem Biophys. 2004;432:252–260. doi: 10.1016/j.abb.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 16.Bradford BU, Kono H, Isayama F, Kosyk O, Wheeler MD, Akiyama TE, et al. Cytochrome P4502E1 but not NADP oxidase is required for ethanol-induced oxidative DNA damage in rodent liver. Hepatology. 2005;41:336–344. doi: 10.1002/hep.20532. [DOI] [PubMed] [Google Scholar]
- 17.Bardag-Gorce F, Yuan QX, Li J, French BA, Fang C, Ingelman-Sundberg M, French SW. The effect of ethanol-induced cytochrome P450 2E1 on the inhibition of proteasome activity by alcohol. Biochem Biophys Res Commun. 2000;279:23–29. doi: 10.1006/bbrc.2000.3889. [DOI] [PubMed] [Google Scholar]
- 18.Morgan K, French SW, Morgan TR. Production of a cytochrome P450 2E1 transgenic mouse and initial evaluation of alcoholic liver damage. Hepatology. 2002;36:122–134. doi: 10.1053/jhep.2002.33720. [DOI] [PubMed] [Google Scholar]
- 19.Butura A, Nilsson K, Morgan K, Morgan TR, French S, Johanssson I, et al. The impact of CYP2E1 on the development of alcoholic liver disease as studied in a transgenic mouse model. J Hepatol. 2008;50:572–583. doi: 10.1016/j.jhep.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 20.Lu Y, Zhuge J, Wang X, Bai J, Cederbaum AI. Cytochrome P4502E1 contributes to ethanol-induced fatty liver in mice. Hepatology. 2008;47:1483–1494. doi: 10.1002/hep.22222. [DOI] [PubMed] [Google Scholar]
- 21.Cheung C, Gonzalez FJ. Humanized mouse lines and their application for prediction of human drug metabolism and toxicological risk assessment. J Pharmacol Exp Ther. 2008;327:288–299. doi: 10.1124/jpet.108.141242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SS, Buter JT, Pineau T, Fernandez-Salguero P, Gonzalez FJ. Role of CYP2E1 in the hepatotoxicity of acetaminophen. J Biol Chem. 1996;271:12063–12067. doi: 10.1074/jbc.271.20.12063. [DOI] [PubMed] [Google Scholar]
- 23.Cheung C, Yu AM, Ward JM, Krausz KW, Akiyama TE, Feigenbaum L, Gonzalez FJ. The CYP2E1-humanized transgenic mouse:role of CYP2E1 in acetaminophen hepatotoxicy. Drug Metab Disp. 2005;33:449–457. doi: 10.1124/dmd.104.002402. [DOI] [PubMed] [Google Scholar]
- 24.Lieber CS, DeCarli LM. Animal models of chronic ethanol toxicity. Methods Enzymol. 1994;233:585–594. doi: 10.1016/s0076-6879(94)33061-1. [DOI] [PubMed] [Google Scholar]
- 25.Wu D, Xu CJ, Cederbaum AI. Role of nitric oxide and nuclear factor kappa B in the CYP2E1 potentiation of TNFα hepatotoxicity in mice. Free Rad Biol Med. 2009;46:480–491. doi: 10.1016/j.freeradbiomed.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Reinke LA, Moyer MJ. p-Nitrophenol hydroxylation. A microsomal oxidation which is highly inducible by ethanol. Drug Metab Dispos. 1985;13:548–552. [PubMed] [Google Scholar]
- 27.Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Rad Biol Med. 2008;44:723–738. doi: 10.1016/j.freeradbiomed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ischiropoulos H, al-Mehdi AB, Fisher AB. Reactive species in ischemic rat lung injury:contribution of peroxynitrite. Am J Physiol. 1995;269:L158–L164. doi: 10.1152/ajplung.1995.269.2.L158. [DOI] [PubMed] [Google Scholar]
- 29.Czaja MJ. The future of GI and liver research: editorial perspectives. III. JNK/AP-1 regulation of hepatocyte death. Am J Physiol Gastrointest Liver Physiol. 2003;284:G875–879. doi: 10.1152/ajpgi.00549.2002. [DOI] [PubMed] [Google Scholar]
- 30.Henderson NC, Pollock K, Frew J, Mackinnon AC, Flavell RA, Davis R, et al. Critical role of c-jun (NH2) terminal kinase in paracetamol-induced acute liver failure. Gut. 2001;56:982–990. doi: 10.1136/gut.2006.104372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131:165–78. doi: 10.1053/j.gastro.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 32.Liu H, Jones BE, Bradham C, Czaja MJ. Increased cytochrome P-450 2E1 expression sensitizes hepatocytes to c-Jun-mediated cell death from TNF-alpha. Am J Physiol Gastrointest Liver Physiol. 2002;282:G257–266. doi: 10.1152/ajpgi.00304.2001. [DOI] [PubMed] [Google Scholar]
- 33.Wu D, Cederbaum AI. Cytochrome p4502E1 sensitizes to tumor necrosis factor alpha-induced liver injury through activation of mitogen-activated protein kinases in mice. Hepatology. 2008;47:1005–1017. doi: 10.1002/hep.22087. [DOI] [PubMed] [Google Scholar]
- 34.Lu Y, Cederbaum AI. Enhancement by pyrazole of lipopolysaccharide-induced liver injury in mice: role of cytochrome P450 2E1 and 2A5. Hepatology. 2006;44:263–274. doi: 10.1002/hep.21241. [DOI] [PubMed] [Google Scholar]
- 35.Adachi A, Ishii H. Role of mitochondria in alcoholic liver injury. Free Rad Biol Med. 2002;32:487–491. doi: 10.1016/s0891-5849(02)00740-2. [DOI] [PubMed] [Google Scholar]
- 36.Yacoub LK, Fogt F, Griniuviene B, Nanji AA. Apoptosis and Bcl-2 Protein expression in experimental alcoholic liver disease in the rat. Alcoholism: Clin Exp Res. 2006;19:854–859. doi: 10.1111/j.1530-0277.1995.tb00958.x. [DOI] [PubMed] [Google Scholar]
- 37.Purohit V, Gao B, Song BJ. Molecular mechanisms of alchoholic fatty liver. Alcoholism: Clin Exp Res. 2009;33:191–205. doi: 10.1111/j.1530-0277.2008.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.You M, Crabb DW. Recent advances in alcoholic liver disease II. Minireview: molecular mechanisms of alcoholic fatty liver. Am J Physiol GI Liver Physiol. 2004;287:G1–G6. doi: 10.1152/ajpgi.00056.2004. [DOI] [PubMed] [Google Scholar]
- 39.You M, Crabb DW. Molecular mechanisms of alcoholic fatty liver: role of sterol regulatory element-binding proteins. Alcohol. 2004;34:39–43. doi: 10.1016/j.alcohol.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 40.Crabb DW, Liangpunsaku S. Alcohol and lipid metabolism. J Gastroent Hepatol. 2006;21:S56–S60. doi: 10.1111/j.1440-1746.2006.04582.x. [DOI] [PubMed] [Google Scholar]
- 41.Sozio M, Crabb DW. Alcohol and lipid metabolism. Am J Physiol Endocrinol Metab. 2008;295:E10–E16. doi: 10.1152/ajpendo.00011.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang SQ, Lin HZ, Lane MD, Clemens M, Diehl AM. Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proc Natl Acad Sci USA. 1997;94:2557–2562. doi: 10.1073/pnas.94.6.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang S, Lin H, Diehl AM. Fatty liver vulnerability to endotoxin-induced damage despite NF-kappaB induction and inhibited caspase 3 activation. Am J Physiol Gastrointest Liver Physiol. 2001;281:G382–G392. doi: 10.1152/ajpgi.2001.281.2.G382. [DOI] [PubMed] [Google Scholar]
- 44.Koteish A, Yang S, Lin H, Huang X, Diehl AM. Chronic ethanol exposure potentiates lipopolysaccharide liver injury despite inhibiting Jun N-terminal kinase and capase 3 activation. J Biol Chem. 2002;277:13037–13044. doi: 10.1074/jbc.M101632200. [DOI] [PubMed] [Google Scholar]
- 45.Ji C, Chan C, Kaplowitz N. Predominant role of SREBP lipogenic pathways in hepatic steatosis in the murine intragastric ethanol feeding model. J Hepatology. 2006;45:717–724. doi: 10.1016/j.jhep.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 46.Ji C, Kaplowitz N. Hyperhomocysteinemia endoplasmic reticulum stress and alcoholic liver injury. World J Gastroenterol. 2004;10:1699–1708. doi: 10.3748/wjg.v10.i12.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wan YY, Cai Y, Li J, Yuan Q, French B, Gonzalez FJ, et al. Regulation of peroxisome proliferator activated receptor alpha-mediated pathways in alcohol fed cytochrome P450 2E1 deficient mice. Hepatol Res. 2001;19:117–130. doi: 10.1016/s1386-6346(00)00089-9. [DOI] [PubMed] [Google Scholar]
- 48.Diluzio NR. Prevention of the acute ethanol-induced fatty liver by the simultaneous administration of antioxidants. Life Sci. 1964;3:113–118. doi: 10.1016/0024-3205(64)90189-4. [DOI] [PubMed] [Google Scholar]
- 49.Wheeler MD, Nakagami M, Bradford BU, Uesugi T, Mason RP, Connor HD, et al. Overexpression of manganese superoxide dismutase prevents alcohol-induced liver injury in the rat. J Biol Chem. 2001;276:36664–36672. doi: 10.1074/jbc.M105352200. [DOI] [PubMed] [Google Scholar]
- 50.Cohen JI, Roychowdhury S, McMullen MR, Stavitsky AB, Nagy LE. Complement and alcoholic liver disease: role of C1q in the pathogenesis of ethanol-induced liver injury in mice. Gastroent. 2010 doi: 10.1053/j.gastro.2010.04.041. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fisher M, You M, Matsumoto M, Crabb DW. PPAR agonist treatment reverses PPAR dysfunction and abnormalities in hepatic lipid metabolism in ethanol-fed mice. J Biol Chem. 2003;278:27997–28004. doi: 10.1074/jbc.M302140200. [DOI] [PubMed] [Google Scholar]
- 52.Nakajima T, Kamijo Y, Tanaka N, Sugiyama E, Tanaka E, Kiyosawa K, et al. Peroxisome proliferator-activated receptor α protects against alcohol-induced liver damage. Hepatology. 2004;40:972–980. doi: 10.1002/hep.20399. [DOI] [PubMed] [Google Scholar]
- 53.Nanji AA, Dannenberg AJ, Jokelainen K, Bass NM. Alcoholic liver injury in the rat is associated with reduced expression of peroxisome proliferator-α regulated genes and is ameliorated by PPAR-α activation. J Pharmacol Exp Ther. 2004;310:417–424. doi: 10.1124/jpet.103.064717. [DOI] [PubMed] [Google Scholar]
- 54.You M, Fisher M, Deeg MA, Crabb DW. Ethanol induces fatty acid synthesispathways by activation of SREBP. J Biol Chem. 2002;277:29342–29347. doi: 10.1074/jbc.M202411200. [DOI] [PubMed] [Google Scholar]
- 55.Ajmo JM, Liang X, Rogers CQ, Pennock BP, You M. Resveratrol alleviates alcoholic fatty liver in mice. Am J Physiol GI Liver Physiol. 2008;295:G833–G842. doi: 10.1152/ajpgi.90358.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miyazaki M, Dobrzyn A, Sampath H, Lee SH, Man WC, Chu K, et al. Reduced adiposity and liver steatosis by stearoyl-CoA desaturase deficiency are independent of peroxisome proliferator activated receptor-alpha. J Biol Chem. 2004;279:35017–35024. doi: 10.1074/jbc.M405327200. [DOI] [PubMed] [Google Scholar]
- 57.Miyazaki M, Flowers MT, Sampath H, Chu K, Otzelberger C, Liu X, et al. Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metabolism. 2006;6:484–496. doi: 10.1016/j.cmet.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 58.Dobrzyn P, Dobrzyn A, Miyazaki M, Cohen P, Asilmaz, Hardie DG, et al. Stearoyl CoA desaturase-1 deficiency increases fatty acid oxidation by activating AMP-protein kinase in liver. Proc Nat’l Acad Sci. 2004;101:6409–6414. doi: 10.1073/pnas.0401627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Korourian S, Hakkak R, Ronis MJ, Shelnutt SR, Waldron J, Ingelman-Sundberg M, et al. Diet and risk of ethanol-induced hepatotoxicity: carbohydrate-fat relationships in rats. Toxicol Sci. 1999;47:110–117. doi: 10.1093/toxsci/47.1.110. [DOI] [PubMed] [Google Scholar]
- 60.Lindros KO, Jarvelainen HA. A new oral low-carbohydrate alcohol liquid diet producing liver lesions: a preliminary account. Alcohol Alcohol. 1998;33:347–353. doi: 10.1093/oxfordjournals.alcalc.a008403. [DOI] [PubMed] [Google Scholar]
- 61.Tiptoe GL, Liong EC, Casey CA, Donohue TM, Eagon PK, So H, et al. A voluntary oral ethanol-feeding rat model associated with necroinflammatory liver injury. Alcoholism: Clin Exp Res. 2008;32:669–682. doi: 10.1111/j.1530-0277.2008.00623.x. [DOI] [PubMed] [Google Scholar]
- 62.Yamamoto K, Ichijo H, Korsmeyer SJ. BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal kinase pathway normally activated at G2/M. Mol Cell Biol. 1999;19:8469–8478. doi: 10.1128/mcb.19.12.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fujino G, Noguchi T, Matsuzawa A, Yamauchi S, Saitoh M, Takeda K, et al. Thioredoxin and TRAF family proteins regulate reactive oxygen species-dependent activation of ASK1 through reciprocal modulation of the N-terminal homophilic interaction of ASK1. Mol Cell Biol. 2007;27:8152–8163. doi: 10.1128/MCB.00227-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu H, Nishitoh H, Ichijo H, Kyriakis JM. Activation of apoptosis signal-regulating kinase 1 (ASK1) by tumor necrosis factor receptor-associated factor 2 requires prior dissociation of the ASK1 inhibitor thioredoxin. Mol Cell Biol. 2000;20:2198–2208. doi: 10.1128/mcb.20.6.2198-2208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 66.Bai J, Cederbaum AI. Adenovirus-mediated expression of CYP2E1 produces liver toxicity in mice. Toxicol Sci. 2006;91:365–371. doi: 10.1093/toxsci/kfj165. [DOI] [PubMed] [Google Scholar]
- 67.Nagy LE. Molecular aspects of alcohol metabolism: transcription factors involved in early ethanol-induced liver injury. Annu Rev Nutr. 2004;24:55–78. doi: 10.1146/annurev.nutr.24.012003.132258. [DOI] [PubMed] [Google Scholar]
- 68.Thurman RG. Mechanisms of hepatic toxicity II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol. 1998;275:G605–G611. doi: 10.1152/ajpgi.1998.275.4.G605. [DOI] [PubMed] [Google Scholar]
- 69.McClain CJ, Barve S, Deaciuc I, Kugelmas M, Hill D. Cytokines in alcoholic liver disease. Semin Liver Dis. 1999;19:205–219. doi: 10.1055/s-2007-1007110. [DOI] [PubMed] [Google Scholar]
- 70.Kamimura S, Tsukamoto H. Cytokine gene expression by Kupffer cells in experimental alcoholic liver disease. Hepatology. 1995;22:1304–1309. [PubMed] [Google Scholar]
- 71.Thurman RG, Bradford BU, Iimuro Y, Knecht KT, Arteel GE, Yin M, et al. The role of gut-derived bacterial toxins and free radicals in alcohol-induced liver injury. J Gastroenterol Hepatol. 1998;13:S39–S50. doi: 10.1111/jgh.1998.13.s1.39. [DOI] [PubMed] [Google Scholar]
- 72.Iimuro Y, Gallucci RM, Luster MI, Kono H, Thurman RG. Antibodies to tumor necrosis factor alfa attenuate hepatic necrosis and inflammation caused by chronic exposure to ethanol in the rat. Hepatology. 1997;26:1530–1537. doi: 10.1002/hep.510260621. [DOI] [PubMed] [Google Scholar]
- 73.Yin M, Wheeler MD, Kono H, Bradford BU, Gallucci RM, Luster MI, et al. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology. 1999;117:942–952. doi: 10.1016/s0016-5085(99)70354-9. [DOI] [PubMed] [Google Scholar]
- 74.Van Rooijen N, Sanders A. Liposome-mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–92. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]