Abstract
Background. Infants born prematurely are highly vulnerable to infections and also exhibit a high susceptibility to organ damage due to inflammation.
Methods. To investigate homeostatic immune control early in life, we used advanced multiparameter flow cytometry to compare responses to multiple Toll-like receptor (TLR) ligands in single cells and mononuclear cell populations in term neonates versus preterm neonates born before 29 weeks of gestation.
Results. Preterm neonates had globally attenuated TLR-stimulated interleukin (IL)-6, interferon-α, and, to a lesser extent, tumor necrosis factor-α responses but demonstrated relative preservation of anti-inflammatory IL10 responses in monocytes and dendritic cell subtypes. Remarkably, preterm neonates were also profoundly deficient in the common IL-12 and IL-23 cytokines' p40 subunit, which is critical for immunity against a wide variety of microbial pathogens in mice. Consistent with the increased susceptibility to infections resulting from the lack of IL-12/IL-23 in human newborns, significantly lower serum p40 concentrations were observed at birth in infants who developed early-onset sepsis.
Conclusion. To our knowledge, this study is the first detailed analysis of multiple TLR function in neonates born extremely premature. Although attenuation of proinflammatory pathways may protect against tissue-damaging immunity early in life, this previously unrecognized p40 immune deficiency appears to result in considerably increased susceptibility to infection in human preterm newborns.
Neonates are highly susceptible to infections and heavily rely on innate immune responses to defend against microorganisms [1]. Among newborns, preterm neonates are most likely to have morbidity and mortality due to infections, and nearly one-third of infants who are born extremely premature (ie, before 29 weeks of gestation) develop serious bloodborne infection during their first weeks of life [2, 3]. Understanding the innate immune mechanisms in preterm neonates is critical for improving outcomes in this patient group.
Previous studies examining innate immune functions in preterm neonates largely focused on lipopolysaccharide (LPS)-induced cytokine responses in whole blood or monocytes [4–14]. Toll-like receptor (TLR) 4, which is the receptor for LPS and a member of a family of at least 10 nonopsonic receptors (TLR1-TLR10) in humans, is predominantly involved in sentinel recognition of pathogens by the innate immune system [15]. In preterm neonates, the proinflammatory cytokine response to LPS [4–8, 16], interleukin (IL)-1 [9], or whole microorganisms [10, 11] is significantly reduced (mainly reported for IL-6 but, also, for tumor necrosis factor [TNF]-α, IL-8, and IL-1β), compared with that in term neonates, whereas data about anti-inflammatory IL-10 or transforming growth factor-1β responses are somewhat conflicting [6, 10, 12]. Part of this attenuation in proinflammatory responses has been attributed to a developmental, gestational age- dependent reduction in the surface expression of TLR4 and its coreceptor CD14 [13, 14, 17], as well as a reduced expression of the downstream intracellular signaling components MyD88 and IRF5 [17]. However, it is unclear whether compensatory responses exist in innate immune cell types other than monocytes or through pathogen recognition by other TLRs. Indeed, recognition of intact pathogens by innate immune cells likely involves multiple TLRs and, to our best knowledge, no studies to date have described responses to stimulation via TLRs other than TLR2 and TLR4 [17].
Dendritic cells are the primary source of IL-12 and IL-23, two immune-regulatory cytokines that are critically important for immune defenses against microorganisms [18]. Both cytokines exist as heterodimers with a common p40 subunit. The p40 subunit mainly functions in conjunction with either p35 (to form IL-12) or p19 (to form IL-23) to support differentiation and maintenance of T helper 1 (Th1) or T helper 17 (Th17) cells, respectively [18]. Th1 and Th17 responses are essential for host protection against multiple intracellular and extracellular pathogens, but they can also induce detrimental autoimmunity in the absence of strict regulatory control [18]. In term neonates, p19 and p40 are expressed, but p35 is barely detectable, resulting in a profound lack of IL-12 that is compensated by the production of IL-23 [19, 20]. Data on the expression of IL-12 or IL-23 in preterm neonates are lacking.
In the present study, we sought to gain new insights into innate immune homeostatic mechanisms in preterm neonates born before 29 weeks of gestation. To achieve this goal, we used multiparameter flow cytometry to directly assess innate responses to well-defined TLR ligands. To account for differences in the proportion of circulating leukocytes throughout gestation, we chose to compare responses in individual innate immune cell types as well as peripheral blood mononuclear cell populations. Our results show that in contrast to term neonates, preterm neonates are profoundly deficient in IL-12/IL-23p40 and provide compelling evidence of a role for this newly recognized preterm p40 deficit in contributing to susceptibility to neonatal sepsis.
Materials And Methods
Sample collection. Cord blood samples were collected in sodium heparin-anticoagulated Vacutainer tubes (BD Biosciences), after written, informed consent was obtained from mothers delivering either prematurely (at <29 weeks of gestation, as determined by ultrasound dating or by the first day of the last menstrual period; n = 12) or at full term (n = 18), at the Children's&Women's Health Centre of British Columbia (Vancouver, British Columbia, Canada) or the Centre Hospitalier Universitaire Sainte-Justine (Montreal, Quebec, Canada). For all preterm subjects, placenta were rigorously examined by a clinical pathologist. Only samples collected from subjects without clinical or histologic evidence of chorioamnionitis (defined as a fetal or maternal stage ⩾1, as determined by use of validated histologic criteria [21]) were included in this study. The research protocol was approved by the institutional ethics review boards of the University of British Columbia, the University of Alberta, and Centre Hospitalier Universitaire Sainte-Justine.
Blood sample processing and in vitro stimulation. Robust standard operating protocols used for the preparation of TLR-stimulation plates and for processing of blood samples have been described elsewhere [22]. In brief, cord blood mononuclear cells (CBMCs) were mixed 1:2 in prewarmed (at 37TC) RPMI 1640 medium (Invitrogen), extracted by ficoll-hypaque gradient centrifugation within 2 h of collection, and stimulated (5 X 105 cells/200 μL) in RPMI 1640 medium supplemented with 10% heat-inactivated human AB serum (Gemini Bio-Products), 100 U penicillin/mL, and 100 μg of streptomycin/ mL (Invitrogen). Cytokine responses were compared after stimulation with PAM3CSK4 (EMC Microcollections; recognized by TLR1/TLR2), FSL (EMC Microcollections; TLR2/TLR6), 0111: B4 LPS (Invivogen; TLR4), 3M-013 (3M; TLR7), 3M-002 (3M; TLR8), 3M-003 (3M; TLR7 and TLR8), and CpG-A type ODN 2336 (Coley; TLR9). CBMCs were added to premade 96-well round-bottom polystyrene plates (VWR) containing different concentrations of 10-fold dilutions of individual TLR agonists, and they were stimulated at 37TC in an atmosphere of 5% CO2. For intracellular cytokine detection, cells were initially incubated for 6 h in the presence of brefeldin A (BFA; final concentration, 10 μg/mL), except in the case of CpG-A stimulation, in which BFA was added only for the final 3 h of culture. For cytokine detection in culture supernatants, cells were stimulated for 24 h. After stimulation (for 6 or 24 h), cells were incubated for 15 min at 37TC after the addition of ethylenediaminetetraacetic acid (final concentration, 2 mmol/L); plates were centrifuged; 120 μL of supernatant was harvested; and cell pellets were resuspended in BD FACS lysing solution (BD Biosciences), sealed, and immediately frozen at -80TC until analysis.
Staining, flow cytometry acquisition, and analysis. Stimulated mononuclear cell samples were analyzed for single-cell cytokine responses in monocytes, conventional dendritic cells (cDCs), and plasmacytoid dendritic cells (pDCs), by means of flow cytometry (for supplementary methods, see the Appendix, which does not appear in the print version of the Journal). CompBeads (BD Biosciences) serving as positive and negative staining controls were stained the same way as cells and were used to standardize voltage settings.
Determination of cytokine secretion in culture supernatants was performed on 10 randomly chosen plates from term neonates and nine 24-h plates from preterm neonates, and it was sufficiently powered to allow sound statistical comparison between term and preterm groups (see below). Supernatants were diluted 1:2 and 1:20 in RPMI, to obtain signals in the linear detection range. Measurements were performed using a “ Flex Kit” multiplex bead array cytokine detection assay (Millipore), which allowed the detection of IL-6, IL-10, TNF-α, IL-12p40 monomers, the IL-12p35/p40 heterodimer (also referred to herein as “ IL-12p70”), and IFN-α-2B with overnight incubation at 4TC. For detection of human IL-23, a human IL-23 enzyme-linked immunosorbent assay specific for the p19/p40 heterodimer (eBioscience) was used. Cytokine detection was performed on a Luminex analyzer (PerkinElmer), except for IL-23, which was analyzed by spectrophotometry at 450 nm with a 570-nm subtraction. IL-23 production with stimulation of TLR9 by CpG is not reported because of background interference on IL-23p19/p40 detection in the assay used (unpublished data, which were also confirmed by the manufacturer). For all cytokines, sigmoid logistic curves were used to generate standard curves. Except for IL-6 levels, cytokine levels in unstimulated culture supernatants were below the limit of detection and therefore are not presented.
Detection of p40 in the serum of neonates at risk of early-onset neonatal sepsis. After parental informed consent was obtained, serum samples were collected at 12–21 h of age from neonates who were born at 24–41 weeks of gestation (n = 425) at the Royal Alexandra Hospital, the Misericordia Hospital, or the Grey Nuns Hospital (Edmonton, Canada) and whose mother had risk factors for early-onset (ie, at <72 h) neonatal sepsis (EONS; risk factors were defined as either a positive maternal vaginal culture for group B streptococcus, preterm labor, prolonged rupture of membranes for >18 h,or clinical chorioamnionitis, which was defined as foul-smelling amniotic fluid, a maternal body temperature >38.0TC, or fetal tachycardia >180 beats/min). Cases with culture-proven infection were compared with a group of randomly selected controls matched by frequency for gestational age and birth weight and in whom sepsis was excluded (ie, those with a negative blood culture result, a C-reactive protein level <5 mg/L, no clinical signs of infection, and a favorable outcome without antibiotic treatment after 48 h). Concentrations of IL-6, IL-10, and IL12/IL-23p40 in serum samples were corroborated using 2 multiplex Luminex assays (Panomics and Medicorp/Invitrogen) on a Luminex analyzer (PerkinElmer), and only data from the former multiplex assay are presented.
Statistical analyses. Proportions of monocytes, cDCs, and pDCs relative to CBMCs were compared using Student's t test as they were normally distributed. Cytokine levels in culture supernatants were compared using the Mann-Whitney U test. The Mann-Whitney U test was also used to compare flow cytometry results in preterm and term neonates. Bonferroni-corrected P values were used to correct for multiple comparisons in the analysis of TLR-stimulated cytokine responses, with P< .01 considered to denote statistical significance. Statistics were calculated using SPSS software for Windows (version 11; Lead Technologies).
Results
Proportions of innate immune circulating cell types in cord blood from term and preterm neonates. Because differential blood counts of leukocytes vary greatly across gestational age and may directly influence the magnitude of cytokine responses, the proportion of monocytes, cDCs, and pDCs in CBMCs was compared between preterm and term neonates. Consistent with previous reports [7, 23], a 2-fold lower proportion of monocytes was detected in CBMCs from preterm neonates, compared with term neonates (mean ± standard deviation [SD], 15% ± 4.3% vs. 8% ± 4.8%) (P = .002). Similarly, the proportions of pDCs were marginally lower (mean ± SD, 0.19% ± 0.16% vs. 0.25% ± 0.11%) (P = .03) in preterm neonates versus term neonates, whereas no difference in the proportions of cDCs was detected (mean ± SD, 0.63% ± 0.39% vs. 0.55% ± 0.24%) (P> .05).
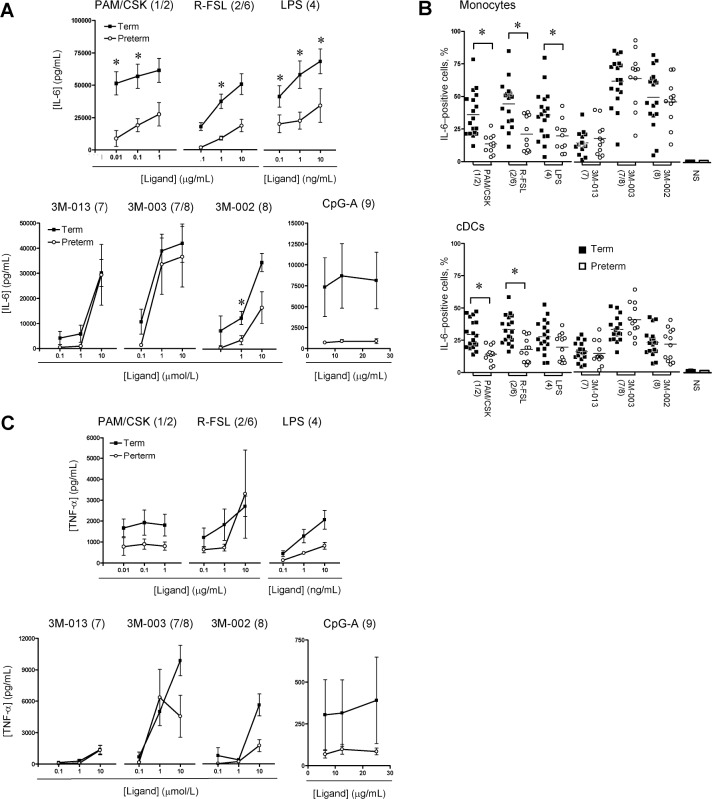
Diminished proinflammatory TLR responses in monocytes and cDCs from preterm neonates. The overall proinflammatory (IL-6) (Figure 1 A) and TNF-α (Figure 1 C) cytokine response generated as a result of TLR stimulation was compared in CBMC culture supernatants. Preterm neonates produced significantly less IL-6 upon stimulation via TLR1, TLR2, TLR4, and TLR6, whereas the response to stimulation via TLR7 and TLR8 was comparable to that in term neonates. For TLR9 stimulation, there was a trend toward higher IL-6 responses in term neonates.
Stimulation with TLR1, TLR2, TLR4, and TLR9 ligands also generally yielded lower TNF-α responses in preterm supernatants; however, these differences did not reach statistical significance. TNF-α responses to stimulation of TLR7/TLR8 using 3M-003 were comparable between preterm and term neonates.
The reduction in IL-6 production in response to stimulation via TLR1, TLR2, TLR4, and TLR6 in preterm neonates was also confirmed at the single-cell level in both monocytes and cDCs (Figure 1 B), when comparisons with term neonates were made. Intracellular TNF-α responses were similar between pre-term and term neonates on a per-cell basis, and when either expressed as a percentage of cytokine-expressing cells (data not shown) or as mean fluorescence intensity (MFI; data not shown). IL-6 and TNF-α were detected in both monocytes and cDCs with similar MFI, indicative of an equal contribution of the 2 cell types to the overall culture supernatant response, both preterm and term newborns (data not shown).
Similar IL-10, but reduced type I IFN, responses in preterm To assess whether preterm neonates in exhibit a deficit in anti-inflammatory cytokine responses, we examined the TLR-induced production of IL-10, a prototypic anti-inflammatory cytokine. In response to all TLR ligands tested, preterm and term neonates demonstrated generally similar IL-10 production (Figure 2), except perhaps for a marginally reduced preterm response to stimulation of TLR2/TLR6 by use of the agonist R-FSL.
Figure 2.
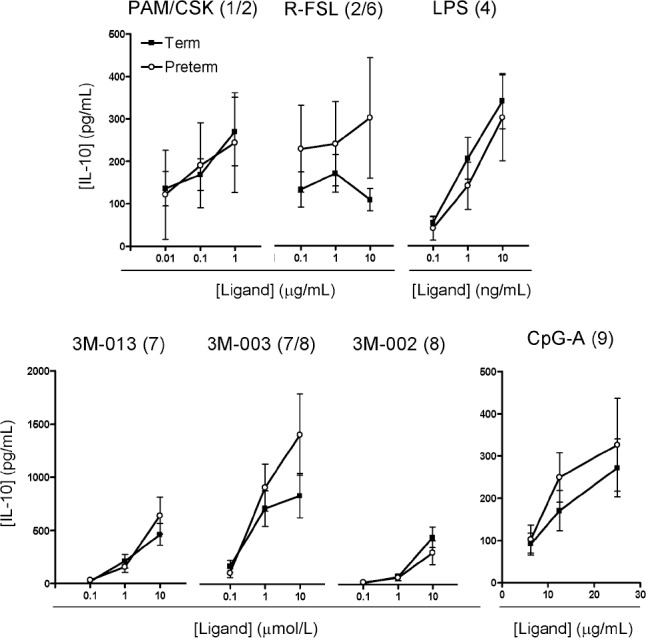
Interleukin (IL)-10 anti-inflammatory cytokine responses in culture supernatants. Data are the mean ± standard error of the mean. Agonists used for stimulation are shown above each graph, with their respective stimulated Toll-like receptor(s) shown in brackets. None of the differences between preterm and term neonates were statistically significant (P< .01). LPS, lipopolysaccharide.
For type I interferon, significant responses were detected only after stimulation with either TLR7 or TLR9 agonists and in pDCs. A trend toward reduced IFN-α production was apparent in preterm neonates when analyzing culture supernatants, but biological variability in this assay precluded differences from reaching a Bonferroni-adjusted threshold for statistical significance within our sample size (Figure 3 A). However, when IFN-α production was compared between preterm and term neonates in a single-cell, flow cytometry-based analysis, the former clearly produced significantly lower responses on a percentage cytokine-producing (pDC) basis (Figure 3 B).
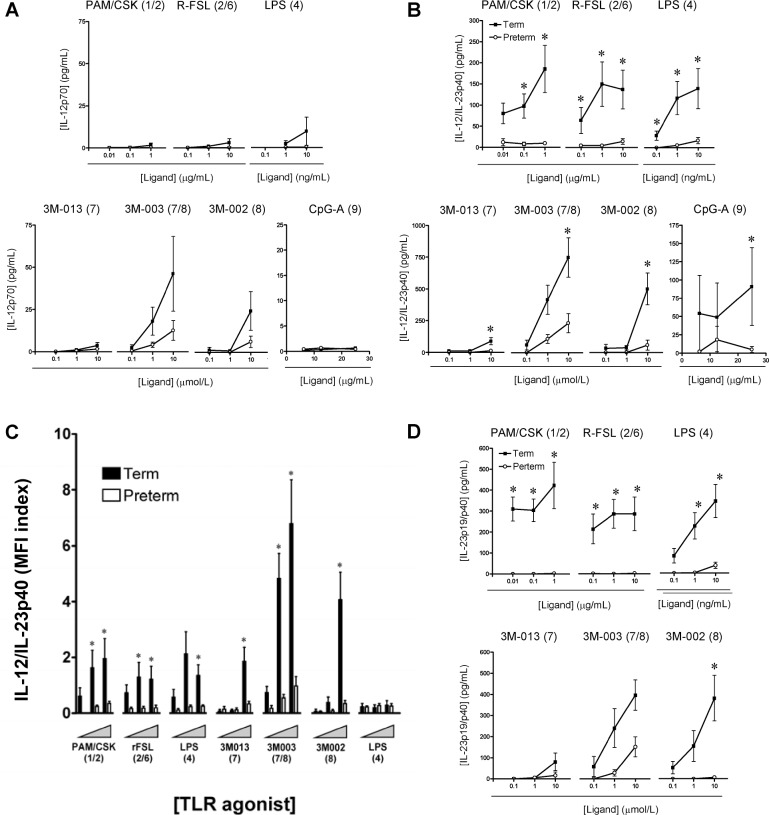
Profound deficiency of IL-12/IL-23p40 in preterm infants. Next, we examined TLR-triggered IL-12/IL-23 cytokine production in monocytes and cDCs. Overall, IL-12/IL-23p40 was mainly detected in cDCs, with 5-to 8-fold greater production, compared with monocytes (as determined based on MFI; not shown), and consistent with previous data [20]. In addition, term neonates were able to produce detectable amounts of IL12/IL-23p40 but negligible amounts of IL-12p70, except after TLR8 stimulation (Figure 4 A), as reported elsewhere [19, 20].
Remarkably, production of IL-12/IL-23p40 was substantially lower in preterm neonates, in response to most TLR agonists tested (Figure 4 B). Although the percentage of IL-12/IL-23p40- producing cDCs was comparable between preterm and term neonates (data not shown), the amount of cytokine produced on a per-cell basis (ie, as measured by MFI) was highly consistent with our results obtained from culture supernatants: levels were markedly reduced in cDCs of preterm neonates, compared with cDCs of term neonates (Figure 4 C). This marked reduction in p40 observed in preterm neonates suggested a profoundly impaired production of the IL-23 cytokine in addition to the lack of IL-12 (Figure 4 A). This was confirmed by direct measurement of the IL-23p19/p40 heterodimer by use of the enzyme-linked immunosorbent assay (Figure 4 D), where no IL-23 was detectable in culture supernatants of preterm neonates, except for a weak response to stimulation of TLR7/TLR8.
IL-12/IL-23p40 is significantly reduced in neonates with early-onset neonatal sepsis. To determine the clinical relevance of IL-12/IL-23p40 for protection against infections in neonates, we conducted a nested case-control analysis in a large prospective cohort of infants (n = 425) at risk for EONS. In this at-risk cohort, the overall incidence of EONS was ∼18 cases per 1000 neonates. Clinical characteristics were similar between the 8 EONS cases (Table 1) and 42 matched controls (mean gestational age ± SD, 32.9 ± 3.8 weeks versus 32.2 ± 3.8 weeks; mean birth weight ± SD, 2038 ± 636 g versus 2010 ± 788 g; frequency of maternal-infant pair exposure to antenatal corticosteroids, 62% versus 48% [95% confidence interval {CI}, -52% to 22%]) (P = .70). In EONS cases, serum concentrations of IL-12/IL-23p40 were significantly lower at birth, compared with gestational age-and birth weight-matched control neonates who did not show any clinical or laboratory signs of infection (P = .018) (Figure 5). The difference in serum p40 levels between cases and controls persisted when considering only neonates without prolonged rupture of membranes over 18 h or clinical signs of chorioamnionitis (28 ± 16 pg/mL versus 74 ± 34 pg/ mL) (P = .02). On the other hand, levels of IL-6 (P = .002), IL-10 (P = .001) (Figure 5), and diagnostic C-reactive protein (mean, 66.5 mg/L versus <5 mg/L) that were simultaneously sampled were higher in cases than in controls, indicating that the reduction in serum levels of IL-12/IL-23p40 was not the result of a generally blunted inflammatory response to infection.
Table 1.
Clinical Characteristics of Infants with Early-Onset Neonatal Sepsis
Figure 5.
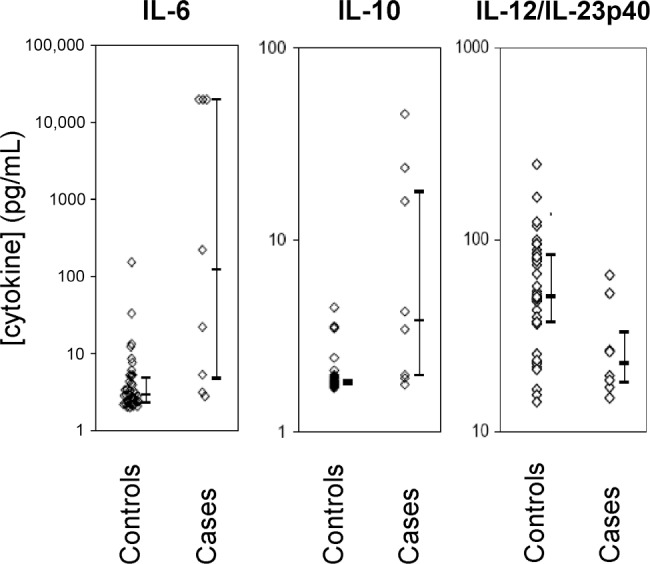
Serum cytokine concentrations in neonates at risk of early-onset neonatal sepsis. Levels of interleukin (IL)-6, IL-10, and IL-12/IL23p40 were measured in serum samples simultaneously obtained from infants with culture-proven sepsis (cases; n = 8) and infants who did not present with any clinical or laboratory signs of infection (controls; n = 42) but were selected from a prospective cohort of neonates at-risk for early-onset neonatal sepsis. Bars denote medians and interquartile ranges.
Discussion
In the present study, we systematically interrogated individual TLR-stimulated innate immune cytokine responses in preterm neonates born most early in gestation. Overall, our results revealed attenuated IL-6 production in monocytes and cDCs from preterm neonates and, to a much lesser extent, a reduction in TNF-α production, after stimulation via TLR1, TLR2, TLR4, TLR6, or TLR9. In contrast, comparison of preterm and term neonates revealed equivalent levels of IL-10 production and similarly weak IL-12p70 and IFN-α responses. Entirely novel was the finding of a profound reduction in IL-12/IL-23p40 production in preterm neonates. The lack of IL-12/IL-23p40 in preterm neonates appears to play a critical role in susceptibility to infections, as demonstrated by the reduction in serum IL-12/IL-23p40 levels in preterm infants with EONS.
Most of the previously published data on TLR-stimulated responses in infants are derived from cord blood samples obtained from term neonates. Studies of term neonates have consistently reported deficits in IFN-α [20, 24, 25] and IL-12p70 responses [20, 26, 27]. In contrast, IL-6 and IL-10 responses were generally comparable or even higher when compared with those in adults [5, 20, 28–32]. However, data are very scarce for preterm neonates, and relatively large discrepancies are reported among studies. This high level of variation could be explained in part by the varying conditions that lead to premature birth. In animal models, chronic antenatal inflammation through repeated LPS exposure induces a state of innate immune tolerance manifested by a blunted response to subsequent TLR stimulation [33], which may affect measurements of TLR responses in cord blood. To avoid this potential pitfall and specifically examine the potential intrinsic developmental difference, we conducted our analysis exclusively in samples from subjects in whom no clinical or histological signs of chorioamnionitis (as defined using rigorous histological criteria) were detected.
In mice, IL-12/IL-23p40 deficiency induces susceptibility to a wide variety of microbial pathogens [18]. In contrast, older p40-deficient humans demonstrate no major disease susceptibility apart from infections involving salmonella and mycobacterium [34]. In term neonates, it was postulated that elevated production of p40 provided sufficient protective immunity via IL-23 [19, 20], but this seemingly is not the case in IL-12- and IL-23-deficient preterm neonates. The relatively mild clinical phenotype in human adults, compared with pre-term neonates, is likely a result of the effect of compensatory immune mechanisms that may mask the contribution of TLRs to the protection conferred by the immune system. Preterm neonates lack these compensatory adaptive immune mechanisms, including maternally transferred antibodies, and therefore uniquely rely on TLR pathways to defend against microorganisms. The significantly lower serum level of p40 in neonates with early-onset neonatal sepsis therefore provides compelling evidence for a critical role of the IL-12 and IL-23 cytokines in humans.
Apart from its role in immune protection, IL-23 clearly plays a pathogenic role in autoimmune disorders through the promotion of proinflammatory, potentially tissue-damaging, IL17-secreting T cells [18]. Attenuation of the IL-23 pathway may be particularly critical to the fetus to prevent Th17 inflammatory-mediated self-organ damage or a potential life-threatening immune response against maternal tissues. Indeed, evidence support an exquisite susceptibility of preterm infants to inflammation-mediated injury to the brain [35–37] or lung [38]. Termlike anti-inflammatory IL-10 responses and reduced IL-6, TNF-α, IL-12p70, and IFN-α responses, together with the almost-complete deficit in IL-12/IL-23p40 reported herein, may thus purposefully serve to protect the fetus (or preterm neonate) against potentially harmful inflammation.
Noticeably, our observations confirm and expand upon the findings of Levy et al [39], who had first reported high levels of activation of the TLR7/TLR8 pathway in term neonates compared with adults. Indeed, the TLR7/8 agonist 3M-003 stimulated a strong TNF-α response and IL-12 production in term neonates but, also, to a lesser extent in preterm neonates. Results suggest a potential therapeutic benefit of using TLR7/8 agonists to promote the development of protective vaccine-induced immunity against pathogens in these age groups, as has also been suggested by others [40]. However, given the damage that poorly regulated inflammation can cause in the preterm infant, one would first need to understand the effect that augmentation of innate immunity may have on susceptibility to inflammation-mediated organ injury in early life. The similarity between responses observed after intracellular (ie, TLR7 and TLR8), but not extracellular, TLR stimulation (ie, TLR1, TLR2, TLR4, and TLR6) on a per-cell basis suggests the existence of differential signaling requirements located proximally along the TLR signaling cascade, an aspect that we are currently investigating in purified cell populations.
Our study has potential limitations. A high proportion of mothers who deliver prematurely received antenatal systemic corticosteroids, which may affect the measurement of immune responses in cord blood. However, although antenatal corticosteroids may partially explain reduced levels of IL-6 and TNF-α in preterm neonates, reductions in IL-10 levels could have been expected as well but were not observed [41]. For production of IL-12/IL-23p40, equivalent concentrations of dexamethasone higher than that persistently observed in the serum of pregnant women after administration of exogenous corticosteroids [42] blunted the response in cord blood cell cultures [41]. However, in our study, the median time from the administration of antenatal corticosteroids to cord blood collection for preterm subjects was 16 days (interquartile range, 6.8- 22 days). All but one mother of preterm infants had received antenatal corticosteroids more than 72 h before delivery, which is longer than the anticipated pharmacological effect of short-course corticosteroid therapy. In addition, we found no correlation between the timing of antenatal corticosteroid administration and the level of the IL-12/IL-23p40 TLR response (data not shown), suggesting that the deficit in p40 is intrinsic to the immune system of the preterm neonate. The exact mechanism by which the IL-12/IL-23p40 response is suppressed in preterm neonates deserves further investigation. Although it is plausible that remote antenatal systemic corticosteroid administration may affect measured preterm neonatal TLR responses, persistence of such an effect is likely of high clinical relevance in determining the susceptibility of preterm neonates to sepsis.
In conclusion, our results identify a novel, profound deficit in IL-12/IL-23p40 production, which may at least in part explain the greater risk of infections clinically observed in preterm infants. On the other hand, the overall attenuation of inflammatory response and relative preservation of anti-inflammatory responses may be part of a remarkably efficient mechanism aimed at protecting the fetus against harmful effects of untoward immunity before the full term of gestation is completed.
Figure 1.
Interleukin (IL)-6 and tumor necrosis factor (TNF)-α proinflammatory cytokine responses. IL-6 (A) and TNF-α (C) responses in cord blood mononuclear cell culture supernatants (mean ± standard error of the mean). Background levels of IL-6 in unstimulated culture supernatants were low (range, 34-6364 pg/mL) and did not differ significantly between term and preterm neonates; therefore, for simplicity, graphs denote levels after subtracting background unstimulated cytokine levels. B, Peak IL-6 responses (expressed as the mean percentage of cytokine-producing cells) in monocytes and conventional dendritic cells (cDCs). Agonists used for stimulation are indicated above each graph, with their respective stimulated TLR(s) in brackets. For monocytes, CD14 staining for one preterm infant was not available for technical reasons, and data from only 11 preterm infants therefore are presented. LPS, lipopolysaccharide; NS, unstimulated (bar graphs). * P< .01.
Figure 3.
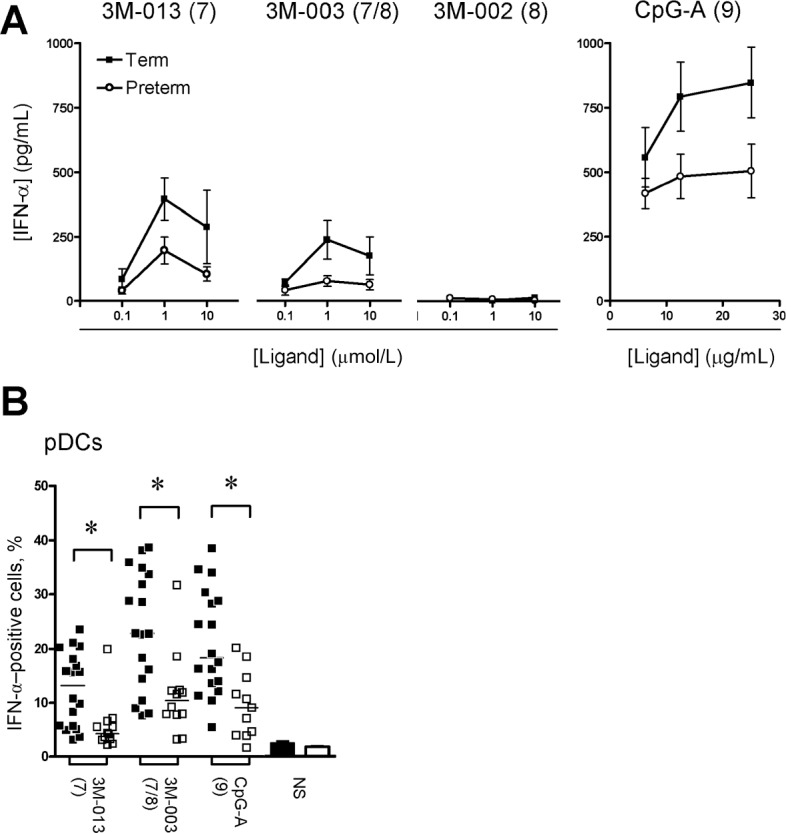
Interferon (IFN)-α cytokine responses. A , Cytokine measured in culture supernatants (mean ± standard error of the mean). B , Peak IFN-α responses (expressed as the percentage of cytokine-producing cells; bars denote the mean) measured in plasmacytoid dendritic cells (pDCs), as determined by flow cytometry. Agonists used for stimulation are shown above each graph, with their respective stimulated Toll-like receptor(s) shown in brackets. * P< .01. NS, unstimulated (bar graphs).
Figure 4.
Interleukin (IL)-12 and IL-23 responses. Data are the mean ± standard error of the mean for IL-12p70 (A) or IL-12/IL-23p40 (B) responses in cord blood mononuclear cell (CBMC) culture supernatants. C , IL-12/IL-23p40 responses in conventional dendritic cells (cDCs). D , IL-23p40/p19 responses in CBMC culture supernatants. Responses to graded concentrations of individual Toll-like receptor agonists in term (black bars) and preterm (white bars) neonates were measured by gating on cDCs and are reported as mean fluorescence index (MFI). Agonists used for stimulation are shown above each graph, with their respective stimulated Toll-like receptor(s) shown in brackets. * P< .01. LPS, lipopolysaccharide.
Acknowledgments
We thank Jennifer Claydon for administrative support with the coordination of this research; Chandra Pham and Sophie Perreault for recruitment of subjects and help with cord blood collection; Deborah McFadden for histological examination of placenta for preterm subjects; Darren Blimkie, Martine Caty, and members of the Wilson Laboratory for experimental assistance; Philippe Chessex for support and mentorship; and parents and nursing staff, who have been invaluable in the success of this study.
Footnotes
Potential conflicts of interest: none reported.
Financial support: P.M.L. and T.R.K. acknowledge support from the Canadian Child Health Clinician Scientist Program, in partnership with the SickKids Foundation, the Child&Family Research Institute (British Columbia), the Women and Children's Health Research Institute (Alberta), and the Manitoba Institute of Child Health. T.R.K. is also supported by a Career Award in the Biomedical Sciences from the Burroughs Wellcome Fund. This research was funded in part by an unrestricted grant from the Division of Neonatology at Children's&Women's Health Centre of British Columbia (to P.M.L.), the British Columbia Lung Association and SickKids Foundation (to P.M.L.), the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant N01 AI50023 to T.R.K.), AllerGen Networks of Centres of Excellence of Canada (grants 07-A1A and 07-B2B to T.R.K.), and the Canadian Institutes for Health Research (grant MOP-53269 to D.P.S.).
References
- 1.Wilson CB, Kollmann TR. Induction of antigen-specific immunity in human neonates and infants. Nestle Nutr Workshop Ser Pediatr Program. 2008;61:183–195. doi: 10.1159/000113493. [DOI] [PubMed] [Google Scholar]
- 2.Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–291. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 3.Moster D, Lie RT, Markestad T. Long-term medical and social consequences of preterm birth. N Engl J Med. 2008;359:262–273. doi: 10.1056/NEJMoa0706475. [DOI] [PubMed] [Google Scholar]
- 4.Yachie A, Takano N, Ohta K, et al. Defective production of interleukin6 in very small premature infants in response to bacterial pathogens. Infect Immun. 1992;60:749–753. doi: 10.1128/iai.60.3.749-753.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schultz C, Rott C, Temming P, Schlenke P, Moller JC, Bucsky P. Enhanced interleukin-6 and interleukin-8 synthesis in term and preterm infants. Pediatr Res. 2002;51:317–322. doi: 10.1203/00006450-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Dembinski J, Behrendt D, Martini R, Heep A, Bartmann P. Modulation of pro-and anti-inflammatory cytokine production in very preterm infants. Cytokine. 2003;21:200–206. doi: 10.1016/s1043-4666(02)00498-2. [DOI] [PubMed] [Google Scholar]
- 7.Strunk T, Temming P, Gembruch U, Reiss I, Bucsky P, Schultz C. Differential maturation of the innate immune response in human fetuses. Pediatr Res. 2004;56:219–226. doi: 10.1203/01.PDR.0000132664.66975.79. [DOI] [PubMed] [Google Scholar]
- 8.Levy E, Xanthou G, Petrakou E, et al. Distinct roles of TLR4 and CD14 in LPS-induced inflammatory responses of neonates. Pediatr Res. 2009;66:179–184. doi: 10.1203/PDR.0b013e3181a9f41b. [DOI] [PubMed] [Google Scholar]
- 9.Liechty KW, Koenig JM, Mitchell MD, omero R, Christensen RD. Production of interleukin-6 by fetal and maternal cells in vivo during intraamniotic infection and in vitro after stimulation with interleukin 1. Pediatr Res. 1991;29:1–4. doi: 10.1203/00006450-199101000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Hartel C, Osthues I, Rupp J, et al. Characterisation of the host inflammatory response to Staphylococcus epidermidis in neonatal whole blood. Arch Dis Child Fetal Neonatal Ed. 2008;93:140–145. doi: 10.1136/adc.2007.124685. [DOI] [PubMed] [Google Scholar]
- 11.Tatad AM, Nesin M, Peoples J, et al. Cytokine expression in response to bacterial antigens in preterm and term infant cord blood monocytes. Neonatology. 2008;94:8–15. doi: 10.1159/000112541. [DOI] [PubMed] [Google Scholar]
- 12.Schultz C, Temming P, Bucsky P, Gopel W, Strunk T, Hartel C. Immature anti-inflammatory response in neonates. Clin Exp Immunol. 2004;135:130–136. doi: 10.1111/j.1365-2249.2004.02313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forster-Waldl E, Sadeghi K, Tamandl D, et al. Monocyte Toll-like receptor 4 expression and LPS-induced cytokine production increase during gestational aging. Pediatr Res. 2005;58:121–124. doi: 10.1203/01.PDR.0000163397.53466.0F. [DOI] [PubMed] [Google Scholar]
- 14.Henneke P, Osmers I, Bauer K, Lamping N, Versmold HT, Schumann RR. Impaired CD14-dependent and independent response of polymorphonuclear leukocytes in preterm infants. J Perinat Med. 2003;31:176–183. doi: 10.1515/JPM.2003.024. [DOI] [PubMed] [Google Scholar]
- 15.Beutler BA. TLRs and innate immunity. Blood. 2009;113:1399–1407. doi: 10.1182/blood-2008-07-019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weatherstone KB, Rich EA. Tumor necrosis factor/cachectin and interleukin-1 secretion by cord blood monocytes from premature and term neonates. Pediatr Res. 1989;25:342–346. doi: 10.1203/00006450-198904000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Sadeghi K, Berger A, Langgartner M, et al. Immaturity of infection control in preterm and term newborns is associated with impaired toll-like receptor signaling. J Infect Dis. 2007;195:296–302. doi: 10.1086/509892. [DOI] [PubMed] [Google Scholar]
- 18.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 19.Vanden Eijnden S, Goriely S, De Wit D, Goldman M, Willems F. Preferential production of the IL-12(p40)/IL-23(p19) heterodimer by dendritic cells from human newborns. Eur J Immunol. 2006;36:21–26. doi: 10.1002/eji.200535467. [DOI] [PubMed] [Google Scholar]
- 20.Kollmann TR, Crabtree J, Rein-Weston A, et al. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol. 2009;183:7150–7160. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2003;6:435–448. doi: 10.1007/s10024-003-7070-y. [DOI] [PubMed] [Google Scholar]
- 22.Jansen K, Blimkie D, Furlong J, et al. Polychromatic flow cytometric high-throughput assay to analyze the innate immune response to Toll-like receptor stimulation. J Immunol Methods. 2008;336:183–192. doi: 10.1016/j.jim.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies NP, Buggins AG, Snijders RJ, Jenkins E, Layton DM, Nicolaides KH. Blood leucocyte count in the human fetus. Arch Dis Child. 1992;67:399–403. doi: 10.1136/adc.67.4_spec_no.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Wit D, Olislagers V, Goriely S, et al. Blood plasmacytoid dendritic cell responses to CpG oligodeoxynucleotides are impaired in human newborns. Blood. 2004;103:1030–1032. doi: 10.1182/blood-2003-04-1216. [DOI] [PubMed] [Google Scholar]
- 25.Danis B, George TC, Goriely S, et al. Interferon regulatory factor 7mediated responses are defective in cord blood plasmacytoid dendritic cells. Eur J Immunol. 2008;38:507–517. doi: 10.1002/eji.200737760. [DOI] [PubMed] [Google Scholar]
- 26.Goriely S, Vincart B, Stordeur P, et al. Deficient IL-12(p35) gene expression by dendritic cells derived from neonatal monocytes. J Immunol. 2001;166:2141–2146. doi: 10.4049/jimmunol.166.3.2141. [DOI] [PubMed] [Google Scholar]
- 27.Aksoy E, Albarani V, Nguyen M, et al. Interferon regulatory factor 3dependent responses to lipopolysaccharide are selectively blunted in cord blood cells. Blood. 2007;109:2887–2893. doi: 10.1182/blood-2006-06-027862. [DOI] [PubMed] [Google Scholar]
- 28.Belderbos ME, van Bleek GM, Levy O, et al. Skewed pattern of Toll-like receptor 4-mediated cytokine production in human neonatal blood: low LPS-induced IL-12p70 and high IL-10 persist throughout the first month of life. Clin Immunol. 2009;133:228–237. doi: 10.1016/j.clim.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Angelone DF, Wessels MR, Coughlin M, et al. Innate immunity of the human newborn is polarized toward a high ratio of IL-6/TNF-α production in vitro and in vivo. Pediatr Res. 2006;60:205–209. doi: 10.1203/01.pdr.0000228319.10481.ea. [DOI] [PubMed] [Google Scholar]
- 30.Dembinski J, Behrendt D, Reinsberg J, Bartmann P. Endotoxin-stimulated production of IL-6 and IL-8 is increased in short-term cultures of whole blood from healthy term neonates. Cytokine. 2002;18:116–119. doi: 10.1006/cyto.2002.0880. [DOI] [PubMed] [Google Scholar]
- 31.Peters AM, Bertram P, Gahr M, Speer CP. Reduced secretion of interleukin-1 and tumor necrosis factor-α by neonatal monocytes. Biol Neonate. 1993;63:157–162. doi: 10.1159/000243926. [DOI] [PubMed] [Google Scholar]
- 32.De Wit D, Tonon S, Olislagers V, et al. Impaired responses to Toll-like receptor 4 and Toll-like receptor 3 ligands in human cord blood. J Autoimmun. 2003;21:277–281. doi: 10.1016/j.jaut.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Kramer BW, Kallapur SG, Moss TJ, Nitsos I, Newnham JP, Jobe AH. Intra-amniotic LPS modulation of TLR signaling in lung and blood monocytes of fetal sheep. Innate Immun. 2009;15:101–107. doi: 10.1177/1753425908100455. [DOI] [PubMed] [Google Scholar]
- 34.Filipe-Santos O, Bustamante J, Chapgier A, et al. Inborn errors of IL12/23-and IFN-g-mediated immunity: molecular, cellular, and clinical features. Semin Immunol. 2006;18:347–361. doi: 10.1016/j.smim.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 35.Edwards AD, Tan S. Perinatal infections, prematurity and brain injury. Curr Opin Pediatr. 2006;18:119–124. doi: 10.1097/01.mop.0000193290.02270.30. [DOI] [PubMed] [Google Scholar]
- 36.Glass HC, Bonifacio SL, Chau V, et al. Recurrent postnatal infections are associated with progressive white matter injury in premature infants. Pediatrics. 2008;122:299–305. doi: 10.1542/peds.2007-2184. [DOI] [PubMed] [Google Scholar]
- 37.Stoll BJ, Hansen NI, Adams-Chapman I, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292:2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 38.Speer CP. Chorioamnionitis, postnatal factors and proinflammatory response in the pathogenetic sequence of bronchopulmonary dysplasia. Neonatology. 2009;95:353–361. doi: 10.1159/000209301. [DOI] [PubMed] [Google Scholar]
- 39.Levy O, Suter EE, Miller RL, Wessels MR. Unique efficacy of Toll-like receptor 8 agonists in activating human neonatal antigen-presenting cells. Blood. 2006;108:1284–1290. doi: 10.1182/blood-2005-12-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Philbin VJ, Levy O. Developmental biology of the innate immune response: implications for neonatal and infant vaccine development. Pediatr Res. 2009;65:98–105. doi: 10.1203/PDR.0b013e31819f195d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bessler H, Kagazanov S, Punsky I, Sirota L. Effect of dexamethasone on IL-10 and IL-12p40 production in newborns and adults. Biol Neonate. 2001;80:262–266. doi: 10.1159/000047154. [DOI] [PubMed] [Google Scholar]
- 42.Tsuei SE, Petersen MC, Ashley JJ, McBride WG, Moore RG. Disporition of synthetic glucocorticoids. II. Dexamethasone in parturient women. Clin Pharmacol Ther. 1980;28:88–98. doi: 10.1038/clpt.1980.136. [DOI] [PubMed] [Google Scholar]
- 43.Perfetto SP, Ambrozak D, Nguyen R, Chattopadhyay P, Roederer M. Quality assurance for polychromatic flow cytometry. Nat Protoc. 2006;1:1522–1530. doi: 10.1038/nprot.2006.250. [DOI] [PubMed] [Google Scholar]
- 44.Lee JA, Spidlen J, Boyce K, et al. MIFlowCyt: the minimum information about a Flow Cytometry Experiment. Cytometry A. 2008;73:926–930. doi: 10.1002/cyto.a.20623. [DOI] [PMC free article] [PubMed] [Google Scholar]