Abstract
Sites involved in central chemoreception (CCR) are widely distributed in the brain. One possible explanation for the existence of multiple central chemoreceptor sites is the vigilance state-dependent hypothesis, that some sites are of greater importance in wakefulness others in sleep. We briefly summarize the evidence for a distributed network of central chemoreceptor sites and a vigilance state-dependent differentiation among them. We then discuss the role of orexin in vigilance state-dependent CCR based on our recent studies using orexin knockout mice and focal microdialysis of an orexin receptor antagonist at the retrotrapezoid nucleus and medullary raphé in rats. Orexin affects CCR in a vigilance state-dependent manner that varies with circadian time. Orexin also contributes to emotional stress- and other state-dependent related regulation of ventilation, e.g., the defense response. Diversity in central chemoreception including orexin neurons and the synaptic control of respiratory and cardiovascular output neurons appears to be necessary for animals to adapt themselves to constantly changing situations and behavioral states.
Keywords: Control of breathing, Chemoreceptor, Sleep, Stress, Circadian
1. Multiple central chemoreception sites
Central chemoreception (CCR) refers here to the detection of CO2/pH at sites within the central nervous system and the resultant effects on ventilation. Therefore, such sites should fulfill at least three criteria; 1) they should be activated by CO2/pH, 2) changes in CO2/pH within a physiological range at the site should affect ventilation, and 3) their destruction or inhibition should result in a diminution of the hypercapia-induced ventilatory augmentation. The detecting element could include glia or neurons or their axons or dendrites.
The first criterion can be examined by measuring the expression of c-fos, a histological marker of neuronal activation, in response to CO2 inhalation in vivo (Sato et al., 1992; Berquin et al., 2000; Okada et al., 2002; Sunanaga et al., 2009) or by electrophysiological measurements using either patch-clamp, Ca2+-sensitive dye, or voltage-sensitive dye in ex vivo slice or block preparations (Williams et al., 2007; Onimaru et al., 2008; Erlichman et al., 2009). Another strategy is to examine the histological distribution of putative CO2/pH-sensing molecules. Many sensing molecules have been proposed to date such as inward rectifier K+ (Kir) channels, tandem pore domain acid-sensing potassium (TASK) channels, gap junctions, transient receptor potential (TRP) channels, and amiloride-sensitive acid-sensing ion channels (ASICs) (Jiang et al., 2005; Dubreuil et al., 2009; Ziemann et al., 2009). These putative CO2/pH sensing molecules do not necessarily function in a mutually exclusive manner but may function synergistically (Jiang et al., 2005). The anatomical studies to date have revealed multiple possible brain sites that could contribute to CCR. In the lower brainstem, they include: 1) ventrolateral medullary surface structures at locations dorsal to the traditional rostral (Mitchell’s are; now thought to be the retrotrapezoid nucleus) and caudal (Loeschke’s) chemosensitive areas and the intermediate (Schlaefke’s) area, 2) the pre-Bötzinger complex, 3) the nucleus tractus solitarii (NTS), 4) the medullary raphe (MR; raphe magnus, obscurus, and pallidus), 5) the locus coeruleus, 6) the lateral parabrachial nucleus. (Berquin et al., 2000; Okada et al., 2002; Jiang et al., 2005; Onimaru et al., 2008; Erlichman et al., 2009), and the adjacent fastigial nucleus of the cerebellum (Martino et al., 2007). Although higher brain structures such as the hypothalamus and cortex can also be activated by CO2 inhalation (Berquin et al., 2000; Ziemann et al., 2009), the lower brainstem has been the focus of special interest in part because decerebration has had only a limited impact on the hypercapnia-induced ventilatory augmentation (Tenney and Ou, 1977).
To examine whether focal stimulation within these candidate sites would affect ventilation, Nattie and colleagues (Li et al., 1999) established a method to produce a focal acidosis by reverse microdialysis of artificial cerebrospinal fluid equilibrated with high CO2 and simultaneously measure ventilation in unanesthetized rats. It is important to note that this technique results in a change in tissue pH like that observed with a ~6.6 mm Hg increase in arterial PCO2, (Li and Nattie, 2002), which means the change is within the physiological range and certainly much less than that accompanying the inhalation of 7% CO2, a commonly used stimulus. From a series of such experiments, 6 central chemoreceptor sites have been described so far in the lower brainstem. 1) Focal acidification of the retrotrapezoid nucleus (RTN) in the rat increased ventilation about 24% due to increases in tidal volume (Li et al., 1999). This site corresponds to the ventrolateral medullary surface structure at a location dorsal to Mitchell’s area. 2) In the rostral MR, focal acidification by dialysis induced an ~20% increase of ventilation mediated by respiratory frequency in the rat (Nattie and Li, 2001). 3) Focal acidification in the caudal MR (raphe obscurus) alone had little effect on ventilation in the rat but if performed simultaneously with focal acidification of the RTN, the response was much greater (51%) than focal RTN acidification alone (24%) (Dias et al., 2008). Focal acidification by dialysis in the MR of the goat also increases ventilation (Hodges et al., 2004a,b). 4) Focal acidification of the caudal NTS of the rat increased ventilation by 20-30% (Nattie and Li, 2002). 5) Focal acidification of the pre-Bötzinger complex increased ventilation in conscious goats (Krause et al., 2009). 6) Focal acidification of the region just deep to the caudal chemsensitive area on the ventrolateral medulla increased ventilation in wakefulness (daSilva et al., 2010). In each experiment, focal dialysis with artificial cerebrospinal fluid equilibrated with 5% CO2 had no effect nor did dialysis of high CO2 at sites adjacent to the areas of interest. Thus, a mild focal acidosis at many, but not all, brainstem sites produces a stimulation of ventilation. The response to the focal acidosis can involve frequency and/or tidal volume (see Fig. 1) and sometimes changes in metabolic rate and heart rate (Hodges et al., 2004a,b).
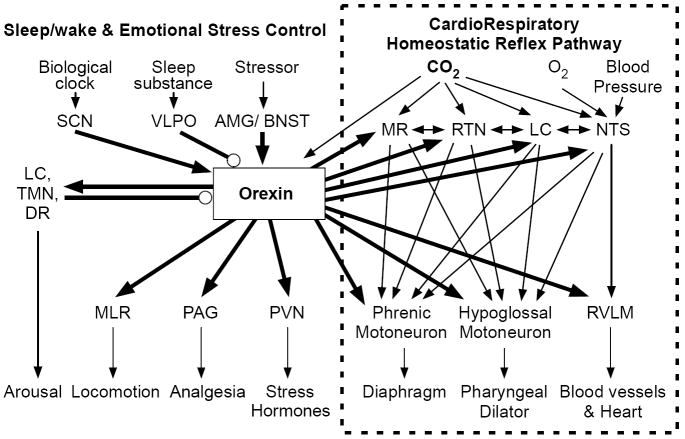
Figure 1. Vigilance-state-dependent central chemoreception.
The top panel shows a schematized view of a medullary cross section at the level of the facial nucleus ~11 mm caudal to bregma. NTS refers to nucleus of the tractus solitarius; RTN to the retrotrapezoid nucleus; VII to the facial nucleus. The rectangles show the approximate size of the dialysis probe placed either in the medullary raphe or in the RTN. The circular shaded areas show the approximate area of decreased pH when the dialysis solution contains 25% CO2, based on pH measurements obtained under anesthesia. The panel at lower left shows the ventilatory responses to focal acidification of the RTN in wakefulness and sleep. Note that ventilation increases only in wakefulness and predominantly via an increase in tidal volume. The panel at lower right shows the ventilatory response to focal acidification of the medullary raphe in wakefulness and sleep. Note that ventilation increases only in sleep and predominantly via an increase in breathing frequency. (Adapted from Nattie 2001)
In earlier experiments under anesthesia, acute destruction of specific chemoreceptor sites including the RTN, the NTS, and the MR severely curtailed the subsequent response to systemic hypercapnia, even when the lesion was unilateral (reviewed by Nattie, 2000). Therefore, under anesthesia all the sites studied seemed to be interrelated and indispensable for the full expression of central chemoreception. However, in recent studies applying focal inhibition or cell specific lesions in unanesthetized animals, the degree of inhibition of the CO2 response was smaller, indicating rather independent and specific roles for the respective central chemoreceptor sites (Nattie and Li, 2009). For example, unilateral microdialysis of muscimol, a GABA-A receptor agonist, into the RTN in conscious rats resulted in a reduction of the systemic CO2 response to inhalation of 7% CO2 by ~20% (Nattie and Li, 2000). And cell-specific lesions of neurokinin-1 receptor expressing cells in the RTN, serotonergic cells in the MR, and of catecholaminergic cells in the locus coeruleus (A6) decreased the CO2 response by 15-30% during both sleep and wakefulness (reviewed by Nattie and Li, 2006). Thus, studied in the absence of anesthesia, each chemosensitive site seems able to contribute uniquely. There is also experimental evidence to indicate that sites can interact in a synergistic manner (Li et al., 2008). We examined in conscious rats the combined function of two central chemoreceptor sites, the MR and the RTN by focal inhibition using microdialysis. Inhibition of neurons within the RTN alone using muscimol, a GABA-A receptor agonist, decreased the ventilatory response to 7% CO2 by 24%. Inhibition of caudal MR serotonergic neurons alone with the 5-HT1A receptor agonist, 8-OH-DPAT, had no significant effect on the CO2 response. But simultaneous inhibition of both sites decreased the CO2 response by 51% indicating the presence of a remarkable synergy between these two central chemoreceptor sites.
Why are there so many central chemoreceptor sites? Multiple sites may play synergistic roles both as a fail-safe mechanism and as an amplification mechanism, which is likely not simply additive. A complementary hypothesis is that central chemoreceptor sites are components of neural circuits that exist for specific but different purposes, which share the property of CO2 sensitivity and ventilatory augmentation. An example of this would be in vigilance state-dependence as shown in Fig. 1 in which focal acidification of the RTN affected ventilation predominantly in wakefulness (Li and Nattie, 2002) while focal acidification of the rostral MR increased breathing predominantly in sleep (Nattie and Li, 2001).
2. Wakefulness/sleep-dependent central chemoreception
Basal ventilation and ventilatory reflex regulation from both central and peripheral (carotid body) chemoreceptors are considerably different during the awake and sleep states (Douglas, 2000; Krieger, 2000). Tidal volume is largest during awake periods, decreases by 20–30% during slow-wave sleep (SWS), and decreases further during rapid-eye-movement (REM) sleep. Respiratory frequency decreases during SWS and is lower than that during quiet wakefulness (QW); however, respiratory frequency does not decrease during REM sleep. Consequently, the rank order of minute ventilation is QW > SWS ≥ REM (Fig. 2). In addition, both the rhythm and amplitude of ventilation are extremely regular during SWS while in REM breathing is much less regular and the CO2 response virtually absent. Reduced metabolic demand during sleep cannot explain the diminished minute ventilation because arterial PCO2 increases slightly during sleep (Krieger, 2000). It is possible that sleep-related neuronal mechanisms actively interfere with the neural or chemical control of breathing, since minute ventilation decreases during sleep even in a hypercapnic environment (Fig. 2) (see also Fig. 2 in daSilva et al., 2010). During SWS, airway resistance markedly increases due to decreased tonus of the upper airway muscles, whereas decreases in the contraction of intercostal muscles and of the diaphragm are small (Krieger, 2000). Therefore, sleep affects the neurons regulating the upper airway and those controlling the thorax in different manners. This notion again supports the hypothesis that the decrease of ventilation during sleep is not a consequence of general depression in central nervous activities.
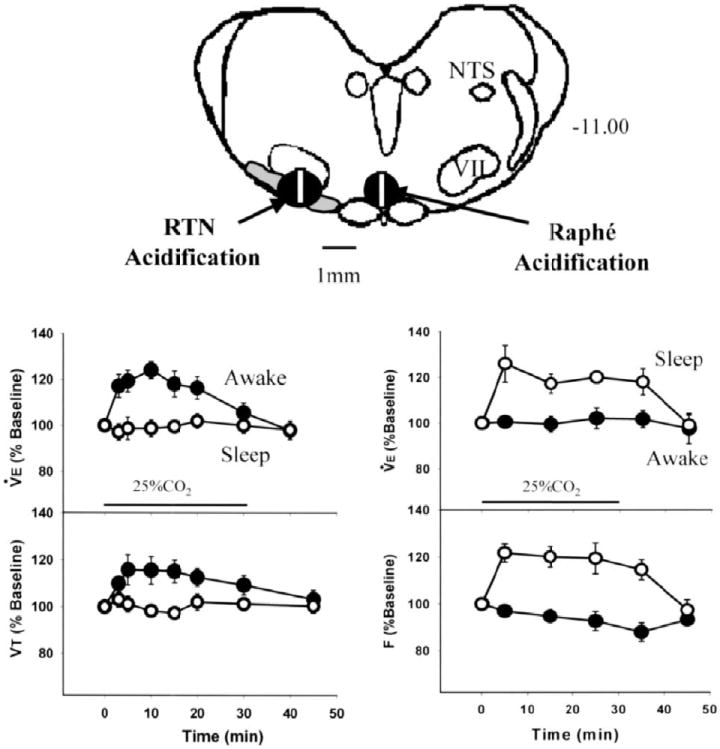
Figure 2. Relationship between vigilance states and minute ventilation under various gas conditions.
Note that vigilance-state-dependent changes in minute ventilation (QW > SWS ≥ REM) is well preserved under hypoxic and hypercapnic conditions. Data are expressed means ± SEM of 5 wild-type mice. * P < 0.05 compared with QW. (Adapted from Kuwaki, 2008)
Hypoxic and hypercapnic ventilatory responses are also vigilance-state dependent (QW > SWS > REM and refer to Fig. 3). The pulmonary stretch receptor reflex and irritant receptor reflex are also suppressed during sleep, and hence, cough develops only after arousal from sleep (Douglas, 2000). Although these phenomena are well known, the underlying mechanisms remain to be elucidated.
Figure 3. Vigilance-state-dependent changes in hypercapnic ventilatory responsiveness in wild-type (WT) mice and prepro-orexin knockout mice (ORX-KO).
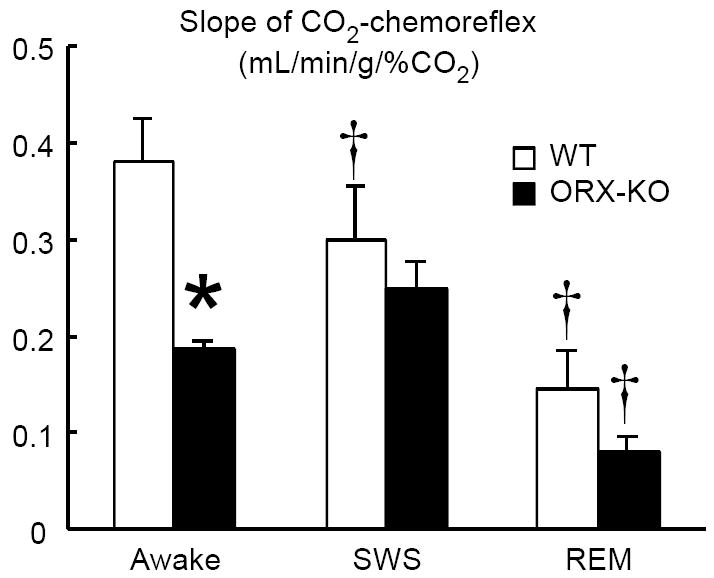
Hypercapnic responsiveness was evaluated by calculating the slope of the relationship between the inspired CO2 concentration (0-10%) and minute ventilation. Data are presented as means ± SEM of 5 WT mice and 5 ORX-KO mice. * P < 0.05 compared with WT mice. † P < 0.05 compared with the data during awake (ANOVA with repeated measures design). Abbreviations: SWS, slow-wave sleep; REM, rapid-eye-movement sleep. (Adapted from (Nakamura et al. 2007))
3. Orexin as an arousal-associated modulator for central chemoreception?
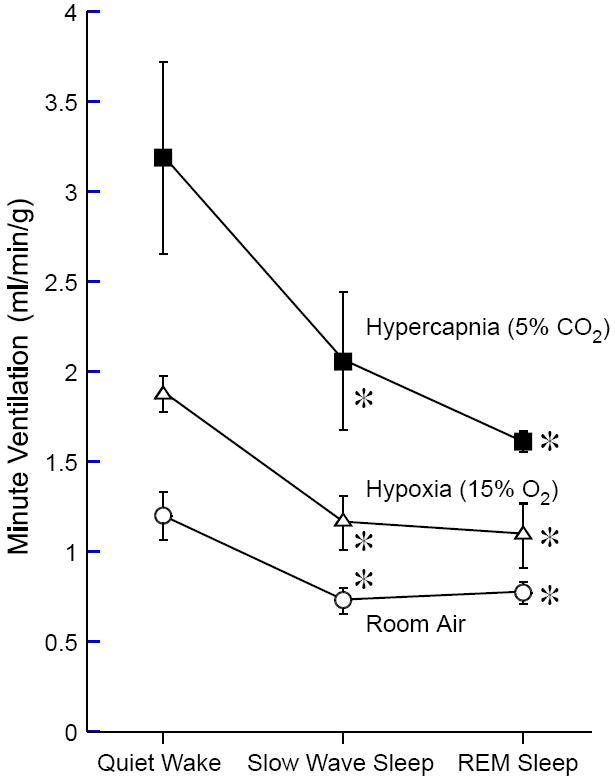
Orexins (orexin-A and orexin-B), also known as hypocretins (hypocretin-1 and hypocretin-2, respectively), were identified as ligands for a G-protein-coupled orphan receptor in 1998 (Sakurai et al., 1998). They are cleaved from a common precursor, prepro-orexin. The orexin-1 receptor (OX1R) has a 10-fold selectivity for orexin-A whereas the orexin-2 receptor (OX2R) binds to orexin-A and -B with equal affinity. In a different perspective, orexin-B has a 10-fold selectivity for OX2R because affinities of orexin-A to bind OX1R (IC50=20 nM) and OX2R (IC50=38 nM) are similar (Sakurai et al., 1998). The location of orexin-containing cell bodies is restricted to the lateral hypothalamus, perifornical area (PFA), and dorsomedial hypothalamus (DMH). Conversely, orexin-containing nerve terminals and receptors are widely distributed in the brain (Elias et al., 1998; Nambu et al., 1999; Marcus et al., 2001). This anatomic feature establishes the basis for the contributions by orexin to the control of multiple physiological functions, such as the control of energy homeostasis, feeding behavior, reward process, sleep-wake states, stress response, cardiovascular and respiratory control (Fig. 4) (Peyron et al., 1998; Saper et al., 2005; Zheng et al., 2005; Sakurai, 2007).
Figure 4. Pivotal role of orexin in connecting state-dependent behavioral regulation system and cardiorespiratory homeostatic reflex pathways.
Among known connections from/to orexinergic neurons in the hypothalamus, selected brain nuclei are depicted that are relevant to this review (thick lines). Many nuclei located at both of input (MR, RTN, LC, NTS) and output (cardiorespiratory motor neurons) interfaces in the homeostatic cardiorespiratory reflex pathway receive projections from orexinergic neurons (right half). Simultaneously, orexinergic connections are engaged in sleep/wake regulation and emotional stress-induced behavioral changes (left half). Thus, orexin can modulate cardiorespiratory homeostasis in a state-dependent manner. Arrows indicate a probable excitatory connection and circles indicate an inhibitory connection. Connections shown in thin lines are either direct or indirect. Abbreviations: AMG, amygdala; BNST, bed nucleus of the stria terminalis; DR, dorsal raphe; LC, locus coeruleus; MLR, medullary locomotor region; MR, medullary raphe; NTS, nucleus tractus solitarius; PAG, periaqueductal gray; PVN, paraventricular nucleus; RTN, retrotrapezoid nucleus; RVLM, rostral ventrolateral medulla where sympathetic premotor neurons are located; SCN, suprachiasmatic nucleus; TMN, tuberomammillary nucleus; VLPO, ventrolateral preoptic nucleus.
Orexins play a key role in the stabilization of wakefulness and are thought to be arousal promoting peptides (Carter et al., 2009). Diminished orexin function can result in a specific clinical syndrome, narcolepsy, in animals and humans (Chemelli et al., 1999; Thannickal et al., 2000). Orexin neurons have state-dependent activity (Lee et al., 2005; Mileykovskiy et al., 2005; Takahashi et al., 2008); orexin levels in cerebrospinal fluid of rats increase just before awakening, remain high during wakefulness (Desarnaud et al., 2004), increase much higher during exercise (Martins et al., 2004) and/or in heightened alertness (Kuwaki, 2008), decrease during the light, inactive period (Desarnaud et al., 2004) and in SWS as measured in the hypothalamus (Kiyashchenko et al., 2002). Orexin neurons receive dense projections from the suprachiasmatic nucleus, a circadian rhythm oscillator (Saper et al., 2005) as well as many other sites (see Fig. 4) and release of orexins into the extracellular fluid in the rat hypothalamus is larger in the dark, active period than in the light, resting period (Yoshida et al., 2001). At the efferent side (Fig. 4), orexin neurons provide excitatory inputs to nuclei that regulate arousal (Peyron et al., 1998; Espana et al., 2001; Sakurai, 2007), and are anatomically connected with neurons involved to the control of breathing (Fung et al., 2001; Young et al., 2005; Zhang et al., 2005; Rosin et al., 2006; Dutschmann et al., 2007; Nakamura et al., 2007; Kuwaki, 2008). A key aspect of orexin physiology is the marked circadian variation of orexin levels in cerebrospinal fluid (Desarnaud et al., 2004) and in hypothalamus (Yoshida et al., 2001) of rats. The circadian change in cerebrospinal fluid orexin is from ~750 pg/mL at the nadir to ~1400 pg/mL at the peak while in the hypothalamus it is from ~300 pg/mL at the nadir to ~ 550 pg/mL at the peak. These measured changes are large compared to those measured in the hypothalamus during a given phase of the circadian cyle comparing wakefulness to slow wave sleep, which change from ~1.4 fmol/20 μL in wakefulness to ~ 1.25 fmol/μL in slow wave sleep (Kiyashchenko et al., 2002). Thus the diurnal changes in orexin levels vary by ~ 2 fold while the changes in orexin levels between wakefulness and sleep within a given diurnal period are much smaller, ~ 11%. In contrast, orexin neuron firing rates do vary by vigilance state even within a circadian period (vida supra) and direct activation of orexin neurons via in vivo photostimulation of channelrhodopsin-2 elicits rapid sleep state transitions regardless of circadian period (Carter et al., 2009). We conclude that orexin must play an important role in circadian variations in cardio-respiratory control (Mortola, 2004; Stephenson, 2007) and that they also likely play a role in vigilance state cardio-respiratory control within circadian periods. One must consider both the vigilance state and the circadian period in the study of orexin function.
In respect to central chemoreception, orexin neurons are activated by CO2/pH in vitro (Williams et al., 2007) and in vivo as measured by c-fos activation (Sunanaga et al., 2009) and prepro-orexin knockout mice have a 50 % decrease in the ventilatory CO2 response measured during the light/inactive phase of the circadian cycle during wakefulness but not during sleep (Fig. 3), an effect that is reversible by administration of orexin-A and orexin-B (Deng et al., 2007). Administration via the cerebral ventricles of an OX1R-selective antagonist (SB-334867) decreased the respiratory chemoreflex by 24% in the wild-type mice also studied during the light/inactive phase of the circadian cycle (Deng et al., 2007).
At the RTN region, where there is evidence for OX1Rs, OX2Rs, and for activation by hypothalamic stimulation (Peyron et al., 1998; Marcus et al., 2001; Ciriello et al., 2003; Fortuna et al., 2009), unilateral microdialysis of OX1R antagonist (SB-334867) in rats during the light/inactive phase of the circadian cycle resulted in a 30% reduction of the ventilatory response to breathing 7% CO2 during wakefulness, while during SWS the inhibitory effect was only 9% (Dias et al., 2009). These results are well in accordance with the results in prepro-orexin knockout mice mentioned above. Thus, orexin contributes to a vigilance state-dependent control of ventilation, at least in a part, through the RTN as measured during the light/inactive phase of the circadian cycle. These effects of antagonism of OX1R in the RTN during wakefulness in the light/inactive phase of the circadian cycle may well be greater if studied during the dark/active phase of the circadian cycle when orexin levels are up to 2 fold higher.
We also examined the possible effect of microdialysing the OX1R antagonist (SB-334867) at the rostral MR, which receives projections from orexin-containing neurons (Peyron et al., 1998) and expresses both OX1R and OX2R (Marcus et al., 2001), during the dark, active and separately, during the light, inactive periods of imposed diurnal cycles (Dias et al., 2010). During wakefulness in the dark period, but not in the light period, OX1R antagonism caused a 16% reduction of the ventilatory response to 7% CO2 compared with vehicle. There was no significant effect in sleep. The fact that focal antagonism of OX1R in the MR was only effective in wakefulness in the dark period of the circadian cycle when orexin levels are high while it was dramatically effective at the RTN during wakefulness in the light period of the diurnal cycle when orexin levels are lower (it was not studied in the RTN in the dark period) suggests a site specific sensitivity to orexin during the circadian cyle with the RTN being much more sensitive. This interpretation requires more evidence but it suggests the hypothesis that different central chemoreceptor sites may vary in function not only by vigilance state but by circadian period.
During preparation of this manuscript, we noted a very recent report examining ventilatory chemoresponsiveness in a large number of narcolepsy-cataplexy patients (Han et al., 2010). Patients (n=130) had higher apnea hypopnea indices in a polysomnographic study and depressed hypoxic responsiveness but normal hypercapnic responsiveness in a daytime study. Possible reasons for the discrepancy in hypercapnic responsiveness between humans and rodents are as follows: 1) the inhalation period of CO2 in our rodent study was relatively long (20 min in rats and 6 hrs in mice) because of a need to collect data in both sleep and wake states while the human data were derived from an acute re-breathing protocol. Such a prolonged stimulus may exaggerate a subtle difference. 2) The animals received no cues as to when the inspired gas mixture was changed. On the other hand, patients would expect and prepare for CO2 in a rebreathing test. Such cognitive and emotional changes may mask possible differences in CO2 responsiveness in humans. 3) Given the diurnal cycle of orexin, high in awake, daytime in man (dark time in rat/mouse), it is possible that the remaining orexin in the patients is enough to activate/excite the chemoreceptor sites but not enough to protect motor tone when sleep state changes or to prevent loss of wakefulness. Although these human data seem not to support our hypothesis of orexin’s contribution to the hypercapnic chemoreflex, they do indicate that orexin seems to contribute to another type of ventilatory regulation; suppression of sleep apnea occurrence. Indeed, we have previously shown, in orexin deficient mice, exaggeration of sleep apnea occurrence (Nakamura et al., 2007) and attenuation of respiratory long term facilitation that was induced by intermittent hypoxia, an experimental model of repetitive sleep apnea (Terada et al., 2008; Toyama et al., 2009). The mechanism by which orexin suppresses sleep apnea is not clear. In that orexin provides an excitatory stimulus to the pre-Bötzinger complex and phrenic motoneurons (Young et al., 2005) and to central chemoreceptor sites, e.g., MR 5-HT neurons (Dias et al., 2010) and the RTN (Dias et al., 2009), its absence via genetic disruption of synthesis or by blockade of receptors could be viewed as removal of a necessary excitatory input during sleep states that promotes neural responses that prevent apnea.
4. State dependency beyond wakefulness/sleep states
In addition to vigilance state-dependent regulation of ventilation, we have also been interested in another type of “state”-dependent regulation, namely, emotional stress-dependent regulation of ventilation and circulation. Localization of orexinergic cell bodies in the PFA and the DMH prompted us to examine a possible role of orexin in the defense response against stressors because stimulation of them elicited behavioral “rage” along with the specific autonomic responses including ventilatory augmentation that was termed the “defense response” (also called “fight or flight response”) (Jordan, 1990; DiMicco et al., 2002).
By using prepro-orexin knockout mice and orexin neuron-ablated mice (Hara et al., 2001), we have several lines of evidence that support our hypothesis of a possible contribution of orexin to the defense response. First, disinhibition of the PFA with the GABA-A receptor antagonist, bicuculline, resulted in a greatly diminished defense response (increases in blood pressure, heart rate, and ventilation) in urethane-anesthetized orexin deficient mice as compared to the wild-type controls (Kayaba et al., 2003; Zhang et al., 2006). Other features of the defense response also attenuated include: a) the blood flow shift from visceral vasculature to the skeletal muscle, and b) the suppression of the baroreceptor reflex to “allow” a blood pressure greater than that in the resting condition. Second, an attenuation of the defense response (circulatory and behavioral responses) was also observed by natural stimulation (application of air jet to the animal’s nose and confrontation to a territorial intruder) in unanesthetized and freely moving animals. Foot shock stress-induced analgesia was also attenuated in the mutant mice (Watanabe et al., 2005). Unfortunately, we did not examine ventilatory parameters in awake animals with the stress paradigms. Finally, in anesthetized orexin neuron-ablated mice, disinhibition of the amygdala or the bed nucleus of the stria terminalis (BNST) by an injection of bicuculline caused little cardiorespiratory activation whereas activation was consistently observed in wild-type control mice (Zhang et al., 2009). From these results, we concluded that orexin-containing neurons in the PFA/DMH mediate at least a part of the amygdala- and BNST-induced cardiorespiratory responses and act as a master switch to activate multiple efferent pathways of the defense response.
Although vigilance state-dependent responses and emotional stress-dependent responses may appear to be independent, we assume that the common feature of these responses is a state-dependent and feedforward adjustment of central ventilatory and autonomic regulation to fit with a bodily demand associated with behavioral and metabolic changes. Animal arousal, or alertness, is minimal during sleep, increases during quiet wakefulness, and further increases during active wakefulness with activities such as exercise, stress, or panic. The level of this arousal activation by orexin in rodents will be greater in the dark, active period of the circadian cycle than in the light, inactive period. Apparently independent regulation of ventilation in a specific “state” seems to be a different facet of a single control system in which orexin play an important role. In line with this notion, orexin was recently shown to play a key role in cardiovascular and behavioral responses associated with panic attack both in animal models and in humans (Johnson et al., 2010).
5. Conclusion
Diversity in central chemoreception and the synaptic control of respiratory and cardiovascular output neurons appears to be necessary for animals to adapt themselves to constantly changing situations and behavioral states. Although further studies in both animals and humans are definitely indispensable, the orexin system is likely to function as one of the essential modulators for orchestrating the circuits that control autonomic functions and behavior.
Acknowledgments
Part of this work was supported by a Grant-in Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan and a grant from the NHLBI, R37 HL 28066.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berquin P, Bodineau L, Gros F, Larnicol N. Brainstem and hypothalamic areas involved in respiratory chemoreflexes: a Fos study in adult rats. Brain Res. 2000;857:30–40. doi: 10.1016/s0006-8993(99)02304-5. [DOI] [PubMed] [Google Scholar]
- Carter ME, Adamantidis A, Ohtsu H, Deisseroth K, de Lecea L. Sleep homeostasis modulates hypocretin-mediated sleep-to-wake transitions. J Neurosci. 2009;29:10939–10949. doi: 10.1523/JNEUROSCI.1205-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, Borg JS, de Lecea L. The brain hypocretins and their receptors: mediators of allostatic arousal. Curr Opin Pharm. 2009;9:39–45. doi: 10.1016/j.coph.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell TE, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Ciriello J, Li Z, de Oliveira CVR. Cardioacceleratory responses to hypocretin-1 injections into rostral ventromedial medulla. Brain Res. 2003;991:84–95. doi: 10.1016/j.brainres.2003.08.008. [DOI] [PubMed] [Google Scholar]
- daSilva GS, Li A, Nattie E. High CO2/H+ dialysis in the caudal ventrolateral medulla (Loeschcke’s area) increases ventilation in wakefulness. Respir Physiol Neurobiol. 2010 doi: 10.1016/j.resp.2010.01.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng B-S, Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T. Contribution of orexin in hypercapnic chemoreflex: Evidence from genetic and pharmacological disruption and supplementation studies in mice. J Appl Physiol. 2007;103:1772–1779. doi: 10.1152/japplphysiol.00075.2007. [DOI] [PubMed] [Google Scholar]
- Desarnaud F, Murillo-Rodriguez E, Lin L, Xu M, Gerashchenko D, Shiromani SN, Nishino S, Mignot E, Shiromani PJ. The diurnal rhythm of hypocretin in young and old F344 rats. Sleep. 2004;27:851–856. doi: 10.1093/sleep/27.5.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias MB, Li A, Nattie EE. Focal CO2 dialysis in raphe obscurus does not stimulate ventilation but enhances the response to focal CO2 dialysis in the retrotrapezoid nucleus. J Appl Physiol. 2008:83–90. doi: 10.1152/japplphysiol.00120.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias MB, Li A, Nattie EE. Antagonism of orexin receptor-1 in the retrotrapezoid nucleus inhibits the ventilatory response to hypercapnia predominantly in wakefulness. J Physiol. 2009;587:2059–2067. doi: 10.1113/jphysiol.2008.168260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias MB, Li A, Nattie EE. The orexin receptor-1 (OX1R) in the rostral medullary raphe contributes to the hypercapnic chemoreflex in wakefulness, during the aactive period of the diurnal cycle. Respir Physiol Neurobiol. 2010;170:96–102. doi: 10.1016/j.resp.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMicco JA, Samuels BC, Zaretskaia MV, Zaretsky DV. The dorsomedial hypothalamus and the response to stress: part renaissance, part revolution. Pharmacol Biochem Behav. 2002;71:469–480. doi: 10.1016/s0091-3057(01)00689-x. [DOI] [PubMed] [Google Scholar]
- Douglas NJ. Respiratory physiology: Control of ventilation. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. W.B. Saunders; Philadelphia: 2000. pp. 221–228. [Google Scholar]
- Dubreuil V, Barhanin J, Goridis C, Brunet JF. Breathing with Phox2b. Phil Trans R Soc B. 2009;364:2477–2483. doi: 10.1098/rstb.2009.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutschmann M, Kron M, Mörschel M, Gestreau C. Activation of Orexin B receptors in the pontine Kölliker-Fuse nucleus modulates pre-inspiratory hypoglossal motor activity in rat. Respir Physiol Neurobiol. 2007;159:232–235. doi: 10.1016/j.resp.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Elias CF, Saper CB, Maratos-Flier E, Tritos NA, Lee C, Kelly J, Tatro JB, Hoffman GE, Ollmann MM, Barsh GS, Sakurai T, Yanagisawa M, Elmquist JK. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol. 1998;402:442–459. [PubMed] [Google Scholar]
- Erlichman JS, Boyer AC, Reagan P, Putnam RW, Ritucci NA, Leiter JC. Chemosensory responses to CO2 in multiple brain stem nuclei determined using a voltage-sensitive dye in brain slices from rats. J Neurophysiol. 2009;102:1577–1590. doi: 10.1152/jn.00381.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana R, Baldo B, Kelley A, Berridge C. Wake-promoting and sleep-suppressing actions of hypocretin (orexin): basal forebrain sites of action. Neuroscience. 2001;106:699–715. doi: 10.1016/s0306-4522(01)00319-0. [DOI] [PubMed] [Google Scholar]
- Fortuna MG, Stornetta RL, H West G, Guyenet PG. Activation of the retrotrapezoid nucleus by posterior hypothalamic stimulation. J Physiol. 2009;587:5121–5138. doi: 10.1113/jphysiol.2009.176875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung SJ, Yamuy J, Sampogna S, Morales FR, Chase MH. Hypocretin (orexin) input to trigeminal and hypoglossal motoneurons in the cat: a double-labeling immunohistochemical study. Brain Res. 2001;903:257–262. doi: 10.1016/s0006-8993(01)02318-6. [DOI] [PubMed] [Google Scholar]
- Han F, Mignot E, Wei YC, Dong SX, Li J, Lin L, An P, Wang LH, Wang JS, He MZ, Gao HY, Li M, Cao ZC, Strohl KP. Ventilatory chemosensiveness, narcolepsy-cataplexy, and HLA-DQB1*0602 status. Eur Resp J. 2010 doi: 10.1183/09031936.00174609. in press. [DOI] [PubMed] [Google Scholar]
- Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, Sakurai T. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Klum L, Leekley T, Brozoski DT, Bastasic J, Davis S, Wenninger JM, Feroah TR, Pan LG, Forster HV. Effects on breathing in awake and sleeping goats of focal acidosis in the medullary raphe. J Appl Physiol. 2004a;96:1815–1824. doi: 10.1152/japplphysiol.00992.2003. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Martino P, Davis S, Opansky C, Pan LG, Forster HV. Effects on breathing of focal acidosis at multiple medullary raphe sites in awake goats. J Appl Physiol. 2004b;97:2303–2309. doi: 10.1152/japplphysiol.00645.2004. [DOI] [PubMed] [Google Scholar]
- Jiang C, Rojas A, Wang R, Wang X. CO2 central chemosensitivity: why are there so many sensing molecules? Respir Physiol Neurobiol. 2005;145:115–126. doi: 10.1016/j.resp.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, Träskman-Bendz L, Goddard AW, Brundin L, Shekhar A. A key role for orexin in panic anxiety. Nature Med. 2010;16:111–115. doi: 10.1038/nm.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan D. Autonomic changes in affective behavior. In: Loewy AD, Spyer KM, editors. Central Regulation of Autonomic Functions. Oxford University Press; New York: 1990. pp. 349–366. [Google Scholar]
- Kayaba Y, Nakamura A, Kasuya Y, Ohuchi T, Yanagisawa M, Komuro I, Fukuda Y, Kuwaki T. Attenuated defense response and low basal blood pressure in orexin knockout mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R581–R593. doi: 10.1152/ajpregu.00671.2002. [DOI] [PubMed] [Google Scholar]
- Kiyashchenko LI, Mileykovskiy BY, Maidment N, Lam HA, Wu M, John J, Peever J, Siegel JM. Release of hypocretin (orexin) during waking and sleep states. J Neurosci. 2002;22:5282–5286. doi: 10.1523/JNEUROSCI.22-13-05282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause KL, Forster HV, Davis SE, Kiner T, Bonis JM, Pan LG, Qian B. Focal acidosis in the pre-Botzinger complex area of awake goats induces a mild tachypnea. J Appl Physiol. 2009;106:241–250. doi: 10.1152/japplphysiol.90547.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger J. Respiratory physiology: Breathing in normal subjects. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. W.B. Saunders; Philadelphia: 2000. pp. 229–241. [Google Scholar]
- Kuwaki T. Orexinergic modulation of breathing across vigilance states. Respir Physiol Neurobiol. 2008;164:204–212. doi: 10.1016/j.resp.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Lee M, Hassani O, Jones B. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Nattie E. CO2 dialysis in one chmoreceptor site, the RTN: stimulus intensity and sensitivity in the awake rat. Respir Physiol Neurobiol. 2002;133:11–22. doi: 10.1016/s1569-9048(02)00134-9. [DOI] [PubMed] [Google Scholar]
- Li A, Randall M, Nattie EE. CO2 microdialysis in retrotrapezoid nucleus of the rat increases breathing in wakefulness but not in sleep. J Appl Physiol. 1999;87:910–919. doi: 10.1152/jappl.1999.87.3.910. [DOI] [PubMed] [Google Scholar]
- Li A, Zhou S, Nattie E. Simultaneous inhibition of caudal medullary raphe and retrotrapezoid nucleus decreases breathing and the CO2 response in conscious rat. J Physiol. 2006;577:307–318. doi: 10.1113/jphysiol.2006.114504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- Martino PF, Davis S, Opansky C, Krause K, Bonis JM, Pan LG, Qian B, Forster HV. The cerebellar fastigial nucleus contributes to CO2-H+ ventilatory sensitivity in awake goats. Respir Physiol Neurobiol. 2007;157:242–251. doi: 10.1016/j.resp.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins PJ, D’Almeida V, Pedrazzoli M, Lin L, Mignot E, Tufik S. Increased hypocretin-1 (orexin-a) levels in cerebrospinal fluid of rats after short-term forced activity. Regul Pept. 2004;117:155–158. doi: 10.1016/j.regpep.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Mileykovskiy B, Kiyashchenko L, Siegel J. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortola JP. Breathing around the clock: an overview of the circadian pattern of respiration. Eur J Appl Physiol. 2004;91:119–129. doi: 10.1007/s00421-003-0978-0. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T. Vigilance state-dependent attenuation of hypercapnic chemoreflex and exaggerated sleep apnea in orexin knockout mice. J Appl Physiol. 2007;102:241–248. doi: 10.1152/japplphysiol.00679.2006. [DOI] [PubMed] [Google Scholar]
- Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999;827:243–260. doi: 10.1016/s0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- Nattie E. Multiple sites for central chemoreception: their roles in response sensitivity and in sleep and wakefulness. Respir Physiol. 2000;122:223–235. doi: 10.1016/s0034-5687(00)00161-4. [DOI] [PubMed] [Google Scholar]
- Nattie EE. Central chemosensitivity, sleep, and wakefulness. Respir Physiol. 2001;129:257–268. doi: 10.1016/s0034-5687(01)00295-x. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. Muscimol dialysis in the retrotrapezoid nucleus ragion inhibits breathing in the awake rat. J Appl Physiol. 2000;89:153–162. doi: 10.1152/jappl.2000.89.1.153. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. CO2 dialysis in the medullary raphe of the rat increases ventilation in sleep. J Appl Physiol. 2001;90:1247–1257. doi: 10.1152/jappl.2001.90.4.1247. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. CO2 dialysis in nucleus tractus solitarius region of rat increases ventilation in sleep and wakefulness. J Appl Physiol. 2002;92:2119–2130. doi: 10.1152/japplphysiol.01128.2001. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. Central chemorecption 2005: A brief review. Autonom Neurosci. 2006;126:332–338. doi: 10.1016/j.autneu.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Nattie E, Li A. Central chemoreception is a complex system function that involves multiple brain stem sites. J Appl Physiol. 2009;106:1464–1466. doi: 10.1152/japplphysiol.00112.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Chen Z, Jiang W, Kuwana S, Eldridge FL. Anatomical arrangement of hypercapnia-activated cells in the superficial ventral medulla of rata. J Appl Physiol. 2002;93:427–439. doi: 10.1152/japplphysiol.00620.2000. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Ikeda K, Kawakami K. CO2-sensitive preinspiratory neurons of the parafacial respiratory group express Phox2b in the neonatal rat. J Neurosci. 2008;28:12845–12850. doi: 10.1523/JNEUROSCI.3625-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol. 2006;499:64–89. doi: 10.1002/cne.21105. [DOI] [PubMed] [Google Scholar]
- Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- Sato M, Severinghaus JW, Basbaum AI. Medullary CO2 chemoreceptor neuron identification by c-fos immunocytochemistry. J Appl Physiol. 1992;73:96–100. doi: 10.1152/jappl.1992.73.1.96. [DOI] [PubMed] [Google Scholar]
- Stephenson R. Circadian rhythms and sleep-related breathing disorders. Sleep Med. 2007;8:681–687. doi: 10.1016/j.sleep.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Sunanaga J, Deng BS, Zhang W, Kanmura Y, Kuwaki T. CO2 activates orexin-containing neurons in mice. Respir Physiol Neurobiol. 2009;166:184–186. doi: 10.1016/j.resp.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Lin JS, Sakai K. Neuronal activity of orexin and non-orexin waking-active neurons during wake–sleep states in the mouse. Neuroscience. 2008;153:860–870. doi: 10.1016/j.neuroscience.2008.02.058. [DOI] [PubMed] [Google Scholar]
- Tenney SM, Ou LC. Ventilatory response of decorticate and decerebrate cats to hypoxia and CO2. Respir Physiol. 1977;29:81–92. doi: 10.1016/0034-5687(77)90119-0. [DOI] [PubMed] [Google Scholar]
- Terada J, Nakamura A, Zhang W, Yanagisawa M, Kuriyama T, Fukuda Y, Kuwaki T. Ventilatory long-term facilitation in mice can be observed both during sleep and wake periods and depends on orexin. J Appl Physiol. 2008;104:499–507. doi: 10.1152/japplphysiol.00919.2007. [DOI] [PubMed] [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama S, Sakurai T, Tatsumi K, Kuwaki T. Attenuated phrenic long-term facilitation in orexin neuron-ablated mice. Respir Physiol Neurobiol. 2009;168:295–302. doi: 10.1016/j.resp.2009.07.025. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Kuwaki T, Yanagisawa M, Fukuda Y, Shimoyama M. Persistent pain and stress activate pain-inhibitory orexin pathways. Neuroreport. 2005;16:5–8. doi: 10.1097/00001756-200501190-00002. [DOI] [PubMed] [Google Scholar]
- Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D. Control of hypothalamic orexin neurons by acid and CO2. Proc Natl Acad Sci USA. 2007;104:10685–10690. doi: 10.1073/pnas.0702676104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y, Fujiki N, Nakajima T, Ripley B, Matsumura H, Yoneda H, Mignot E, Nishino S. Fluctuation of extracellular hypocretin-1 (orexin A) levels in the rat in relation to the light–dark cycle and sleep–wake activities. Eur J Neurosci. 2001;14:1075–1081. doi: 10.1046/j.0953-816x.2001.01725.x. [DOI] [PubMed] [Google Scholar]
- Young JK, Wu M, Manaye KF, Kc P, Allard JS, Mack SO, Haxhiu MA. Orexin stimulates breathing via medullary and spinal pathways. J Appl Physiol. 2005;98:1387–1395. doi: 10.1152/japplphysiol.00914.2004. [DOI] [PubMed] [Google Scholar]
- Zhang W, Fukuda Y, Kuwaki T. Respiratory and cardiovascular actions of orexin-A in mice. Neurosci Lett. 2005;385:131–136. doi: 10.1016/j.neulet.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Zhang W, Sakurai T, Fukuda Y, Kuwaki T. Orexin neuron-mediated skeletal muscle vasodilation and shift of baroreflex during defense response in mice. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1654–R1663. doi: 10.1152/ajpregu.00704.2005. [DOI] [PubMed] [Google Scholar]
- Zhang W, Zhang N, Sakurai T, Kuwaki T. Orexin neurons in the hypothalamus mediate cardiorespiratory responses induced by disinhibition of the amygdala and bed nucleus of the stria terminalis. Brain Res. 2009;1262:25–37. doi: 10.1016/j.brainres.2009.01.022. [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Berthoud H-R. Orexin-A projections to the caudal medulla and orexin-induced c-Fos expression, food intake, and autonomic function. J Comp Neurol. 2005;485:127–142. doi: 10.1002/cne.20515. [DOI] [PubMed] [Google Scholar]
- Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, Lynch CM, Faraci FM, Howard MA, III, Welsh MJ, Wemmie JA. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139:1012–1021. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]