Hepatic allografts are unusually resistant to hyper-acute rejection, and they function well despite the presence of antidonor lymphocytotoxic antibody (positive crossmatch) with very few exceptions.1–6 We have previously reported that 1- and 2-year hepatic graft survivals were not adversely affected by the lymphocytotoxic antibody and that a positive crossmatch should not be considered as a contraindication for hepatic allotransplantation.4,5 It is true, however, that crossmatch-positive hepatic grafts have been lost repeatedly for inadequately explained reasons at our center as well as at other centers.7–10
The improvement in organ preservation, provided by the University of Wisconsin (UW) solution, might have nearly eliminated the intrinsic graft-related causes of loss from the analysis.11–13
At our center the incidence of so-called primary nonfunction of liver graft used to be between 3.5% and 7.4% with Euro-Collins solution,5–11 but it has been less than 2.0% with UW solution for the last 3 years.11 The incidence of early hepatic retransplantation has also decreased from 20% to 11.3%.11 Thus, we decided to examine, for the third time, the effect of antidonor lymphocytotoxic antibody upon graft survival.
CASE MATERIALS AND METHODS
Cyclosporine (CyA) Group
During the 2-year period between October 1987 and September 1989, 631 adult patients (18 yean old or older) received their first orthotopic liver transplantation under CyA-steroid therapy at the University Health Center of Pittsburgh. Three of the 631 patients received ABO blood group incompatible grafts and 28 others were not tested for lymphocytotoxic antibody against a specific donor. These 31 patients were excluded from this study. Thus, the patients of the CyA group consisted of a total of 600 first-graft recipients.
FK 506 Group
During the 9-month period between August 1989 and April 1990, 166 adult patients received primary hepatic allografts under FK 506 and low-dose steroid therapy. Eleven patients were excluded from the study because the crossmatch test was not performed. There was no ABO blood group incompatible transplant in this group. Thus, the FK S06 group consisted of 155 adult patients (18 years old or older).
Crossmatch Test
The recipients sera obtained immediately before liver transplantation were tested for cytotoxic antibody against donor lymphocytes (unfractionated) at room temperature or 37°C by trypan blue dye exclusion, with a 30-minute incubation with serum and 60-minute incubation with complement. The crossmatch test was interpreted as positive (+) when more than 30% of lymphocytes were killed. and it was considered negative (−) when less than 10% of cells were killed. When 10% to 30% of donor lymphocytes were killed by recipient serum, the crossmatch was interpreted as weakly positive (±).
Organ Preservation
All of the liver allografts in this study were preserved with the UW solution,13 and not with Euro-Collins solution which was used in our previous reports.4,5
Graft Survival, Follow-Up, and Statistics
Hepatic allograft was considered “lost” when the recipient died with or without good graft function or when the graft was replaced by another because of poor or nonfunction. The survival rates were calculated by the life table method of Kaplan-Meier. The results were summarized as of June 30, 1990. with a minimum follow-up of 9 months in the CyA group and of 2 months in the FK 506 group.
Statistical comparisons were made by the method of Breslow, by student t test and by chi-square test. The difference was considered statistically significant when P < .05.
RESULTS
Incidence of Positive Crossmatch
Of the 600 patients in the CyA group, the cross match was positive (greater than 30% killing) against the first hepatic allograft donors in 72 (12.0%) recipients, weakly positive (10% to 30% killing) in 75 (12.5%) recipients, and negative (less than 10% killing) in 453 (75.5%) recipients.
In the FK 506 group, the crossmatch was positive against the first hepatic donor in 21 (13.5%) of the 155 recipients, weakly positive in 12 (7.8%) recipients, and negative in 122 (78.7%) recipients. The incidence of positive crossmatch for the first hepatic allograft was similar between the CyA group and the FK 506 group.
Graft Survival
The survival of first hepatic allografts under CyA-steroid therapy was compared among the crossmatch-positive grafts, the weakly positive (10% to 30% killing) grafts, and the negative grafts listed in Table 1. There was no statistically significant difference in the survival curves among the three groups of grafts (P = .07 by Breslow method). However, 1-month and 3-month survival rates of the crossmatch positive grafts were significantly lower than those of the negative grafts and those of the weakly positive grafts (by Student’s t test). At 6 months, the survival rate of the crossmatch-positive graft was still significantly lower than that of the crossmatch-negative graft (Table 1).
Table 1.
Actuarial Graft Survival in the CyA Group
| % Graft Survival (Mean ± SE) |
|||||
|---|---|---|---|---|---|
| 1 Month | 3 Months | 6 Months | 1Year | 2 Years | |
| crossmatch | |||||
| Positive (n = 72) | 70.8 ± 5.4 (n = 49) | 66.7 ± 5.6 (n = 46) | 62.5 ± 5.7 (n = 44) | 59.7 ± 5.8 (n = 22) | 54.9 ± 6.0 (n = 15) |
| Weak positive (n = 75) | 84.0 ± 4.2 (n = 63) | 81.3 ± 4.5 (n = 58) | 76.0 ± 4.9 (n = 54) | 73.2 ± 5.1 (n = 25) | 69.9 ± 5.4 (n = 21) |
| Negative (n = 453) | 83.9 ± 1.7 (n = 378) | 78.4 ± 1.9 (n = 353) | 74.2 ± 2.1 (n=311) | 69.9 ± 2.2 (n = 139) | 66.8 ± 2.3 (n = 83) |
| Total (n = 600) | 82.2 ± 1.6 (n = 490) | 77.3 ± 1.7 (n = 457) | 72.9 ± 1.8 (n = 409) | 69.1 ± 1.9 (n = 186) | 65.7 ± 2.0 (n=119) |
p < .05; 70.8 ± 5.4 VS 84.0 ± 4.2; 70.8 ± 5.4 VS 83.9 ± 1.7; 66.7 ± 5.6 VS 78.4 ± 1.9; 66.7 ± 5.6 VS 81.3 ± 4.5; 62.5 ± 5.7 VS 74.2 ± 2.1.
One-, 3-, and 6-month survival rates of primary hepatic allografts under FK 506 and low-dose steroid therapy are shown in Table 2. The survivals of the crossmatch-positive grafts were lower than those of the negative grafts and those of the weakly positive grafts, but the differences were not statistically significant.
Table 2.
Actuarial Graft Survival In FK 508 Group
| % Graft Survival (Mean ± SE) |
|||
|---|---|---|---|
| 1 Month | 3 Months | 6 Months | |
| Crossmatch | |||
| Positive (n = 21) | 75.0 ± 10.0 (n = 16) | 69.4 ± 10.0 (n = 14) | 69.4 ± 10.0 (n = 6) |
| Weak positive (n = 12) | 91.7 ± 8.0 (n = 11) | 82.9 ± 11.0 (n = 9) | 622 ± 15.0 (n = 5) |
| Negative (n = 122) | 91.7 ± 3.0 (n = 111) | 87.5 ± 3.0 (n = 96) | 86.4 ± 3.0 (n = 51) |
| Total (n = 155) | 89.5 ± 2.0 (n = 138) | 84.8 ± 3.0 (n =119) | 82.3 ± 3.0 (n = 62) |
Main Causes of Graft Loss
Liver grafts were often lost to multiple causes and it was difficult to select a single main cause of graft loss. However, the main cause of graft loss was categorized to the best of our knowledge in the CyA group as shown in Table 3.
Table 3.
Main Cause of Graft Loss in the CyA Group
| 0–1 Month |
1–3 Months |
3–6 Months |
After 6 Months |
Total |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crossmatch (%) | CP | WP | N | CP | WP | N | CP | WP | N | CP | WP | N | CP | WP | N |
| Primary nonfunction | 7 (9.7) | 3 (4.0) | 29 (6.4) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 (9.7) | 3(4.0) | 29 (6.4) |
| Technical failure | 5(6.9) | 6(8.0) | 22 (4.9) | 1 (2.0) | 0 | 4 (1.1) | 0 | 0 | 2(0.5) | 0 | 1 (1.9) | 4 (1.2) | 6 (8.3) | 7(9.3) | 32 (7.1) |
| Rejection | 6(8.3) | 1 (1.3) | 8 (1.8) | 1 (2.0) | 2 (3.2) | 3 (0.8) | 2 (4.3) | 0 | 3 (0.8) | 0 | 0 | 6 (1.8) | 9 (12.5) | 3 (4.0) | 20(4.4) |
| Infection | 1 (1.4) | 1 (1.3) | 4 (0.9) | 1 (2.0) | 0 | 9 (2.4) | 0 | 3 (5.2) | 1 (0.8) | 1 (2.3) | 1 (1.9) | 2 (0.6) | 3 (4.2) | 5 (6.7) | 16 (3.5) |
| Others | 4 (5.6) | 1 (1.3) | 11 (2.4) | 0 | 0 | 9 (2.4) | 1 (2.2) | 1 (1.7) | 11(3.1) | 2 (4.7) | 1 (1.9) | 10 (3.0) | 7 (9.7) | 3 (4.0) | 41 (9.1) |
| Undetermined | 0 | 0 | 1 (0.2) | 0 | 0 | 0 | 0 | 1 (1.7) | 1 (0.3) | 0 | 0 | 1 (0.3) | 0 | 1 (1.3) | 3 (0.7) |
| Total graft loss | 23 (31.9) | 12 (16.0) | 75 (16.6) | 3 (6.1) | 2 (3.2) | 25(6.6) | 3 (6.5) | 5 (B.6) | 18 (5.1) | 3 (7.0) | 3 (5.7) | 23 (6.9) | 32 (4) | 22 (29.3) | 141 (31.1) |
| Total grafts | 72 | 75 | 453 | 49 | 63 | 378 | 46 | 58 | 353 | 43 | 53 | 335 | 72 | 75 | 453 |
Abbrivation: CP, Crossmatch positive; WP, weak positive; N. negative, P < .05: 6 (8.3) vs 8 (1.8); 9 (12.5) VS 20 (4.4).
Rejection was the most common cause of primary liver allograft failure when the crossmatch was positive. Actually within 1 month after transplantation, 6 (8.3%) of the 72 crossmatch-positive grafts were lost to rejection, but only 1 (1.3%) of the 75 crossmatch weakly positive grafts and only 8 (1.8%) of the 453 crossmatch-negative grafts were lost to the same cause. During the entire observation period, 9 (12.5%) of the 72 crossmatch-positive grafts and 20 (4.4%) of the 453 crossmatch-negative grafts were lost to rejection. The incidence of graft loss to rejection was significantly higher in the crossmatch-positive group than in the negative group (P < .05), and it was most remarkable during the first month as shown in Table 3 and Figure 1. There was no difference in the incidence of graft loss to other various causes among the three groups (Table 3).
Fig 1.
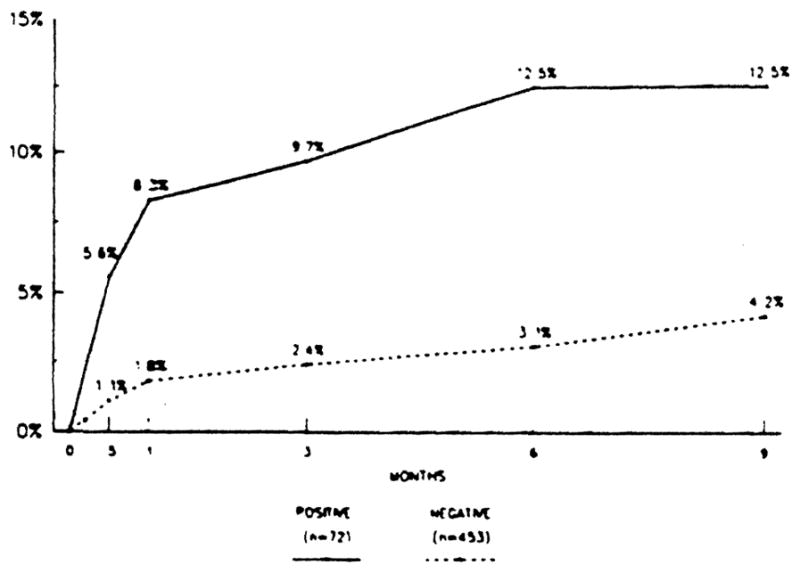
The cumulative incidence of graft loss to rejection was compared between the crossmatch-positive grafts and the negative grafts. The incidence in the former was significantly higher than in the latter for the first 9 months after liver transplantation (P < .05).
DISCUSSION
This study demonstrated for the first time that anti donor lymphocytotoxic antibody (positive crossmatch) adversely affected the survivals of primary hepatic allografts during the first 6 months after transplantation (Table 1). The reason appears to be immunological, as the grafts were lost to rejection significantly more often in positive crossmatch than in negative crossmatch transplant (Table 2 and Figure 1).
Our previous reports4,5 had failed to show any significant effect of positive crossmatch upon the hepatic graft survivals. The graft survivals were essentially equal between the crossmatch-positive group and the negative group. In our early report, the primary graft survival rate was only 50% at 1 year, and the mortality related to operation or graft loss due to technical problems were 17% without the use of veno-venous bypass. In our last report,5 the primary graft survival rate increased to 63% at 1 year with the routine use of vena-venous bypass. In both reports, the liver grafts were preserved with Euro-Collins solution.
In the present study, the liver grafts were preserved with UW solution, the primary graft survival further increased to 70% at 1 year, and the operative mortality and graft loss due to technical problems decreased to 8%. The rate of graft primary nonfunction has decreased to less than 2% with UW solution, and the rate of early retransplantation has been reduced to 11.3% in recent years.11
Although the basic immunosuppressive therapy in these studies was the combination of CyA and steroid, supplemented by ALG, and/or azathioprine, the rate of graft loss to rejection and to infectious death decreased from 13% and 9% to 5.4% and 4%, respectively. Only after these significant advances in surgical techniques, organ preservation, and perioperative patient care, including the treatment of infection and rejection, were the adverse effects of positive crossmatch finally disclosed in the hepatic transplantation.
The method of antidonor lymphocytotoxic antibody assay (crossmatch test) used in this study is primitive, but it has been a practical and dependable test in clinical transplantation over a long period of time. Even with this rather primitive crossmatch test, this study finally demonstrated the significant deterious effects of cytotoxic antibody upon early hepatic allograft survivals. Since November 1989, we have adapted antidonor T-lymphocytotoxic antibody assay with dithiothreitol (DTT) treatment of recipient sera at 37°C (T-warm crossmatch with DTT treatment).14–16 Although the results are still preliminary, the presence of high titer antibody with this new method of assay appears to predict severe early hepatic graft malfunction. Timely treatment of this severe immunological insult with a bolus of steroid injection and/or OKT3 usually avoids the early retransplantation of the liver.
Antidonor lymphocytotoxic antibody assay has become an important laboratory lest for immunosuppressive therapy in the earliest posttransplant period, if not yet for the selection of the hepatic recipient.
Acknowledgments
Supported by research grants from the Veterans Administration and project grant no. OK 29961 from the National Institutes of Health. Bethesda. Maryland.
References
- 1.Starzl TE, Ishikawa M, Putnum CW, et al. Transplant Proc. 1974;6:129. [PMC free article] [PubMed] [Google Scholar]
- 2.Iwatsuki S, Iwaki Y, Kano T, et al. Transplant Proc. 1981;13:286. [PMC free article] [PubMed] [Google Scholar]
- 3.Starzl TE, Iwatsuki S, Van Thiel DH, et al. Hepatology. 1982;2:614. [Google Scholar]
- 4.Iwatsuki S, Rabin BS, Shaw BW, Jr, et al. Transplant Proc. 1984;16:1427. [PMC free article] [PubMed] [Google Scholar]
- 5.Gordon RD, Fung J, Markcus B, et al. Surgery. 1986;100:705. [PMC free article] [PubMed] [Google Scholar]
- 6.Moore SB, Wiesner RH, Perkins JD, et al. Transplant Proc. 1987;19:2390. [PubMed] [Google Scholar]
- 7.Knechtle SJ, Kolbeck PC, Tsuchimoto S, et al. Transplantation. 1987;43:8. doi: 10.1097/00007890-198701000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Hanto DW, Snober DC, Sibley RK, et al. Clin Transplantation. 1987;1:304. [Google Scholar]
- 9.Bird G, Friend P, Donaldson P, et al. Transplant Proc. 1989;21:3742. [PubMed] [Google Scholar]
- 10.Starzl TE, Demetris AJ, Todo S, et al. Clin Transplantation. 1989;3:37. [PMC free article] [PubMed] [Google Scholar]
- 11.Todo S, Nery J, Yanaga K, et al. JAMA. 1989;261:711. [PMC free article] [PubMed] [Google Scholar]
- 12.D’Alessandro AM, Kalayoglu M, Sollinger HW, et al. Transplant Proc. 1990;22:474. [PubMed] [Google Scholar]
- 13.Jamieson NV, Sundberg R, Lindell S, et al. Transplant Proc. 1988;20:945. [PubMed] [Google Scholar]
- 14.Okuno T, Kondelis N. J Clin Pathol. 1978;31:1152. doi: 10.1136/jcp.31.12.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chapman JR, Taylor CJ, Ting A, et al. Transplantation. 1986;42:608. doi: 10.1097/00007890-198612000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Iwaki Y, Lau M, Terasaki PI. Clin Transplantation. 1988;2:81. [Google Scholar]


