Abstract
FK506 and cyclosporine were used for the prevention of acute graft-versus-host disease. Acute GVHD was induced in Lewis rats by total-body irradiation and subsequent reconstitution with allogeneic (ACI) bone marrow and spleen cells (BMTx). GVHD was assessed by both clinical and histologic parameters during the experiment duration of 60 days, and longer for selected animals. All untreated BM recipients died within 26 days from severe acute GVHD. GVHD was prevented with CsA during the period of immunosuppressive therapy, but it appeared within a few days afterward. FK506-treated BM recipients were also protected, but they had a markedly prolonged GVHD-free period after therapy was discontinued. Most such animals eventually developed GVHD but with notable exceptions. Maintenance therapy with doses of FK506 as low as 0.1 mg/kg every other day (1/20 of daily induction dose) was infallible insurance against delayed GVHD. The relevance of these findings to GVHD caused by lymphoid-containing solid organs such as the intestine was discussed.
Graft-versus-host disease is a systemic manifestation of alloactivation of transplanted lymphoid cells to recipient alloantigens (1, 2). Cutaneous, gastrointestinal, pulmonary, and hepatic manifestations of GVHD can be subdivided into acute and chronic forms, which represent a continuum of immune-regulated injury. Various immunosuppressive strategies have been used to prevent or treat GVHD in humans, but none are completely satisfactory.
We have evaluated the new immunosuppressive drug, FK506 (3), as well as cyclosporine, for the prevention of acute GVHD after transplantation of fully allogeneic bone marrow plus spleen cells in a rat model (BMTx). The ACI to Lewis donor/recipient strain combination is fully MHC and non-MHC disparate, and this technique of lymphoid transplantation results in uniform mortality from GVHD in untreated recipients.
MATERIALS AND METHODS
Experimental procedures
Male Lewis (RT11) and male ACI (RT1a) rats weighing 200–225 g (Harlan Sprague Dawley, Indianapolis, IN) were maintained in a laminar-flow caging system. They were given acidified water containing neomycin sulfate (10 mg/L) and were fed rat chow, ad libitum. Bone marrow was harvested from the tibias and femurs of ACI donor rats, washed, and resuspended in Hanks’ balanced salt solution supplemented with gentamicin (50 μg/ml). In addition, single-cell suspensions were prepared from ACI donor spleens. A total of 60×106 ACI BM cells and 30×106 ACI spleen cells were infused via the penile vein of Lewis recipients, 2 hr after they had been given 1000 rads total-body irradiation from a 137Cs source. After the bone marrow–splenocyte transplantation (referred to as BMTx), the animals were given penicillin/streptomycin/gentamicin on day 0, followed by gentamicin injections on days 1 and 2.
The FK506 and CsA were given by intramuscular injection once a day unless noted otherwise. FK506 powder (Fujisawa Pharmaceutical Company, Osaka, Japan) with carrier solvent HCO-60 and D-mannitol was diluted in normal saline. Cyclosporine (Sandoz Pharmaceuticals, Hanover, NJ) was dissolved in Intralipid, to a concentration of 15 mg/ml or 25 mg/ml.
With either drug, daily treatment was for 7 or 14 days, starting on day 3 after BMTx (Table 1). One group of animals also received a prolonged course of every-other-day therapy with FK506 out to 60 days; at which time, some of the animals were sacrificed to search for histologic evidence of GVHD. Controls are summarized in Table 1. These included irradiation alone (1000 rads TBI, without BM rescue), syngeneic BMTx (1000 rads TBI plus syngeneic Lewis BM), and allogeneic BMTx without drug treatment (1000 rads TBI plus allogeneic ACI BM).
Table 1.
Experimental groups
| Group | Reconstitution | Immunosuppression | mg/kg | Days of therapy | No. |
|---|---|---|---|---|---|
| 1 | None | None | — | — | 5 |
| 2 | Lewis BM | None | — | — | 5 |
| 3 | ACI BM | None | — | — | 10 |
| 4 | ACI BM | CsA | 15 | 3–9 | 10 |
| 5 | ACI BM | CsA | 25 | 3–9 | 10 |
| 6 | ACI BM | FK506 | 1 | 3–9 | 10 |
| 7 | ACI BM | FK506 | 1 | 3–9 | 10 |
| 0.1 q.o.d. | 12–60 | ||||
| 8 | ACI BM | FK506 | 0.1 | 3–16 | 10 |
| 9 | ACI BM | FK506 | 1.0 | 3–16 | 10 |
| 10 | ACI BM | FK506 | 1.5 | 3–16 | 10 |
Determination of GVHD
Assessment of GVHD was done with formal criteria (Table 2), with body-weight measurement every 3 days and surveillance daily for clinical signs of GVHD. Clinical GVHD was diagnosed when at least 3 of the physical findings described in Table 2 were present. Weekly ear biopsies were taken from 3 or 4 animals in each experimental group. When the terminal stages of GVHD were reached, all such animals were sacrificed, and samples were obtained of skin, tongue, liver, and small bowel. The histopathologic grading (Table 2) was done independently by two pathologists (AJD, WM) without knowledge of the experimental group.
Table 2.
Grading of graft-versus-host diseasea
| Severity | Histopathologic parameters |
||
|---|---|---|---|
| Ear/tongue | Liver | Small intestine | |
| + |
|
|
|
| ++ |
|
|
|
| +++ |
|
|
|
Three parameters required to be graded positive for GVHD. Clinical GVHD parameters include the following: erythematous ear, hyperkeratosis of the footpad, dermatitis, weight loss, unkempt appearance, and diarrhea.
Determination of chimerism
Four weeks after BMTx, ACI (donor) and Lewis (original recipient) phenotypes were assessed by surface-marker analysis of 5×105 lymphoid cells from the recipient peripheral blood of 3 rats in each group following Ficoll-Hypaque gradient centrifugation and resuspension of the cells in HBSS. Three different monoclonal antibodies were used to detect MHC class I antigens. MAb 163 (IgG2b) is a monoclonal antibody that is specific for the RT1.A1 haplotype expressed on Lewis cells, while MAb 211 (IgG2b) is specific for the RT1.Aa haplotype expressed on ACI cells. MAb 42 is a monoclonal directed against RT1.An haplotype and served as the control. All antibodies were kindly provided by H. W. Kunz, University of Pittsburgh, Department of Pathology, Pittsburgh, PA; they have been previously characterized (4, 5). These primary biotinylated monoclonal antibodies were used at a 1:50 dilution, incubated for 60 min at 4°C, washed twice with HBSS containing 0.1% gelatin, and resuspended with FITC-avidin as a secondary marker. After 1 hr incubation at 4°C, the cells were washed 3 times with HBSS plus 0.1% gelatin with a final suspension in 1 % paraformaldehyde. Triplicate cell suspensions were analyzed on a fluorescence-cell analyzer (Coulter Epics, Hialeah, FL).
Statistical analysis
Survival was calculated by the life-table method, with the mean survival time (MST)* being derived from that at which 50% of the animals were surviving. In this study, animals living >60 days were considered long-term survivors, and the survival time was defined as 60 days. Comparisons of the MST were analyzed using a two-tailed Student’s t test, with unequal variances. P values <0.05 were considered statistically significant.
RESULTS
Chimerism
By 3–4 weeks after allogeneic BMTx, more than 95% of the lymphocytes in the Lewis recipients bore ACI phenotypes in all tested animals (Fig. 1). After more than 60 days, 4 long survivors were randomly phenotyped with the same results indicating the stability of the chimeric state. As controls, Lewis animals reconstituted with syngeneic BM cells showed only Lewis RT1.A1 phenotypes (data not shown).
Figure 1.
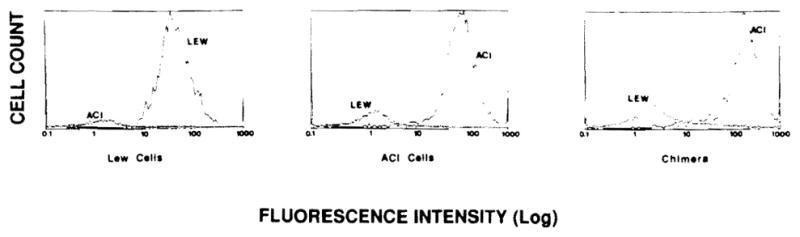
Flow cytometric analysis of cell phenotypes from control (Lew, left and ACI, middle) and a representative chimeric bone marrow transplant rat (right).
Survival and clinical GVHD
The mean survival of 12 days after irradiation alone (group 1) was increased by allogeneic BMTx (group 3), but all these animals died of acute GVHD in less than 26 days (Table 1). Syngeneic BMTx rescued all animals (group 2).
In allogeneic BMTx recipients, a 7-day course of 15 or 25 mg/kg/day CsA for 7 days delayed the appearance of GVHD, but all animals developed this complication after stopping treatment (Table 3) and died soon after (groups 4 and 5). FK506 at doses of 1 mg/kg/day for the same interval was more effective (group 6). Even using 1/10 this dose for 14 days, amelioration of GVHD was evident but of brief duration after the drug was stopped (group 8). Doses of 1 or 1.5 mg/kg/day for 14 days extended the GVHD-free interval even more, as well as survival (groups 9 and 10), and at the higher dose 90% of animals did not have GVHD during the 60 days of the study. The GVHD-free interval after stopping therapy was related to the induction dose and was significantly longer with each dose increment (P<0.005) in groups 8–10. However, when animals had only a 1-week course of 1 mg/kg/day plus continued supplementary maintenance doses (0.1 mg/kg) every other day for 60 days (group 7), all survived throughout the study without GVHD (Table 1).
Table 3.
Onset of GVHD and survival
| Group | Therapy | Mg/kg/day i.m. | Days of therapy after BM | % With GVHD | GVHD median days to onset | Days surviving | Mean survival time | P | Versus group |
|---|---|---|---|---|---|---|---|---|---|
| 1 | None | — | — | — | — | 11, 11, 12, 13, 13 | 12.0 | ||
| 2 | None | — | — | — | — | all >60 | >60 | ||
| 3 | None | — | — | 100 | 10 | 17, 19, 19, 20, 20, 20, 22, 24, 25, 25 | 21.1 | ||
| 4 | CsA | 15 | 3–9 | 100 | 17 | 22, 31, 31, 31, 31, 31, 31, 31, 31, 32 | 30.2 | <0.005 | 3 |
| 5 | CsA | 25 | 3–9 | 100 | 25 | 25, 28, 32, 32, 32, 32, 32, 32, 32, 39 | 31.6 | <0.005 | 3 |
| 6 | FK506 | 1 | 3–9 | 70 | 50 | 38, 51, 52,a 52, 52, 52, | 52.3 | <0.005 | 4 |
| 52, 54, >60, >60 | <0.005 | 5 | |||||||
| 7 | FK506 | 1 | 3–9, then 0.1 mg/kg | 0 | — | ALL >60 (n = 3) | >60 | <0.005 | 6 |
| 8 | FK506 | 0.1 | 3–16 | 100 | 25 | 31, 31, 31, 31, 31, 31, 31, 31, 31, 31 | 31 | <0.005 | 3 |
| 9 | FK506 | 1.0 | 3–16 | 90 | 52 | 46, 52,a58,a 58,a 58, 58, 58, 58, 58, >60 | 56 | <0.005 | 7 8 |
| 10 | FK506 | 1.5 | 3–16 | 10 | 58 | 58, >60, >60, >60, >60, >60, >60, >60, >60, >60 | >60 | — | 11 |
Animals died without clinical symptoms of GVHD.
At the conclusion of the 60-day study, 22 rats without clinical evidence of GVHD were alive. Two were sacrificed for tissue studies, leaving 20. During the next 40 days off all therapy, 17 of the residual group died, and delayed GVHD was responsible for the mortality in nearly all the animals. By 100 days, 3 animals were left, 2 from groups 7 and one from group 10.
Histopathologic studies
Both CsA and FK506 prevented histopathologic evidence of GVHD during active treatment (Table 4), but afterward lesions began to appear, even in some animals without clinical evidence of GVHD. The greatest freedom from GVHD lesions was in the continuous FK506-therapy rats of group 7 and the high 14-day dose FK506 rats of group 10 (Fig. 2).
Table 4.
Histologic grading of GVHD during and following cessation of immunosuppression
| Group | Therapy | mg/kg/day i.m. | Days of therapy after BM | Grading of GVHD during treatment—eara | Grading of GVHD at autopsy |
|||
|---|---|---|---|---|---|---|---|---|
| Ear | Tongue | Liver | Intestine | |||||
| 1 | None | — | — | — | — | — | — | — |
| 2 | None | — | — | 0 | 0 | NAc | NA | NA |
| 3 | None | — | — | 2d | 2d | 3d | 2d | 2d |
| 4 | CsA | 15 | 3–9 | 0 | 1d | 2d | 0 | 1d |
| 5 | CsA | 25 | 3–9 | 0 | 0–1d | 1–2d | 0 | 0–1d |
| 6 | FK506 | 1 | 3–9 | 0 | 0–1d | 1–2d | 0 | 0–1d |
| 7b | FK506 | 1 | 3–9, then 0.1 q.o.d. | 0 | 0 | 1d | 0 | 0 |
| 8 | FK506 | 0.1 | 3–16 | 0 | 1–2d | 2d | 2d | 1d |
| 9 | FK506 | 1.0 | 3–16 | 0 | 0–1d | 1–2d | 0 | 0–1d |
| 10 | FK506 | 1.5 | 3–16 | 0 | 0 | NA | NA | NA |
Biopsies taken on the last day of treatment.
Two animals were sacrificed at 60 days for tissue analysis, reported in the autopsy grading of GVHD. The remaining animals were followed until 100 days.
NA: not available.
Animals died without clinical symptoms of GVHD.
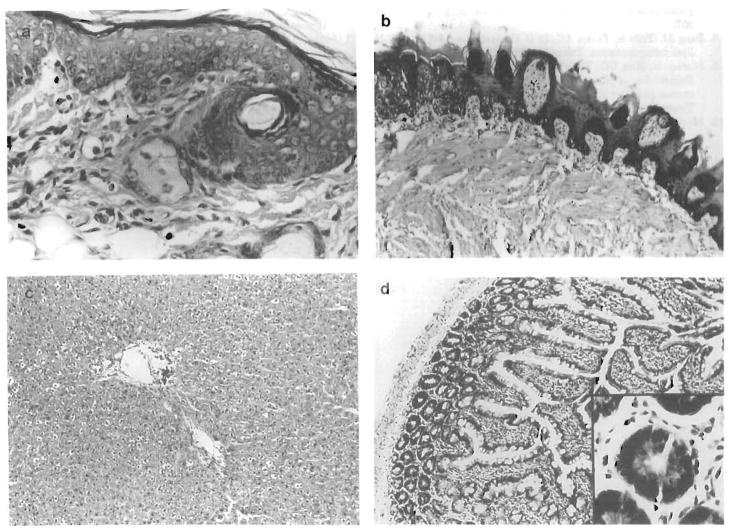
Figure 2.
Effect of low-dose continuous treatment with FK506 on the development of GVHD. Histology of target organs from an animal treated with FK506 at 1.0 mg/kg followed by 0.1 mg/kg/q.o.d. (group 7). Biopsies of (A) ear, (B) tongue, (C) liver, and (D) small bowel were taken 60 days after BMTx. There was no histological evidence of GVHD, except for foci of lymphocytic exocytosis, seen in the tongue.
DISCUSSION
The effectiveness of CsA in preventing GVHD in rats was described in 1979, using a less-severe model than that of the present study (6). In the earlier work, BM cells alone were transplanted in contrast to the bone marrow–splenocyte combination that we employed. The mortality from GVHD with our modification of BMTx was uniform in untreated animals, with well-documented histologic GVHD found in all organ systems analyzed. A short course of CsA did not lead to tolerance, and GVHD was noted in all animals within two weeks after cessation of induction therapy. All died from GVHD within 40 days.
FK506 given for the same period of time appeared to be more effective, but here also the delayed appearance of GVHD was the rule after discontinuance of therapy. However, administration of small doses of maintenance FK506 every other day was capable of suppressing GVHD once the induction period was completed. It is noteworthy that FK506 can reverse established graft-versus-host disease in the same experimental model (7) and ameliorate chronic GVHD in humans (8).
With any immunosuppressive regimen, the difficulty of achieving permanent graft acceptance of bone marrow without continuing therapy is similar to that with heart (9) and most other kinds of solid organ grafts. In contrast is the ease in the same rat-strain combination with which livers can be transplanted under FK506 (9). Nevertheless, the seemingly permanent acceptance of BM in a small number of our rats suggests that the objective of permanent graft acceptance across a strong histocompatibility barrier could be within grasp in animals or in patients.
Insight about how to achieve a stable chimeric state may come from a better understanding of the events following transplantation of “difficult” solid organs such as the intestine, which contain a large lymphoid mass. It was shown recently in rats (10) and humans (11) treated with FK506 that the lymphoreticular population of intestinal grafts was replaced completely over several postoperative weeks with cells of recipient phenotype while the epithelial cells remained of donor origin. At the same time in the patients (11), donor lymphocytes in large numbers were detectable in the peripheral blood, meaning that the traffic in the graft lymphoid tissue was two way. The absence of either graft rejection or GVHD during this extraordinary cell repopulation or afterward under FK506 was not thought to be drug specific but rather a function of the drug’s potency. How to control the repopulation process reliably is the practical question under investigation. In the meanwhile, it seems clear that a drug like FK506 can reduce the jeopardy of GVHD from solid organ grafts in the same way as it can do so after BMTx.
Footnotes
This work was supported by Research Grant No. DK 29961 from the National Institutes of Health, Bethesda, Maryland and grants from the Veterans Administration.
Abbreviation: mean survival time.
References
- 1.Deeg HJ, Storb R. Graft-versus-host disease: pathophysiological and clinical aspects. Annu Rev Med. 1984;35:11. doi: 10.1146/annurev.me.35.020184.000303. [DOI] [PubMed] [Google Scholar]
- 2.Gale RP. Graft-versus-host disease. Immunol Rev. 1985;88:193. doi: 10.1111/j.1600-065x.1985.tb01159.x. [DOI] [PubMed] [Google Scholar]
- 3.McCloud AM, Thomson AW. FK 506: immunosuppressant for the 1990’s. Lancet. 1991;337:25. doi: 10.1016/0140-6736(91)93341-6. [DOI] [PubMed] [Google Scholar]
- 4.Gill TJ, Kunz HW, Misra DW, Cortese-Hassett AL. The major histocompatibility complex of the rat. Transplantation. 1987;43:773. [PubMed] [Google Scholar]
- 5.Inomato T, Kunz HW, Gill TJ. Immunogenetic analysis of rat strains with recombinations in different regions of MHC. Immunogenetics. 1988;23:133. doi: 10.1007/BF00377975. [DOI] [PubMed] [Google Scholar]
- 6.Tutschka PJ, Beschorner WE, Allison AC, Burns WH, Santos GW. Use of cyclosporine A in allogeneic bone marrow transplantation in the rat. Nature. 1979;20:148. doi: 10.1038/280148a0. [DOI] [PubMed] [Google Scholar]
- 7.Markus PM, Cai X, Ming W, Demetris AJ, Fung JJ, Starzl TE. FK 506 reverses acute graft-versus-host disease following allogeneic bone marrow transplantation in rats. Surgery. 1991;110:357. [PMC free article] [PubMed] [Google Scholar]
- 8.Fung JJ, Todo S, Tzakis AG, et al. Current status of FK 506 in liver transplantation. Transplant Proc. 1991;23:1902. [PMC free article] [PubMed] [Google Scholar]
- 9.Murase N, Kim DG, Todo S, Cramer DV, Fung JJ, Starzl TE. Suppression of allograft rejection with FK 506: I. Prolonged cardiac and liver survival in rats following short-course therapy. Transplantation. 1990;50:186. doi: 10.1097/00007890-199008000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murase N, Demetris AJ, Matsuzaki T, et al. Long survival in rats after multivisceral versus isolated small bowel allotransplantation under FK 506. Surgery. 1991;110:87. [PMC free article] [PubMed] [Google Scholar]
- 11.Iwaki Y, Starzl TE, Yagihashi A, et al. Replacement of donor lymphoid tissue in human small bowel transplants under FK 506 immunosuppression. Lancet. 1991;337:818. doi: 10.1016/0140-6736(91)92517-6. [DOI] [PMC free article] [PubMed] [Google Scholar]