Abstract
The feasibility of composite tissue allografts (CTA) has been demonstrated by the successful transplantation of the hand, abdomen and face. However, the survival of these transplants is dependent on immunosupression. Our laboratory is interested in achieving tolerance in order to decrease the risks associated with the use of chronic immunosuppression. The purpose of this experiment was to develop a large animal model for CTA. Four canine flaps were auto-transplanted to examine the use of a myocutaneous rectus flap based on the deep inferior epigastric vessels. Five CTA transplants were performed between Dog Leukocyte Antigen (DLA) identical littermates without any therapy. The allografts were followed clinically and underwent routine biopsies. The anatomic dissections and auto-transplants were all successful and revealed that the flap could be divided into two separate components. Skin is routinely perfused by the superficial epigastric artery. Rectus muscle is perfused by the deep inferior epigastrc system. This allows the allografts to be transplanted as muscle, skin or with both components based on the external iliac artery and veins. The DLA-identical littermates rejected the allografts in 15 to 30 days. This study demonstrates the versatility of the myocutaneous rectus flap for use in canines as composite tissue allograft models.
Keywords: Composite Tissue Allograft, Large Animal Model, Skin rejection, Muscle rejection, Canine
INTRODUCTION
The recent clinical applcations of composite tissue allotransplantation to reconstruct the hand, face and abdomen represent a significant advance in the field of reconstructive surgery. Composite tissue allotransplantation could allow the reconstruction of complex deformities from trauma, congenital defects, burn injury, or cancer ablation with the exact tissue lost without any donor site issues.1 These transplants allow for reconstruction of severe injuries that were previously thought to be unreconstructable in a single stage. During the past 10 years there have been over 43 hand transplants2 and 7 partial face transplants performed worldwide. However, the survival of these transplants is dependent on chronic immunosupression. Even with the use of chronic immunosuppression, all of the patients have experienced episodes of rejection of the skin that required additional therapy and often increases in the overall level of immunosuppression.3
The complications of immunosuppression include infection, malignancy, cardiovascular disease, and diabetes.1,4 Although, such risks may be justifiable for life preservation in end stage solid organ disease, these risks limit the current application of composite tissue allograft (CTA). A large body of literature addresses the nature of complications secondary to high doses of lifelong immunosuppression and a number of severe adverse events have actually been observed after human hand and face transplantation.5-11 The central goal in this field of research is to establish a CTA protocol that would reduce or eliminate the need for chronic immunosuppressive drugs.
Developing a clinical protocol that reduces or eliminates the need for chronic immunosuppresion requires the use of a reliable pre-clinical animal model. The data obtained in tolerance protocols performed in small animals have advanced our basic understanding of the unique challenges presented by composite tissue transplantation. While these small animal models are useful for the initial evaluation of protocols and the derivation of mechanistic concepts, the results derived from these experiments are not easily translatable to human clinical trials due to inherent differences in the human immune system.1 In fact, the small animal protocols that have led to tolerance to skin have not been successful in large animal models.
Our goal was to develop a large animal pre-clinical model for CTA that can best represent the human immune system in order to develop clinically relevant protocols. Most importantly this model should be directly translatable to humans, for which outbred and genetically defined animals are advantageous, as well as have a reliable and versatile experimental protocol. Consequently, we have focused our efforts on the canine model. Over the past 40 years, our laboratory has successfully translated hematopoietic stem cell transplantation (HCT) regimen that have been based directly on studies performed in the preclinical canine model.12-17 This report demonstrates the technical aspects of using canines for composite tissue allotransplantation and provides an overview of other commonly used animal models used in CTA research.
MATERIALS AND METHODS
Dogs
Litters of beagles and mini-mongrel cross-breeds were either raised at the Fred Hutchinson Cancer Research Center (FH-CRC), Seattle, WA, or purchased from commercial kennels. The dogs weighed from 7.0 to 13.3 (median 9.6) kg and were 7 to 27 (median 8) months old. They were observed for disease for at least 60 days before study. All were immunized for leptospirosis, papillomavirus, distemper, hepatitis, and parvovirus. Research was conducted according to the principles outlined in the Guide for Laboratory Animal Facilities and Care prepared by the National Academy of Sciences, National Research Council. The Institutional Animal Care and Use Committee of the FHCRC approved the research protocols and the American Association for Accreditation of Laboratory Animal Care certified the kennels.
Ten littermate donor/recipient pairs were dog leukocyte antigen-(DLA) identical on the basis of matching for highly polymorphic major histocompatibility complex (MHC) class I and class II micro- satellite markers.18 In addition, specific DLA DRB1 allelic identity was confirmed by direct sequencing.19
Surgical Technique
Four autologous rectus flap transfers were performed in the canine model to demonstrate the technical viability of the flap design. Following demonstation of the technical feasibility of the rectus myocutaneous flap, 5 transplants were performed in the absence of any immunosuppression between DLA-identical littermates.
The composite tissue allograft was derived from a myocutaneous rectus abdominus flap initially based on the deep inferior epigastric artery and vein. Food was withheld for 24 hours before surgery. The dogs received premedication with fentanyl patches before halothane general anesthesia. We continuously monitored the animal's electrocardiographic tracing and transcutaneous oxygen saturation while under general anesthesia.
The surgery was carried out under sterile conditions. The skin portion of the flap was outlined after the perforating vessels were located on the skin with the portable doppler. Once the skin paddle was outlined, we incised the edges and dissected down through the rectus fascia to the rectus muscle. At the superior end the muscle was isolated and the superior epigastric artery divided. The flap was then dissected towards the main pedicle (the deep inferior epigastric artery, DIEA). In the initial flap design, the flap was based solely on the DIEA system. During the initial autologus flap transfers the consistent presence of a superficial epigastric arterial system (SIEA) supplying the skin and subcutaneous tissues was noted. Thus, the flap dissection was modified to preserve the SIEA system as well. The distal end of the muscle was divided and vascular pedicle followed both the DIEA and SIEA branches into the retroperitoneal space where they joined with the iliac artery. Finally, a small portion of the iliac artery and vein were included in the flap to facilitate the transplant.
The flap was brought to the back table and after irrigation with 100ml of cold saline containing heparin sulfate (5000u/liter). A penrose drain was placed into the donor wound and incision was closed in layers. The recipient dog was then brought into the operating room and anesthesia was used as outlined above. The femoral artery and vein were isolated and then the transplanted flap was anastomosed to the femoral vessels using the operating microsope. The flap was inset and the incisions closed with continuous nonabsorbable suture and the skin reapproximated with an absorbable subcuticular suture.
Postoperative Care
Postsurgical recovery included warming lights and parenteral analgesia with oxymorphone (0.04 mg/kg). An Elizabethan collar was used to protect the incision site. Food and water were reinstituted on the first day after transplantation. The flap was followed closely by clinical inspection as well as daily Doppler checks.
Postoperative Analysis
The skin and muscle were followed via clinical examination for color of the skin and hair growth. The animals were also followed by open biopsies of the skin and muscle. The tissue was fixed in 10% formalin, imbedded in paraffin, and cut sections were stained with hematoxylinand-eosin and Jones’ stains. A pathologist evaluated the presence or absence of rejection.
RESULTS
Autologous Flap Transfer
Four autologus flaps were successfully transferred from the left abdomen to the right groin (Figure 1). These flaps demonstrated good perfusion of both the skin and the muscle after the anastomosis of the deep inferior epigastric artery and vein to the femoral artery and vein (Figure 2a). Intercostal nerves were also anastomosed (Figure 2b). The initial flap design were were based on the deep inferior epigastric artery and vein (Figure 3a). However, after further exploration of the model we found that canines have reliable and separate deep and superficial epigastric systems (Figure 3c). These two systems join together and drain into the external iliac artery and vein. This allows for versatility in the use of the flap. It can be transferred based on this blood supply as a muscle or skin only transplant. We modified our flap design to capture both systems and proceeded to harvest the flap using the external iliac artery and vein as the donor vessels (Figure 3b).
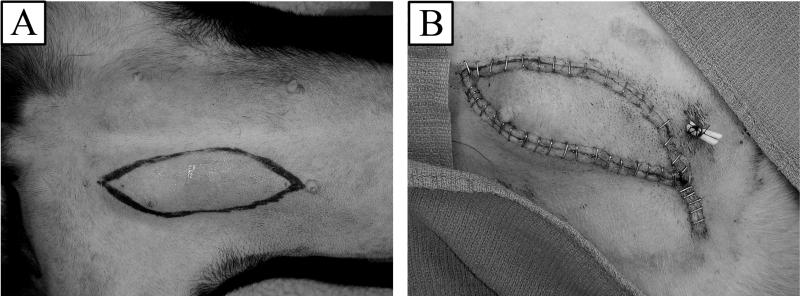
Figure 1.
(A) Preoperative markings on donor dog abdomen. (B) Donor flap inset into recipient dog's groin.
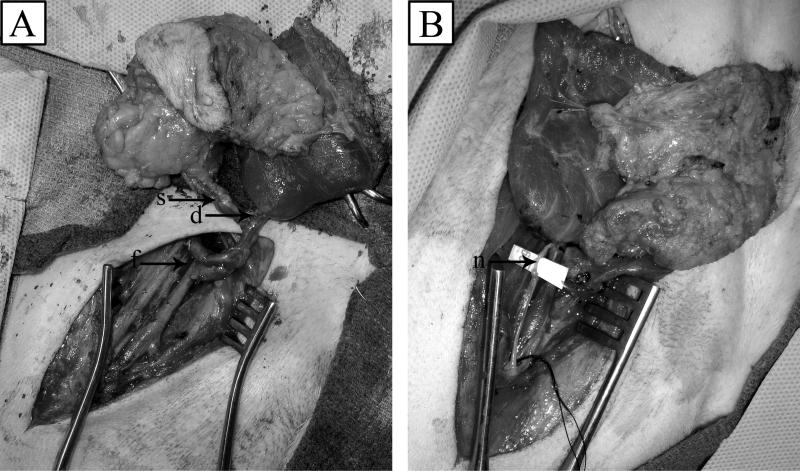
Figure 2.
(A) Vascular anastamosis of recipient femoral artery and vein (f) to deep inferior (d) and superficial (s) epigastric vessels of allograft. (B) Intercostal nerve (n) attachment.
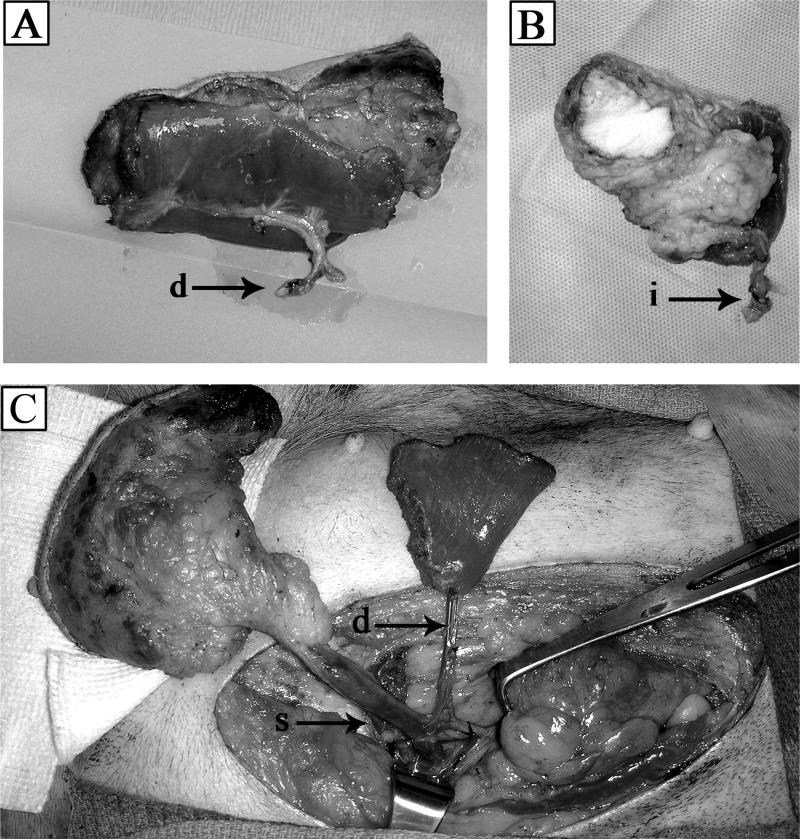
Figure 3.
(A) Composite tissue allograft harvested on deep inferior epigastric (d) artery and vein. (B) Alternate blood vessels from external iliac artery and vein (i). (C) Deep inferior epigastric and superior inferior epigastric (s) vasculature supplying graft tissue of donor dog.
Experimental transplants without immunosuppression
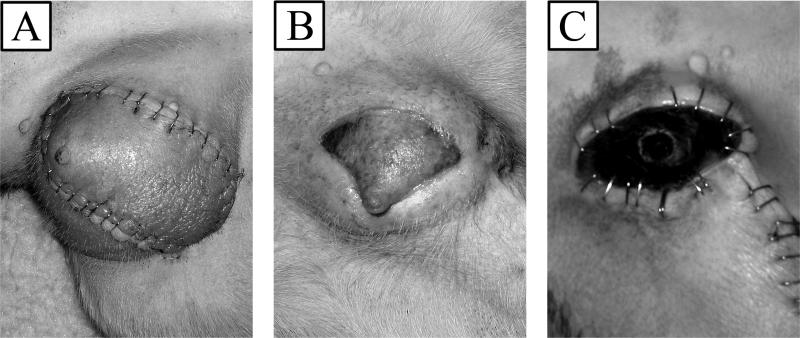
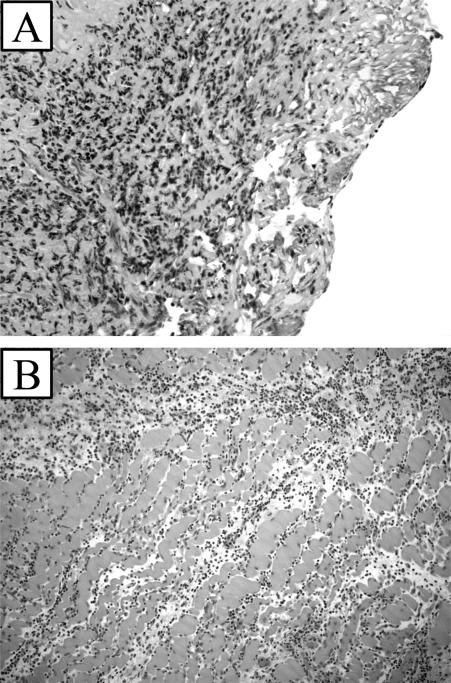
Five transplants were performed between DLA identical littermates without any additional treatment. The allografts were designed as described above based on the external iliac artery and vein. All of the allografts were successful with good blood flow demonstrated at the end of the case. The flaps continued to demonstrate good color and flow to the skin which was documented on a daily basis by an audible Doppler signal. The flaps demonstrated good color until they underwent a process of rejection. The initial sign of rejection was increased erythema and swelling in the transplant seen at day 12 (Figure 4a). This progressed to thrombosis and loss of the allograft (Figure 4b). Complete rejection of the allograft occurred from days 15 to 30 (Figure 4c). Rejection was also demonstrated by histologic evaluation of biopsies of the skin and muscle (Figure 5). Pathologic evaluation demonstrated severe cellular rejection as evidenced by the massive lymphocytic infiltrate and destruction of native cellular elements.
Figure 4.
Progression of rejecting allotransplant in recipient dog. (A) Signs of early rejection and swelling 12 days postoperatively. (B) Further evidence of acute rejection on day 22. (C) Complete necrosis on day 30.
Figure 5.
Histology of allograft skin (A) and muscle (B) showing lymphocytic infiltration and necrosis taken on postoperative day 22.
DISCUSSION
Currently, the reconstructive procedures used for major tissue loss are inadequate to reconstruct a lost hand or substantial facial deformities. Those that are reconstructed, such as severe facial injury patients, often suffer from poor functional and aesthetic outcomes. They require multiple revision procedures, prolonged rehabilitation, high costs of multiple surgeries, numerous hospitalizations, and they experience donor site morbidity resulting from the use of autologous tissues. One potential solution to the overwhelming need for tissue reconstruction is the use of composite tissue allografts (CTA). While composite tissue allotransplantation is now a clinical reality, its application is limited by its dependence on chronic immunosuppression.
During the period between September 1998 and March 2009, there have been over 41 hand transplants performed worldwide.2 CTAs have also been performed to reconstruct the face, larynx, and abdominal wall. All of the patients have experienced episodes of rejection despite the use of multi-drug immunosuppression regimens.3 In addition to the problem of acute rejection and the possibility of chronic rejection, the patients have experienced complications related to the immunosuppression. These have included infections such as cytomegalovirus reactivation, ulnar osteitis by Staphylococcus aureus, and Clostridium difficile enteritis.3,4 Moreover, several metabolic complications have been reported such as Cushing's syndrome and increased creatinine levels.3 Transient hyperglycemia occurred in 50% of hand transplant recipients,4 demonstrating an increased risk for developing diabetes mellitus.3 There have also been reports of allograft rejection leading to amputation of the transplanted hand due to both non-compliance to the immunosuppressive drug regimen and secondary to vascular rejection. Recently, the recipient of the first face and double hand transplant died in June of 2009 secondary to sepsis.20
Immunologic tolerance, however, would allow for the long-term survival of these organs without the need for chronic immunosuppression. This would significantly impact the risk-benefit ratio and allow for the more widespread use of CTA in the reconstruction of lost limbs and severe facial deformities. Thus, further research is needed to develop protocols that can eliminate or reduce the need for chronic use of immunosuppression. The transfer of most research developments from the laboratory to the clinic and the clinical relevance of a protocol require careful evaluation in animal models. CTA research has relied on both small and large animal models. The ideal model for this research should be conducive to the development of tolerance protocols that can be applied directly to a clinical setting, be available in infinite genotypes that are well defined, and have a protocol that is reliable.
Much of the initial research in CTA has been performed in small animal models. The attractive qualities of rodents, including mice and rats, are the relatively lower costs to acquire and maintain them, the ability to genetically manipulate the animals, and the many reagents available to explore certain mechanistic questions.21 Having access to knockout mice and unlimited supplies of syngeneic animal can facilitates studies aimed at understanding select aspects of the mechanisms of tolerance. Furthermore, tolerance to CTAs have been induced in certain rodent transplant models.22-23
However, the limitations of small animals in CTA research are numerous. It has been difficult to replicate the protocols developed in rodent models in large animals. In addition their short lifespan can make it difficult to truly assess long-term graft survival. Whereas, survival for the generally accepted amount of 100 days has been claimed to suggest tolerance in mice, a minimum of a year is required before such claims can be concluded in large animals.21 Also, being brought up in a pathogen-free environment and studied while only 5 - 11 weeks old contributes to a very naïve immune system, much different from that of humans who would ultimately be benefiting from CTA transplants.24 Most importantly, a lack of class II antigen expression on the vascular endothelium of rodents means that their immune systems are fundamentally different from that of humans.1 Therefore, while small animal models are useful for proof of concepts, large animal models are central in the determination of a protocols practicality for application in human transplantation. Large animals are excellent preclinical model as they allow for assessment of a hypothesis in a complex system that shares strong similarities in anatomic structures, histology and immune responses to the human.26 There are three main large animal models that are commonly used in transplantation research today: swine, non-human primates (NHPs), and canines.
Similarly to small animal models, the ease of breeding and genetic control is an advantage for the use of pigs as a research model,21 in addition to the fact there is little public resentment towards their involvement in scientific studies. Otherwise the main concerns with pigs used in CTA research is the extent of inbreeding, common complications with allotransplant procedures, and their cost. Inbreeding is common particularly within the MHC-defined mini-swine population,27 which makes it difficult to assess true recipient responses to allotransplants of minor antigen mismatched pairs. Furthermore, the swine has a higher tendency of contracting posttransplant lymphoproliferative disease (PTLD), shown in a study by Huang et al., with an incidence of 43% after allogeneic hematopoietic cell transplantation28 compared to experiments in canine experiments29 and clinical trials30 showing no evidence of the disease after the same procedure. Also, as are other large animals, they are costly to purchase and maintain and require a considerable amount of living space and reagents due to their size, which is not cost effect for the overall benefit of their use relative to other models.
Nonhuman primates are an extensively used model for CTA research because of their genetic and anatomical similarity to humans.26,31 A key feature is their strong MHC homology to humans31-32 making them useful for testing human-specific immunosuppressants. They are the most commonly used animal models for studies in biologics such as human monoclonal antibodies,33 and unlike with other models do not require the production of animal specific antibodies and immunosuppressive drugs.34 Some of the drawbacks of this model include limited translatability to humans, a lack of out-bred animals, and genetic definition. Drug metabolisms for certain immunosuppressive drugs (such as FK-506) are vastly different from humans, thus, compromising their translatability. Some drug regimens that have been greatly therapeutic in primates have been shown to be ineffective in humans35 and much larger doses of tacrolimus and sirolimus are needed compared to humans.36 A lack of outbreeding in NHPs results in poor genetic variability and since most animals are not genetically defined, they would require extensive typing before using them in tolerance experiments, which is not currently feasible. Unpredicatable and frequent rejections are common in primates,26 secondary to incomplete typing, as are incidents of PTLD,32 making NHPs a challenge to work with at times.
The canine model is a common research animal for transplantation studies. It was the original model used for limb transplantation by Goldwyn etal in 1966. Our canine model, in particular, has several advantages that make it ideal for developing CTA protocols. The dogs are outbred, leading to a diversity of MHC antigens available for research. In addition, their MHC has been well characterized, and DLA (Dog Leukocyte Antigen) defined species are available for studies that require specific genotypes.37 While the dogs are still a large animal species, those used in research are generally smaller in size than both pigs and NHPs, requiring less space and smaller drug doses, thereby reducing expense. Furthermore, there is also a tendency to use more mature dogs in transplant research,21 giving the animals time to develop their immune systems as they encounter pathogens throughout their life, simulating a more realistic representation of the human immune system. A difficulty of using this model is the necessity for stringent ethical regulations in the development of research studies; however these stringent ethical considerations also apply to studies with monkeys. Also, the same breadth of reagents available for rodents is absent for dogs.
In this paper we have outlined a new CTA model that offers multiple benefits to the development of clinical tolerance protocol for CTA. The myocutaneous rectus transplant, based on the external iliac artery and vein, can be modified to transplant an individual tissue type (such as vascualized skin or muscle alone) or transplanted with all of the tissue types. The transplant does not require the death of the donor as is seen in limb transplantation or in many of the primate models. This allows the donor to be maintained for additional studies. Finally, chimerism and allograft infiltrating cells can be tracked by PCR (Polymerase Chain Reaction) in recipient dogs by tracking the expression of FoxP3, chemokines, and cytokines.
The most critical aspect of our model is its translatability to humans. The dog responds to immunosuppressive drugs in a manner similar to humans.38 Our laboratory has successfully translated a non-myeloablative hematopoietic stem cell (HSC) transplant regimen to the clinic that is based directly on studies performed in the preclinical canine model.39-41 The regimen has been successfully translated into the clinic to treat human patients with both malignant and nonmalignant diseases by grafts from human leukocyte antigen (HLA)-matched related and unrelated donors.42-43 Our current studies are working on the application of this protocol to CTA transplantation.
CONCLUSION
We have developed a relevent pre-clinical model for composite tissue allotransplantation in the canine. The unique benefits of our model are its track record of translation from the laboratory to the clinic, its defined genetic make up, and the immunologic similarity to human composite tissue allografts.
Acknowledgments
Supported in part by NIH grant CA78902.
REFERENCES
- 1.Siemionow M, Nasir S. Chimerism and bone marrow based therapies in transplantation. Microsurgery. 2007;27:510–521. doi: 10.1002/micr.20395. [DOI] [PubMed] [Google Scholar]
- 2. [August 1, 2009];International Registry on Hand and Composite Tissue Transplantation. Available at: http://www.handregistry.com/page.asp?page=4.
- 3.Lanzetta M, Petruzzo P, Dubernard JM, et al. Second Report (1998–2006) of the International Registry of Hand and Composite Tissue Transplantation. Transpl Immunol. 2007;18:1–6. doi: 10.1016/j.trim.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Whitaker IS, Duggan EM, Alloway RR, et al. Composite tissue allotransplantation: a review of relevant immunological issues for plastic surgeons. Plastic, Reconstructive & Aesthetic Surgery. 2008;61:481–492. doi: 10.1016/j.bjps.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Mihatsch MJ, Kyo M, Morozumi K, Yamaguchi Y, Nickeleit V, Ryffel B. The side-effects of ciclosporine-A and tacrolimus. Clin Nephrol. 1998;49:356. [PubMed] [Google Scholar]
- 6.Philip AT, Gerson B. Toxicology and adverse effects of drugs used for immunosuppression in organ transplantation. Clin Lab Med. 1998;18:755. [PubMed] [Google Scholar]
- 7.Mele TS, Halloran PF. The use of mycophenolate mofetil in transplant recipients. Immunopharmacology. 2000;47:215. doi: 10.1016/s0162-3109(00)00190-9. [DOI] [PubMed] [Google Scholar]
- 8.Fardet L, Kassar A, Cabane J, Flahault A. Corticosteroid-induced adverse events in adults: frequency, screening and prevention. Drug Saf. 2007;30:861. doi: 10.2165/00002018-200730100-00005. [DOI] [PubMed] [Google Scholar]
- 9.Fishman JA, Rubin RH. Infection in organ-transplant recipients. N Engl J Med. 1998;338:1741. doi: 10.1056/NEJM199806113382407. [DOI] [PubMed] [Google Scholar]
- 10.Schneeberger S, Lucchina S, Lanzetta M, et al. Cytomegalovirus-related complications in human hand transplantation. Transplantation. 2005;80:441. doi: 10.1097/01.tp.0000168454.68139.0a. [DOI] [PubMed] [Google Scholar]
- 11.Baumeister S, Kleist C, Dohler B, Bickert B, Germann G, Opelz G. Risks of allogeneic hand transplantation. Microsurgery. 2004;24:98. doi: 10.1002/micr.20003. [DOI] [PubMed] [Google Scholar]
- 12.Storb R, Yu C, Wagner JL, et al. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood. 1997;89:3048. [PubMed] [Google Scholar]
- 13.Yu C, Seidel K, Nash RA, et al. Synergism between mycophenolate mofetil and cyclosporine in preventing graft-versus-host disease among lethally irradiated dogs given DLA-nonidentical unrelated marrow grafts. Blood. 1998;91:2581. [PubMed] [Google Scholar]
- 14.Yu C, Storb R, Mathey B, et al. DLA-identical bone marrow grafts after low-dose total body irradiation: effects of high-dose corticosteroids and cyclosporine on engraftment. Blood. 1995;86:4376. [PubMed] [Google Scholar]
- 15.Storb R, Raff RF, Appelbaum FR, et al. FK-506 and methotrexate prevent graft-versus-host disease in dogs given 9.2 Gy total body irradiation and marrow grafts from unrelated dog leukocyte antigen-nonidentical donors. Transplantation. 1993;56:800. doi: 10.1097/00007890-199310000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Storb R, Epstein RB, Rudolph RH, Thomas ED. Allogeneic canine bone marrow transplantation following cyclophosphamide. Transplantation. 1969;7:378. doi: 10.1097/00007890-196905000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Storb R, Epstein RB, Graham TC, Thomas ED. Methotrexate regimens for control of graft-versus-host disease in dogs with allogeneic marrow grafts. Transplantation. 1970;9:240. doi: 10.1097/00007890-197003000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Wagner JL, Burnett RC, DeRose SA, Francisco LV, Storb R, Ostrander EA. Histocompatibility testing of dog families with highly polymorphic microsatellite markers. Transplantation. 1996;62:876. doi: 10.1097/00007890-199609270-00032. [DOI] [PubMed] [Google Scholar]
- 19.Wagner JL, Works JD, Storb R. DLA-DRB1 and DLA-DQB1 histocompatibility typing by PCR-SSCP and sequencing. Tissue Antigens. 1998;52:397. doi: 10.1111/j.1399-0039.1998.tb03063.x. [DOI] [PubMed] [Google Scholar]
- 20. [August 15, 2009];World's first face and double-hand transplant patient dies in France. The Daily Telegraph June 16, 2009. Available at: http://www.dailytelegraph.com.au/news/world/worlds-first-face-and-and-double-hand-transplant-patient-dies-in-france/story-e6frev00-1225736077918.
- 21.Kirk AD. Crossing the bridge: large animal models in translational transplantation research. Immunological Reviews. 2003;196:176–196. doi: 10.1046/j.1600-065x.2003.00081.x. [DOI] [PubMed] [Google Scholar]
- 22.Ikeguchi R, Sacks JM, Unadkat JV, et al. Long-term survival of limb allografts induced by pharmacologically conditioned, donor alloantigen-pulsed dendritic cells without maintenance immunosuppression. Transplantation. 2008;85:237–246. doi: 10.1097/TP.0b013e31815e870e. [DOI] [PubMed] [Google Scholar]
- 23.Demir Y, Ozmen S, Klimczak A, Mukherjee AL, Siemionow M. Tolerance Induction in Composite Facial Allograft Transplantation in the Rat Model. Plast Reconstr Surg. 2004 Dec;114:1790–801. doi: 10.1097/01.prs.0000142414.92308.ab. [DOI] [PubMed] [Google Scholar]
- 24.Blattman JN, Antia R, Sourdive DJ, et al. Estimating the precursor frequency of naive antigen-specific CD8 T cells. J Exp Med. 2002;195:657–664. doi: 10.1084/jem.20001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siemionow M, Unal S. Strategies for Tolerance Induction in Nonhuman Primates. Annals of Plast Surg. 2005;55:545–553. doi: 10.1097/01.sap.0000182657.35457.8b. [DOI] [PubMed] [Google Scholar]
- 26.Cendales LC, Xu H, Bacher J, Eckhaus MA, Kleiner DE, Kirk AD. Composite tissue allotransplantation: development of a preclinical model in nonhuman primates. Transplantation. 2005;80:1447–1454. doi: 10.1097/01.tp.0000183292.57349.27. [DOI] [PubMed] [Google Scholar]
- 27.Singer DS, Ehrlich R, Satz L, et al. Structure and expression of class I MHC genes in the miniature swine. Vet Immunol Immunopathol. 1987;17:211–221. doi: 10.1016/0165-2427(87)90141-3. [DOI] [PubMed] [Google Scholar]
- 28.Huang CA, Fuchimoto Y, Gleit ZL, et al. Posttransplantation lymphoproliferative disease in miniature swine after allogeneic hematopoietic cell transplantation: similarity to human PTLD and association with a porcine gammaherpesvirus. Blood. 2001;97:1467. doi: 10.1182/blood.v97.5.1467. [DOI] [PubMed] [Google Scholar]
- 29.Kuhr CS, Allen MD, Junghanss C, et al. Tolerance to vascularized kidney grafts in canine mixed hematopoietic chimeras. Tranplantation. 2002;73:1487–1493. doi: 10.1097/00007890-200205150-00020. [DOI] [PubMed] [Google Scholar]
- 30.McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 31.Siemionow M. Analysis of historical outcomes of composite tissue allograft transplants in nonhuman primates. Transplantation. 2005;80:1374–1375. doi: 10.1097/01.tp.0000183960.06766.ae. [DOI] [PubMed] [Google Scholar]
- 32.Barth RN, Bluebond-Langner R, Nam A, et al. Facial Subunit Composite Tissue Allografts in Nonhuman Primates: I. Technical and Immunosuppressive Requirements for Prolonged Graft Survival. Plast Reconstr Surg. 2009;123:493–501. doi: 10.1097/PRS.0b013e3181954edd. [DOI] [PubMed] [Google Scholar]
- 33.Kirk AD. Transplant tolerance: a look at the non-human primate literature in the view of modern tolerance theories. Crit Rev Immunol. 1999;19:349–388. [PubMed] [Google Scholar]
- 34.Impellizeri JA, Howell K, McKeever KP, Crow SE. The role of rituximab in the treatment of canine lymphoma: an ex vivo evaluation. Vet J. 2006;171(3):556–558. doi: 10.1016/j.tvjl.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 35.Kirk AD, Knechtle SJ, Sollinger H, Vincenti FG, Stecher S, Nadeau K. Preliminary results of the use of humanized anti-CD154 in human renal allotransplantation. Am J Transplant. 2001;1:S191. [Google Scholar]
- 36.Montgomery SP, Mog SR, Xu H, et al. Efficacy and toxicity of a protocol using sirolimus, tacrolimus, and daclizumab in a nonhuman primate renal allotransplant model. Am J Transplant. 2002;2:381–385. doi: 10.1034/j.1600-6143.2002.20415.x. [DOI] [PubMed] [Google Scholar]
- 37.Wagner JL, Burnett RC, Storb R. Organization of the canine major histocompatibility complex: current perspectives. J Hered. 1999;90:35–38. doi: 10.1093/jhered/90.1.35. [DOI] [PubMed] [Google Scholar]
- 38.Ochiai T, Nagata M, Nakajima K, et al. Studies of the effects of FK506 on renal allografting in the beagle dog. Transplantation. 1987;44:729–733. doi: 10.1097/00007890-198712000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Storb R, Yu C, Wagner JL, et al. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood. 1997;89:3048. [PubMed] [Google Scholar]
- 40.Yu C, Seidel K, Nash RA, et al. Synergism between mycophenolate mofetil and cyclosporine in preventing graft-versus-host disease among lethally irradiated dogs given DLA-nonidentical unrelated marrow grafts. Blood. 1998;91:2581. [PubMed] [Google Scholar]
- 41.Storb R, Raff RF, Appelbaum FR, et al. FK-506 and methotrexate prevent graft-versus-host disease in dogs given 9.2 Gy total body irradiation and marrow grafts from unrelated dog leukocyte antigen-nonidentical donors. Transplantation. 1993;56:800. doi: 10.1097/00007890-199310000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Luznik L, O'Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maris MB, Niederwieser D, Sandmaier BM, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood. 2003;102:2021. doi: 10.1182/blood-2003-02-0482. [DOI] [PubMed] [Google Scholar]