Abstract
CD8+ T cells responding to intracellular infection give rise to cellular progeny that become terminally differentiated effector cells and self-renewing memory cells. T-bet and Eomesodermin are key transcription factors of cytotoxic lymphocyte lineages. We now show that CD8+ T cells lacking Eomesodermin compete poorly in contributing to the pool of antigen-specific central memory cells. Eomesodermin-deficient CD8+ T cells undergo primary clonal expansion but are defective in long-term survival, populating the bone marrow niche, and re-expanding after re-challenge. The phenotype of Eomesodermin-deficient CD8+ T cells supports the hypothesis that T-bet and Eomesodermin can act redundantly to induce effector functions, but can also act to reciprocally promote terminal differentiation versus self-renewal of antigen-specific memory cells.
Introduction
Memory CD8+ T cells can be categorized into at least two groups, effector-memory and central-memory, distinguishable by cell surface marker expression, anatomic location, and functional properties (1, 2). Effector-memory CD8+ T cells are more prevalent in peripheral, non- lymphoid tissues, rapidly exert effector functions upon antigen encounter, and have limited proliferative capacity. Central-memory cells retain greater capacity for secondary re-expansion and greater long-term persistence, enabled by efficient homeostatic self-renewal. Central-memory CD8+ T cells are more prevalent in lymphoid tissues including the bone marrow, spleen, and lymph nodes. Of these tissues, the bone marrow is thought to provide a niche that supports homeostatic self-renewal and acts as a reservoir for memory CD8+ T cells (3–6).
Prior work supports critical roles for two members of the T-box transcription factor family, T-bet and Eomesodermin (Eomes), in the formation of CD8+ T cell effector and memory subsets (7–9). The observation of enhanced central-memory differentiation in CD8+ T cells lacking T-bet suggests that T-box factors may serve as regulators of CD8+ T cell propensity for terminal effector differentiation versus persistence as long-lived memory cells (10, 11). In this study we have evaluated terminal differentiation versus memory cell development in CD8+ T cells lacking Eomes. We observe diminished capacity to compete for the antigen-specific memory compartment in CD8+ T cells lacking Eomes. Eomes deficient memory CD8+ T cells have defects in long-term persistence and secondary expansion after re-challenge, two hallmark properties of central-memory CD8+ T cells. We further observe diminished ability to compete effectively for the bone marrow memory niche in memory CD8+ T cells lacking Eomes. These results suggest that Eomes confers competitive fitness to memory cells and support a role for Eomes in promoting persistence as long-lived memory versus terminal effector differentiation of antigen-specific CD8+ T cells.
Materials and Methods
Mice
Mice were used in accordance with the University of Pennsylvania Institutional Animal Care and Use Guidelines. C57BL/6 mice, P14 TCR transgenic mice, Tbx21−/− (T-bet KO) mice, Eomesfl/flCD4 -Cre (Eomes KO) mice (12), Thy1.1 mice, CD45.1 mice, and RAG1−/− mice were back-crossed to C57BL/6 for at least ten generations. For adoptive transfer experiments, 5×104 Thy1.1 P14 CD8+ T cells were mixed with 5×104 Eomes KO P14 CD8+ T cells were transferred intravenously into CD45.1 recipients, with viral infection the following day.
Infections
Mice were infected with 2×105 plaque forming units (initial challenge) or 1×106 plaque forming units (re-challenge) LCMV Armstrong strain by intra-peritoneal injection. For rechallenges, 5×105Listeria monocytogenes expressing GP33 were injected intravenously.
RAG1−/− Bone Marrow Chimeras
Recipient RAG1−/− mice were subjected to sub-lethal irradiation (400 rads) and injected intravenously with 5 × 106 Thy1.1 bone marrow cells mixed with 5 × 106 Eomes KO bone marrow cells harvested on the same day. 8–10 weeks post transplant, peripheral blood from each recipient was analyzed for presence and relative numbers of CD8+ T cells, CD4+ T cells, and B cells derived from each background.
Flow cytometry and T cell stimulation
Surface staining, peptide stimulations, intracellular cytokine staining, H-2Db GP33–41 (GP33) and NP396–404 (NP396) tetramer staining and flow cytometry were done as described (12). Antibodies used for flow cytometry were purchased from BD biosciences (CD44, CD62L, CD122, CD127, CD27, IFN-γ, KLRG1, Thy1.2, BrdU), or eBioscience (Eomes, CXCR4, integrin α4, integrin β1).
BrdU Incorporation
Mice received 0.2 ml PBS containing 2 mg BrdU by intra-peritoneal injection daily for seven days prior to analysis.
Quantitative RT-PCR
Quantitative real-time PCR primer and probe set used for HPRT was previously described (10). Presynthesized Taqman Gene Expression Assays (Applied Biosystems) were used to amplify CXCR4, CXCR3, and Bcl-2. Sample gene values are expressed relative to HPRT, with the lowest value standardized at 1.
Results and Discussion
Diminished central-memory CD8+ T cell population in the absence of Eomes
To evaluate the expression of Eomes at the single cell level in CD8+ T cells responding to infection, we infected C57BL/6 mice with LCMV Armstrong and analyzed the expression of Eomes using intracellular flow cytometry in LCMV-specific CD8+ T cells. Specificity of Eomes staining was validated using Eomes KO cells (Fig. S1). Compared to CD8+ T cells 8 days after LCMV infection, the vast majority of which are effector cells, LCMV specific memory CD8+ cells 90 days after initial infection express higher levels of Eomes (Fig. 1A). Our results support a correlation between Eomes protein expression and preservation as LCMV-specific memory.
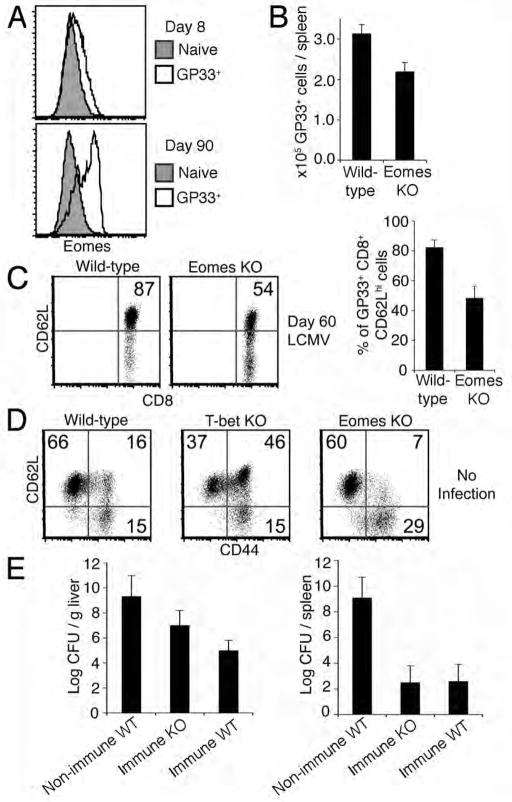
Figure 1. Diminished central-memory CD8+ T cell compartment in the absence of Eomes.
(A) Eomes expression in effector (8 days post LCMV infection, GP33+) and memory (90 days post LCMV infection, GP33+) CD8+ T cells relative to naïve (CD44lo GP33-) CD8+ T cells from spleens of C57BL/6 mice.
(B) Quantification of CD8+ GP33+T cells harvested from spleens of wild-type and Eomes KO mice 60 days after infection with LCMV. Data in (A) and (B) are representative of three independent experiments.
(C) GP33-specific central-memory CD8+ T cells in spleens of wild-type versus Eomes KO mice 60 days after infection with LCMV. Plots show CD8+CD44 +GP33 + cells. Numbers within plots refer to percentage of cells in the upper right quadrant. Graph shows mean +/− SEM from six mice of each genotype.
(D) Central-memory (CD62Lhi CD44hi), effector-memory (CD62LloCD44 hi) and naïve (CD62Lhi CD44lo) CD8+ T cell compartments in spleens of 6–12 month old naïve, non-infected wild-type, T-bet KO, or Eomes KO mice. Numbers within plots refer to percentage of cells in the respective quadrant. Data are representative of three independent experiments.
(E) Listeria monocytogenes-GP33 (LMgp33) burden in liver or spleen 3 days after infection with 5×105 LMgp33 organisms in non-immune mice (non-immune WT), immunized (by LCMV infection 100 days prior) wild-type mice (immune WT), and immunized Eomes KO mice (immune KO) with three mice per group. Data is representative of two independent experiments.
We infected Eomes KO mice and Eomes-proficient control mice (hereafter referred to as wild-type) with LCMV. 60 days after infection, we observed a modest deficit in memory CD8+ T cells specific to the LCMV epitope GP33 in Eomes KO mice (Fig. 1B). We found that Eomes KO LCMV GP33-specific memory CD8+ T cells have a substantial reduction in percentage of cells that are CD62Lhi compared to wild-type, suggesting that fewer antigen-specific CD8+ T cells differentiate into central-memory CD8+ T cells in the absence of Eomes (Fig. 1C).
We next examined the effector-memory (CD44hiCD62Llo) and central-memory (CD44hiCD62Lhi) CD8+ T cell compartments in Eomes KO mice compared to T-bet KO and wild-type mice that were aged at least six months and unchallenged (no LCMV infection) (Fig. 1D). In contrast to the increased central-memory population in the absence of T-bet, central-memory CD8+ T cells are less abundant in Eomes KO mice compared to wild-type.
To assess the ability of Eomes deficient CD8+ T cells to mount a secondary memory response, we challenged Eomes KO or wild-type mice that had been infected with LCMV 100 days prior with 10-times the 50% lethal dose of Listeria monocytogenes expressing GP33. Eomes KO mice had a mild defect in clearance of bacteria from the liver, but no defect in the spleen, suggesting preserved functional protective capacity in Eomes KO memory CD8+ T cells (Fig. 1E). Overall our findings suggest that there may not be an absolute requirement for Eomes in central-memory CD8+ T cell development. Nonetheless, the data are compatible with a role for Eomes in promoting central-memory-like cell persistence.
Eomes-deficient CD8+ T cells contribute poorly to the memory cell pool when competing with normal cells
The relative defect in LCMV specific CD8+ T cell memory in Eomes KO mice indicates that while Eomes is not essential in memory development, its expression in individual CD8+ T cells may provide a competitive advantage to persist as a memory cell. We employed a mixed bone marrow chimeric system to assess memory development in Eomes KO cells in direct competition with wild-type CD8+ T cells. Sub-lethally irradiated RAG1 deficient mice were reconstituted with bone marrow from an Eomes KO (Thy1.2+) mouse mixed in equal proportion with Thy1.1+ CD4 Cre+ (hereafter referred to as WT) bone marrow. Eomes deficiency did not appear to confer an advantage or defect in naïve T cell homeostasis, as relative numbers of naïve CD8+ T cells from Eomes KO and WT backgrounds were roughly equivalent (not shown). We infected these chimeras (Eomes KO/WT BM chimeras) with LCMV and followed the development of GP33- and NP396- specific memory CD8+ T cells derived from each genotype (Fig. 2A).
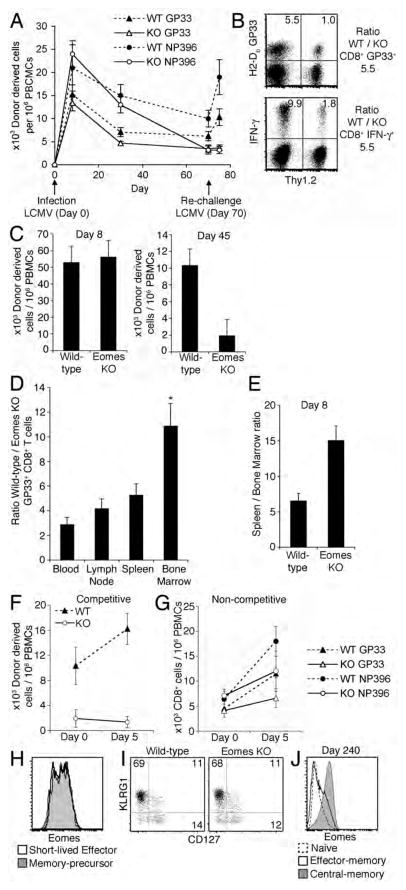
Figure 2. Defective memory CD8+ T cell persistence and re-expansion in the absence of Eomes in competitive models.
(A) Serial flow cytometric analysis of peripheral blood from Eomes KO/WT bone marrow chimeras, showing CD8+GP33 + and CD8+ NP396+ cells derived from Eomes KO bone marrow or WT bone marrow, as labeled, versus time in relation to initial LCMV infection. Data are representative of three independent experiments with four chimeric mice each.
(B) Ratio of wild-type to Eomes KO CD8+ GP33+ T cells (top plot) and IFN-γ+ CD8+ T cells (bottom plot) in the spleen of a Eomes KO/WT bone marrow chimera spleen five days after rechallenge with LCMV (65 days after primary infection). Numbers within quadrants represent percentage of cells within that quadrant. Data are representative of five independent experiments.
(C) Relative number of Eomes KO (CD8+ Thy1.2+ CD45.1−) and wild-type (CD8+ Thy1.2− CD45.1−) P14 cells in the blood of recipients 8 days or 45 days after primary LCMV infection. Data are mean +/− SEM and are derived from four chimeric mice in each of three independent experiments.
(D) Ratio of WT versus Eomes KO CD8+ GP33+ cells in Eomes KO/WT bone marrow chimeras 60 days after LCMV infection. Data presented as mean and SEM from four individual chimeras. * P < .01 vs. blood, P < .05 vs. lymph node or spleen; Student’s two-tailed T test
(E) Ratio of P14 cells in spleen relative to bone marrow 8 days after LCMV infection for both wild-type and Eomes KO populations in a competitive adoptive transfer setting. Data are representative of 7 co-transfer recipients over two independent experiments.
(F) Relative number of Eomes KO (CD8+ Thy1.2+ CD45.1−) and wild-type (CD8+ Thy1.2− CD45.1−) P14 cells in the blood of recipients on the day of LCMV re-challenge (45 days after primary infection) and five days after re-challenge. Data are mean +/− SEM and are representative of three independent experiments with four mice per experiment.
(G) Relative number of GP33+ or NP396+ CD8+ cells in the blood of Eomes KO or Wild-type mice at least 90 days after initial LCMV infection on the day of LCMV re-challenge and five days after re-challenge. Data are mean +/− SEM with four mice per group.
(H) Eomes expression in short-lived effector (KLRG1hi CD127lo CD8+ GP33+) and memory-precursor (KRLG1lo CD127hi CD8+ GP33+) populations in spleens from mice 8 days after LCMV infection. Data is representative of four independent experiments.
(I) KLRG1hi CD127lo cells (number in left upper quadrant) and KLRG1lo CD127hi cells (number in right lower quadrant) in wild-type (CD8+ Thy1.2− CD45.1−) and Eomes KO (CD8+ Thy1.2+ CD45.1−) P14 cells in a competitive adoptive transfer setting 8 days after LCMV infection. Data is representative of 2 independent experiments with a total of 7 co-transfer recipients.
(J) Eomes expression in naïve (CD44lo CD62Lhi GP33−), central-memory (CD44hi CD62Lhi GP33+) and effector-memory (CD44hi CD62Llo GP33+) CD8+ T cell populations from the spleen of a mouse 240 days after LCMV infection. Data are representative of two independent experiments.
Eomes KO CD8+ T cells responded comparably to control CD8+ T cells after acute LCMV infection (Day 8) (Fig. 2A), but at time points post viral clearance (Days 30 and 70) the number of Eomes KO GP33-specific and NP396-specific CD8+ T cells declined relative to WT. On reinfection with LCMV on Day 70 after primary infection, GP33-specific and NP396-specific CD8+ T cells derived from WT bone marrow underwent more robust re-expansion than those derived from Eomes KO bone marrow (Fig. 2A). While most subsequent results are shown in GP33-specific populations, similar findings were found in NP396-specific CD8+ T cells. Eomes KO GP33-specific memory CD8+ T cells were not defective in the expression of IFN-γ following activation with GP33 peptide (Fig. 2B).
To reduce variability resulting from TCR heterogeneity, we also employed a competitive adoptive transfer model using TCR transgenic T cells. We combined naïve WT P14 (LCMV GP33-specific) TCR transgenic CD8+ T cells (hereafter referred to as WT P14 and marked by Thy1.1) with an equal number of Eomes KO P14 CD8+ T cells (marked by Thy1.2) and injected the combination into CD45.1 non-TCR transgenic recipients. We infected recipient animals with LCMV and followed the relative abundance of Eomes KO P14 and WT P14 CD8+ T cells. We found no consistent differences between Eomes KO and WT effector CD8+ T cells expansion 8 days after infection (Fig. 2C). After viral clearance (Day 45), the relative percentage of memory Eomes KO P14 cells declined precipitously (Figure 2C). We analyzed the relative prevalence of Eomes KO versus wild-type GP33 specific memory cells in the blood, lymph nodes, spleen, and bone marrow of Eomes KO/WT bone marrow chimeric mice 60 days after infection with LCMV (Fig. 2D). We observed a consistent hierarchy among different tissues, with the least amount of skewing in the blood, modestly increased skewing in the spleen and lymph nodes, and markedly higher skewing in the bone marrow (Fig. 2D). While much of the data shown is derived from blood, similar observations were made in almost all lymphoid organs (not shown). In the setting of acute infection, we observed a 2.3-fold defect in bone marrow localization in Eomes KO P14 cells 8 days after LCMV infection in the competitive adoptive transfer model (Fig. 2E), suggesting a role for Eomes in bone marrow localization in both effector and memory CD8+ T cells.
Upon re-infection of immune mice 45 days after initial infection, WT P14 cells underwent re-expansion, while Eomes KO P14 cells underwent virtually no expansion and remained barely detectable (Fig. 2F). The defect in Eomes KO memory CD8+ T cell re-expansion when competing with wild-type cells was more severe than differences observed in non-chimeric Eomes KO and control mice re-challenged with LCMV 90 days after initial infection (Fig. 2F, G). The results suggest that Eomes enables CD8+ T cells to compete for niches and signals that promote memory differentiation rather than being an absolute regulator of memory differentiation.
The results raise the possibility of a relationship between Eomes expression and the development of memory-precursor (KLRG1lo CD127hi) CD8+ T cells (11). In the setting of acute LCMV infection, we observed similar levels of Eomes in memory-precursors (KLRG1lo CD127hi) and short-lived effector cells (KLRG1hi CD127lo) (Fig. 2H). Similarly, 8 days after infection, short-lived effector and memory-precursor population frequencies do not significantly differ within Eomes-deficient and -sufficient populations in a competitive setting (Fig. 2I). Several months after viral clearance, we observed significantly higher levels of Eomes in central-memory (CD44hi CD62Lhi) relative to effector-memory (CD44hi CD62Llo) CD8+ T cells (Fig. 2J). Taken together, these data do not support a role for Eomes in promoting the memory-precursor fate. Instead, the results provide evidence that Eomes is involved in bone marrow localization, long-term persistence, and re-expansion capacity of memory cells.
Defective population of the bone marrow niche by Eomes KO memory CD8+ T cells
The different ratios of wild-type to Eomes KO memory CD8+ T cells in different lymphoid tissues raises possibilities of altered bone marrow survival, proliferation, or trafficking in CD8+ T cells lacking Eomes. Molecules associated with memory T cell bone marrow localization include the chemokine receptor CXCR4, and VLA-4, which is composed of integrin α4 and integrin β1 (5). We observed diminished expression of CXCR4, but neither integrin α4 nor integrin β1, in Eomes KO GP33-specific memory CD8+ T cells from Eomes/WT bone marrow chimeric mice compared to WT 60 days after LCMV infection (Fig. 3A). Sorted Eomes KO central memory (CD44hiCD62Lhi) CD8+ T cells from Eomes/WT bone marrow chimeric mice 60 days after LCMV infection were found to have less CXCR4 and CXCR3 mRNA compared to their WT counterparts (Fig. 3B). Currently there does not appear to be a known role for CXCR3 in bone marrow homing.
Figure 3. Defective bone marrow localization and proliferation Eomes KO memory CD8+ T cells.

(A) CXCR4, integrin α4, and integrin β1 expression on CD8+ GP33+T cells from spleens of Eomes KO/WT bone marrow chimeras 60 days after LCMV infection. Plots are representative results from three chimeric mice.
(B) Quantitative RT-PCR of CXCR4 or CXCR3 mRNA from wild-type versus Eomes KO central-memory (CD44hi CD62Lhi) CD8+ cells sorted from an individual Eomes KO/WT bone marrow chimera 60 days after infection with LCMV. Data are representative of two independent experiments.
(C) BrdU uptake in Eomes KO versus wild-type CD8+ GP33+ T cells from the bone marrow of Eomes KO/WT bone marrow chimeras 60 days after LCMV infection. Data are derived from four chimeric animals.
(D) Quantitative RT-PCR of Bcl-2 mRNA as in (B).
(E) CD122, CD127, and CD27 expression on CD8+ GP33+ T cells from spleens of wild-type and Eomes KO mice 60 days after infection with LCMV. Data are representative of three independent experiments.
Diminished persistence of Eomes KO memory CD8+ T cells could be related to defects in homeostatic proliferation, survival or both. The highest rate of homeostatic proliferation of memory CD8+ T cells is found in the bone marrow, and in bone marrow memory CD8+ T cells we observed a modest reduction in proliferation in Eomes KO cells compared to wild-type as measured by BrdU incorporation (Fig. 3C). In addition to their proliferation defect, a survival disadvantage is suggested by our observation of reduced expression of Bcl-2 mRNA in Eomes KO memory CD8+ T cells (Fig. 3D). Proliferation and survival of memory CD8+ T cells are supported by cytokine signals, including IL-15 and IL-7, as well as CD27-CD70 interactions (1). We found a modest, but reproducible defect in CD122 (IL-15Rβ) expression but no deficit in expression of CD127 (IL-7Rα) or CD27 on Eomes KO memory CD8+ T cells (Fig. 3E and Fig. S2). These data suggest that reduced proliferation and survival might both be involved in the defective persistence of Eomes KO memory CD8+ T cells.
In summary, we provide evidence that CD8+ T cells lacking Eomes are less fit for preservation as memory and population of the bone marrow memory niche. These observations are in keeping with a model in which the relative expression levels of Eomes and T-bet may contribute to the adoption of a memory fate versus terminal effector differentiation in CD8+ T cells. Given the ability to manipulate the relative expression of T-bet and Eomes with agents including rapamycin and IL-12 (13, 14), the data provide rationale for novel approaches to generating long-lasting immunity.
Supplementary Material
Acknowledgments
We are grateful to M. Ciocca, L. Rupp, and J. Chaix for assistance and discussion.
This work was supported by National Institute of Health (grants AI061699, AI076458, AI071309, AI007324, AI055428, CA076931, and CA09140) and the Abramson Family Cancer Research Institute.
References
- 1.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 2.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 3.Becker TC, Coley SM, Wherry EJ, Ahmed R. Bone marrow is a preferred site for homeostatic proliferation of memory CD8 T cells. J Immunol. 2005;174:1269–1273. doi: 10.4049/jimmunol.174.3.1269. [DOI] [PubMed] [Google Scholar]
- 4.Letsch A, Knoedler M, Na IK, Kern F, Asemissen AM, Keilholz U, Loesch M, Thiel E, Volk HD, Scheibenbogen C. CMV-specific central memory T cells reside in bone marrow. Eur J Immunol. 2007;37:3063–3068. doi: 10.1002/eji.200636930. [DOI] [PubMed] [Google Scholar]
- 5.Mazo IB, Honczarenko M, Leung H, Cavanagh LL, Bonasio R, Weninger W, Engelke K, Xia L, McEver RP, Koni PA, Silberstein LE, von Andrian UH. Bone marrow is a major reservoir and site of recruitment for central memory CD8+ T cells. Immunity. 2005;22:259–270. doi: 10.1016/j.immuni.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Parretta E, Cassese G, Barba P, Santoni A, Guardiola J, Di Rosa F. CD8 cell division maintaining cytotoxic memory occurs predominantly in the bone marrow. J Immunol. 2005;174:7654–7664. doi: 10.4049/jimmunol.174.12.7654. [DOI] [PubMed] [Google Scholar]
- 7.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, Reiner SL. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 8.Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA, Shen H, Cereb N, Yang SY, Lindsten T, Rossant J, Hunter CA, Reiner SL. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan BM, Juedes A, Szabo SJ, von Herrath M, Glimcher LH. Antigen-driven effector CD8 T cell function regulated by T-bet. Proc Natl Acad Sci U S A. 2003;100:15818–15823. doi: 10.1073/pnas.2636938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Intlekofer AM, Takemoto N, Kao C, Banerjee A, Schambach F, Northrop JK, Shen H, Wherry EJ, Reiner SL. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J Exp Med. 2007;204:2015–2021. doi: 10.1084/jem.20070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Intlekofer AM, Banerjee A, Takemoto N, Gordon SM, Dejong CS, Shin H, Hunter CA, Wherry EJ, Lindsten T, Reiner SL. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science. 2008;321:408–411. doi: 10.1126/science.1159806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 32:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, Reiner SL. Cutting Edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J Immunol. 2006;177:7515–7519. doi: 10.4049/jimmunol.177.11.7515. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.