Abstract
After fear conditioning (e.g., by pairing a tone to a shock), memory retrieval typically leads to fear expression (e.g., freezing to the tone). Here, we examined the effect of a conditioned rat's fear memory retrieval on a naïve cage-mate's behavior to the conditioned stimulus. We show that rats exposed to a novel tone in the presence of a cage-mate previously conditioned to that same tone selectively showed increased freezing to the stimulus the next day (fear conditioning by-proxy). In addition, fear conditioning by-proxy experienced prior to pairing the tone to a mild shock increased freezing during presentation of that tone the next day. Our results suggest that, during memory retrieval, fear of a stimulus can be socially transmitted to a cage-mate. These findings may have implications for models of phobias.
Keywords: Social transmission of fear, fear conditioning, memory retrieval, observational learning
In fear conditioning, the pairing of an initially neutral conditioned stimulus (e.g., an auditory tone) to an aversive unconditioned stimulus (e.g., a footshock) leads to the formation of a long-lasting fear memory, such that when the tone is later presented on its own, it elicits fear expression [11], [22], [13]. This simple paradigm has been extensively studied, since the pathways engaged during the formation of fear memories are thought to overlap greatly with those that are involved in anxiety-related disorders [11]. Certain types of anxiety-related disorders, for instance post-traumatic stress (PTSD), involve, by definition, a previous experience that led to the formation of a memory now at the source of the disorder symptoms [14]. Re-experiencing the traumatic episode, via internal or external triggering of the memory, is a key aspect of PTSD [14]. In the case of specific phobias, however, attributions of fear to stimuli is not necessarily preceded by a traumatic episode encoded in memory [21]. For example, an individual may have an extreme fear of spiders, and yet have no recollection of having ever experienced a situation in which a spider inflicted them harm.
One plausible explanation for such a case may be that the individual simply does not recall the episode that would have caused the phobia initially. Another possibility would suggest that fear can be socially passed on, and thus acquired indirectly, through transmission of verbal information or vicarious learning in response to an otherwise neutral stimulus [21].
Acquiring information about stimuli that predict danger is extremely important for individuals' ability to generate appropriate behaviors in response to threats. Much is known about the ability to learn about stimuli that directly predict fear, and several studies have described observational learning of fear in primates [7], [15], [16], [18], [19], [7]; yet, literature on rodents' ability to learn about danger indirectly through observing another's reaction has only recently started to emerge [9], and [10]. Through a series of elegant studies, Knapska and colleagues developed a model in which rats were housed in pairs, and one of the two was fear conditioned to a context. After interacting with the conditioned cagemate, a rat shows increases in amygdala activity (as evidenced by increased c-Fos labeling) [9], interacts differently with its cagemate, and shows enhanced fear learning [10]. Other groups have shown that the presence of a non-fearful conspecific can help reduce fear experienced when rodents are placed in a novel context [6].
Here, we sought to examine whether fear could be socially transmitted in rats during retrieval of a discrete memory. Specifically, we asked whether a specific cue (a tone) could come to elicit fear expression simply by having a rat observing a conspecific's conditioned response in the presence of that otherwise benign stimulus. We next tested whether this aforementioned procedure would modulate a subsequent tone-shock pairing fear association.
Methods
Subjects
Male Sprague-Dawley rats (250-275g) (Harlan Lab Animals Inc) were used for all experiments. Forty-two rats were used for experiment 1, and 18 rats for experiment 2. Procedures were conducted in compliance with the National Institutes of Health Guide for the Care and Use of Experimental Animals and were approved by the University of Texas at Austin Animal Care and Use Committee.
Housing
Rats were housed in triads in temperature and humidity-controlled transparent polyethylene cages and were maintained on a 12h/12h light/dark cycle with food and water ad libitum for the duration of the experiments. Groups were housed together for a minimum of 10 days prior to the beginning of experiments. Rats were randomly assigned to each triad as soon as they arrived to the University of Texas at Austin from Harlan. All rats from this experiment arrived to campus from the same shipment. Each triad consisted of one rat to be fear conditioned (FC), one rat to be fear conditioned by-proxy (FCbP), and one rat that would not be conditioned (naïve control). Rats were not handled, other than to perform cage changes, prior to the start of the experiements.
Behavior
Experimental design
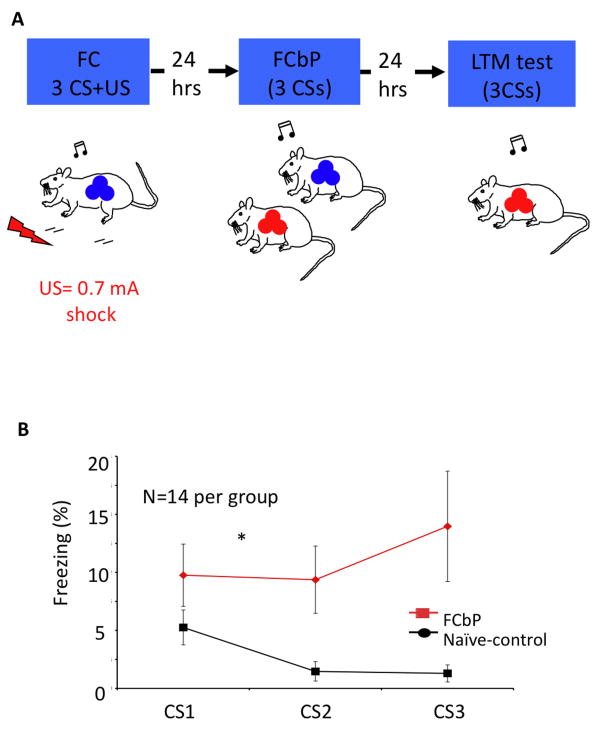
Two experiments were conducted. In the first experiment, we fear conditioned one of the rats from each triad with 3 tone-shock pairings. The next day, each conditioned rat returned to the conditioning context along with one of its cagemates (FCbP), and 3 tones were presented. All rats were then returned to their home cage, and tested the next day for fear memory expression. The fear conditioning by-proxy paradigm is presented in Figure 1A.
Figure 1.
(A) Experimental design diagram illustrating the fear conditioning by-proxy paradigm. One rat from the triad was conditioned to 3 tone-shock pairings (0.7 mA footshock) (Blue). The next day, the conditioned rat and one of his cage-mates (FCbP rat, red) were placed in the conditioning chamber and 3 tones were played. No shock US was delivered at this time. Twenty-four hours later, all rats were tested for their freezing response to the CS (FCbP rat is shown here). (B) Effect of fear conditioning by proxy (FCbP) on freezing to a tone. FCbP rats showed significantly (p<0.05) more freezing to the tone than their naïve control cagemates when tested in the context in which they observed conditioned fear responses 24 h earlier.
In the second experiment, one rat from the triad was fear conditioned, and one rat was fear conditioned by-proxy. Four hours after conditioning by-proxy, the FCbP and naïve control rats were conditioned to a mild shock (20 second tone, coterminating with a 500 ms, 0.4 mA footshock). All rats were tested for their freezing to the tone the next day.
Apparatus
Rats were trained and tested in yoked fear conditioning chambers (Rat Test Cage, Coulbourn Instruments, Allentown, PA) equipped with metal stainless-steel rod flooring connected to a shock generator (Model H13-15; Coulbourn Instruments). Each chamber was individually enclosed and sound-insulated (Model H10-24A; Coulbourn Instruments). There was no barrier between the observer and the demonstrator animal during the fear conditioning by proxy; the rats could freely interact with one another. Behavior was digitally recorded on the computer hard drive using infrared digital cameras mounted within each unit. Stimulus presentation was automated using Freeze Frame software (Coulbourn Instruments). All equipment was rinsed with water between each session.
Fear conditioning
Rats were first habituated for 10 minutes in fear conditioning chambers lit with white house lights, and then were fear conditioned with three 20-second, 5kHz, 80dB tones (CS), each coterminating with a 500ms, 0.7mA footshock (US) (for experiment 1), or a 500ms, 0.4mA footshock (for experiment 2). The interval between each CS was 180 seconds on average. After conditioning, each rat was returned to its home cage.
Fear conditioning by-proxy (FCbP)
One day after conditioning, one rat per triad was acclimated to the conditioning chambers for 10 minutes, and then received 3 CS presentations (as described above) in the presence of their previously conditioned cage-mate. The interval between each CS was 180 seconds on average.
Long-term memory—fear expression test
Twenty-four hours after FCbP, all rats received an LTM test (3 CSs) in the FCbP context to assess fear expression to the tone presentation.
Behavioral Scoring
Freezing
Behavior was manually scored by 2 individual raters blind to experimental conditions. Freezing was defined as the absence of any movement, excluding breathing and whisker twitching. The total number of seconds of CS-induced freezing, expressed as % from CS duration (20 seconds), was used as a measure of fear.
Social interactions
Social contact was defined as any physical contact or interaction (qualitatively defined below), excluding accidental contact made in passing, measured as the percentage of time that the FCbP rat spent in contact with the fear conditioned (FC) rat throughout the duration of each CS, and during the 20 seconds preceding the first CS presentation. This contact was subdivided into six unique behavior types: grooming partner, paw contact, body contact, sniffing, nose-to-nose contact, and play. ‘Grooming partner’ occurred when the FCbP rat groomed the FC rat while the tone was playing. ‘Paw contact’ occurred when the FCbP rat placed one or both of his paws on the FC rat excluding both accidental contact from trying to get around the FC rat or using the FC rat as a support to reach a different area of the chamber. ‘Body contact’ occurred when the FCbP rat maintained close contact with the FC rat by either leaning against the FC rat or huddling against him. ‘Sniffing’ occurred when the FCbP rat actively sniffed at the FC rat while in the FC rat's immediate vicinity. ‘Nose-to-nose contact’ occurred when the two rats touched noses while facing one another. ‘Play’ occurred when the two rats engaged in any mode of playful behavior, including wrestling, pouncing, biting, or chasing.
Data Analysis
Tests were performed for the relevant phase of the experiment (LTM). For the first experiment, initial analyses compared freezing between the FCbP and naïve control using a repeated measures ANOVA. Additional analyses were conducted for the first experiment after dividing the FCbP group into 2 using a median split. All other comparisons were statistically analyzed using a repeated-measures ANOVA with group as a factor, and long-term memory CSs as a repeated measures factor. Significant main effects and interactions were followed by further tests (Fisher's PLSD) to identify the source of those effects. To examine the potential contributing factors to freezing in the FcbP group, we also performed a stepwise multiple regression with ‘FC partner freezing’ and ‘social interaction’ as independent variables, and ‘FcbP freezing’ as a dependent variable. Only independent variables that contributed significantly to the variance accounted for in the dependent variable were entered in the final regression equation. Differences were considered significant if p < 0.05.
Results
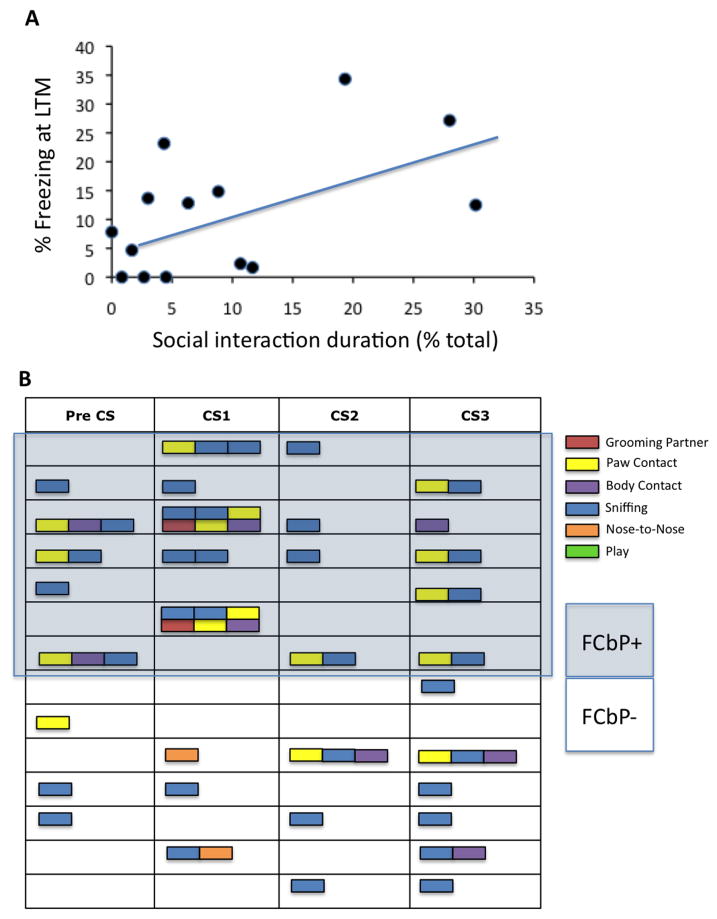
In our first experiment, we sought to examine whether rats could come to fear a tone simply by being present during a previously conditioned cagemate's exposure to that tone. As shown in figure 1B, the FCbP rats, on average, froze significantly more to the tone than their naïve control cagemates, F(1,26)=8.486, p<.05. The effect size was modest, and there was more variability in the FCbP group than the naïve control (FCbP SD=3.45%, naïve control SD=1.02%). Qualitative assessment of the raw data suggested that 50% of the rats in the FCbP group did not show any freezing to the tone during testing. We thus decided to perform a median split for the FCbP group (and labeled these FCbP+ and FCbP-), and to run additional analyses comparing those groups to the naïve control and the FC groups. A between group ANOVA revealed a significant main effect of group, F(3,37)=51.946, p<.05 Follow-up tests (Tukey) showed that the FCbP+ and the FC groups froze significantly more to the tone than the naïve control and FCbP- (p<.05, all comparisons). In addition, the FC group froze significantly more than the FCbP+ (p<.05) (Figure 2). Pre-CS freezing (20 seconds prior to first CS presentation) was minimal, and was not significantly different between groups (p>.05), suggesting that subsequent freezing was specific to the CS (respective mean ± SEM freezing (%) were as follows: Naive controls= 1.57±0.74; FCbP+= 0±0; FCbP-= 0±0; FC= 7.65±2.52). We next tried to identify potential factors that could contribute to the differential freezing in the FCbP rats, by examining the effects of ‘FC partner freezing’ and ‘social interaction duration’ on FCbP freezing using a step-wise multiple regression analysis. Our results suggest that ‘FC partner freezing’ did not significantly correlate with FCbP freezing (p<.05). ‘Social interaction duration’ significantly correlated with FCbP freezing, R(12)=.533, p<.05, accounting for 28.5% of the variance in that group (R2=.285) (Figure 3A). The qualitative breakdown of the social interactions between the FC and FCbP rats is shown in figure 3B.
Figure 2.
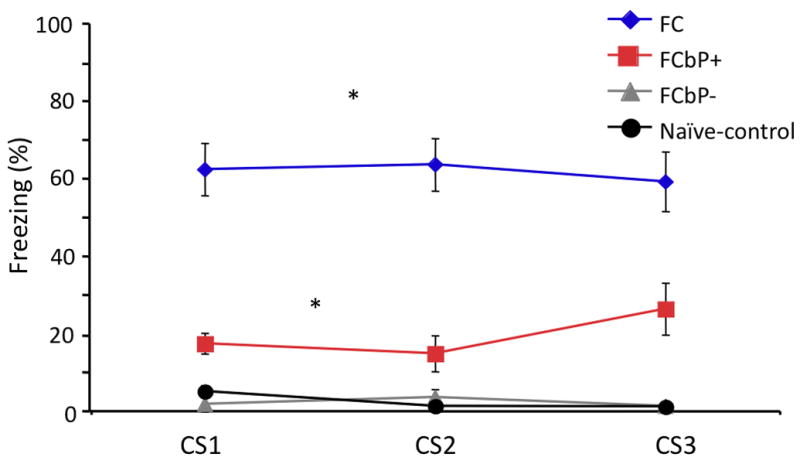
FCbP median split shows that only half of FCbP rats displayed freezing behavior when tested 24 h after the FCbP training session. The upper half of the FCbP rats (FCbP+) froze more than the lower half (FCbP-) of the FCbP and the naïve control rats (p<.05). Fear-conditioned rats froze significantly (p < 0.05) more than either FCbP or naïve rats.
Figure 3.
Social interaction duration and type between the FC and FCbP rats before and during CS exposure. (3A) Relationship between social interaction duration with an FC cagemate during CS, and FCbP freezing the next day. A multiple regression analysis revealed that the duration of social interactions between the FC and FCbP rats significantly accounted for the variance accounted for in FCbP freezing the next day, R(12)=.533, p<.05. (3B) Qualitative depiction of social interaction types between the FC and FCbP rats during each CS, and for 20 seconds prior to the first CS presentation.
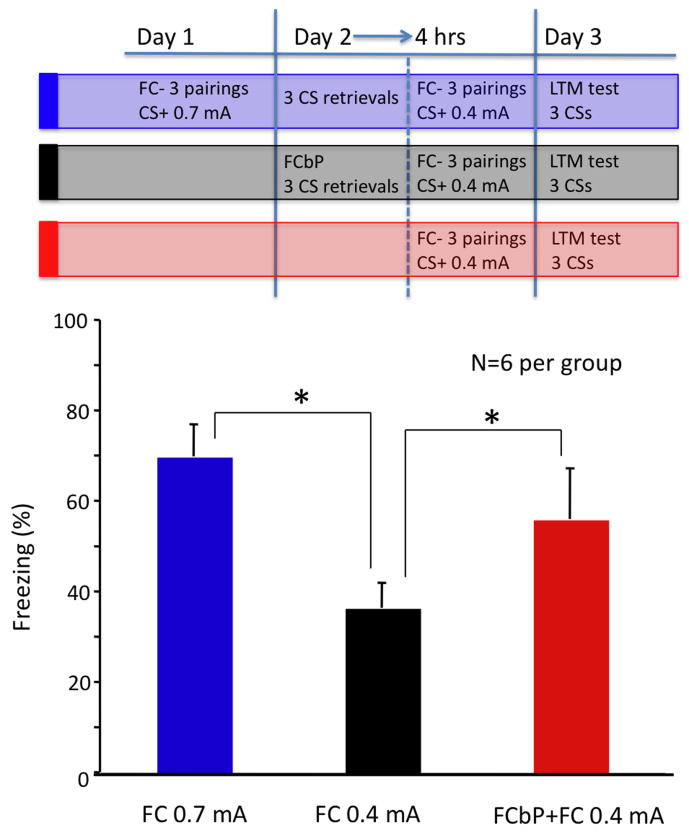
Next, we examined whether FCbP treatment would affect freezing induced by subsequent conditioning to a milder shock (0.4 mA). Three groups were compared: FC 0.7 mA, FC 0.4 mA, and FCbP+FC 0.4 mA. The experimental design is illustrated in figure 4A. A between-subject ANOVA revealed a significant main effect of group, F(2,17)=10.9, p<.05. Follow-up tests (Fisher's PLSD) were conducted to compare the groups, and as shown in figure 4B, the FC 0.7 mA group froze significantly more than the FC 0.4 mA (p<.05); the FCbP+FC 0.4 mA froze significantly more than the FC 0.4 mA (p<.05). Figure 4B shows average freezing across the 3 CS presentations at the LTM test. There was no significant difference between the FC 0.7 mA and the FCbP+FC 0.4 mA groups (p>.05).
Figure 4.
(A) Experimental design diagram illustrating methodology for experiment 2. First, one rat from the triad was conditioned to 3 tone-shock pairings (0.7 mA footshock). The next day, the conditioned rat and one of his cage-mates (FCbP rat) were placed in the conditioning chamber and 3 tones were played. No shock US was present at this time. Four hours later, the third rat (until then, naive), as well as the FCbP rat, individually received 3 CS-US pairing (0.4 mA US). All rats (FC-0.7 mA [Blue], FC-0.4 mA [black], and FCbP+FC-0.4 mA [Red]) were tested 24 h later for their freezing response to the CS. (B) Effect of fear conditioning by proxy (FCbP) on subsequent conditioning. Rats conditioned using 0.7 mA shock US (Blue) froze significantly more than the rats conditioned using 0.4 mA shock US (Black) (p<0.05). Rats that were fear-conditioned by-proxy (Red) prior to being conditioned to tone shock pairings (0.4 mA US) froze significantly (p<0.05) more than the previously naïve rats conditioned using the same parameters. There was no significant difference between the rats conditioned with 0.7 mA shock US and the rats conditioned with a milder shock US (0.4 mA) who underwent FCbP prior to the CS-US pairing (p>.05).
Discussion
The ability to acquire and retain information about potentially threatening stimuli is important to individuals' survival. Much work has been done in understanding how direct danger information is acquired, mostly through studies on fear conditioning [11], [13]. Recent work in rodents has emerged suggesting that interactions with a conspecific that recently experienced fear increases another's fear response following conditioning [10], [3]. Here, we extend these findings and show that retrieval of a cued fear memory can induce fear conditioning in a cagemate observer. The effect is modest, but clearly impacts subsequent fear learning. To our knowledge, this study is the first study to directly assess the effects of a conspecific's fear reaction to an otherwise benign stimulus on an observer's response to that stimulus at a later time. Most previous studies employed observational learning paradigms in which observers witnessed a conspecifc reacting fearfully to either an unconditioned stimulus (e.g. Mineka and Cook, 1993) or observed conditioning episodes in which the CS was paired with the US (e.g. Ollson and Phelps, 2004). The present findings demonstrate that rats can acquire fear of a neutral stimulus simply by being exposed to a previously conditioned rat's response to that stimulus.
Only a portion of rats that experienced fear conditioning by proxy showed fear expression to the tone the next day. Our analysis of the social interaction that took place during FCbP showed that the duration of social contact between the FC and FCbP rats was positively correlated with subsequent freezing in response to the tone stimulus. FCbP rats that spent more time interacting with a conditioned rat during FCbP showed higher freezing to the tone CS the next day. This effect was not modulated by the amount of freezing displayed by the FC rat. Future studies will examine possible factors that contribute to enhanced physical contact (and FCbP acquisition) from some rats but not others. It would be interesting to examine whether individual differences in the rats' behavior toward their conditioned cagemates are modulated by their hierarchical status within a triad, and whether the effects we observed in the current study would be enhanced by housing the triads together for a longer period of time (from weaning age, for example). This model may prove useful for future studies of empathy in rodents, and could further our understanding of the relationship between empathy and anxiety-related disorders, which has only recently started being explored [5].
Mineka et al. (1987) previously found that rhesus monkeys can more readily acquire observational learning of fear-relevant stimuli, such as snakes, than neutral stimuli, such as flowers. In the present study, rats learned, through observation of the previously conditioned cage mate, that a neutral stimulus (tone) can acquire aversive value. Some anxiety disorders (e.g., phobias) are thought, in some cases, to be acquired through observational learning rather than from a direct experience with a fearful stimulus [14]. An animal model which could account for observational learning of not only species-specific fear-relevant stimuli, but also of fear associated with any stimulus, would expedite study of root causes of certain anxiety disorders, such as phobias.
It has been speculated that learning through observation may be supported by an evolutionarily old system that predates the emergence of language [19]. Results presented here support that notion, but also suggest that not all rats subjected to fear conditioning by-proxy acquire fear to the tone. It will be important, in future studies, to isolate potential factors that might be contributing to individuals' susceptibility to acquiring fear by-proxy. A recent study, conducted in mice, also reports individual differences in social transmission of fear [3]. In that case, the differences could be accounted for by differences in genetic background: the more social strain showed fear transmission, and the less social strain did not [3]. Fear was transmitted by having the mice observe their conspecific be conditioned to tone-shock pairings. Interestingly, another group found that exposure to a recently conditioned familiar mouse impaired the subsequent acquisition of fear in the observer mouse [2]. In that experiment, interactions between the conditioned mouse and the observer occurred in the home cage. A different group also found that, whereas mice pre-exposed to a novel context in the presence of a fearful conspecific acquired contextual fear to a level comparable to controls, those that experienced the pre-exposure in the presence of a non-fearful conspecific showed a profound reduction in fear [6]. Together, these findings suggest a possible context modulation in social transmission of fear.
Recent fMRI studies [19], [7] suggest that observational conditioning activates the amygdala and the hippocampus and that personality factors modulate neural mechanisms of observational learning. It will also be important to assess the neurobiological mechanisms that underlie susceptibility and resistance to fear conditioning by proxy. An important finding from the present study is that fear conditioning by-proxy influences subsequent Pavlovian conditioning to a tone-CS. A similar effect was previously found to occur with contextual fear conditioning [10], strengthening the notion that observational learning impacts subsequent behavior, which could play a role in the development of anxiety-related disorders. Together, these results may indicate that FCbP recruits mechanisms that overlap with those engaged during fear conditioning (e.g., the amygdala).
Observational fear learning is a well-documented phenomenon in primates [8], [15], [16], [18], [19] and developing similar models in rodents would facilitate the study of neural mechanisms underlying indirect fear conditioning. Together with recent findings from other groups [10] and [3], the present results broaden our understanding of fear modulation to include indirect acquisition: attributing aversive value to a stimulus by virtue of the fact that it impacts another. Use of this paradigm in future studies may improve our understanding of the development of specific phobias.
Acknowledgments
We acknowledge the following NIH funding grant: R21 MH086805 to MHM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blair HT, Schafe GE, Bauer EP, Rodrigues SM, LeDoux JE. Synaptic plasticity in the lateral amygdala: a cellular hypothesis of fear conditioning. Learn Mem. 2001;8:229–242. doi: 10.1101/lm.30901. [DOI] [PubMed] [Google Scholar]
- 2.Bredy T, Barad M. Social modulation of associative learning by pheromonone communication. Learn Mem. 2009;16:12–18. doi: 10.1101/lm.1226009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Q, Panksepp JB, Lahvis GP. Empathy is moderated by genetic background in mice. PLoS One. 2009;4(2):e4387. doi: 10.1371/journal.pone.0004387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook M, Mineka S. Second-order conditioning and overshadowing in the observational conditioning of fear in monkeys. Behav Res Ther. 1987;25(5):349–64. doi: 10.1016/0005-7967(87)90013-1. [DOI] [PubMed] [Google Scholar]
- 5.Fontenelle LF, Soares ID, Miele F, Borges MC, Prazeres AM, Rangé BP, Moll J. Empathy and symptoms dimensions of patients with obsessive-compulsive disorder. J Psychiatr Res. 2009;43(4):455–63. doi: 10.1016/j.jpsychires.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Guzmán YF, Tronson NC, Guedea A, Huh KH, Gao C, Radulovic J. Social modeling of conditioned fear in mice by non-fearful conspecifics. Behav Brain Res. 2009;201(1):173–8. doi: 10.1016/j.bbr.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hooker CI, Verosky SC, Miyakawa A, Knight RT, D'Esposito M. The influence of personality on neural mechanisms of observational fear and reward learning. Neuropsychologia. 2008;46(11):2709–24. doi: 10.1016/j.neuropsychologia.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hygge S, Ohman A. Modeling processes in the acquisition of fears: vicarious electrodermal conditioning to fear-relevant stimuli. J Pers Soc Psychol. 1978;36(3):271–9. doi: 10.1037//0022-3514.36.3.271. [DOI] [PubMed] [Google Scholar]
- 9.Knapska E, Nikolaev E, Boguszewski P, Walasek G, Blaszczyk J, Kaczmarek L, Werka T. Between-subject transfer of emotional information evokes specific pattern of amygdala activation. Proc Natl Acad Sci U S A. 2006;103(10):3858–62. doi: 10.1073/pnas.0511302103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knapska E, Mikosz M, Werka T, Maren S. Social modulation of learning in rats. Learn Mem. 2010;17(1):35–42. doi: 10.1101/lm.1670910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 12.Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- 13.McGaugh JL. Memory: a century of consolidation. Science. 2000;287(5451):248–51. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 14.Michael T, Ehlers A, Halligan SL, Clark DM. Unwanted memories of assault: What intrusion characteristics are associated with PTSD? Behaviour Research and Therapy. 2005;43:613–628. doi: 10.1016/j.brat.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Mineka S, Cook M. Mechanisms involved in the observational conditioning of fear. Journal of Experimental Psychology: General. 1993;122:23–38. doi: 10.1037//0096-3445.122.1.23. [DOI] [PubMed] [Google Scholar]
- 16.Mineka S, Davidson M, Cook M, Keir R. Observational conditioning of snake fear in rhesus monkeys. J Abnorm Psychol. 1984;93(4):355–72. doi: 10.1037//0021-843x.93.4.355. [DOI] [PubMed] [Google Scholar]
- 17.Mineka S, Cook M. Immunization against the observational conditioning of snake fear in rhesus monkeys. J Abnorm Psychol. 1986;95(4):307–18. doi: 10.1037//0021-843x.95.4.307. [DOI] [PubMed] [Google Scholar]
- 18.Cook M, Mineka S. Second-order conditioning and overshadowing in the observational conditioning of fear in monkeys. Behav Res Ther. 1987;25(5):349–64. doi: 10.1016/0005-7967(87)90013-1. [DOI] [PubMed] [Google Scholar]
- 19.Ollson R, Phelps E. Learned fear of “unseen” faces after Pavlovian, observational, and instructed fear. Psychol Sci. 2004 Dec;15(12):822–8. doi: 10.1111/j.0956-7976.2004.00762.x. [DOI] [PubMed] [Google Scholar]
- 20.Pavlov IV, editor; Anrep GV, translator. Conditioned reflexes. New York: Liveright publishing; 1927. [Google Scholar]
- 21.Rachman BJ. Fear and courage. San Francisco: W H Freeman; 1978. [Google Scholar]
- 22.Squire L, Davis S. The pharmacology of memory: a neurobiological perspective. Annu Rev Pharmacol Toxicol. 1981;21:323–56. doi: 10.1146/annurev.pa.21.040181.001543. [DOI] [PubMed] [Google Scholar]