Abstract
OBJECTIVE
Adverse drug events(ADEs) are a common complication of medical care, but few pediatric data are available describing the frequency or epidemiology of these events. We estimated the national incidence of pediatric ADEs requiring medical treatment, described the pediatric population seeking care for ADEs, and characterized the events in terms of patient symptoms and medications implicated.
METHODS
Data were obtained from the National Center for Health Statistics, which collects information on patient visits to outpatient clinics and emergency departments throughout the United States. We analyzed data for children 0 to 18 years of age seeking medical treatment for an ADE between 1995 and 2005.
RESULTS
The mean annual number of ADE-related visits was 585 922 (95%confidence interval [CI]:503 687–668 156) of which 78% occurred in outpatient clinics and 12% occurred in emergency departments. Children 0 to 4 years of age had the highest incidence of ADE-related visits, accounting for 43.2% (95% CI: 35.6%–51.2%) of visits. The most common symptom manifestations were dermatologic conditions (45.4% [95% CI: 36.9%–54.1%]) and gastrointestinal symptoms (16.5% [95% CI: 11.1%–23.8%]). The medication classes most frequently implicated in an ADE were antimicrobial agents (27.5% [95% CI: 21.5%–34.5%]), central nervous system agents (6.5% [95% CI: 4.0%–10.5%]), and hormones (6.1% [95% CI: 3.1%–11.6%]). While ADEs related to antimicrobial agents were most common among children 0 to 4 years old and decreased in frequency among older children, ADEs resulting from central nervous system agents and hormones increased in frequency among children 5 to 11 and 12 to 18 years old.
CONCLUSIONS
ADEs result in a substantial number of health care visits, particularly in outpatient clinics. The incidence of ADEs and medications implicated vary by age, indicating that age-specific approaches for monitoring and preventing ADEs may be most effective.
Keywords: adverse drug events, ambulatory health care
Pharmacologic therapy is provided for children in almost 70% of ambulatory care encounters.1 As many as 56% of children receive at least 1 medication during the preceding week, with 26% using 2 or more medications and >20% using a prescription medication.2,3 Adverse drug events (ADEs), which are defined as injuries resulting from a drug taken for medical intervention,4,5 have been linked to a large number of medications and studies have begun to describe the large scope of this medical care complication.4–7 Estimates indicate that 4.7% of all hospitalizations are the result of an ADE and up to 6.5% of inpatients suffer an ADE during their hospitalization.5,8,9 Most studies have documented ADEs in the adult and elderly population with relatively less information available on ADEs among pediatric patients.
Children receive the majority of their medical care, including pharmacologic therapy, in the ambulatory setting and are likely to seek treatment for ADEs in clinics and emergency departments. Few data are available on the frequency or epidemiology of these events. One study investigating pediatric clinic visits found that up to 16% of all outpatient prescriptions are associated with an ADE.10 Another study examining ADEs treated in emergency departments nationally found that there are nearly 160 000 yearly visits for ADEs among children 0 to 18 years old.11 Additional information on the burden of ADEs among pediatric patients, including age-specific incidences and the prevalence of specific medications, could help identify areas for targeted prevention strategies.
For this study, we used a national database to examine outpatient pediatric ADEs resulting in a health care visit over an 11-year study period. Our objective was to provide estimates of the incidence of ADEs, describe the pediatric population seeking care for ADEs, and characterize the ADEs in terms of patient symptoms and medications implicated.
METHODS
Data Sources
Data on patient visits to outpatient clinics and emergency departments for the treatment of ADEs were obtained from the National Ambulatory Medical Care Survey (NAMCS) and the National Hospital and Ambulatory Medical Care Survey (NHAMCS) from 1995 to 2005.12 These surveys are national probability-sample surveys conducted by the National Center for Health Statistics (NCHS),a branch of the Centers for Disease Control and Prevention. The NAMCS collects information on patient visits to nonfederally employed office-based physicians and the NHAMCS collects information on visits to hospital-based clinics and emergency departments of noninstitutional, general, and short-stay hospitals. The NAMCS employs a 3-stage probability design of geographic areas, physician practices within these areas, and patient visits within practices. The NHAMCS consists of a 4-stage probability design with geographic areas, hospitals within these areas, clinics or emergency departments within hospitals, and patient visits to these sites. A random sample of patient visits to the selected clinics or emergency departments are surveyed during a randomly assigned time period to generate national estimates of ambulatory medical care use in the United States.
Standard patient record forms are completed by physicians, office and hospital staff, and NCHS field representatives. Data are collected on patient demographics, reasons for the visit (coded according to the NCHS reason-for-visit classification), diagnostic and screening services provided, treatments and procedures performed, medications prescribed or provided at the visits (coded by using the National Drug Code Directory), diagnoses (coded according to the International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM]), and disposition. If the visit is related to an injury, poisoning, or adverse effect of medical treatment, including an ADE, a brief text description with the details of the event is included. This information is used by the NCHS to assign up to 3 supplementary classification of external causes of injury and poisoning codes (E-codes, found in the ICD-9-CM) that provide information on the medications implicated in the ADE. In 2005, the clinic record forms were modified and event description was replaced with a question asking whether the visit was related to the adverse effect of medicinal/surgical care or a medicinal drug.
During the study period, the average physician participation rate in the NAMCS was 66% with a total of 292 121 surveys completed. The average hospital participation rate in the NHAMCS was 94% with 340 108 surveys collected in outpatient clinics and 319 227 surveys collected in the emergency departments. Multiple quality control measures are used by the NCHS including a 2-way independent verification procedure of 10% of the records. Error rates for items requiring medical coding averaged <2% for the NAMCS and <3% for the NHAMCS. Additional details on the sample design, field quality control, data collection procedures, data processing, and estimation procedures used in the NAMCS and NHAMCS are available on the NCHS Web site (www.cdc.gov/nchs/ahcd.htm).
Study Population
We examined visits by children 0 to 18 years old and identified ADE-related visits to outpatient clinics and emergency departments from data on adverse medical events and from physician diagnoses. Visits were classified as an ADE visit if they included an E-code for “drugs, medicinal, and biological substances causing adverse effects in therapeutic use” (E-codes E930–E949), or a diagnosis of anaphylactic shock due to an adverse effect of correct medicinal substance properly administered (ICD-9-CM code 995.0), an unspecified adverse effect to correct medicinal substance properly administered (ICD-9-CM code 995.2), shock due to anesthesia in which the correct substance was properly administered(ICD-9-CMcode945.4), aspirin gastritis (ICD-9-CM code 535.4), drug dermatitis (ICD-9-CM codes 692.3 and 693.0), drug reaction in newborn (ICD-9-CM code 779.4), drug psychoses (ICD-9-CM codes 292.1–292.9), allergic urticaria (ICD-9-CM code 708.0), and neuropathy due to drugs (ICD-9-CM code 357.6). In addition, visits associated with an adverse medical event of accidental poisoning by drugs (E-codes E850–E858) or a diagnosis of poisoning by drugs (ICD-9-CM codes 960–979) were included if the verbatim text description was available and did not describe an event resulting from the administration of the wrong medication (eg, a child ingesting a family member’s medication), an intentional drug overdose, or use of an illicit substance. From this sample we excluded all visits associated with an adverse medical event code or diagnosis of drug dependence or abuse, use of an illicit substance, drug withdrawal, intentional self-harm, or assault by poisoning.
Data and Statistical Analysis
We calculated national annual estimates of ADE-related visits to outpatient clinics and emergency departments and described the visits in terms of patient demographics and clinic or hospital characteristics. We calculated age-specific population rates of ADEs by using 2000 US Census data.13 Up to 3 symptom manifestations were assigned from the reasons for the visit, the description of the adverse medical event, and the diagnoses. Medications implicated in the ADE were identified by using the adverse medical event E-codes and diagnoses, which include the names of the medications responsible for the ADE or poisoning. Medications were grouped into medication classes based on the National Drug Code Directory. To calculate ADE visits for the different medication classes, cases with missing medication data were imputed assuming that the proportion of cases in each medication class was the same as the proportion of cases without missing data. The number of ADE visits for each medication class was then divided by the number of outpatient visits (both to clinics and emergency departments in the NAMCS and NHAMCS) in which a medication in the corresponding medication class was listed as part of the patient’s therapy. These medications include those provided, prescribed, or continued at the time of the visit.
All estimates and rates were calculated with 95% confidence intervals (CIs) by using SUDAAN 9.0.1 statistical software (Research Triangle Institute, Research Triangle Park, NC). This software takes into account the multistage sampling design used in the NAMCS and NHAMCS and is recommended by the NCHS for the computation of SEs. Conditional probability rules were used to account for imputations in variance estimates of total ADE visits.
RESULTS
During the 11-year study period the mean annual number of ADE-related visits was 585922 (95% CI: 503687–668 156) with 454 780 (95% CI: 360 464–549 095) visits to outpatient clinics, and 370 763 (95% CI: 284 651–456 875) visits to general practice clinics, specifically (Table 1). The highest proportion of visits was by children 0 to 4 years old who accounted for 43.2% (95% CI: 35.6%–51.2%) of visits, followed by children 15 to 18 years old who comprised 22.6% (95% CI: 16.7%–29.7%) of ADE-related visits. The population rate of ADEs was greatest among the youngest age group with 13.2 visits per 1000 children (95% CI: 10.5–15.9). ADE rates for the other age groups were 4.5 visits (95% CI: 2.8–6.3), 5.2 visits (95% CI: 3.0–7.4), and 8.2 visits (95% CI: 5.4–11.0) per 1000 persons for children 5 to 9, 10 to 14, and 15 to 18 years old, respectively. In both the clinic and emergency department settings, the greatest proportion of ADE visits were by non-Hispanic white children, those with private insurance, and those residing in the South. In outpatient clinics, ADEs accounted for 0.3% of all visits, whereas in the emergency department 0.5% of visits were the result of an ADE. There was no significant increase in the incidence of ADEs over the study period (P = .2).
TABLE 1.
National Annual Estimates and Demographic Characteristics of Pediatric Patients With ADEs Treated in US Outpatient Clinics and Emergency Departments
| Patient Characteristic | National Annual Estimate of ADE Visits, N (%) |
||
|---|---|---|---|
| Outpatient Clinic (N = 293) | Emergency Department (N = 402) | Total | |
| Age, y | |||
| 0–4 | 197 176 (43.4) | 55 925 (42.6) | 253 101 (43.2) |
| 5–9 | 71 457 (15.7) | 21 878 (16.7) | 93 335 (15.9) |
| 10–14 | 90 058 (19.8) | 17 212 (13.1) | 107 270 (18.3) |
| 15–18 | 96 089 (21.1) | 36 127 (27.6) | 132 216 (22.6) |
| Gender | |||
| Female | 199 447 (43.9) | 84 713 (64.6) | 284 160 (48.5) |
| Male | 255 332 (56.1) | 46 428 (35.4) | 301 761 (51.5) |
| Race/ethnicitya | |||
| Non-Hispanic white | 301 319 (73.9) | 70 539 (61.4) | 371 859 (71.2) |
| Non-Hispanic black | 38 904 (9.6) | 21 533 (18.7) | 60 438 (11.6) |
| Hispanic | 43 433 (10.7) | 15 686 (13.7) | 59 120 (11.3) |
| Other | 23 919 (5.9) | 7162 (6.2) | 31 081 (6.0) |
| Payment sourcea | |||
| Private insurance | 330 437 (77.1) | 70 004 (55.9) | 400 441 (72.3) |
| Government | 65 561 (15.3) | 43 853 (35.0) | 109 414 (19.8) |
| Self-pay | 8046 (1.9) | 7938 (6.3) | 15 983 (2.9) |
| Other | 24 307 (5.7) | 3422 (2.7) | 27 728 (5.0) |
| Clinical setting | |||
| General practice clinic | 370 763 (81.5) | NA | 370 763 (81.5) |
| Subspecialty clinic | 84 017 (18.5) | NA | 84 017 (18.5) |
| Region | |||
| Northeast | 125 127 (27.5) | 19 172 (14.6) | 144 298 (24.6) |
| Midwest | 116 074 (25.5) | 37 112 (28.3) | 153 186 (26.1) |
| South | 139 502 (30.7) | 43 798 (33.4) | 183 300 (31.3) |
| West | 74 076 (16.3) | 31 060 (23.7) | 105 136 (17.9) |
| Total | 454 780 | 131 142 | 585 922 |
NA indicates not applicable.
Among outpatient clinic cases there were 34 cases with missing race/ethnicity data and 12 cases with missing payment data; among emergency department cases, 42 and 20 cases were missing race/ethnicity and payment data, respectively.
Dermatologic conditions were the most common ADE manifestation, present in 45.4% (95% CI: 36.9%–54.1%) of cases, followed by gastrointestinal symptoms in 16.5% (95% CI: 11.1%–23.8%) of cases (Table 2). All other symptom categories were present in <5% of ADE cases. The distribution of the most common presenting symptoms was similar for the 3 age groups of 0 to 4, 5 to 11, and 12 to 18 years old (Fig 1A).
TABLE 2.
Symptom Manifestations of Pediatric ADEs Treated in US Outpatient Clinics and Emergency Departments
| Symptom Manifestation | National Annual Estimate of ADE Visits, n (%) |
||
|---|---|---|---|
| Outpatient Clinic (N = 293) | Emergency Department (N = 402) | Total | |
| Dermatologic | 205 878 (45.3) | 59 899 (45.7) | 265 777 (45.4) |
| Gastrointestinal | 70 613 (15.5) | 25 933 (19.8) | 96 547 (16.5) |
| Neurological | 22 629 (5.0) | 5032 (3.8) | 27 661 (4.7) |
| Unspecified allergy | 12 630 (2.8) | 6793 (5.2) | 19 423 (3.3) |
| General malaise/fever | 15 823 (3.5) | 3243 (2.5) | 19 066 (3.2) |
| Psychological | 13 464 (3.0) | 3466 (2.6) | 16 929 (2.9) |
| Edema/swelling | 7327 (1.6) | 9125 (7.0) | 16 452 (2.8) |
| Syncope/dizziness | 10 788 (2.4) | 3660 (2.8) | 14 449 (2.5) |
| Unspecified toxicity | 5880 (1.3) | 7102 (5.4) | 12 982 (2.2) |
| Endocrine | 8461 (1.9) | 2634 (2.0) | 11 096 (1.9) |
| Respiratory | 3445 (0.8) | 6253 (4.8) | 9698 (1.7) |
| Cardiovascular | 2326 (0.5) | 4099 (3.1) | 6424 (1.1) |
All symptoms representing >1% of cases in outpatient clinics or emergency departments are included. Selected symptoms accounted for 76% of clinic ADE cases and 93% of emergency department ADE cases. Up to 3 presenting symptoms were assigned to each case.
FIGURE 1.
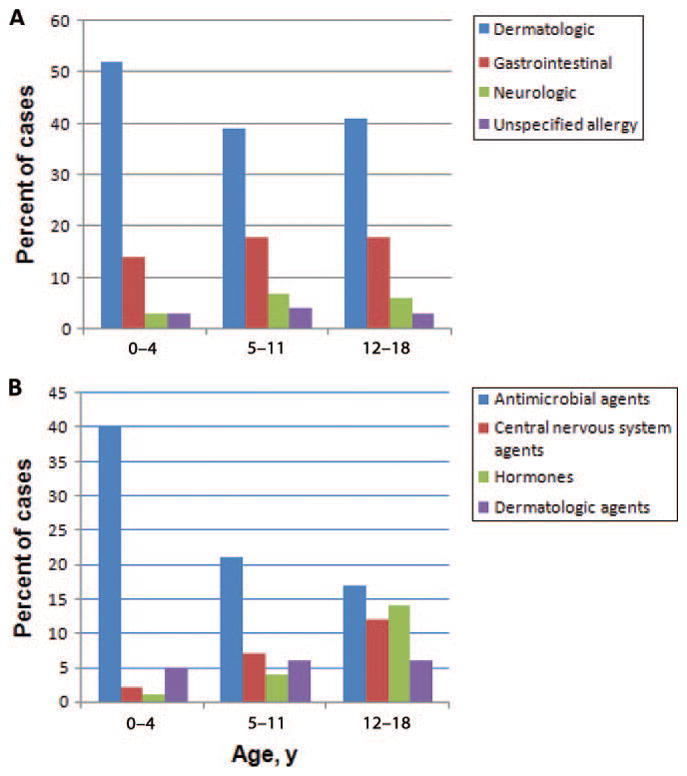
Symptoms (A) and medications (B) associated with pediatric ADEs according to age groups. The 4 most common symptom types and medication classes are shown.
The medication classes most frequently implicated in an ADE were antimicrobial agents (27.5% [95% CI: 21.5%–34.5%]), central nervous system agents (6.5% [95% CI: 4.0%–10.5%]), and hormones (6.1% [95% CI: 3.1%–11.6%]). Among ADEs related to antimicrobial agents, more than half were the result of a penicillin (40%) or cephalosporin (15%). Adverse events to central nervous system agents were most often related to stimulants (37%) or antidepressants (29%), and the majority of ADEs involving hormones were associated with estrogens and progesterones (73%).
The age-specific distribution of medications implicated in ADEs is displayed in Fig 1B. Among the youngest age group, antimicrobial agents were responsible for 40% of events, of which penicillins comprised 48% and cephalosporins comprised 19%. There was an increase in ADEs related to central nervous system agents and hormones among older children, with antidepressants and stimulants the most common central nervous system agents (38% and 31%, respectively), and ovarian hormones responsible for the majority of hormone-related ADEs (82%).
The highest rate of visits for ADEs resulted from antineoplastic and immunosuppressive agents, with 19.7 ADE-related visits per 1000 outpatient visits associated with this medication class (95% CI: 7.6–50.7 ADE visits per 1000 medication visits) (Table 3). Other medication classes with high rates of ADEs were hormones (7.2 ADE visits per 1000 medication visits; 95% CI: 3.6–14.5 ADE visits per 1000 medication visits) and antimicrobial agents (5.1 ADE visits per 1000 medication visits; 95% CI: 3.7–7.1 ADE visits per 1000 medication visits). Antibiotics, specifically, were associated with an ADE rate of 4.2 visits per 1000 medication visits (95% CI: 2.9–6.1 ADE visits per 1000 medication visits). The overall rate of ADE visits per 1000 medication visits was 4.2 (95% CI: 3.5–5.1 per 1000 medication visits).
TABLE 3.
Medications Associated With Pediatric ADEs Treated in US Outpatient Clinics and Emergency Departments
| Medication Class | National Annual Estimate of ADE Visits, N (%) |
Rate of ADE Visits, Total No. per 1000 Medication Visits (95% CI)a | ||
|---|---|---|---|---|
| Outpatient Clinic (N = 293) | Emergency Department (N = 402) | Total | ||
| Antimicrobial agents | 111 515 (24.5) | 49 716 (37.9) | 161 231 (27.5) | 5.1 (3.7–7.1) |
| Central nervous system agents | 26 266 (5.8) | 12 002 (9.2) | 38 268 (6.5) | 1.7 (1.0–2.8) |
| Hormones | 29 392 (6.5) | 6420 (4.9) | 35 812 (6.1) | 7.2 (3.6–14.5) |
| Agents affecting skin and mucous membranes and topical ear, nose, and throat agents | 28 707 (6.3) | 2641 (2.0) | 31 348 (5.4) | 3.7 (1.7–8.4) |
| Vaccines | 18 702 (4.1) | 9360 (7.1) | 28 062 (4.8) | 2.4 (1.4–4.1) |
| Antihistamines, antitussives, expectorants, cold remedies, and respiratory agents | 11 761 (2.6) | 5273 (4.0) | 17 034 (2.9) | 0.8 (0.3–2.1) |
| Analgesic agents | 1469 (0.3) | 9279 (7.1) | 10 748 (1.8) | 0.7 (0.4–1.1) |
| Cardiovascular agents | 6188 (1.4) | 1372 (1.0) | 7560 (1.3) | 3.1 (0.9–10.2) |
| Antineoplastic and immunosuppressive agents | 4174 (0.9) | 731 (0.6) | 4905 (0.8) | 19.7 (7.6–50.7) |
| Other agent | 6324 (1.4) | 7106 (5.4) | 13 430 (2.3) | 1.0 (0.5–1.9) |
| Unspecified agent | 211 458 (46.5) | 30 811 (23.5) | 242 269 (41.1) | NA |
Up to 3 medications can be associated with an ADE based on E-codes. There were 99.5% of clinic cases and 96.4% of emergency department cases associated with a single medication class. NA indicates not applicable.
Medication visits included all visits to outpatient clinics and emergency departments that involved the initiation or continuation of a medication.
Among ADE cases in outpatient clinics, 2.2% were treated with an antidote (eg, naloxone) or ADE-specific medication (eg, diphenhydramine) and 0.7% were admitted to the hospital. Emergency department ADE visits were associated with a medication treatment rate of 46.3% and an admission rate of 5.0%.
DISCUSSION
On the basis of 11 years of national data on patient visits to US ambulatory health care facilities, we found that ADEs are a common complication of medication use among pediatric outpatients with more than half a million children seeking care annually in the outpatient setting. Of these, 43% occur in children <5 years old. While the majority of children are treated in outpatient clinics (78%), those children seen in emergency departments require greater medical intervention. The distribution of presenting symptoms is similar for the different age groups, but the medications implicated in ADEs vary according to age, with a large proportion of ADEs resulting from antimicrobial agents in the youngest children and increasing frequencies of ADEs related to central nervous system agents and hormones and synthetic substitutes among the older age groups. Overall, we found that chemotherapeutic agents were most likely to result in an ADE, followed by hormones and antimicrobial agents.
We defined ADEs as injuries occurring during therapeutic use of a medication, including adverse effects (eg, allergic reactions or nausea), accidental overdoses (eg, dosing errors or elevated drug levels associated with proper use of a medication), and secondary effects (eg, injuries due to medication-induced dizziness).5 This differs from the definitions used by some other studies which have also included overdoses resulting from taking the wrong medication, such as when a child finds and ingests a medication.11,14 We chose to exclude these cases and to focus only on ADEs that occur in patients taking a drug for medical therapy because this provides data that can inform clinical decisions by health care providers prescribing medications.
To our knowledge, this is the first study to examine ADE-related ambulatory visits comprehensively, including visits to office-based clinics, hospital outpatient clinics, subspecialty clinics, and emergency departments. A previous study examined ADEs among pediatric patients followed in 6 office practices and found an ADE rate of 16% among patients receiving medication prescriptions.10 Twelve percent of these were deemed to be serious events and, overall, 3% were preventable (eg, errors in drug administration) and 13% nonpreventable (eg, allergic reactions). Another study used the National Electronic Injury Surveillance System-Cooperative Adverse Event Surveillance Project to examine pediatric ADE visits to emergency departments nationally.7 The authors of this study estimated that a total of 158 520 (95% CI: 117 745–199 295) children seek care for an ADE in emergency departments every year. This number is slightly larger than our figure (131 142 emergency department visits) which may in part be because of our exclusion of unintentional ingestions. Similar to our findings, they report the highest proportion of cases among children <5 years old and found that antimicrobial agents were the most frequently implicated drug.
Another study examined emergency department ADE visits for antibiotics specifically.14 Although they did not include age-specific rates, they did report that the highest rate of antibiotic associated ADEs was among children younger than 1 year, with 15.9 visits per 10 000 medication visits. This finding is consistent with our data, which yields an estimated rate for this age group of 16.1 visits per 10 000 medication visits.
Children 0 to 4 years old comprised the largest group of ADE visits and had the highest case incidence with 13.2 ADE visits per 1000 persons. Although we cannot ascertain from our data the specific drug reactions resulting in these visits, 56% of these children presented with dermatologic symptoms, edema or swelling, or unspecified allergic symptoms, indicating that a large number of reactions were allergic in nature. This supports previous findings demonstrating rates of allergic reactions as high as 72% among children 0 to 4 years old with an ADE.11 Thus, the high frequency of ADEs among young children is likely related to first-time medication exposures revealing allergic reactions and underscores the importance of anticipatory guidance concerning these types of reactions by prescribing clinicians. Another potential contributor to the large number of ADEs in this age group are medication errors, because young children frequently receive medications available in multiple formulations and concentrations and are more sensitive to dosing errors.10
Among children 12 to 18 years of age, we found an increase in the number of ADEs related to central nervous system agents and hormones and synthetic substitutes. The rise in visits related to central nervous system agents likely reflects the increase in medication therapy for depression and other emotional and behavioral disorders during adolescent years.15,16 Adverse effects commonly associated with psychotropic medications include headache, agitation and other behavioral disturbances, and gastrointestinal symptoms.17 At the same time, teenaged girls are prescribed contraceptive medications, which are associated with dys-menorrhea, nausea and vomiting, and dermatologic conditions.18 Clinicians should be cognizant of these potential adverse effects and council patients appropriately when initiating therapy with these agents.
There are several limitations to the use of NAMCS and NHAMCS data for the measurement of ADEs. First, the information is limited to the data collected by using standard records, and we could not obtain additional information on the type of drug reaction, the preventability of the event, or the patient outcome. We were also unable to determine the specific medications implicated in ADEs in many cases, which limited our ability to calculate exact frequencies for medication classes associated with ADEs. Second, the identification of ADEs is subject to underreporting and misclassification. Physicians may not recognize a symptom as an adverse effect of a medication or may fail to include the ADE in the list of adverse effects or diagnoses, particularly for patients presenting with multiple problems. It is also possible that some cases were incorrectly classified as an ADE, although we were careful to exclude all cases which might be related to drug abuse, use of an illicit substance, intentional overdose, or administration of the wrong medication. Third, we were unable to perform additional age-specific analyses because of the small sample size and could not determine age-specific frequencies of ADEs for all the symptom types and medication classes or for specific medications. Finally, as is the case with all survey data, these data are retrospective and subject to incompleteness, inaccuracies, and coding errors, although the NCHS uses rigorous quality control measures at multiple stages of the data collection and preparation process.
CONCLUSIONS
ADEs are a common medical complication among children in the outpatient setting and represent a large burden to the health care system, particularly in outpatient clinics. Children <5 years old are most likely to suffer an ADE requiring medical treatment which seems to be related to high rates of allergic reactions with first-time exposures to medications. Medications implicated in ADEs vary according to age, indicating that age-specific approaches to monitoring and preventing ADEs are warranted.
WHAT’S KNOWN ON THIS SUBJECT
Pharmacologic therapy is used extensively for children in the outpatient setting. Among pediatric inpatients, ADEs have been found to be a frequent complication of medical care.
WHAT THIS STUDY ADDS
ADEs result in a large number of health care visits to outpatient clinics and emergency departments with age-specific variation in the incidence of events and medications implicated.
Acknowledgments
This work was supported by R01LM007970 from the National Library of Medicine and 5 T32 HD40128 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
ABBREVIATIONS
- ADE
adverse drug event
- NAMCS
National Ambulatory Medical Care Survey
- NHAMCS
National Hospital and Ambulatory Medical Care Survey
- NCHS
National Center for Health Statistics
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- E-codes
Supplementary Classification of External Causes of Injury and Poisoning Codes
- CI
confidence interval
Footnotes
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
References
- 1.Cherry DK, Woodwell DA, Rechtsteiner EA. National Ambulatory Medical Care Survey: 2005 summary. Adv Data. 2007;387:1–39. [PubMed] [Google Scholar]
- 2.National Center for Health Statistics. [Accessed August 3, 2009];Health, United States, 2007, with chartbook on trends in the health of Americans. Available at: www.cdc.gov/nchs/hus.htm. [PubMed]
- 3.Slone Epidemiology Center. [Accessed August 3, 2009];Patterns of medication use in the United States, 2006: a report from the Slone Survey. Available at: www.bu.edu/slone/SloneSurvey/SloneSurvey.htm.
- 4.Gurwitz JH, Field TS, Harrold LR, et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA. 2003;289(9):1107–1116. doi: 10.1001/jama.289.9.1107. [DOI] [PubMed] [Google Scholar]
- 5.Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events: implications for prevention. ADE Prevention Study Group. JAMA. 1995;274(1):29–34. [PubMed] [Google Scholar]
- 6.Gandhi TK, Weingart SN, Borus J, et al. Adverse drug events in ambulatory care. N Engl J Med. 2003;348(16):1556–1564. doi: 10.1056/NEJMsa020703. [DOI] [PubMed] [Google Scholar]
- 7.Budnitz DS, Pollock DA, Weidenbach KN, Mendelsohn AB, Schroeder TJ, Annest JL. National surveillance of emergency department visits for outpatient adverse drug events. JAMA. 2006;296(15):1858–1866. doi: 10.1001/jama.296.15.1858. [DOI] [PubMed] [Google Scholar]
- 8.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279(15):1200–1205. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 9.Kaushal R, Bates DW, Landrigan C, et al. Medication errors and adverse drug events in pediatric inpatients. JAMA. 2001;285(16):2114–2120. doi: 10.1001/jama.285.16.2114. [DOI] [PubMed] [Google Scholar]
- 10.Kaushal R, Goldmann DA, Keohane CA, et al. Adverse drug events in pediatric outpatients. Ambul Pediatr. 2007;7(5):383–389. doi: 10.1016/j.ambp.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Cohen AL, Budnitz DS, Weidenbach KN, et al. National surveillance of emergency department visits for outpatient adverse drug events in children and adolescents. J Pediatr. 2008;152(3):416–421. doi: 10.1016/j.jpeds.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 12.National Center for Health Statistics. Ambulatory health care data. [Accessed August 3, 2009]; Available at: www.cdc.gov/nchs/ahcd.htm.
- 13.US Census Bureau Census 2000. [Accessed August 3, 2009];Sex by age for the population under 20 years (table P14) Available at: http://factfinder.census.gov/servlet/DTSubjectShowTablesServlet?_ts=267357437064.
- 14.Shehab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47(6):735–743. doi: 10.1086/591126. [DOI] [PubMed] [Google Scholar]
- 15.Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch Gen Psychiatry. 2003;60(8):837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- 16.Thomas CP, Conrad P, Casler R, Goodman E. Trends in the use of psychotropic medications among adolescents, 1994 to 2001. Psychiatr Serv. 2006;57(1):63–69. doi: 10.1176/appi.ps.57.1.63. [DOI] [PubMed] [Google Scholar]
- 17.Bezchlibnyk-Butler KZ, Virani AS, editors. Clinical Handbook of Psychotropic Drugs for Children and Adolescents. 2. Cambridge, MA: Hogrefe; 2007. [Google Scholar]
- 18.Thorogood M, Villard-Mackintosh L. Combined oral contraceptives: risks and benefits. Br Med Bull. 1993;49(1):124–139. doi: 10.1093/oxfordjournals.bmb.a072592. [DOI] [PubMed] [Google Scholar]


