Abstract
Background
Endogenous carbon monoxide (CO) at physiologic concentrations is cytoprotective, whereas excess levels reflect underlying oxidative stress, inflammation, and vascular pathology and portend adverse clinical sequelae. However, the relation of exhaled CO to metabolic/vascular risk in the community is unknown.
Methods and Results
We related exhaled CO, a surrogate measure of blood CO concentration, to the risk of developing new-onset metabolic syndrome and incident cardiovascular disease (CVD) following 14,943 routine examinations (4,139 unique participants; mean age 46 years, 53% women) in the Framingham Heart Study. Baseline exhaled CO was associated with the presence of cardiometabolic risk factors (including smoking) and prevalent metabolic syndrome (odds ratio [OR] 1.08 per log-CO; 95% confidence interval [CI] 1.02–1.16; P=0.01). During up to 4 years of follow up, 1,458 participants developed new-onset metabolic syndrome, and 416 experienced a first CVD event. Compared to individuals in the lowest quartile of exhaled CO, those in the highest quartile were more likely to develop metabolic syndrome (OR 1.48, 95% CI 1.25–1.76; P<0.0001) and CVD events (hazards ratio 1.66, 95% CI 1.14–2.40; P=0.008) in multivariable analyses that included adjustment for smoking status.
Conclusion
In our community-based sample, higher exhaled CO levels predicted the development of metabolic syndrome and future CVD events, underscoring the importance of this endogenous second messenger in the pathogenesis of metabolic and vascular risk.
Keywords: carbon monoxide, risk factors, metabolic syndrome, cardiovascular disease
BACKGROUND
Carbon monoxide (CO) is a ubiquitous gas that is produced both exogenously, by the incomplete combustion of hydrocarbons, and endogenously by the enzymatic degradation of heme and non-heme compounds.1 Endogenous CO is produced predominantly as an essential byproduct of heme oxygenase (HO) activity. Accumulating evidence suggests that endogenous CO is not only structurally but also biologically similar to nitric oxide (NO) in its ability to modulate vascular function.1–3 In light of numerous studies highlighting the fundamental role of NO as a signaling molecule in both physiological and disease states, CO has now also been identified as a key gaseous second messenger of special relevance to the cardiovascular system.1,2 At physiologic levels, endogenous CO is cytoprotective and exerts anti-oxidant, anti-inflammatory, and anti-apoptotic properties.2,4 In addition, experimental studies indicate that endogenous CO has the ability to vasodilate arteries,5 regulate vascular smooth muscle cell growth,6 and inhibit platelet aggregation.7 Conversely, excess endogenous CO – even at low levels – can reflect underlying inflammatory, oxidative, and vascular pathology. Clinical studies indicate that exhaled CO, which closely reflects total blood CO concentration, is increased among individuals with respiratory infections,8 hyperglycemia,9 and critical illness,10 irrespective of smoking status. Functionally, excess endogenous CO can lead to the formation of reactive oxygen species,11 can impair NO-mediated vasodilation,3 and can promote adverse vascular remodeling.12 Thus, despite exhibiting protective activity at physiologic concentrations, chronically elevated levels of CO may reflect underlying oxidative stress and inflammation as well as more specific vascular processes that predispose to cardiovascular disease (CVD).13–16
Exhaled CO closely reflects an individual’s total blood CO, which is largely reflects the activity of pathways involved in endogenous CO cycling.13,17 Therefore, we hypothesized that exhaled CO is associated with both metabolic and cardiovascular risk in the general population. We tested this hypothesis by investigating the relation of exhaled CO to the presence of cardiometabolic risk factors cross-sectionally, and to the incidence of metabolic syndrome and cardiovascular disease longitudinally in a large community-based sample.
METHODS
Study Sample
The sampling design and enrollment criteria of the Offspring cohort (N=5,124) of the Framingham Heart Study have been described previously.18 The present investigation included Offspring cohort participants who attended at least one quadrennial examination from cycles 2 (1979–1982) through 6 (1996–1997). Of the 19,086 participant visits at these examinations with available follow-up data, we excluded 1,618 visits with prevalent cardiovascular disease (CVD), and 1,635 with prevalent lung disease (chronic bronchitis, emphysema, or asthma, and 890 visits with unavailable exhaled CO measures). Individuals with and without exhaled CO measures were similar with respect to age, sex, BMI, and smoking status (P>0.24). After the exclusions, 14,943 participant visits (4,139 individual participants) were included in the analysis. At each Heart Study examination, participants undergo standardized measurements of blood pressure, anthropometry, medical history, physical examination, and laboratory assessment of risk factors.19 All participants gave written informed consent and the study protocol was approved by the Institutional Review Board at Boston University Medical Center.
Exhaled Carbon Monoxide Assessment
In the absence of acute or chronic pulmonary disease, exogenous and endogenous CO concentrations effectively equilibrate across the alveolar-capillary barrier and exhaled CO accurately reflects blood concentrations of carboxyhemoglobin (COHb).17 Exhaled CO was measured in each participant in a resting state, using the Ecolyzer (2000 series) instrument (Energetics Science Inc., Elmsford, NY). This device employs an electrochemical sensor to quantify the level of CO gas in samples, with a range from 1 to 100 parts per million (ppm). On each day of testing, a canister of CO gas containing exactly 50 ppm was used to calibrate the Ecolyzer to the midpoint of the scale. Two readings were obtained from each participant and averaged. The averaged Ecolyzer readings minus the base rate of the ambient CO level in the testing room constituted the exhaled CO level, a measure shown to be reproducible20 and predominantly reflective of endogenous CO with minimal contamination by ambient exposure.13
Clinical Assessment of Risk Factors
Systolic (SBP) and diastolic (DBP) blood pressure were defined as the average of 2 physician-obtained measurements (using a mercury sphygmomanometer) on participants who had rested for over 5 minutes in a sitting position. Hypertension was defined as a SBP ≥140 mm Hg or DBP≥90 mm Hg, or the use of antihypertensive medication. Current smoking was self-reported and defined as regular cigarette smoking within the year preceding the Heart Study examination. Serum triglycerides, total and HDL cholesterol, and blood glucose were measured after an overnight fast. Diabetes was defined by fasting blood glucose≥126 mg/dL or the use of oral hypoglycemic agents or insulin. Among participants without diabetes, metabolic syndrome was defined as the presence of ≥3 of the following: waist circumference ≥40 inches in men or ≥35 inches in women; serum triglycerides ≥150 mg/dL or taking medication for hypertriglyceridemia; HDL cholesterol <40 mg/dL in men or <50 mg/dL in women or taking medication for reduced HDL cholesterol; SBP ≥130 mm Hg or DBP ≥85 mm Hg or taking antihypertensive medication; and, fasting glucose ≥100 mg/dL.21 Body mass index (BMI) ≥30 kg/m2 was substituted for increased waist circumference at examinations 2 and 3, where measurements of waist were not obtained. As previously described,22 high-sensitivity C-reactive protein (CRP) was measured on examination cycle 2 and 6 specimens with a Dade Behring nephelometer; CRP was measured on examination 5 specimens using the Hemagen assay.
Follow Up and Outcomes
The outcomes of interest included incidence of metabolic syndrome and a first cardiovascular disease (CVD) event during a follow-up period of 4 years after the index examination. Major CVD events were fatal and nonfatal coronary heart disease (myocardial infarction, coronary insufficiency, and angina pectoris), stroke or transient ischemic attack, intermittent claudication, and heart failure. Criteria for these events have been described previously.19,21 CVD events were adjudicated by a panel of 3 experienced investigators based on a review of medical histories, physical examinations at the Framingham Heart Study, and hospitalization and personal physician records. The incidence of metabolic syndrome was defined based on values for the components at the Heart Study examination.
Statistical Analyses
Exhaled CO values above or equal to 50 ppm were censored and the remaining values were log-transformed for all analyses, due to a right skewed distribution.
In analyses of prevalent disease and risk factor burden, we used multivariable linear regression to examine the relation of exhaled CO with the following variables: age, sex, BMI, SBP, DBP, total/HDL cholesterol ratio, log triglycerides, diabetes, self-reported smoking status (never, prior, current), and examination cycle. Among individuals without diabetes, we also examined the relations of exhaled CO with the presence of metabolic syndrome in analyses adjusting for age. All analyses were performed using generalized estimating equations23 based on an autoregressive correlation structure to account for correlations within each participant in the study sample (SAS PROC GENMOD). We performed these analyses in both the total sample and in the sample of non- smokers (including never and prior smokers) to elucidate correlates uninfluenced by the effects of active smoking. For all analyses, exhaled CO was analyzed as a continuous log-transformed variable and as quartiles, with the lowest quartile as the referent. We also examined the correlation of exhaled CO with serum iron (a byproduct of HO activity), serum total bilirubin (a powerful anti-oxidant24), and high sensitivity C-reactive protein (an inflammatory marker) at examination 2, the cycle at which these measurements were available contemporaneously with exhaled CO.
In analyses of incident disease, we used the method of pooling repeated observations25 to assess the relation of exhaled CO with outcomes over consecutive 4-year intervals, after confirming that the assumption of proportionality of hazards was met. Thus, Cox regression26 models were constructed for each 4-year follow-up interval, and participants were eligible to re-enter the analyses if they remained free of events and did not meet the exclusion criteria at each examination visit. Individuals with prevalent metabolic syndrome or diabetes were additionally excluded from analyses of incident metabolic syndrome (leaving 9,573 eligible attendees) but remained eligible for analyses of incident CVD (where participants with prevalent CVD were excluded at the outset). Multivariable analyses of incident metabolic syndrome adjusted for age (due to collinearity of metabolic syndrome with other conventional risk factors). In multivariable analyses of incident CVD, Model 1 adjusted for age and sex; Model 2 further adjusted for BMI, SBP, DBP, total/HDL cholesterol ratio, diabetes, and examination cycle; and, Model 3 adjusted for the covariates in Model 2 in addition to self-reported smoking status. All analyses were performed in both the total sample and in a sample of non-smokers. To assess for any potential non-linearity of relations between exhaled CO and CVD incidence, existing above or below any particular quartile cut-point, we also examined multivariable generalized additive models using penalized splines.
In secondary analyses, we repeated analyses in the sample of participants with BMI in the narrower range of ≥22 and ≤28 kg/m2. To assess the role of inflammation on the association of CO with cardiometabolic risk, we also repeated the main analyses while adjusting for circulating CRP concentrations. For analyses of incident metabolic syndrome, additional adjustment was made for BMI, SBP and smoking status.
To determine the relative contributions of CO and metabolic syndrome to CVD risk, we performed analyses that included both CO and metabolic syndrome as covariates in the same model; we also used a multiplicative interaction term to assess for effect modification of each covariate on the relation of the other to incident CVD.
All analyses were performed using SAS version 9.1.3 (SAS, Cary, NC). The display of multivariable-adjusted hazards ratio of CVD risk versus CO was generated using S-Plus. A two-sided value of P<0.05 was considered significant. The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
RESULTS
Correlates of Exhaled CO
The baseline characteristics of our sample are shown in Table 1. A rug plot displaying the distribution of exhaled CO in our sample is shown in Figure 1. In the total sample, exhaled CO was associated positively with diabetes, total/HDL cholesterol, and smoking status and inversely associated with age and examination cycle (Table 2). In the subset of non-smokers, CO was directly associated with diabetes and BMI, while also inversely associated with age and examination cycle. At examination cycle 2, exhaled CO was modestly correlated with serum iron (r=0.125; P<0.001), serum total bilirubin (r=−0.07 [P<0.001]; r=0.10 [P=0.001] in non-smokers and r=−0.11 [P<0.001] in current/past smokers), and C-reactive protein (r=0.18; P<0.001) in unadjusted Pearson correlation analyses of log-transformed values of each biomarker.
Table 1.
Baseline Characteristics of Study Participants (N=4,139)
| Characteristic | Total Sample | 1st CO quartile | 2nd CO quartile | 3rd CO quartile | 4th CO quartile |
|---|---|---|---|---|---|
| Age, years | 45.6±10.2 | 46.5±10.4 | 46.2±10.6 | 45.8±10.1 | 44.6±10.0 |
| Women, % | 53 | 71 | 53 | 45 | 47 |
| Body mass index, kg/m2 | 25.9±4.7 | 25.1±4.6 | 26.1±4.5 | 26.6±4.9 | 25.6±4.5 |
| Waist circumference, cm | 35.2±5.8 | 33.4±5.5 | 35.1±5.6 | 36.0±5.8 | 35.8±5.7 |
| Blood pressure, mmHg | |||||
| Systolic | 122±17 | 122±17 | 124±17 | 124±16 | 121±17 |
| Diastolic | 78±10 | 77±9 | 79±10 | 79±10 | 77±10 |
| Hypertension, % | 25 | 22 | 27 | 28 | 22 |
| Antihypertensive therapy, % | 11 | 10 | 11 | 13 | 10 |
| Serum cholesterol, mg/dL | |||||
| Total cholesterol | 206.3±40.2 | 203.7±40.8 | 205.4±39.4 | 206.7±40.2 | 208.2±40.3 |
| HDL cholesterol | 49.8±14.7 | 53.9±15.0 | 50.6±14.8 | 50.0±14.3 | 46.8±14.1 |
| Total/HDL ratio | 4.5±1.7 | 4.1±1.5 | 4.4±1.6 | 4.5±1.7 | 4.8±1.8 |
| Serum triglycerides, mg/dL (median, IQR) | 90.0 (54.8, 125.2) | 80.3 (48.9, 111.7) | 86.5 (55.0, 118.0) | 91.2 (54.5, 128.0) | 96.2 (57.4, 135.0) |
| Blood glucose, mg/dL | 94.7±22.5 | 93.2±19.5 | 95.0±23.1 | 95.3±24.6 | 95.1±22.1 |
| Metabolic syndrome, % | 25 | 19 | 25 | 26 | 27 |
| Diabetes, % | 3 | 2 | 4 | 3 | 3 |
| Smoking status, % | |||||
| Current | 32 | 7 | 7 | 12 | 78 |
| Past | 33 | 43 | 46 | 43 | 12 |
| Never | 34 | 50 | 46 | 44 | 10 |
CVD, cardiovascular disease; CO, carbon monoxide; IQR, interquartile range.
Values are presented as mean±SD or percentages unless otherwise indicated.
Figure 1.
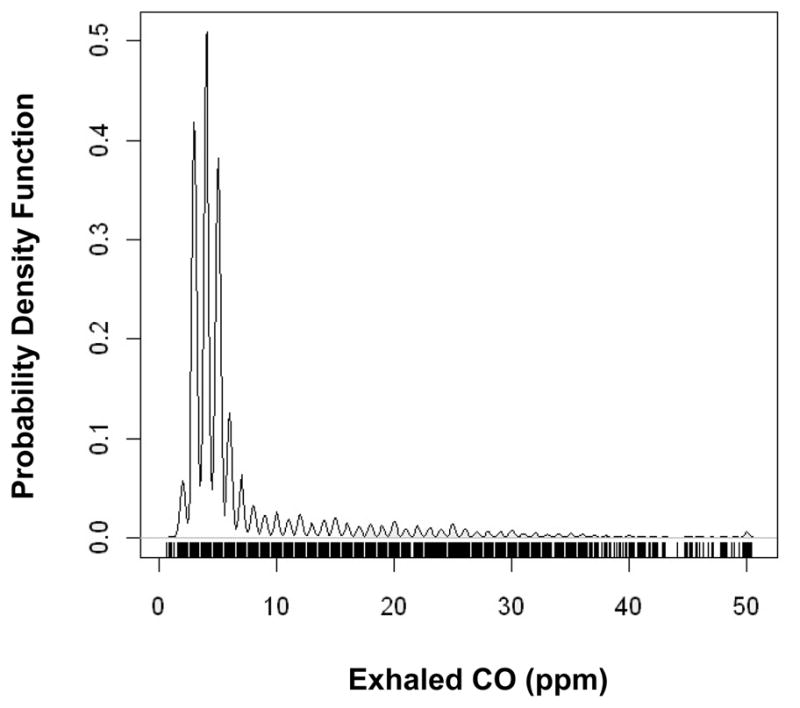
A rug plot displaying the frequency distribution of exhaled CO in the study sample.
Table 2.
Multivariable-Adjusted Cross-Sectional Correlates of Exhaled Carbon Monoxide*
| Variable | Total Sample | Non-Smokers | ||
|---|---|---|---|---|
| Regression Coefficient† (standard error) | P value | Regression Coefficient (standard error) | P value | |
| Age (per 10 years) | −0.025 (0005) | <0.0001 | −0.018 (0.004) | <0.0001 |
| Women | −0.133 (0.010) | <0.0001 | −0.143 (0.008) | <0.0001 |
| BMI (per 5 kg/m2) | −0.001 (0.005) | 0.88 | −0.028 (0.004) | <0.0001 |
| Total/HDL cholesterol | 0.031 (0.006) | <0.0001 | 0.003 (0.005) | 0.56 |
| Log triglycerides | −0.008 (0.010) | 0.43 | 0.011 (0.009) | 0.21 |
| Diabetes | 0.045 (0.018) | 0.014 | 0.039 (0.014) | 0.006 |
| Current smoker | 1.088 (0.018) | <0.0001 | … | … |
| Former smoker | 0.036 (0.007) | <0.0001 | ||
BMI, body mass index; total/HDL, total/high-density lipoprotein cholesterol ratio.
Exhaled carbon monoxide is log transformed and all models are run according to the generalized estimating equation. The model for the total sample adjusted for age, sex, BMI, systolic blood pressure, diastolic blood pressure, total/HDL cholesterol, log triglycerides, diabetes, smoking status, and examination cycle. The model for the non-smokers sample is adjusted for these same covariates except smoking status.
Regression coefficients represent the change in natural log CO for an increase in the value of the predictor variables shown. Thus, women had a 13% lower CO (in ppm) relative to men (e−0.134 = 0.87).
Relations of Exhaled CO with Metabolic Syndrome
Higher CO was positively associated with prevalent metabolic syndrome, both in the total sample (Table 3) as well as in the subset of non-smokers (odds ratio 1.38; P<0.001). In longitudinal analyses, higher CO also conferred a greater risk for incident metabolic syndrome (hazards ratio [HR] 1.23, 95% confidence interval [CI] 1.13–1.34; P<0.0001). Individuals with the highest quartile of baseline exhaled CO had a 1.48 times higher rate of developing metabolic syndrome over the follow-period than those with the lowest quartile of CO (P<0.0001). This relation of baseline CO and incident metabolic syndrome was also observed among the subset of non-smokers, for whom increasing CO was associated with a 1.41 hazards ratio (95% CI, 1.17–1.71) for incident metabolic syndrome (P=0.0003). The association of CO with metabolic syndrome was incrementally higher across increasing CO quartiles (Table 3).
Table 3.
Relation of Exhaled Carbon Monoxide with Metabolic Syndrome
| Exhaled CO Variable | Prevalent Metabolic Syndrome |
Incident Metabolic Syndrome |
|||
|---|---|---|---|---|---|
| Odds Ratio (95% CI) | P value | No. Events/Individuals at Risk* | Odds Ratio (95% CI) | P value | |
| Log CO† | 1.09 (1.02–1.17) | 0.013 | 1458/3199 | 1.23 (1.13–1.34) | <0.0001 |
| 1st quartile‡ | Referent | … | 333/663 | Referent | … |
| 2nd quartile | 1.10 (1.01–1.20) | 0.028 | 390/778 | 1.21 (1.03–1.42) | 0.021 |
| 3rd quartile | 1.11 (1.01–1.22) | 0.029 | 404/769 | 1.45 (1.23–1.70) | <0.0001 |
| 4th quartile | 1.18 (1.05–1.33) | 0.005 | 331/989 | 1.48 (1.25–1.76) | <0.0001 |
CO, carbon monoxide; CI, confidence interval.
All analyses are adjusted for age, and analyses of prevalent metabolic syndrome are performed using the general estimating equation.
For incident metabolic syndrome, number of attendees at risk: 9573 in the total sample; 2442, 2569, 2419, and 2143 across increasing quartiles of exhaled CO, respectively.
Risk estimates correspond to 1 unit change in log CO (in ppm).
Exhaled CO quartiles are defined as 0–<4, ≥4–<5, ≥5–<6, and ≥6 ppm, respectively.
Relations of Exhaled CO with Incident CVD
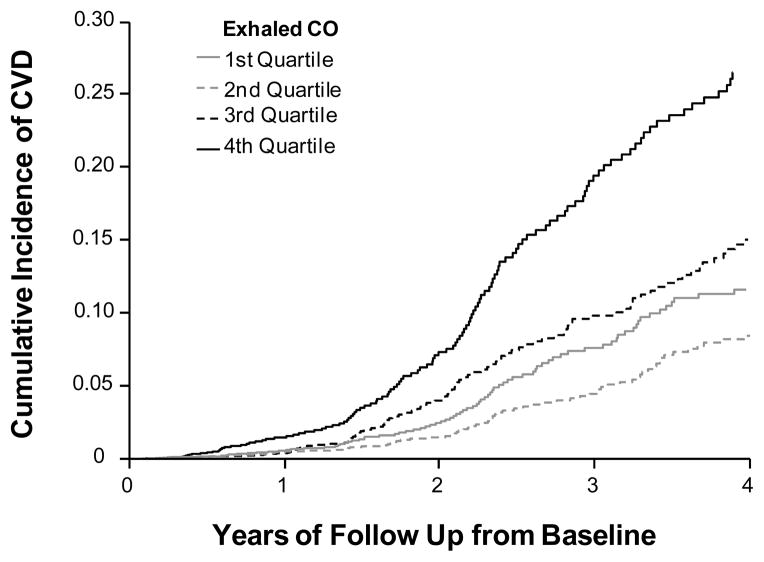
Over the follow-up period, there were a total of 416 incident CVD events (128 in women) among 14,943 individuals at risk. A total of 125 individuals were censored due to death (N=124) or being lost to follow up (N=1). Higher CO was independently associated with higher CVD incidence in the total sample, even after adjusting for smoking status (Table 4). This trend was particularly evident in the highest compared to lowest quartile of CO (Figure 2). In the subset of non-smokers, increasing CO was similarly associated with greater risk for CVD in multivariable analyses (adjusted HR 1.50, 95% CI 1.07–2.10; P=0.018). Accordingly, non-smokers with the highest compared to lowest quartile of CO had a 1.64 adjusted hazards for CVD (95% CI 1.06–2.54; P=0.027). Regression splines (Figure 3) suggested a progressive escalation in CVD risk with increasing values of CO.
Table 4.
Relation of Exhaled Carbon Monoxide with Incident Cardiovascular Disease
| Exhaled CO Variable | No. Events/Individuals at Risk* | Model 1 HR (95% CI) | P value | Model 2 HR (95% CI) | P value | Model 3 HR (95% CI) | P value |
|---|---|---|---|---|---|---|---|
| Log CO† | 416/4139 | 1.72 (1.50–1.98) | <0.0001 | 1.62 (1.40–1.88) | <0.0001 | 1.46 (1.18–1.82) | 0.0006 |
| 1st quartile‡ | 92/836 | Referent | … | Referent | … | Referent | … |
| 2nd quartile | 80/922 | 0.76 (0.56–1.02) | 0.070 | 0.75 (0.55–1.01) | 0.057 | 0.74 (0.55–1.00) | 0.052 |
| 3rd quartile | 109/991 | 1.12 (0.84–1.48) | 0.43 | 1.05 (0.78–1.39) | 0.76 | 1.03 (0.77–1.37) | 0.84 |
| 4th quartile | 135/1390 | 2.19 (1.66–2.89) | <0.0001 | 1.94 (1.45–2.58) | <0.0001 | 1.66 (1.14–2.40) | 0.008 |
CO, carbon monoxide; HR, hazards ratio; CI, confidence interval.
Model 1 is adjusted for age and sex. Model 2 is adjusted for age, sex, systolic and diastolic blood pressure, total/HDL cholesterol, diabetes, and examination cycle. Model 3 is adjusted for age, sex, BMI, systolic and diastolic blood pressure, total/HDL cholesterol, diabetes, examination cycle, and smoking status.
Number of attendees at risk: 14943 in the total sample; 3674, 4037, 3871, and 3361 across increasing quartiles of exhaled CO, respectively.
Risk estimates correspond to 1 unit change in log CO (in ppm).
Exhaled CO quartiles are defined as 0 to <4, ≥4–<5, ≥5–<6, and ≥6 ppm, respectively.
Figure 2.
Kaplan-Meier curves showing the cumulative incidence of cardiovascular disease by exhaled CO quartiles over the follow-up period from the baseline examination (in years).
Figure 3.
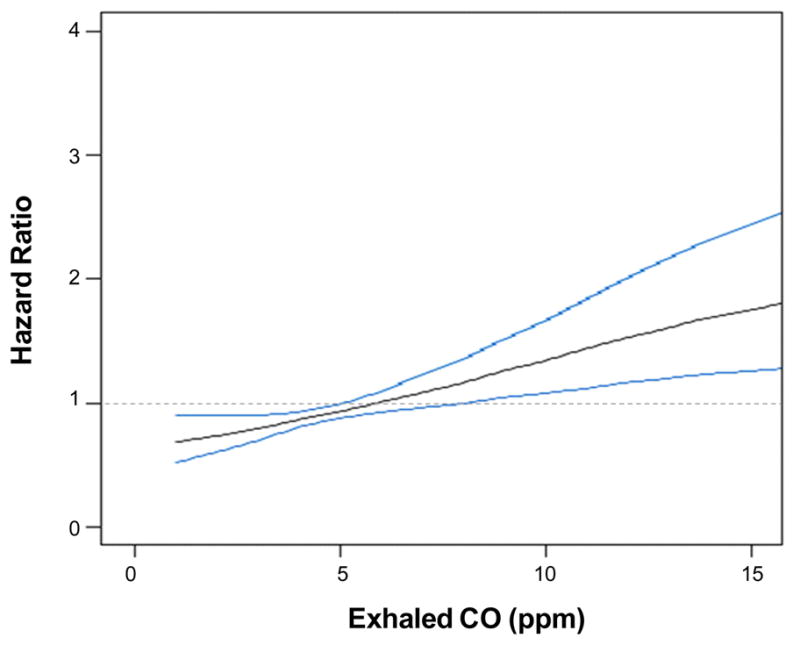
Multivariable-adjusted spline graphs displaying the relation of exhaled CO with cardiovascular events. Bold gray lines show the association between of exhaled CO with event risk; 95% confidence intervals are plotted as fine blue lines. The horizontal dashed line is the line of no association.
Secondary Analyses
In analyses restricted to participants with BMI 22–28 kg/m2, results of the main analyses were unchanged (data not shown). In analyses adjusting for CRP in the total sample, exhaled CO remained significantly associated with prevalent (OR 1.15, 95% CI 1.05–1.25; P=0.003) and with incident (OR 1.17, 95% CI 1.03–1.33; P<0.0001) metabolic syndrome. Exhaled CO also remained significantly associated with incident CVD in multivariable analyses adjusting for CRP in addition to traditional risk factors (HR 1.54, 95% CI 1.26–1.88; P<0.0001), and smoking status (HR 1.34, 95% CI 1.00–1.79; P=0.05). The relation of CO with incident metabolic syndrome remained significant in analyses adjusting for age, BMI, SBP, and smoking status (OR 1.17, 95% CI 1.02–1.34; P=0.03).
The presence of metabolic syndrome as a covariate did not significantly alter the relation of exhaled CO to CVD risk in multivariable analyses adjusting for traditional risk factors (HR 1.64, 95% CI 1.41–1.90; P<0.0001) and additionally for smoking status (HR 1.48, 1.18–1.85; P=0.0006). Metabolic syndrome was a significant predictor of CVD in analyses adjusting for age, sex, smoking status, and examination cycle (HR 2.22, 95% CI 1.81–2.73; P<0.0001), and this relation remained statistically significant in analyses additionally adjusting for exhaled CO (HR 2.21, 95% CI 1.80–2.71; P<0.0001). The multiplicative term representing the interaction between metabolic syndrome and exhaled CO was statistically non-significant in analyses of incident CVD adjusting for traditional risk factors including smoking status (P=0.45). Accordingly, the association of exhaled CO with incident CVD was similar between individuals with (HR 1.40, 95% CI 1.03–1.89; P=0.03) and without (HR 1.54, 95% CI 1.10–2.16; P=0.01) the metabolic syndrome.
DISCUSSSION
Our principle findings were three-fold. First, exhaled CO was inversely associated with age but positively associated with several conventional cardiometabolic risk factors. Second, higher exhaled CO was directly related cross-sectionally to prevalence of the metabolic syndrome. Third, higher CO was associated with both future development of the metabolic syndrome and CVD incidence. Notably, individuals with the highest quartile of exhaled CO had a ~1.7-fold higher risk for CVD than those with the lowest quartile of CO exposure. Overall, these findings suggest that inter-individual variation in CO is associated with metabolic derangements as well as the development of manifest CVD.
Clinical Correlates of Exhaled CO
In our community-based sample, exhaled CO levels were significantly higher in younger compared to older individuals and in men compared to women. These age- and sex-based differences are consistent with findings from smaller samples,27,28 and may be related to demographic variations in chronic exposure to microenvironmental CO.29 The inverse association of CO with age, and differences in CO between men and women, may also be related to the dependence of endogenous CO production on heme oxygenase activity. In animal models, heme oxygenase activity is known to be reduced in older age and more so in males compared to females,30 potentially due to decreased constitutive expression as well as inducibility of HO as a result of yet undetermined mechanisms.31
Cardiometabolic Correlates of Exhaled CO
Importantly, exhaled CO remained significantly associated with several specific cardiovascular risk factors after adjusting for age, sex, examination cycle, and even smoking status. Notably, each of these risk factors is also considered a metabolic trait: increased total/HDL cholesterol, diabetes, and greater BMI. Accordingly, exhaled CO was also significantly associated with both prevalent and incident metabolic syndrome. The association of CO with metabolic syndrome and its components may be due to several mechanisms. In particular, oxidative stress and inflammatory cytokines are known to increase expression of HO, which has a wide tissue distribution and is the primary contributor of enzymatic heme degradation to form endogenous CO, in addition to biliverdin and iron products.1 This corresponds with the observed positive correlation of exhaled CO with serum iron and CRP in our sample. We also observed a correlation between exhaled CO and bilirubin that was positive in non-smokers although negative in current and past smokers. Consistent with the inverse relation of bilirubin to smoking status seen in prior studies,32 it is possible that excess levels of oxidative stress in smokers leads to a relative decrease in conversion of biliverdin to bilirubin via reduction, in favor of increased bilirubin-to-biliverdin conversion via oxidation.33
The HO dependent response to oxidative stress, in particular, may be a main common pathway by which CO is associated with metabolic traits. In a small physiologic study of 24 individuals with diabetes and 37 healthy controls,9 Paredi and colleagues observed that exhaled CO was higher in the presence of diabetes, regardless of smoking status. Both hyperglycemia and the progressive accumulation of advanced glycation end-products in diabetes can be drivers of increased oxidative activity and, in turn, chronic activation of HO.34,35 Similarly, the relation of dyslipidemia with excess CO levels may be related to the fact that oxidized lipids also activate HO.35 This association could also be part of a regulatory pathway whereby the non-CO byproducts of HO activity, biliverdin and bilirubin, are capable of preventing oxidative modification of LDL cholesterol.36 Among the non-smokers in our sample, exhaled CO was also strongly associated with BMI even after adjusting for diabetes. This association could be driven by the presence of metabolic derangements that precede overt diabetes but similarly predispose to oxidative stress. Notably, chronic elevations in endogenous CO may be the both the product and cause of oxidative stress, since excess levels of CO can disrupt the mitochondrial electron transport chain, resulting in the generation of reactive oxygen species.11
Relations of Exhaled CO and Incidence of Cardiovascular Disease
Beyond its relation with metabolic traits, exhaled CO was also significantly associated with incident CVD. Elevated CO may be related to CVD risk in a dose-dependent fashion through a variety of mechanisms. At low physiologic levels, endogenous CO production reflects vasoprotective HO activity.3 In response to stress, endothelial and vascular smooth muscle cells increased HO expression, leading to increased production of CO, which acts as a potent vasodilator.37 Endogenous CO can also inhibit platelet aggregation, vascular cell apoptosis, and inflammatory cytokine release.3 Conversely, overexpression of HO with excess CO production may decrease NO-mediated vasodilation and also promote adverse vascular remodeling by inhibiting vascular proliferation.3,12 Recent experimental data suggest that unopposed oxidative stress may cause endogenous CO to exert vasoconstrictive rather than vasodilatory activity.38 In addition to these vascular effects, it is well recognized that excess total CO exposure can lead to tissue hypoxia by preferentially binding circulating hemoglobin to form COHb.39,40
Taken together, the association of excess CO with both metabolic syndrome and CVD highlights the possibility that CO-related pathways are involved in the pathogenesis of both conditions. Indeed, experimental evidence suggests that CO cycling plays a central role in regulating the endogenous response to a variety of stresses including oxidative stress, inflammation, and disruption of vascular integrity. Accordingly, inter-individual variation in circulating CRP accounted for only a portion of the cardiometabolic risk associated with CO in our analyses. Given its involvement in multiple pathways, an excessive rise in CO could represent a common pathologic factor contributing to the clustering of cardiometabolic disorders. Notably, CO was associated with risk for metabolic syndrome at a lower concentration threshold than for CVD, consistent with the hypothesis that accrual of metabolic derangements likely precedes the development of overt vascular disease.41 Thus, in addition to increasing risk for metabolic syndrome, elevated CO could also promote the progression of metabolic syndrome to overt CVD. Also, exhaled CO and metabolic syndrome appeared to have largely independent effects on increasing cardiovascular risk in our sample. Indeed, elevated CO was associated with incident CVD among individuals with as well as without baseline metabolic syndrome.
Limitations
Several limitations of the present study merit consideration. Although the reference standard for estimating individual CO concentrations is gas chromatography, exhaled CO is a validated13 surrogate measure that can similarly capture spatial and temporal variation in CO levels (unlike ambient monitors). Although, exhaled CO measurements in this study were obtained at a single point in time per visit, the use of a standardized protocol and routinely calibrated instrumentation likely served to reduce excess intra- as well as inter-individual variability. We did not consider data on current number of cigarettes per day or time between the last cigarette and the CO measurement; however, the consistency of the findings in non-smokers is reassuring in this regard. Individuals in our study sample did not have concurrent measures of exhaled or endogenous NO, a theoretical potential confounder in the present analyses.1 Residual confounding from additional unmeasured covariates is also possible. Generalized estimating equations were based on an autogressive correlation structure to account for multiple observations within each participant, but not for between sibling correlations since these correlations have been associated with minimal effects on outcomes in similar prior Framingham studies. Lastly, our sample was predominantly white and of European ancestry, limiting the generalizability of our findings to other racial/ethnic groups.
Conclusion
In our community-based sample, higher exhaled CO levels were associated with the development of metabolic syndrome and future CVD events. The association of exhaled CO with adverse cardiovascular outcomes was maintained after adjustment for traditional cardiovascular risk factors including smoking status. Taken together, the relation of CO with both metabolic traits and overall CVD risk suggests that excess CO exposure is associated with cardiovascular risk via mechanisms beyond those related to tissue hypoxia. Abnormal activation of endogenous CO and HO-related pathways could predispose to both metabolic and cardiovascular diseases through a variety of mechanisms. Similar to the importance of physiologic NO in maintaining vascular homeostasis, endogenous pathways involving the cycling of CO may be critical for modulating vasoactive but also oxidative as well as inflammatory activity. Further investigations of such endogenous CO-related pathways could provide insights into the pathogenesis of metabolic syndrome and CVD.
Clinical Summary.
Carbon monoxide (CO) is widely recognized as a potentially toxic byproduct of hydrocarbon combustion. More recently, CO has been recognized as a key second messenger molecule that is normally present in the body at low concentrations and that serves to protect the cardiovascular system against oxidative, inflammatory, and vascular stresses. Thus, elevated levels of endogenous CO may reflect the presence of underlying metabolic and cardiovascular pathology. We assessed the association of exhaled CO (a marker of endogenous CO) with the presence of cardiometabolic risk factors cross-sectionally, and with the risk of developing future metabolic syndrome as well as overt cardiovascular disease (CVD) prospectively among 4,139 individuals (contributing 14,934 person-examinations) at the Framingham Heart Study. We observed that exhaled CO was associated with the presence of metabolic syndrome (P=0.01) in addition to many of its component risk factors. During up to 4 years of follow-up, baseline CO was also associated with future cardiometabolic events, with individuals in the highest quartile of exhaled CO having a ~1.5 odds of developing metabolic syndrome (P<0.0001) and ~1.7 risk of developing CVD (P=0.0008) compared to lowest quartile, serving as referent. Overall, the findings of this study suggest that elevated CO is an important marker for the development of both metabolic disease and CVD. Additional research is warranted to elucidate how CO-related pathways may contribute to cardiometabolic disorders and their associated outcomes.
Acknowledgments
Funding Support: This work was supported in part by the National Heart, Lung and Blood Institute’s Framingham Heart Study (Contract No. N01-HC-25195) and the American College of Cardiology/Merck Research Fellowship in Cardiovascular Disease and Cardiometabolic Disorders (SC).
Footnotes
Conflict of Interest/Disclosures: None.
References
- 1.Wu L, Wang R. Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol Rev. 2005;57:585–630. doi: 10.1124/pr.57.4.3. [DOI] [PubMed] [Google Scholar]
- 2.Dulak J, Deshane J, Jozkowicz A, Agarwal A. Heme oxygenase-1 and carbon monoxide in vascular pathobiology: focus on angiogenesis. Circulation. 2008;117:231–41. doi: 10.1161/CIRCULATIONAHA.107.698316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durante W. Carbon monoxide and bile pigments: surprising mediators of vascular function. Vasc Med. 2002;7:195–202. doi: 10.1191/1358863x02vm424ra. [DOI] [PubMed] [Google Scholar]
- 4.Bauer I, Pannen BH. Bench-to-bedside review: Carbon monoxide - from mitochondrial poisoning to therapeutic use. Crit Care. 2009;13:220. doi: 10.1186/cc7887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sabaawy HE, Zhang F, Nguyen X, ElHosseiny A, Nasjletti A, Schwartzman M, Dennery P, Kappas A, Abraham NG. Human heme oxygenase-1 gene transfer lowers blood pressure and promotes growth in spontaneously hypertensive rats. Hypertension. 2001;38:210–5. doi: 10.1161/01.hyp.38.2.210. [DOI] [PubMed] [Google Scholar]
- 6.Togane Y, Morita T, Suematsu M, Ishimura Y, Yamazaki JI, Katayama S. Protective roles of endogenous carbon monoxide in neointimal development elicited by arterial injury. Am J Physiol Heart Circ Physiol. 2000;278:H623–32. doi: 10.1152/ajpheart.2000.278.2.H623. [DOI] [PubMed] [Google Scholar]
- 7.Wagner CT, Durante W, Christodoulides N, Hellums JD, Schafer AI. Hemodynamic forces induce the expression of heme oxygenase in cultured vascular smooth muscle cells. J Clin Invest. 1997;100:589–96. doi: 10.1172/JCI119569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biernacki WA, Kharitonov SA, Barnes PJ. Exhaled carbon monoxide in patients with lower respiratory tract infection. Respir Med. 2001;95:1003–5. doi: 10.1053/rmed.2001.1196. [DOI] [PubMed] [Google Scholar]
- 9.Paredi P, Biernacki W, Invernizzi G, Kharitonov SA, Barnes PJ. Exhaled carbon monoxide levels elevated in diabetes and correlated with glucose concentration in blood: a new test for monitoring the disease? Chest. 1999;116:1007–11. doi: 10.1378/chest.116.4.1007. [DOI] [PubMed] [Google Scholar]
- 10.Scharte M, Bone HG, Van Aken H, Meyer J. Increased carbon monoxide in exhaled air of critically ill patients. Biochem Biophys Res Commun. 2000;267:423–6. doi: 10.1006/bbrc.1999.1936. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Piantadosi CA. Mitochondrial oxidative stress after carbon monoxide hypoxia in the rat brain. J Clin Invest. 1992;90:1193–9. doi: 10.1172/JCI115980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peyton KJ, Reyna SV, Chapman GB, Ensenat D, Liu XM, Wang H, Schafer AI, Durante W. Heme oxygenase-1-derived carbon monoxide is an autocrine inhibitor of vascular smooth muscle cell growth. Blood. 2002;99:4443–8. doi: 10.1182/blood.v99.12.4443. [DOI] [PubMed] [Google Scholar]
- 13.Kharitonov SA, Barnes PJ. Exhaled markers of pulmonary disease. Am J Respir Crit Care Med. 2001;163:1693–722. doi: 10.1164/ajrccm.163.7.2009041. [DOI] [PubMed] [Google Scholar]
- 14.Sato S, Nishimura K, Koyama H, Tsukino M, Oga T, Hajiro T, Mishima M. Optimal cutoff level of breath carbon monoxide for assessing smoking status in patients with asthma and COPD. Chest. 2003;124:1749–54. doi: 10.1378/chest.124.5.1749. [DOI] [PubMed] [Google Scholar]
- 15.Zayasu K, Sekizawa K, Okinaga S, Yamaya M, Ohrui T, Sasaki H. Increased carbon monoxide in exhaled air of asthmatic patients. Am J Respir Crit Care Med. 1997;156:1140–3. doi: 10.1164/ajrccm.156.4.96-08056. [DOI] [PubMed] [Google Scholar]
- 16.Horvath I, Donnelly LE, Kiss A, Paredi P, Kharitonov SA, Barnes PJ. Raised levels of exhaled carbon monoxide are associated with an increased expression of heme oxygenase-1 in airway macrophages in asthma: a new marker of oxidative stress. Thorax. 1998;53:668–72. doi: 10.1136/thx.53.8.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones RH, Ellicott MF, Cadigan JB, Gaensler EA. The relationship between alveolar and blood carbon monoxide concentrations during breathholding; simple estimation of COHb saturation. J Lab Clin Med. 1958;51:553–64. [PubMed] [Google Scholar]
- 18.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–90. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 19.Kannel WB, Wolf PA, Garrison RJ, editors. Section 34: Some Risk Factors Related to the Annual Incidence of Cardiovascular Disease and Death in Pooled Repeated Biennal Measurements: Framingham Heart Study, 30 Year Follow-Up. Bethesda, MD: US Department of Health and Human Services; 1987. [Google Scholar]
- 20.Hedblad B, Ogren M, Engstrom G, Wollmer P, Janzon L. Heterogeneity of cardiovascular risk among smokers is related to degree of carbon monoxide exposure. Atherosclerosis. 2005;179:177–83. doi: 10.1016/j.atherosclerosis.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 22.Benjamin EJ, Dupuis J, Larson MG, Lunetta KL, Booth SL, Govindaraju DR, Kathiresan S, Keaney JF, Jr, Keyes MJ, Lin JP, Meigs JB, Robins SJ, Rong J, Schnabel R, Vita JA, Wang TJ, Wilson PW, Wolf PA, Vasan RS. Genome-wide association with select biomarker traits in the Framingham Heart Study. BMC Med Genet. 2007;8 (Suppl 1):S11. doi: 10.1186/1471-2350-8-S1-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.SAS/STAT User’s Guide, Version 8. Cary, NC: SAS Institute Inc; 2000. [Google Scholar]
- 24.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–6. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 25.D’Agostino RB, Lee ML, Belanger AJ, Cupples LA, Anderson K, Kannel WB. Relation of pooled logistic regression to time dependent Cox regression analysis: the Framingham Heart Study. Stat Med. 1990;9:1501–15. doi: 10.1002/sim.4780091214. [DOI] [PubMed] [Google Scholar]
- 26.Cox DR. Regression models and life tables (with discussion) J Royal Stat Soc. 1972;34 (series B):187–220. [Google Scholar]
- 27.Jones AY, Lam PK. End-expiratory carbon monoxide levels in healthy subjects living in a densely populated urban environment. Sci Total Environ. 2006;354:150–6. doi: 10.1016/j.scitotenv.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 28.Cunnington AJ, Hormbrey P. Breath analysis to detect recent exposure to carbon monoxide. Postgrad Med J. 2002;78:233–7. doi: 10.1136/pmj.78.918.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chau CK, Tu EY, Chan DW, Burnett J. Estimating the total exposure to air pollutants for different population age groups in Hong Kong. Environ Int. 2002;27:617–30. doi: 10.1016/s0160-4120(01)00120-9. [DOI] [PubMed] [Google Scholar]
- 30.Ariyoshi T, Tsuboi K, Hamasaki K. Effects of age and sex on microsomal heme oxygenase and cytochrome P-450 content in liver of rats. J Pharmacobiodyn. 1981;4:664–9. doi: 10.1248/bpb1978.4.664. [DOI] [PubMed] [Google Scholar]
- 31.Barnes CJ, Cameron IL, Puleo-Scheppke B, Lee M. Age alters expression and inducibility of heme oxygenase isoenzymes in mice. Age. 1998;21:123–128. doi: 10.1007/s11357-998-0019-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Hoydonck PG, Temme EH, Schouten EG. Serum bilirubin concentration in a Belgian population: the association with smoking status and type of cigarettes. Int J Epidemiol. 2001;30:1465–1472. doi: 10.1093/ije/30.6.1465. [DOI] [PubMed] [Google Scholar]
- 33.Baranano DE, Rao M, Ferris CD, Snyder SH. Biliverdin reductase: a major physiologic cytoprotectant. Proc Natl Acad Sci U S A. 2002;99:16093–8. doi: 10.1073/pnas.252626999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan SD, Schmidt AM, Anderson GM, Zhang J, Brett J, Zou YS, Pinsky D, Stern D. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J Biol Chem. 1994;269:9889–97. [PubMed] [Google Scholar]
- 35.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–12. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 36.Neuzil J, Stocker R. Free and albumin-bound bilirubin are efficient co-antioxidants for alpha-tocopherol, inhibiting plasma and low density lipoprotein lipid peroxidation. J Biol Chem. 1994;269:16712–9. [PubMed] [Google Scholar]
- 37.Siow RC, Sato H, Mann GE. Heme oxygenase-carbon monoxide signalling pathway in atherosclerosis: anti-atherogenic actions of bilirubin and carbon monoxide? Cardiovasc Res. 1999;41:385–94. doi: 10.1016/s0008-6363(98)00278-8. [DOI] [PubMed] [Google Scholar]
- 38.Lamon BD, Zhang FF, Puri N, Brodsky SV, Goligorsky MS, Nasjletti A. Dual pathways of carbon monoxide-mediated vasoregulation. Circ Res. 2009;105:775–783. doi: 10.1161/CIRCRESAHA.109.197434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coburn RF. Endogenous carbon monoxide production and body CO stores. Acta Med Scand Suppl. 1967;472:269–82. doi: 10.1111/j.0954-6820.1967.tb12633.x. [DOI] [PubMed] [Google Scholar]
- 40.Rodkey FL, O’Neal JD, Collison HA. Oxygen and carbon monoxide equilibria of human adult hemoglobin at atmospheric and elevated pressure. Blood. 1969;33:57–65. [PubMed] [Google Scholar]
- 41.Deedwania PC. Metabolic syndrome and vascular disease: is nature or nurture leading the new epidemic of cardiovascular disease? Circulation. 2004;109:2–4. doi: 10.1161/01.CIR.0000110642.73995.BF. [DOI] [PubMed] [Google Scholar]