Abstract
Background
We previously used a three-dimensional (3D) reconstituted basement membrane (rBM) assay to demonstrate that tumorigenic HMT-3522 T4–2 human breast cells can be induced to form morphologically normal structures (“reversion”) by treatment with inhibitors of β1 integrin, the epidermal growth factor receptor (EGFR), or mitogen-activated protein kinase (MAPK). We have now used this assay to identify reversion and/or death requirements of several more aggressive human breast cancer cell lines.
Methods
Breast tumor cell lines MCF7, Hs578T, and MDA-MB-231 were cultured in 3D rBM and treated with inhibitors of β1 integrin, MAPK, or phosphatidylinositol 3-kinase (PI3K). MDA-MB-231 cells, which lack E-cadherin, were transfected with an E-cadherin cDNA. The extent of reversion was assessed by changes in morphology and polarity, growth in 3D rBM or soft agar, level of invasiveness, and tumor formation in nude mice.
Results
All three cell lines showed partial reversion (MCF7 the greatest and Hs578T the least) of tumorigenic properties treated with a single β1 integrin, MAPK, or PI3K inhibitor. Combined inhibition of β1 integrin and either PI3K or MAPK resulted in nearly complete phenotypic reversion (MDA-MB-231, MCF7) or in cell death (Hs578T). E-cadherin-transfected MDA-MB-231 cells showed partial reversion, but exposure of the transfectants to an inhibitor of β1 integrin, PI3K, or MAPK led to nearly complete reversion.
Conclusion
The 3D rBM assay can be used to identify signaling pathways that, when manipulated in concert, can lead to the restoration of morphologically normal breast structures or to death of the tumor cells, even highly metastatic cells. This approach may be useful to design therapeutic intervention strategies for aggressive breast cancers.
Epithelial tissue structure and function depend on coordinated cues from the extracellular matrix (ECM), neighboring cells, and growth factors (1,2). The integrin family of cell–ECM adhesion receptors, the cadherin family of cell–cell adhesion receptors, and the epidermal growth factor receptor (EGFR) family participate in mediating these signals. Misregulation of these signaling pathways results in a loss of tissue organization and can contribute to tumor formation and progression (3,4). We have developed a three-dimensional (3D) assay that uses a gel of reconstituted basement membrane (rBM) proteins in which phenotypically normal and malignant human breast cells can be distinguished from each other by differences in structural organization and growth behavior (5), and we have used this assay to investigate alterations in signaling pathways that accompany the acquisition of malignancy in a progression model (6,7) of human breast cancer development. When cultured in 3D rBM, nonmalignant, early-passage HMT-3522 cells (called S1 cells) develop into growth-arrested, phenotypically normal structures that are reminiscent of terminal ductal lobular units (or acini) with functional E-cadherin-containing cell–cell junctions, integrins with polarized localization, and basal secretion of basement membrane components. The malignant HMT-3522 cells (called T4–2 cells), derived after removal of EGF from the culture medium (7), form disorganized colonies with compromised cell–cell adhesion, and these cells are tumorigenic in nude mice. Comparison of S1 and T4–2 cells revealed that the latter cells express elevated levels and activities of β1 integrins, EGFR, and mitogen-activated protein kinase (MAPK). Nevertheless, the T4–2 cells can undergo a phenotypic reversion to a growth-arrested and polarized structure in response to treatment with an inhibitory antibody against β1 integrin, an EGFR inhibitor, or an MKK1 (mitogen kinase kinase 1) inhibitor (8,9). Consequently, the phenotype associated with the unbalanced signaling resulting from activation of MAPK, likely mediated by increased levels of β1 integrins and EGFR, can be restored to normal in this malignant cell line with a single inhibitor. These experiments show that the 3D rBM assay is a tractable model that allows molecular events leading to malignant behavior can be systematically dissected.
In this study, we asked whether other breast cancer cell lines, including metastatic and invasive lines, could be induced to revert to a normal phenotype. For these experiments, MCF7 cells were chosen as representative of rapidly growing tumor cells that are E-cadherin positive, vimentin negative, and non-invasive (10). (E-cadherin is an adhesion molecule and a tumor suppressor. Vimentin is an intermediate filament protein.) MDA-MB-231 and Hs578T breast tumor cells were chosen as examples of invasive and metastatic tumor cells that express vimentin and lack E-cadherin (11,12). All cell lines examined show constitutive deregulation of growth factor/cell adhesion signaling pathways due, in part, to mutation and/or overexpression of downstream ras guanosine 5′-triphosphatases (GTPases) (13–15) and elevated levels of β1 integrins, phosphatidylinositol 3-kinases (PI3Ks), and MAPK (16–18).
Materials and Methods
Cell Culture
The human breast cancer cell lines MCF7 and MDA-MB-231 were obtained from the American Type Culture Collection (Manassas, VA), and Hs578T cells were a gift from Dr. J. Mott (Lawrence Berkeley National Laboratory, Berkeley, CA). E-cadherin-positive MDA-MB-231 cells were obtained by transfecting parental MDA-MB-231 cells with a full-length mouse E-cadherin cDNA in pBATEM2 plasmid under the control of the chicken β-actin promoter (11). E-cadherin-transfected MDA-MB-231 cells were referred to as MDA-E-cad cells. All cell lines were maintained in Dulbecco’s modified Eagle medium (DMEM)/F12 (Life Technologies [Gibco BRL], Rockville, MD) with 5% fetal bovine serum and penicillin/streptomycin. MDA-MB-231 cells transfected with vector (clone MR) or E-cadherin (clone M) (19) were cultured in DMEM/F12 containing 5% fetal bovine serum and G418 (500 μg/mL). HMT-3522 mammary epithelial cells were cultured in H14 medium (DMEM/F12 containing insulin at 250 ng/mL, transferrin at 10 μg/mL, sodium selenite at 2.6 ng/mL, 1 × 10−10 M estradiol, 1.4 × 10−6 M hydrocortisone, and prolactin at 5 μg/mL). The nonmalignant S1 cells were grown on plastic in medium containing EGF at 10 ng/mL, and the malignant T4–2 cells were propagated on collagen type I-coated dishes in the absence of EGF (7–9). The 3D rBM cultures were prepared by growing cells to confluence as monolayers, followed by trypsinization and embedding (S1 and T4–2 cells at 8.5 × 105 cells/mL, MDA-MB-231 cells at 5 × 105 cells/mL) inside a commercially prepared rBM that is produced by Englebreth–Holm–Swarm tumors (Matrigel, growth factor reduced; Collaborative Research, Inc., Waltham, MA) (5,8,9). S1 and T4–2 cells grown in 3D rBM were maintained in medium as described above, and MDA-MB-231 cells were cultured in DMEM/F12 medium. All cultures were analyzed after 10 days of cultivation in Matrigel.
Blocking Antibodies and Inhibitors
The β1 integrin function-blocking monoclonal antibody AIIB2 (a gift from C. Damsky, University of California at San Francisco) (20) was introduced into the cell-embedded substratum as purified rat immunoglobulin G (IgG) at a concentration of 10 μg/mL at the time of Matrigel gelation (8). The human EGFR-blocking monoclonal antibody mAb225 (Oncogene Research Products, San Diego, CA) was added as purified mouse IgG at a concentration of 4 μg/mL (8). Nonspecific rat IgG was obtained from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA). Tyrphostin AG 1478 (a potent protein tyrosine kinase inhibitor; Calbiochem Corp., La Jolla, CA), PD98059 (a specific inhibitor of MAPK; New England Biolabs, Inc., Beverly, MA), and LY294002 (an inhibitor of PI3K; Biomol, Plymouth Meeting, PA) were dissolved in dimethyl sulfoxide. PD98059 and LY294002 were used at 4 μM for the single treatment of MDA-MB-231 cells and at 2 μM for combined treatment of these cells. All inhibitors were added to the medium on alternate days. The concentration of inhibitors used for assays permitted 50% growth inhibition under standard culture conditions without apparent cell death. The optimal inhibitor concentrations for the MDA-MB-231 cells were used with the other aggressive breast cancer cells for comparison. Control cultures were treated with vehicle only.
5-Bromo-2-deoxyuracil Incorporation Index
The proliferative rate of cells grown in 3D rBM was measured by assaying 5-bromo-2-deoxyuracil (BrdUrd) incorporation with a commercially available labeling and detection kit (Boehringer Mannheim GmbH, Mannheim, Germany). Briefly, after 9 days, cells were pulse-labeled with BrdUrd for 24 hours, released from Matrigel with Dispase (Collaborative Biomedical Products, Bedford, MA), and then allowed to spread on gelatin-coated coverslips before fixation. Labeled nuclei were detected, according to the manufacturer’s instructions (Boehringer Mannheim GmbH). BrdUrd-labeled indices were determined by visually scoring nuclei stained with 4′,6-diamidino-2-phenylindole in 200–400 cells and thereafter scoring BrdUrd-positive cells as a percentage of the total cell number (8,9). Each experiment was repeated at least three times, and each point was in triplicate. Values given are the results of one representative experiment, all with similar results.
Immunoblotting
Cells grown as monolayers were lysed in situ in RIPA buffer (1% Nonidet P-40, 0.5% deoxycholate, 0.2% sodium dodecyl sulfate, 150 mM sodium chloride, and 50 mM Tris–HCl [pH 7.4] containing 2 mM sodium fluoride, 1 mM sodium orthovanadate, leupeptin at 10 μg/mL, pepstatin at 10 μg/mL, aprotinin at 10 μg/mL, E64 at 10 μg/mL, and 1 mM Pefabloc [Sigma Chemical Co., St. Louis, MO]). Cells grown in 3D rBM cultures were first isolated as colonies in ice-cold PBS/EDTA (0.01 M sodium phosphate [pH 7.2] containing 138 mM sodium chloride and 5 mM EDTA), as previously described (8,9), and thereafter lysed in RIPA buffer. Equal amounts of protein lysates were loaded on reducing Laemmli gels (21) and analyzed on immunoblots. Positive bands were detected with an enhanced chemiluminescence (ECL) system (Amersham Biosciences, Piscataway, NJ). For immunoblot analysis of EGFR, β1 integrin, and E-cadherin, antibody clones 13, 18, and 36 were used, respectively (BD Biosciences, San Jose, CA). For immunoblot analysis of cadherin 11 and β-actin, we used antibody clones cad113H (a gift from M. Bussemakers, University Hospital Nijmegen, The Netherlands) (22) and PT-66 (Sigma Chemical Co.), respectively.
Soft Agar Assays
Approximately 1 × 104 cells were first plated in 1 mL of DMEM/F12 containing 5% fetal bovine serum and 0.3% agarose and then overlaid with 1 mL of 1% agarose, with or without an inhibitor treatment. Cultures were maintained for 10 days. Duplicate wells were scored positive when colonies exceeded the minimum diameter of 80 μm (9). Experiments for MDA-MB-231 and MDA-E-cad cells were done in triplicate and repeated three times. One representative experiment is shown.
Invasion Assays
Invasion assays were performed in a modified Boyden chamber with filter inserts (8-μm pores) for 24-well plates (Collaborative Biomedical Products) (23). Filters were coated on ice with 10 μL of Matrigel protein at 6–8 mg/mL. Approximately 1 × 105 cells were plated into the upper chamber in 200 μL of DMEM/F12 containing 5% fetal bovine serum. The lower chamber was filled with 300 μL of medium. Blocking antibodies and inhibitors were added immediately after cell plating at the concentrations described above. After 18 hours in culture, cells were fixed with 5% glutaraldehyde in phosphate-buffered saline and stained with 0.5% toluidine blue (Sigma Chemical Co.) in 2% Na2CO3. Cells on the upper side of the filter, including those in the Matrigel, were removed with paper towels, and cells on the lower side of the filter were visualized and counted. Each experiment was repeated four times in duplicate, and one representative experiment is shown.
Tumor Formation In Vivo
Approximately 5 × 105 MDA-MB-231 cells were plated on 100-mm2 tissue culture dishes (Falcon; BD Biosciences) and treated with 4 μM LY 294002, 4 μM PD 98059, 10 μg/mL AIIB2 alone, or 10 μg/mL AIIB2 plus 2 μM LY 294002 4 hours after plating. Cells were propagated for 3–4 days, trypsinized, washed three times in DMEM/F12, and incubated with the above reagents for 2 hours. Control cells were treated with mock antibody (nonspecific rat IgG) and dimethyl sulfoxide. Approximately 1 × 106 cells were then injected subcutaneously into the rear flanks of 6- to 7-week-old BALB/c nu/nu mice. Tumor nodules were measured 4 weeks after injection.
Results
Reversion and Inhibition of β1 Integrin, PI3K, or MAPK
Malignant T4–2 cells (HMT-3522), grown in 3D rBM, are reverted phenotypically by treatment with anti-integrin antibodies, EGFR antibodies or inhibitors, or an MKK1 inhibitor (9). This reversion is paralleled by a reduction in malignant behavior and tumorigenicity (9). More recently, we have found that inhibition of PI3K has a similar effect (24). To determine whether similar reversion can be obtained with other breast cancer cells, we chose the rapidly growing MCF7 and the highly invasive and metastatic MDA-MB-231 and Hs578T breast tumor cell lines. We assessed the effect of individual inhibitors of β1 integrin (AIIB2), PI3K (LY294002), or MAPK (PD98059) pathways on the morphology of all three cell lines grown in 3D rBM and on their invasiveness in Boyden chambers. In 3D rBM, MCF7 cells formed large, loose aggregates similar to T4–2 cells (Fig. 1, A), and the addition of AIIB2, LY294002, or PD98059 equally reduced the size of aggregates (Fig. 1, A and B, and data not shown). Both MDA-MB-231 and Hs578T cells formed stellate structures in 3D rBM (Fig. 1, A), but treatment of either cell line with AIIB2 altered the growth rate and the morphology of colonies, leading to the formation of compact aggregates that were reminiscent of the colonies formed by untreated T4–2 cells in 3D rBM [Fig. 1, A and B, and (8,9)]. Although the PI3K inhibitor LY294002 (Fig. 1) and the MAPK inhibitor PD98059 (data not shown) reduced the size of cell colonies (25), neither inhibitor strongly affected the morphology of cell aggregates (Fig. 1, A). All three inhibitors reduced cell growth by 60%–70% (Fig. 1, B). Similar to findings for HMT-3522 T4–2 cells, treatment of MDA-MB-231 cells and Hs578T cells with these inhibitors did not induce cellular apoptosis (data not shown).
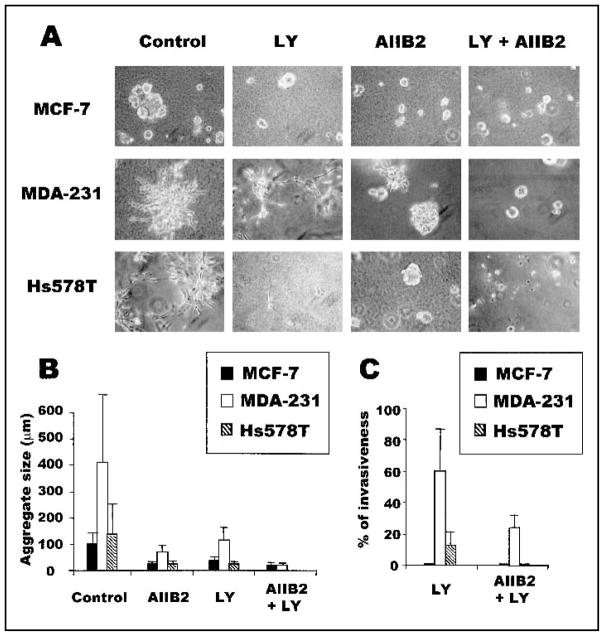
Fig. 1.
Inhibiting the combination of β1 integrin and phosphatidylinositol 3-kinase (PI3K) promotes a greater alteration in morphology, aggregate size, and invasiveness than inhibiting either PI3K or β1 integrin alone. A) MCF7, MDA-MB-231 (MDA-231), and Hs578T breast tumor cells grown in three-dimensional (3D) reconstituted basement membrane (rBM) cultures in the presence of anti-β1 integrin antibody (AIIB2) and/or PI3K inhibitor LY294002 (LY). All cultures were analyzed after 10 days of rBM culture. B) Size of the colonies formed by the three breast tumor cell lines grown in 3D rBM in the presence of inhibitors (error bars indicate 95% confidence intervals of triplicate samples). Experiments were repeated four times with similar results. C) Invasiveness of treated and untreated MDA-MB-231 (MDA-231) and Hs578T breast cancer cells in Matrigel-coated Boyden chamber assays. The invasiveness of these cells treated with AIIB2, LY294002, or AIIB2 plus LY294002 is shown as a percentage of control (error bars indicate 95% confidence intervals of triplicate samples; experiments were repeated four times with similar results). MCF7 cells were not invasive in this assay. Experiments were repeated three times with similar results.
The invasive behavior of cell lines was analyzed as another marker of a tumorigenic property and quantified by Matrigel-coated Boyden chamber assays (Fig. 1, C). MCF7 cells did not invade through Matrigel-coated filters, but as previously reported (26), both MDA-MB-231 and Hs578T breast tumor cells were invasive. The addition of AIIB2 or LY294002 inhibited, but did not completely block, the invasive behavior of MDA-MB-231 and Hs578T cells (Fig. 1, C). PD98059 had an effect similar to that of LY294002 (data not shown).
The response of MDA-MB-231 tumor cells to the addition of single inhibitors EGFR (mAb225), AIIB2, LY294002, and PD98059 was then analyzed and compared with the responses of T4–2 cells (8,9). Treatment of MDA-MB-231 cells with the EGFR-neutralizing antibody mAb225 had no detectable effect on either morphology in 3D rBM or rate of growth (Fig. 2, A and B). This result is consistent with previous observations that MDA-MB-231 cells respond poorly to the mitogenic effects of EGF or EGFR-neutralizing antibodies when grown as monolayers (13–15). The AIIB2 antibody, PD98059, and LY294002 inhibited growth in 3D rBM to a similar extent (Fig. 2, B). Because AIIB2 antibody had the strongest effect on the morphology of MDA-MB-231 tumor cells grown in 3D rBM, the response of the MDA-MB-231 cells to AIIB2 was investigated in more detail and compared with the phenotype of both S1 (nonmalignant) and AIIB2-treated T4–2 tumorigenic cells. The AIIB2-treated MDA-MB-231 cells formed larger colonies than S1 cells (Fig. 2, A and C [inset]) and exhibited a partially organized cortical actin cytoskeleton as compared with IgG-treated MDA-MB-231 cells, which showed a disorganized actin cytoskeleton (Fig. 2, C). S1 and antibody-treated T4–2 cells formed well-organized, cortical actin cytoskeletons [Fig. 2, C (inset) and (8,9)]. Neither AIIB2-treated MDA-MB-231 cells nor control IgG-treated cells showed distinct polarized β4 integrin (data not shown). Collectively, these experiments demonstrate that, although individual inhibition of MAPK or PI3K reduced growth of MDA-MB-231 cells grown in 3D rBM and although AIIB2 antibody also induced a limited degree of structural organization, none of these treatments alone was sufficient to produce a full phenotypic reversion.
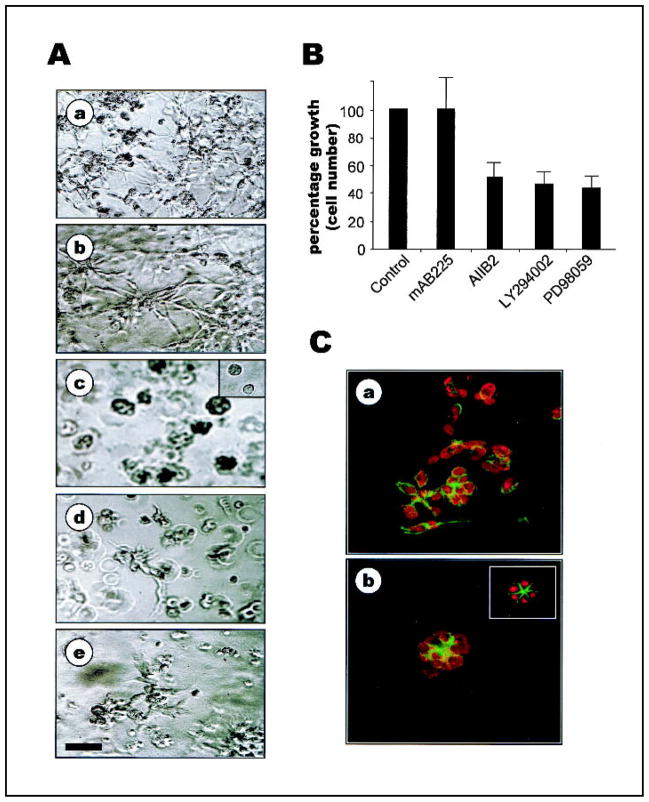
Fig. 2.
Inhibition of epidermal growth factor receptor (EGFR), β1 integrin, phosphatidylinositol 3-kinase (PI3K), or mitogen-activated protein kinase (MAPK) alone is not sufficient to induce phenotypic reversion of MDA-MB-231 cells. A) Phase-contrast micrographs of control untreated cells (a) or cells treated with EGFR inhibitory antibody mAb225 (b), β1 integrin inhibitory antibody AIIB2 (c), PI3K inhibitor LY294002 (d), or MAPK inhibitor PD98059 (e) in three-dimensional (3D) reconstituted basement membrane (rBM) cultures. Cb, inset) Phase-contrast micrograph of S1 cells in 3D rBM. Bar = 60 μm. B) Growth of MDA-MB-231 cells treated with mAb225, AIIB2, LY294002, or PD98059, as indicated by cell number shown as percentage of control (error bars indicate 95% confidence intervals of triplicate samples; experiments were repeated three times with similar results). C) Confocal fluorescence microscopy images of filamentous actin (fluorescein isothiocyanate = green) and nuclei (propidium iodide = red) in 5-μm cryosections of MDA-MB-231 cells in the presence (b) or absence (a) of AIIB2. Inset, Confocal fluorescence microscopy image of S1 cells. All cultures in A–C were analyzed after 10 days of rBM culture.
Reversion and Reduced Malignant Behavior With Combined Inhibition of β1 Integrins and PI3K or β1 Integrin and MAPK
Treatment of T4–2 cells with any one inhibitor was sufficient to allow a reversion to a nonmalignant phenotype in 3D rBM (9). Because this mode of treatment was not sufficient to produce a similar phenotypic conversion in the more aggressive cell lines, simultaneous inhibition of more than one pathway was assessed for synergistic effects. The three cell lines were grown in 3D rBM with the AIIB2 antibody combined with either LY294002 or PD98059. We also tested the response of MDA-MB-231 cells to a combination of LY294002 and PD98059 in the absence of AIIB2. In MCF7 and MDA-MB-231 tumor cells, treatment with AIIB2 and either LY294002 or PD98059 reduced the colony size to nearly that of S1 or reverted T4–2 acini (Figs. 1, B, and 3, A). In contrast, combined use of AIIB2 with either LY294002 or PD98059 resulted in death of Hs578T tumor cells (Fig. 1, A). These combinations of inhibitors also strongly reduced the invasiveness of MDA-MB-231 tumor cells in Boyden chamber assays (Fig. 1, C). The combination of LY294002 and PD98059 did not cause the morphology of MDA-MB-231 tumor cells to revert to normal (Fig. 3, A), although the growth of these cells in monolayer and cell treatment with the AIIB2 antibody plus LY294002 or PD98509 were similar (Fig. 3, C, and data not shown). Thus, these results show that morphologic reorganization and inhibition of proliferation are distinct phenomena.
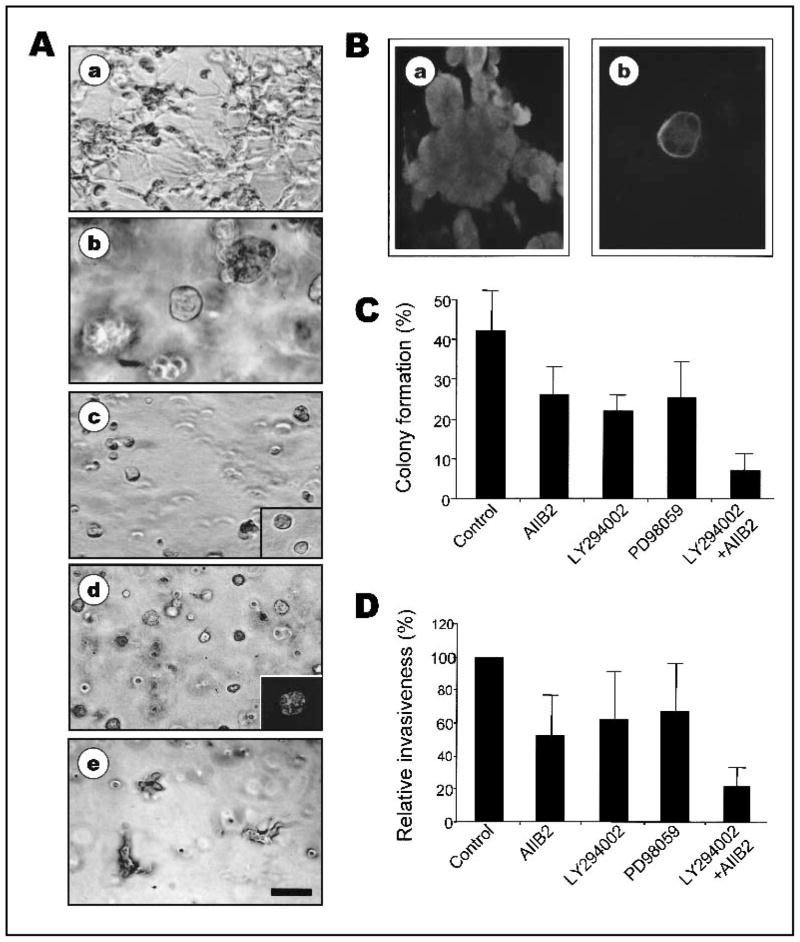
Fig. 3.
Inhibition of β1 integrin plus phosphatidylinositol 3-kinase (PI3K) or inhibition of β1 integrin plus mitogen-activated protein kinase (MAPK) is sufficient to induce phenotypic reversion of MDA-MB-231 cells. A) Phase-contrast micrographs of control untreated MDA-MB-231 cells (a), MDA-MB-231 cells treated with β1 integrin inhibitory antibody AIIB2 (b), AIIB2 plus PI3K inhibitor LY294002 (c), AIIB2 plus MAPK inhibitor PD98059 (d), or LY294002 plus PD98059 (e) in three-dimensional (3D) reconstituted basement membrane (rBM) cultures. Bar = 50 μm. c, inset) Phase-contrast micrograph of S1 cells in 3D rBM. d, inset) Confocal fluorescence microscopy image of filamentous actin (F-actin) in 5-μm cryosections of MDA-MB-231 cells treated with AIIB2 plus PD98059 (×2 image). Bar = 25 μm. Similar pattern of staining of F-actin and nuclei was obtained with cells treated with AIIB2 plus LY294002. B) Confocal immunofluorescence microscopy images of β4 integrin in untreated control (a) and AIIB2 plus LY294002 treated (b) MDA-MB-231 cells. C) Anchorage-independent growth of untreated and treated MDA-MB-231 cells in soft agar. Colony formation by MDA-MB-231 cells treated with AIIB2, LY294002, PD98059, or AIIB2 plus LY294002 shown as percentage of colony formation (mean ± 95% confidence intervals; experiments were repeated three times with similar results). D) Relative invasiveness of untreated and treated MDA-MB-231 cells, treated as in (B), shown as percentage of control (± 95% confidence intervals; experiments were repeated three times with similar results).
We targeted the MDA-MB-231 tumor cell line for further evaluation of phenotypic reversion induced by a combination of inhibitors. Treatment of MDA-MB-231 cells with AIIB2 and either PD98059 or LY294002 promoted a polarized, basal distribution of β4 integrins (Fig. 3, B) and the formation of a well-organized cortical actin cytoskeleton (Fig. 3, Ad, inset). Thus, the phenotypic reversion of MDA-MB-231 cells treated with two inhibitors was similar to that of T4–2 tumor cells treated with one inhibitor. Growth rates of MDA-MB-231 tumor cells, measured by BrdUrd incorporation, were also substantially reduced by these treatments (data not shown).
To investigate whether dual inhibition of β1 integrin and PI3K or MAPK was sufficient to reduce other indicators of malignant behavior, we assessed anchorage-independent growth in vitro and tumor formation in nude mice. Compared with untreated MDA-MB-231 cells, MDA-MB-231 cells treated with AIIB2, LY294002, or PD98059 alone exhibited a 40% decrease in anchorage-independent growth, whereas MDA-MB-231 cells treated with combinations of AIIB2 and LY294002 or PD98059 exhibited an 80% decrease in anchorage-independent growth (Fig. 3, C, and data not shown). Nude mice injected with MDA-MB-231 cells treated with AIIB2, LY294002, or PD98059 produced fewer and smaller tumors than mice injected with untreated tumor cells (Table 1). MDA-MB-231 cells treated with a combination of AIIB2 and LY294002, however, produced no detectable tumors in any of the injected mice during the time frame of the experiment (Table 1). These results demonstrate that reversion of MDA-MB-231 cells in 3D rBM results in long-term inhibition of tumorigenic behavior, despite the fact that the treated cells retain their original mutated genome.
Decreased Levels of β1 Integrin and Cadherin 11 After Combined Inhibition of β1 Integrin and PI3K or MAPK
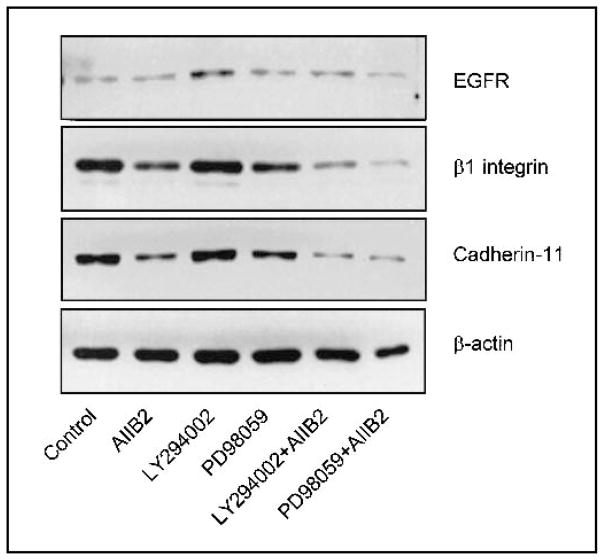
We have previously noted that a decrease in the expression of β1 integrins and EGFR in T4–2 cells is cross-regulated when cells are treated with inhibitory anti-β1 integrin antibody and grown in 3D rBM (8,9). Consequently, we were not surprised to find that the more aggressive MDA-MB-231 cells also show some cross-modulation. β1 integrin, cadherin 11, and EGFR levels were measured in MDA-MB-231 cells treated with AIIB2, LY294002, or PD98059 alone or with combinations of AIIB2 and either LY294002 or PD98059. Treatment with LY294002 or PD98059 alone did not strongly alter the expression of β1 integrin. In contrast, treatment with AIIB2 alone reduced β1 integrin expression slightly, and treatment with AIIB2 plus LY294002 or AIIB2 plus PD98059 strongly decreased the expression of β1 integrin (Fig. 4). EGFR protein expression, which is low in MDA-MB-231 cells, was not measurably altered by any of these treatments (Fig. 4).
Fig. 4.
Immunoblot of epidermal growth factor receptor (EGFR), β1 integrin, and cadherin 11 protein levels as a function of inhibitor treatment. Control MDA-MB-231 cells and MDA-MB-231 cells treated with AIIB2, LY294002, PD98059, AIIB2 plus LY294002, or AIIB2 plus PD98059. β-Actin was used as the loading control.
In addition to the expression of integrin and growth factor receptor, the levels and localization of cell–cell adhesion proteins are important for maintaining the normal phenotype of epithelial cells. Loss of E-cadherin in malignant breast epithelial cells is associated with an invasive phenotype, and its restoration reduces invasion (27–30). Although the expression of E-cadherin is frequently decreased or absent in malignant breast epithelial cell lines, cells often express other cadherins, including N- and P-cadherins, as well as cadherin 11 (26). MDA-MB-231 cells do not express N-cadherin [data not shown and (26)], but they do express cadherin 11, which is associated with increased motility and invasive potential of some breast cancer lines (22,25,27). Treatment of MDA-MB-231 cells with AIIB2, AIIB2 plus LY294002, or AIIB2 plus PD98059 decreased the expression of cadherin 11 (Fig. 4), which may account for the reduced invasiveness associated with these treatments (Fig. 1, C). These treatments did not lead to re-expression of E-cadherin in MDA-MB-231 cells (data not shown).
Reversion of MDA-MB-231 Cells With E-Cadherin Expression and Treatment With a Single Inhibitor
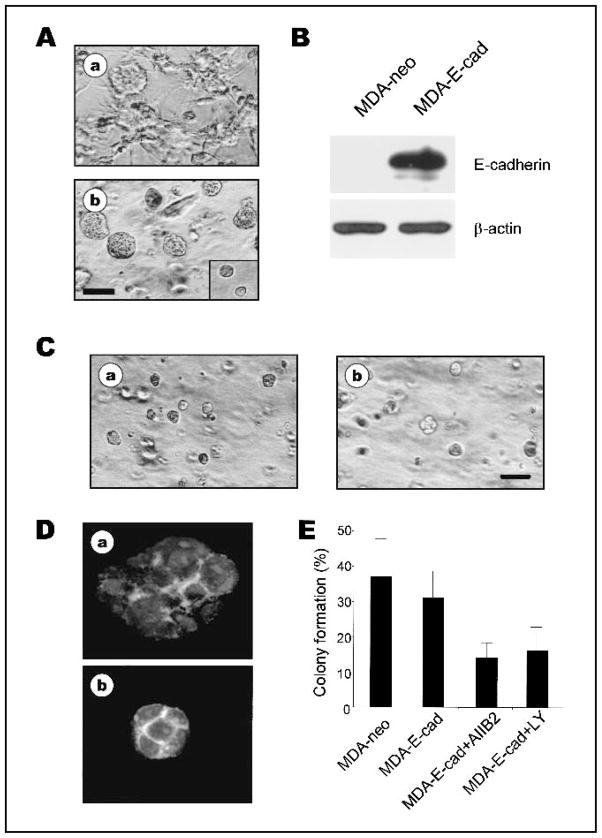
Exogenous expression of E-cadherin in MDA-MB-231 cells has been shown to promote the formation of prominent cell–cell adhesions, an altered cellular morphology, and a marked decrease in osteolytic bone metastasis in nude mice (19,28). When grown in 3D rBM, colonies of MDA-MB-231 cells transfected with E-cadherin (MDA-E-cad) were compact, similar to parental cells treated with AIIB2 (compare Fig. 5, Ab, with Fig. 1, A). Transfected cells were only partially reverted, as indicated by a 50% growth reduction (data not shown), larger colony size relative to S1 cells, and a partially organized, cortical, filamentous actin cytoskeleton (compare Fig. 5 with Figs. 2 and 3). BrdUrd-labeling indices of MDA-E-cad cells were similar to those of MDA-MB-231 cells treated with AIIB2 (data not shown), but MDA-E-cad colonies were larger (compare Fig. 2, A, with Fig. 5, A).
Fig. 5.
Expression of E-cadherin plus a single inhibitor is sufficient to induce phenotypic reversion of MDA-MB-231 cells. A) Phase-contrast micrographs of control vector-transfected MDA-MB-231 cells (MDA-neo, a) and MDA-MB-231 cells expressing E-cadherin (MDA-E-cad, b) in three-dimensional (3D) reconstituted basement membrane (rBM) cultures. Bar = 60 μm. B) Immunoblot of E-cadherin levels in MDA-neo and MDA-E-cad cells. β-Actin was used as a loading control. C) Phase-contrast micrographs of MDA-E-cad cells treated with β1 integrin inhibitory antibody AIIB2 (a), or phosphatidylinositol 3-kinase (PI3K) inhibitor LY294002 (b), in 3D rBM. Scale bar = 60 μm. D) Confocal fluorescence microscopy image of filamentous actin and nuclei in 5-μm cryosections of MDA-E-cad cells (a) and MDA-E-cad cells treated with LY294002 (b). E) Anchorage-independent growth of untreated and treated MDA-E-cad cells in soft agar. Percentage of colony formation by MDA-neo cells, MDA-E-cad cells, or MDA-E-cad cells treated with AIIB2 or LY294002 (mean ± 95% confidence intervals of duplicate samples; experiments were repeated three times).
To determine whether inhibition of additional pathways, in combination with forced expression of E-cadherin, would achieve a more complete reversion, we treated MDA-E-cad cells with AIIB2, LY294002 (Fig. 5, C), or PD98059 (data not shown) alone. We found that these treatments produced small colonies similar in size and BrdUrd labeling to normal S1 acini (Fig. 5, C, and data not shown). These treated colonies also exhibited a well-organized, cortical, filamentous actin cytoskeleton (Fig. 5, D) similar to that in S1 cells or reverted T4–2 tumor cells (8,9).
Finally, we assessed the effect of E-cadherin expression combined with inhibition of β1 integrin, PI3K, or MAPK on the behavior of MDA-MB-231 cells grown on soft agar. The anchorage-independent growth of MDA-E-cad cells (E-cadherin-transfected MDA-MB-231 cells) was not substantially lower than that of empty vector-transfected parental cells. When MDA-E-cad cells were treated with AIIB2, LY294002 (Fig. 5, E), or PD98059 (data not shown), alone or with AIIB2 plus LY294002 or PD98059, anchorage-independent cell growth was inhibited to a similar extent (compare Fig. 3, C, with Fig. 5, E). Although expression of E-cadherin in the absence of treatment with signaling inhibitors (or vice versa) was insufficient to inhibit the malignant phenotype of MDA-MB-231 cells, the combination of E-cadherin transfection and treatment with signaling inhibitors led to phenotypic reversion.
Discussion
In this article, we describe the ability of signaling inhibitors to either revert invasive and metastatic breast cancer cells to a near-normal phenotype or to cause cell death. Reversion to a near normal phenotype was defined by the formation of small, growth-arrested, well-differentiated, and polarized acinar structures in 3D rBM. We previously found that when HMT-3522 T4–2 cells, a tumorigenic but less invasive breast tumor cell line than the cell lines used in this study, were grown in 3D rBM, inhibition of EGFR, β1 integrin, PI3K, or MAPK alone was sufficient to restore formation of normal breast structures (8,9). These inhibitors also promoted phenotypic reversion in 3D rBM of MCF7 breast tumor cells, which proliferate rapidly but, like HMT-3522 T4–2 cells, are not invasive. The single-treatment regimens produced neither complete phenotypic reversion nor cell death of invasive MDA-MB-231 or Hs578T tumor cells in 3D rBM. However, altering adhesion receptor levels (by inhibiting β1 integrin or by expressing E-cadherin) while simultaneously inhibiting one of the intracellular protein kinase signaling pathways (by inhibiting PI3K or MAPK) effectively reverted the more aggressive tumor cells (e.g., MDA-MB-231 cells) or caused cell death (e.g., Hs578T cells). Therefore, tumor cells such as MDA-MB-231 cells, despite having a mutated genome, still retain the ability to be restored to a nonmalignant phenotype when they are grown in an appropriate 3D context. Because this treatment prevents tumor formation in vivo, transient reconfiguration of these signaling pathways has a lasting effect on cell phenotype. These results provide further evidence that balanced integration of signals from cell–ECM, cell–cell, and growth factor pathways is required for the formation of differentiated tissue-like structures and that this integration can be achieved even when key intracellular signaling pathways are constitutively deregulated by mutations. We predict that the combined inhibition of aberrant ECM and/or cell adhesion receptors and intracellular signaling pathways is a potential therapeutic approach that may be a more effective treatment for aggressive breast tumors than the use of single inhibitors (8,9,24), and that assaying these treatments in 3D rBM may be a useful method to identify the optimal combination for a particular tumor cell.
Previous studies have shown that β1 integrins play a prominent role in the invasiveness and metastatic potential of MDA-MB-231 cells (29,30). β1 integrins, which are required for growth, differentiation, and prevention of apoptosis of normal mammary epithelial cells (5), coordinate signaling through EGFR and other growth factor receptors (3,4,31). Overexpression and/or defective signaling through this cell–ECM receptor and the growth factor receptor EGFR affect characteristics associated with tumor invasiveness and metastatic potential (31–34).
Unlike the effect in T4–2 cells (7), inhibition of EGFR activity had no measurable effect on the phenotype and the tumorigenic properties of MDA-MB-231 cells in our assay. A previous study showed that EGF promotes the migration of MDA-MB-231 cells in vitro (35), but inhibition of EGFR did not block invasion of MDA-MB-231 cells in 3D rBM. The lack of effect of EGFR function-blocking antibodies on the phenotype of MDA-MB-231 cells noted in this article is consistent with previous work demonstrating that MDA-MB-231 cells lack a proliferative response to EGF (11,13), a signaling defect that may result from the presence of an activating mutation in Ras (13,15).
In addition to rendering MDA-MB-231 cells less responsive to the mitogenic effects of EGF than cells with a wild-type Ras gene, the presence of a ras mutation would also constitutively activate signaling through effector pathways that activate PI3K and MAPK. These kinases act downstream of both β1 integrins and EGFR, and their constitutive activation in MDA-MB-231 and Hs578T cells partially explains the requirement for inhibiting these pathways to achieve reversion or cell death. However, the requirement for a β1 integrin blockade in addition to the blockade of either PI3K or MAPK suggests that β1 integrin signaling provides critical regulatory input to these pathways. Regulation could involve coupling or partial uncoupling of these kinases with other signaling pathways downstream of β1 integrins or with those pathways originating from other cell surface ECM receptors, such as dystroglycan [Mushler J, Bissell MJ: unpublished results, and (36,37)] or E-cadherin. Certainly, one important difference between T4–2 cells and either MDA-MB-231 or Hs578T cells is that the latter cells express no measurable E-cadherin.
E-cadherin is a Ca2+-dependent cell–cell adhesion protein, the loss of which is strongly associated with breast tumor progression (38) and enhanced tumor cell motility and invasion in vitro (39). Although some malignant cells, including T4–2 cells, still express E-cadherin, expression of this protein does not always result in functional cell adhesion sites (8,9,40). E-cadherin is coupled to signaling pathways regulated by ras, and activation of ras–MAPK or ras–PI3K pathways can result in disassembly of E-cadherin-mediated cell–cell junctions (41–45). Conversely, suppression of the ras–erk pathway can permit the expression of E-cadherin and/or reassembly of cadherins and other proteins required for functional cell–cell adhesion sites (44,46). Consistent with these reports, we have previously shown that inhibition of the MAPK pathway promotes E-cadherin-mediated cell–cell adhesion in T4–2 tumor cells (9). In MDA-MB-231 cells, however, we found that inhibition of MAPK or PI3K did not result in re-expression of E-cadherin; this defect may thus underlie the requirement for inhibition of both β1 integrin and ras effector pathways to achieve reversion. An important consequence of exogenous E-cadherin expression in MDA-MB-231 cells was the resultant alteration in reversion requirements: E-cadherin expression permitted reversion to occur in the presence of any one of the three inhibitors used in this study, even though the genome of the MDA-MB-231 cells was otherwise unaffected. Previous investigations have shown that E-cadherin signaling is integrated with signaling from integrins and growth factor receptors through MAPK (8,9) and PI3K, and that alterations in cadherin 11 can also affect the level and activity of integrins (and vice versa) (40,47), although the exact alteration is highly cell type-dependent (48–50).
In summary, we have shown that alteration of adhesion receptors and intracellular signaling pathways can be used to successfully revert the malignant phenotype of aggressive breast cancer cell lines that bear activating mutations in key regulatory genes, if these manipulations are performed in a physiologic context. Clearly, correction of β1 integrin, EGFR, and E-cadherin signaling is not the only way to achieve phenotypic reversion. However, these results show that the 3D rBM assay can be used to identify which particular combinations of inhibitors may be the most effective for a given subset of breast cancers (4). Thus, this assay may be of use for designing tumor-specific therapy regimens with the potential to kill cancer cells or to revert breast cancer progression.
Acknowledgments
This work was supported by contract DE-AC03-76SF00098 from the U.S. Department of Energy, Office of Biological and Environmental Research (to M. J. Bissell); Public Health Service grants CA64786 (to M. J. Bissell and O. W. Petersen) and CA63528 (to T. Yoneda) from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services; the Novo Foundation; grant 9503681 from the Danish Medical Research Council (to O. W. Petersen); Hollaender postdoctoral fellowship DE-AC03-SF00098 (to D. Radisky); and Canadian Institutes of Health research grant MT-9416 (to E. Turley).
We thank C. Damsky for the AIIB2 antibody; M. Wheelock for the suggestion to test the cadherin 11 levels; and R. Schwarz, S. Muthuswamy, and Fu-Sheng Wang for helpful comments. We thank A. Ukena and N. Jelliffe for administrative support and S.-Y. Moonlee for expert technical assistance.
Contributor Information
Fei Wang, Life Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA.
Rhonda K. Hansen, Life Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA
Derek Radisky, Life Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA.
Toshiyuki Yoneda, University of Texas Health Science Center, Department of Medicine, San Antonio, TX.
Mary Helen Barcellos-Hoff, Life Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA.
Ole W. Petersen, Structural Cell Biology Unit, Institute of Medical Anatomy, The Panum Institute, Copenhagen, Denmark
Eva A. Turley, London Regional Cancer Center, London, Ontario, Canada
Mina J. Bissell, Life Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA
References
- 1.Bissell MJ, Weaver VM, Lelievre SA, Wang F, Petersen OW, Schmeichel KL. Tissue structure, nuclear organization, and gene expression in normal and malignant breast. Cancer Res. 1999;59(7 Suppl):1757s–63s. discussion 1763s–4s. [PubMed] [Google Scholar]
- 2.Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–57. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 3.Werb Z, Vu TH, Rinkenberger JL, Coussens LM. Matrix-degrading proteases and angiogenesis during development and tumor formation. APMIS. 1999;107:11–8. doi: 10.1111/j.1699-0463.1999.tb01521.x. [DOI] [PubMed] [Google Scholar]
- 4.Bissell MJ, Radisky D. Putting tumours in context. Nature Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells [published erratum appears in Proc Natl Acad Sci U S A 1993;90:2556] Proc Natl Acad Sci U S A. 1992;89:9064–8. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briand P, Petersen OW, Van Deurs B. A new diploid nontumorigenic human breast epithelial cell line isolated and propagated in chemically defined medium. In Vitro Cell Dev Biol. 1987;23:181–8. doi: 10.1007/BF02623578. [DOI] [PubMed] [Google Scholar]
- 7.Briand P, Nielsen KV, Madsen MW, Petersen OW. Trisomy 7p and malignant transformation of human breast epithelial cells following epidermal growth factor withdrawal. Cancer Res. 1996;56:2039–44. [PubMed] [Google Scholar]
- 8.Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, et al. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–45. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, Briand P, et al. Reciprocal interactions between β1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci U S A. 1998;95:14821–6. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schiemann S, Schwirzke M, Brunner N, Weidle UH. Molecular analysis of two mammary carcinoma cell lines at the transcriptional level as a model system for progression of breast cancer. Clin Exp Metastasis. 1998;16:129–39. doi: 10.1023/a:1021941203905. [DOI] [PubMed] [Google Scholar]
- 11.Kirschmann DA, Seftor EA, Nieva DR, Mariano EA, Hendrix MJ. Differentially expressed genes associated with the metastatic phenotype in breast cancer. Breast Cancer Res Treat. 1999;55:127–36. doi: 10.1023/a:1006188129423. [DOI] [PubMed] [Google Scholar]
- 12.Paciotti GF, Tamarkin L. Interleukin-1 directly regulates hormone-dependent human breast cancer cell proliferation in vitro. Mol Endocrinol. 1988;2:459–64. doi: 10.1210/mend-2-5-459. [DOI] [PubMed] [Google Scholar]
- 13.Davidson NE, Gelmann EP, Lippman ME, Dickson RB. Epidermal growth factor receptor gene expression in estrogen receptor-positive and negative human breast cancer cell lines. Mol Endocrinol. 1987;1:216–23. doi: 10.1210/mend-1-3-216. [DOI] [PubMed] [Google Scholar]
- 14.Ennis BW, Lippman ME, Dickson RB. The EGF receptor system as a target for antitumor therapy. Cancer Invest. 1991;9:553–62. doi: 10.3109/07357909109018953. [DOI] [PubMed] [Google Scholar]
- 15.Kato K, Ueoka Y, Tamura T, Nishida J, Wake N. Oncogenic Ras modulates epidermal growth factor responsiveness in endometrial carcinomas. Eur J Cancer. 1998;34:737–44. doi: 10.1016/s0959-8049(97)10124-1. [DOI] [PubMed] [Google Scholar]
- 16.Aoudjit F, Vuori K. Integrin signaling inhibits paclitaxel-induced apoptosis in breast cancer cells. Oncogene. 2001;20:4995–5004. doi: 10.1038/sj.onc.1204554. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen DH, Catling AD, Webb DJ, Sankovic M, Walker LA, Somlyo AV, et al. Myosin light chain kinase functions downstream of Ras/ERK to promote migration of urokinase-type plasminogen activator-stimulated cells in an integrin-selective manner. J Cell Biol. 1999;146:149–64. doi: 10.1083/jcb.146.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castoria G, Migliaccio A, Bilancio A, Di Domenico M, de Falco A, Lombardi M, et al. PI3-kinase in concert with Src promotes the S-phase entry of oestradiol-stimulated McF-7 cells. EMBO J. 2001;20:6050–9. doi: 10.1093/emboj/20.21.6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mbalaviele G, Dunstan CR, Sasaki A, Williams PJ, Mundy GR, Yoneda T. E-cadherin expression in human breast cancer cells suppresses the development of osteolytic bone metastases in an experimental metastasis model. Cancer Res. 1996;56:4063–70. [PubMed] [Google Scholar]
- 20.Werb Z, Tremble PM, Behrendtsen O, Crowley E, Damsky CH. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989;109:877–89. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Pishvaian MJ, Feltes CM, Thompson P, Bussemakers MJ, Schalken JA, Byers SW. Cadherin-11 is expressed in invasive breast cancer cell lines. Cancer Res. 1999;59:947–52. [PubMed] [Google Scholar]
- 23.Lochter A, Srebrow A, Sympson CJ, Terracio N, Werb Z, Bissell MJ. Misregulation of stromelysin-1 expression in mouse mammary tumor cells accompanies acquisition of stromelysin-1-dependent invasive properties. J Biol Chem. 1997;272:5007–15. doi: 10.1074/jbc.272.8.5007. [DOI] [PubMed] [Google Scholar]
- 24.Wang F, Yoneda T, Barcellos-Hoff MH, Bissell MJ. Combinatorial modifications of multiple pathways reverts the malignant phenotype of mammary carcinoma cells MDA-MB231. Mol Biol Cell. 1999;10(Suppl):2024. [Google Scholar]
- 25.Sebolt-Leopold JS, Dudley DT, Herrera R, Becelaere KV, Wiland A, Gowan RC, et al. Blockade of the MAP kinase pathway suppresses growth of colon tumors in vivo. Nat Med. 1999;5:810–6. doi: 10.1038/10533. [DOI] [PubMed] [Google Scholar]
- 26.Nawrocki Raby B, Polette M, Gilles C, Clavel C, Strumane K, Matos M, et al. Quantitative cell dispersion analysis: new test to measure tumor cell aggressiveness. Int J Cancer. 2001;93:644–52. doi: 10.1002/ijc.1380. [DOI] [PubMed] [Google Scholar]
- 27.Nieman MT, Prudoff RS, Johnson KR, Wheelock MJ. N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J Cell Biol. 1999;147:631–44. doi: 10.1083/jcb.147.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zantek ND, Azimi M, Fedor-Chaiken M, Wang B, Brackenbury R, Kinch MS. E-cadherin regulates the function of the EphA2 receptor tyrosine kinase. Cell Growth Differ. 1999;10:629–38. [PubMed] [Google Scholar]
- 29.Trusolino L, Cavassa S, Angelini P, Ando M, Bertotti A, Comoglio PM, et al. HGF/scatter factor selectively promotes cell invasion by increasing integrin avidity. FASEB J. 2000;14:1629–40. doi: 10.1096/fj.14.11.1629. [DOI] [PubMed] [Google Scholar]
- 30.Morini M, Mottolese M, Ferrari N, Ghiorzo F, Buglioni S, Mortarini R, et al. The alpha 3 beta 1 integrin is associated with mammary carcinoma cell metastasis, invasion, and gelatinase B (MMP-9) activity. Int J Cancer. 2000;87:336–42. [PubMed] [Google Scholar]
- 31.Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, et al. FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol. 2000;2:249–56. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- 32.Tsynes BB, Haugland HK, Bjerkvig R. Epidermal growth factor and laminin receptors contribute to migratory and invasive properties of gliomas. Invasion Metastasis. 1997;17:270–80. [PubMed] [Google Scholar]
- 33.Mariotti A, Kedeshian PA, Dans M, Curatola AM, Gagnoux-Palacios L, Giancotti FG. EGF-R signaling through Fyn kinase disrupts the function of integrin alpha6beta4 hemidesmosomes: role in epithelial cell migration and carcinoma invasion. J Cell Biol. 2001;155:447–58. doi: 10.1083/jcb.200105017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pouliot N, Nice EC, Burgess AW. Laminin-10 mediates basal and EGF-stimulated motility of human colon carcinoma cells via alpha(3)beta(1) and alpha(6)beta(4) integrins. Exp Cell Res. 2001;266:1–10. doi: 10.1006/excr.2001.5197. [DOI] [PubMed] [Google Scholar]
- 35.Price JT, Tiganis T, Agarwal A, Djakiew D, Thompson EW. Epidermal growth factor promotes MDA-MB-231 breast cancer cell migration through a phosphatidylinositol 3′-kinase and phospholipase C-dependent mechanism. Cancer Res. 1999;59:5475–8. [PubMed] [Google Scholar]
- 36.Losasso C, Di Tommaso F, Sgambato A, Ardito R, Cittadini A, Giardina B, et al. Anomalous dystroglycan in carcinoma cell lines. FEBS Lett. 2000;484:194–8. doi: 10.1016/s0014-5793(00)02157-8. [DOI] [PubMed] [Google Scholar]
- 37.Colognat H, Winkelmann DA, Yurchenco PD. Laminin polymerization induces a receptor-cytoskeleton network. J Cell Biol. 1999;145:619–31. doi: 10.1083/jcb.145.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berx G, Van Roy F. The E-cadherin/catenin complex: an important gatekeeper in breast cancer tumorigenesis and malignant progression. Breast Cancer Res. 2001;3:289–93. doi: 10.1186/bcr309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takeichi M. Cadherins in cancer: implications for invasion and metastasis. Curr Opin Cell Biol. 1993;5:806–11. doi: 10.1016/0955-0674(93)90029-p. [DOI] [PubMed] [Google Scholar]
- 40.Zhu AJ, Watt FM. Expression of a dominant negative cadherin mutant inhibits proliferation and stimulates terminal differentiation of human epidermal keratinocytes. J Cell Sci. 1996;109:3013–23. doi: 10.1242/jcs.109.13.3013. [DOI] [PubMed] [Google Scholar]
- 41.Sander EE, van Delft S, ten Klooster JP, Reid T, van der Kammen RA, Michiels F, et al. Matrix-dependent Tiam1/Rac signaling in epithelial cells promotes either cell–cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase. J Cell Biol. 1998;143:1385–98. doi: 10.1083/jcb.143.5.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamei T, Matozaki T, Sakisaka T, Kodama A, Yokoyama S, Peng YF, et al. Coendocytosis of cadherin and c-Met coupled to disruption of cell–cell adhesion in MDCK cells—regulation by Rho, Rac and Rab small G proteins. Oncogene. 1999;18:6776–84. doi: 10.1038/sj.onc.1203114. [DOI] [PubMed] [Google Scholar]
- 43.Expada J, Perex-Moreno M, Braga VM, Rodriguez-Viciana P, Cano A. H-ras activation promotes cytoplasmic accumulation and phosphoinositide 3-OH kinase association of beta-catenin in epidermal keratinocytes. J Cell Biol. 1999;146:967–80. doi: 10.1083/jcb.146.5.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu Q, Paredes M, Zhang J, Kosik KS. Basal extracellular signal-regulated kinase activity modulates cell–cell and cell-matrix interactions. Mol Cell Biol. 1998;18:3257–65. doi: 10.1128/mcb.18.6.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Potempa S, Ridley AJ. Activation of both MAP kinase and phosphatidylinositide 3-kinase by Ras is required for hepatocyte growth factor/scatter factor-induced adherens junction disassembly. Mol Biol Cell. 1998;9:2185–200. doi: 10.1091/mbc.9.8.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen YH, Lu Q, Schneeberger EE, Goodenough DA. Restoration of tight junction structure and barrier function by down-regulation of the mitogen-activated protein kinase pathway in ras-transformed Madin-Darby canine kidney cells. Mol Biol Cell. 2000;11:849–62. doi: 10.1091/mbc.11.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moorehead RA, Fata JE, Johnson MB, Khokha R. Inhibition of mammary epithelial apoptosis and sustained phosphorylation of Akt/PKB in MMTV-IGF-II transgenic mice. Cell Death Differ. 2001;8:16–29. doi: 10.1038/sj.cdd.4400762. [DOI] [PubMed] [Google Scholar]
- 48.Gimond C, van Der Flier A, van Delft S, Brakebusch C, Kuikman I, Collard JG, et al. Induction of cell scattering by expression of beta1 integrins in beta1-deficient epithelial cells requires activation of members of the rho family of GTPases and downregulation of cadherin and catenin function. J Cell Biol. 1999;147:1325–40. doi: 10.1083/jcb.147.6.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilding J, Vousden KH, Soutter WP, McCrea PD, Del Buono R, Pignatelli M. E-cadherin transfection down-regulates the epidermal growth factor receptor and reverses the invasive phenotype of human papilloma virus-transfected keratinocytes. Cancer Res. 1996;56:5285–92. [PubMed] [Google Scholar]
- 50.Hazan RB, Norton L. The epidermal growth factor receptor modulates the interaction of E-cadherin with the actin cytoskeleton. J Biol Chem. 1998;273:9078–84. doi: 10.1074/jbc.273.15.9078. [DOI] [PubMed] [Google Scholar]