Summary
The extracellular matrix (ECM) is an important regulator of mammary epithelial cell function both in vivo and in culture. Substantial remodeling of ECM accompanies the structural changes in the mammary gland during gestation, lactation and involution. However, little is known about the nature of the enzymes and the processes involved. We have characterized and studied the regulation of cell-associated and secreted mammary gland proteinases active at neutral pH that may be involved in degradation of the ECM during the different stages of mammary development. Mammary tissue extracts from virgin and pregnant CD-1 mice resolved by zymography contained three major proteinases of 60K (K=103Mr), 68K and 70K that degraded denatured collagen. These three gelatinases were completely inhibited by the tissue inhibitor of metalloproteinases. Proteolytic activity was lowest during lactation especially for the 60K gelatinase which was shown to be the activated form of the 68K gelatinase. The activated 60K form decreased prior to parturition but increased markedly after the first two days of involution. An additional gelatin-degrading proteinase of 130K was expressed during the first three days of involution and differed from the other gelatinases by its lack of inhibition by the tissue inhibitor of metalloproteinases. The activity of the casein-degrading proteinases was lowest during lactation. Three caseinolytic activities were detected in mammary tissue extracts. A novel 26K cell-associated caseinase – a serine arginine-esterase – was modulated at different stages of mammary development. The other caseinases, at 92K and a larger than 100K, were not developmentally regulated. To find out which cell type produced the proteinases in the mammary gland, we isolated and cultured mouse mammary epithelial cells. Cells cultured on different substrata produced the full spectrum of gelatinases and caseinases seen in the whole gland thus implicating the epithelial cells as a major source of these enzymes. Analysis of proteinases secreted by cells grown on a reconstituted basement membrane showed that gelatinases were secreted preferentially in the direction of the basement membrane. The temporal pattern of expression of these proteinases and the basal secretion of gelatinases by epithelial cells suggest their involvement in the remodelling of the extracellular matrix during the different stages of mammary development and thus modulation of mammary cell function.
Keywords: gelatinase, caseinase, extracellular matrix (ECM), mammary gland, proteinase
Introduction
Two classes of enzymes have been implicated in a proteolytic cascade for extracellular matrix (ECM) degradation: metalloproteinases and plasminogen activators. The metalloproteinases appear to be the rate-limiting enzymes in the degradation of ECM components, while plasminogen activators which are serine proteinases are implicated indirectly in the remodelling of the ECM by participating in the activation of the metalloproteinases (Alexander and Werb, 1989). The effect of ECM on the different aspects of cell function including cell adhesion, migration, morphology and differentiation (Talhouk et al. 1991; Stoker et al. 1990; Thiery et al. 1988) has led to increasing interest in recent years in factors that modify or remodel the ECM. However, most of the literature so far has concentrated on correlation between growth of cancer cells and metastasis, and the ability of these cells to secrete increased levels of ECM-degrading proteinases (Sloane et al. 1986; Liotta, 1986; Ossowski, 1988; Lyons et al. 1989; Matrisian, 1990). Except for few reports on embryo development (Frisch and Werb, 1989; Brenner et al. 1989), branching morphogenesis in the developing salivary gland (Fukuda et al. 1988) and ovulation (Beers et al. 1975), no information is available on the nature and the role of ECM-degrading proteinases in normal developmental processes.
The mammary gland is one of the few organs that develops in fetal life and undergoes structural, compositional and functional changes during the lifetime of an individual (Anderson, 1985; Lascelles and Lee, 1978; Russo and Russo, 1987; Forsyth, 1982; Dembinski and Shiu, 1987). Whereas the importance of cell–ECM interaction and deposition of ECM components on the functional differentiation of mammary cells have been well documented (Daniel and DeOme, 1965; Emerman and Pitelka, 1977; Wicha et al. 1980; Lee et al. 1984; Wiens et al. 1987; Blum et al. 1987; Li et al. 1987; Bissell and Barcellos-Hoff, 1987; Chen and Bissell, 1989; Streuli and Bissell, 1990), the nature of ECM-degrading proteinases and their role in the development and involution of the mammary gland has received little attention (Martinez-Hernandez et al. 1976; Ossowski et al. 1979).
Given the importance of ECM in the regulation of mammary-specific functions, and the implication from earlier studies (Chin et al. 1985; Unemori and Werb, 1986; Unemori et al. 1987; Matrisian, 1990) that metalloproteinases are important in the remodelling of the ECM, we determined which ECM-degrading proteinases are expressed in vivo during the various stages of mammary growth, differentiation and involution, and in isolated mammary epithelial cells in culture.
Materials and methods
Reagents
Medium 199, Ca2+-free Dulbecco’s modified minimum essential medium (DMEM), gentamicin and fetal calf serum were purchased from Gibco Laboratories (Grand Island, NY). Prolactin was obtained from the National Hormone and Pituitary Program (contracted to NIADDK, Baltimore, MD). Gelatin (from porcine skin), α-casein (from bovine milk), p-aminophenylmercuric acetate (APMA), hydrocortisone, insulin (from bovine pancreas), 7-amino-4-methylcoumarin (AMC), Nα-benzoyl L-arginine-7-amido-4 methylcoumarin (BAAMC), 1,10 o-phenanthroline (o-PHE), phenylmethane-sulfonylfluoride (PMSF), EDTA, trypsin (type XIII, TPCK treated, from bovine pancreas), phosphoramidon, leupeptin, ε-amino-n-caproic acid (EACA), benzamidine hydrochloride, N-α-p-tosyl-L-lysine chloromethyl ketone hydrochloride (TLCK), and pepstatin A were purchased from Sigma Chemical Co. (St Louis, MO). Trasylol and tosyl-L-phenylalanyl chloromethane were obtained from Calbiochem (La Jolla, CA) and p-nitrophenyl-p′-guanidinobenzoate hydrochloride (NPGB) was purchased from ICN (Costa Mesa, CA). Recombinant human tissue inhibitor of metalloproteinases (TIMP) was a gift of D. Carmichael (Synergen Corporation, Boulder, CO). [14C]Casein was prepared by acetylating casein with [1-14C] acetic anhydride according to the procedure described by Cawston et al. (1981). EHS-matrix, a reconstituted basement membrane from ‘Engelbreth-Holm-Swarm’ tumor (Kleinman et al. 1986), and rat tail collagen were prepared and used as described by Li et al. (1987) and Lee et al. (1984), respectively.
Mammary tissue
Mammary tissues obtained from female CD-1 mice (Charles River, Wilmington, MA) were used in all experiments described. For proteinase analysis, tissue was collected from the mostly fatty stroma mammary gland of virgin mice, from pregnant mice at various stages of gestation, and from lactating mice when the lobulo-alveolar development was complete. After weaning, tissue was also collected during involution of the lobulo-alveolar structures and prepared for proteinase analysis as described below. Mammary tissue for preparation of primary cell culture of mouse mammary epithelial cells (MMEC) was obtained from 11 to 14 days pregnant mice and prepared according to the procedure described by Lee et al. (1985).
SDS–substrate gels
Zymograms of proteinases implicated in ECM degradation were prepared from gelatin and casein substrate gels as described previously (Unemori and Werb, 1986; and Brenner et al. 1989). Zymogram gels consisted of 7% or 12% polyacrylamide impregnated with gelatin or casein, respectively, at 1 mg ml−1. After electrophoresis the gels were washed twice for 30 min each in 2.5 % Triton X-100 solution at ambient temperature, then incubated for 24 to 48 h in substrate buffer (50 mM Tris–HCl, 5 mM CaCl2, 0.02 % NaN3, pH8.0) at 37°C. The gels were then stained in Coomasie Blue R250 for 1 h and destained in water overnight. Gelatinase and caseinase activities were visualized as clear bands, indicating proteolysis of the substrate protein. Members of the ECM-degrading metalloproteinases are secreted in a latent form and require a peptide cleavage for proteolytic activation. However, incubation of the tissue extracts with sample buffer (see below) prior to loading on the gels activates the latent enzyme without proteolytic cleavage. The 72K (K=103Mr) gelatinase (Collier et al. 1988) migrates at 72K under reducing conditions, but at 68K under non-reducing substrate gel conditions. To avoid confusion this enzyme is designated the 68K gelatinase throughout. Photographs of SDS–substrate gels are shown as negative images.
For sample preparation, freshly isolated mammary tissue was immediately frozen in liquid nitrogen. The tissue was then pulverized into a fine powder, weighed and either stored at −70°C or suspended 1:5 (wt/vol) in extraction buffer (1% Triton X-100 in 500 mM Tris–HCl buffer, pH7.6, containing 200 mM NaCl and 10 mM CaCl2). The suspension was frozen on dry ice, thawed 4 times and microfuged (12 000g for 30 min at 4°C). The supernatant was removed and stored at −70°C until needed for zymography. Conditioned medium, EGTA-extractable fraction, and cellular fraction from MMEC cultures were collected and stored at −70°C as described below. Fresh milk from 9 days lactating mice was collected and stored at −70°C until needed for zymography. Before analysis on zymogram gels, samples were diluted with 4×SDS sample buffer without mercaptoethanol and loaded onto gels. Analysis of proteinase activity was based on equal amounts of protein (15 μg) in tissue extracts and milk or equal numbers of cells in culture.
To characterize the proteinases, enzyme inhibitors were incubated with mammary tissue extracts and the activity assayed on zymogram gels, or by a fluorescent assay described below. For zymogram gel analysis, various proteinase inhibitors were added to samples for 30 min at 37°C before mixing with 4×SDS sample buffer, and added again during incubation with substrate buffer. Inhibitors and concentrations used are described where appropriate. Similarly, 10 mM APMA dissolved in 0.01M NaOH and adjusted with HCl to pH7.5 was used to characterize further the gelatinases. APMA is an organomercurial compound that activates ECM-degrading metalloproteinases. The activation of the proteinases in the presence of APMA involves the conversion of the pro-enzyme form to its active lower molecular weight form by autoproteolytic cleavage of a peptide fragment from their amino-terminus (Stetler-Stevenson et al. 1989; Grant et al. 1987; Whitham et al. 1986). APMA was mixed with samples to a final concentration of 1 mM and incubated for 16 h at ambient temperature. Samples were then mixed with 4×SDS sample buffer and analyzed on zymogram gels.
Fluorescent assay for the determination of arginine esterase activity
Arginine esterase activity was determined by a modification of an assay developed by Zimmerman et al. (1978). Mammary tissue from virgin or pregnant mice was extracted in 500 mM Tris–HCl, pH7.6 with 200 mM NaCl and 1 % Triton X-100 as described above for SDS–substrate gels. An aliquot was incubated at 37°C for 60 min in 0.5 ml reaction volume containing 50 mM Tris–HCl. pH7.6, 10 mM CaCl2 and 5% DMSO with 200 μM of the fluorogenic substrate, N-α-benzoyl-L-arginine-7-amido-4-methylcoumarin. The release of the fluorogenic product 7-amino-4-methylcoumarin was measured in a Turner model 430 spectrofluorometer at 383 nm excitation and 455 nm emission. Trypsin (50–100 ng) and extraction buffer were included as positive and negative controls and 3–200 nM AMC was used for the standard curve. For enzyme characterization studies, inhibitors were preincu-bated with the mammary extract for 30min at ambient temperature prior to addition of the substrate.
Quantitation of [14C]casein-degrading activity
Casein-degrading proteinase activity was quantified by the ability of mammary tissue extracts to degrade [14C]casein (Cawston et al. 1981; Chin et al. 1985). Briefly, a 100 μl sample of tissue extract was added to an equal volume of 100 mM Tris–HCl, pH7.6, containing 30 mM CaCl2. After addition of 50 μl of [14C]casein (2 mg ml−1), the mixture was incubated at 37°C for 2 h. The reaction was stopped with 50 μl of cold 36 % (w/v) trichloroacetic acid solution, vortexed and incubated at 4°C for 30 min. Samples were microfuged (12 000g), the supernatant was removed and radioactivity was determined as cts min−1. Tissue samples were collected from 2 mice per stage of development and those in turn were run in duplicate. Trypsin (1mg ml−1) served as the positive control. The 2 mg ml−1 of [14C]casein used in this assay far exceeded the levels of endogenous casein in tissue extracts from a lactating gland. This was confirmed by SDS–PAGE and by western blots and immunodetection of milk caseins in the tissue extracts used in the assay. Similar results were obtained when 20 mg ml−1 of [14C]casein was used.
Cell culture
Mouse mammary epithelial cells, obtained from 11 to 14 days pregnant CD-1 mice, cultured on EHS-matrix were prepared according to the procedure described by Lee et al. (1984). Medium (representing basal secretion) and EGTA-extractable fraction (representing apical or intra-lumenal secretion) were sampled (Barcellos-Hoff et al. 1989) at daily intervals and centrifuged, and the supernatant was frozen at −70 °C until needed for zymography. The cell fraction was collected by removing cells from the EHS-matrix by continuous gentle swirling with trypsin at 37°C. Cells were washed 3 times with medium, and resuspended in a volume equal to that in which they were grown. An aliquot was removed for cell counts by a hemocytometer, and the remainder was stored at −70°C until needed for zymography. Cells were also cultured on type I collagen gels that were floated on day 2 of culture (FG) and on plastic as described previously (Li et al. 1987).
Results
Developmental regulation of proteinases in vivo Gelatin-degrading proteinases
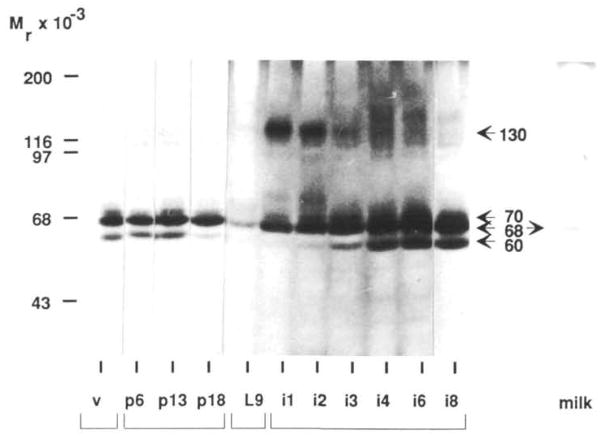
We first used zymography on gelatin-impregnated SDS-gels to resolve the proteinases present in the mammary gland at various stages of development. Three major proteinases at 60K, 68K and 70K were detected in mammary tissue extracts of 11 to 12 week old virgin and pregnant CD-1 mice (Fig. 1). Although gelatinase activities were comparable in virgin and early- to mid-pregnant mice, the activity of the 60K gelatinase, which is the activated form of the 68K gelatinase (see below), decreased before parturition and remained absent during lactation. The 70K gelatinase was also absent during lactation, and the amount of the 68K gelatinase decreased dramatically. Despite a marked decrease in the 68K activity in the whole gland during lactation, it could be detected in milk.
Fig. 1.
Gelatinase activity at the various stages of mammary development. Extracts of freshly isolated mammary tissue from 11-to 12-week-old virgin (v), pregnant (p), lactating (L), and involuting (i) CD-1 mice and milk from 9-day lactating mice were assayed for gelatin-degrading activity by SDS–substrate gel zymography. The specific day within each stage of mammary development is indicated in arabic numerals. Lanes were loaded with 15 μg of protein per lane.
As early as one day after involution, initiated by separating the pups from the mother, the 68K gelatinase increased markedly (Fig. 1). The 60K gelatinase activity began to reappear on the third day of involution. Gelatinase activities beyond day 4 of involution and up to day 8 included all three bands (60K, 68K, and 70K) that were observed in virgin and mid-pregnant mammary extracts. In addition, a 130K gelatinase appeared during the early stages, between day 1 and day 3 of involution, and several other high molecular weight bands were seen in this general region between day 4 and 8 of involution (Fig. 1).
Casein-degrading proteinases
Because gelatin does not detect all proteinases, we also used casein substrate zymograms to study the expression of proteinases in the mammary gland. Two major caseinases of 26K and 92K and a minor high relative molecular mass (>100K) caseinase were observed (Fig. 2). The caseinases were prominent in virgin, mid-pregnant and involuting gland (day 4–8) but were low in extracts from lactating tissue, a pattern similar to that of gelatinases. During lactation and the first 3 days of involution, the 26K caseinase activity was absent altogether. In contrast, the 92K and the high relative molecular mass activities were present and could be detected in mouse milk. The decline of gelatinases and caseinases during lactation was noted whether the substrate gels were loaded on the basis of equal weight of tissue or equal amount of DNA (data not shown), demonstrating that the variation of the proteinase activities during lactation and early involution could not be attributed to the dilution effect caused by the accumulation of milk proteins in the gland during lactation.
Fig. 2.
Caseinase activity at the various stages of mammary development. Tissue samples, milk, conditions of the gel and all abbreviations are as described in the legend to Fig. 1.
Because zymography separates the enzymes from each other and from their inhibitors, any interactions among the proteinases or between the proteinases and their inhibitors are obscured. We therefore analyzed the ability of the whole mammary tissue extracts to degrade [14C]casein. The casein-degrading activity measured with this assay was still lowest in tissue extracts from lactating gland (Fig. 3) and highest in tissue extracts from mid-pregnant and 3-day involuting gland. During these two latter stages, the activity was ~7–10 times the activity in lactation and ~2–3 times that in virgin. The activity during early involution (1 day) before the degeneration of alveolar structures (Knight and Peaker, 1982; Hurley, 1989) was ~2 times that in lactation.
Fig. 3.

Quantitative analysis of caseinase activity across mammary gland development. Caseinolytic activity in whole tissue extracts from freshly isolated mammary glands from virgin, mid-pregnant (11–14 days), lactating, 1 day (1d Inv.) and 3 days (3d Inv.) involuting mice, was measured in a soluble [14C]casein-degradation assay. Values are the average caseinase activity of duplicate mammary tissue samples from two mice per stage of development.
Expression of proteinases by mouse mammary epithelial cells in culture
Mammary tissue consists of a complex mixture of mammary epithelial cells (MMEC) and mesenchymal cells such as fat cells and fibroblasts. To determine whether or not mammary epithelial cells produce any of the proteinases seen in extracts of gland, we isolated MMEC from mid-pregnant CD-1 mice and cultured them on different substrata. Extracts of freshly isolated MMEC contained essentially the same proteinases as the whole mammary gland (data not shown). MMEC cultured on EHS-matrix aggregated after plating and underwent remodelling and by day 4 the cell aggregates organized into hollow spheres lined by MMEC with the apical side of cells oriented toward the lumen of the sphere, and the basal side delineated by a basement membrane (Fig. 4) as described previously by Barcel-los-Hoff et al. (1989). In contrast, MMEC grown on plastic or a floating gel spread into sheets of cells and by day 4 covered the substratum (Emerman and Pitelka, 1977; and Li et al. 1987).
Fig. 4.
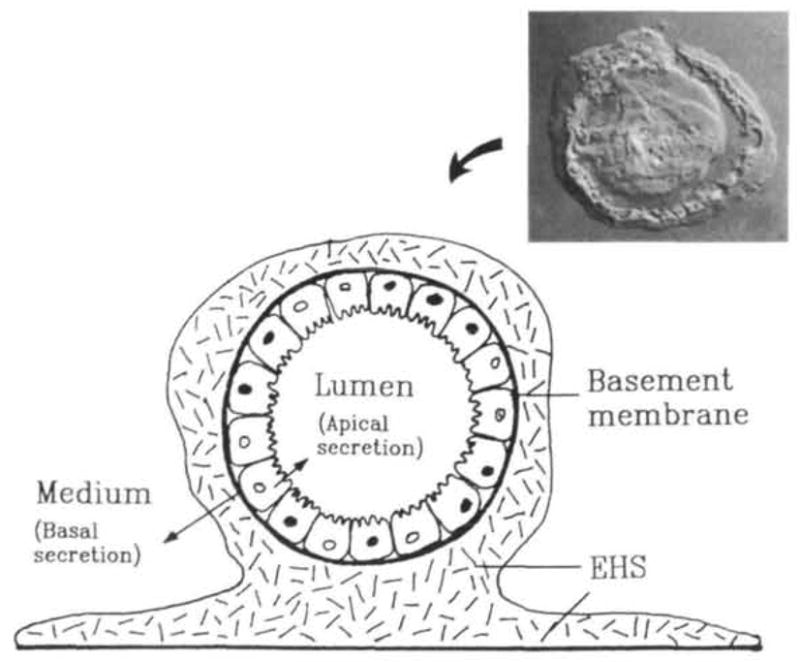
Schematic diagram of MMEC cultured on EHS-matrix. The cells aggregate into alveolar-like structures with their apical side toward the lumen and their basal side underlined by a basement membrane. Cells in this aggregate retract the EHS-matrix and are engulfed within it. The insert shows a Nomarski image of a thick transverse section of such an alveolus-like structure. With this cell arrangement, the cells secrete basally into the medium (basal compartment) and apically into the lumen (apical compartment). Diagram and inset courtesy of Sally Nash and Charles Streuli respectively.
MMEC in culture secreted abundant gelatinolytic activity (Fig. 5). The 68K and 70K gelatinases were prominent and were secreted on all substrata; however, the 60K gelatinase was secreted only by cells on floating collagen and plastic. Another gelatinase of 77K was secreted, at low levels, by cells on floating gels and plastic. Several additional gelatinases between 70K and 200K resembling some of the activities seen during involution were secreted by cells on all three substrata with no discernable substratum-dependence.
Fig. 5.
Effect of the ECM on the secretion of gelatinases by MMEC in culture. MMEC were grown on EHS-matrix, floating collagen (FG), or plastic under serum-free conditions, and conditioned medium was collected every 24 h and samples from day (d) 4 to 7 were analyzed by SDS–gelatin zymography. Lanes were loaded at equal cell number (5.5×103 cells per lane).
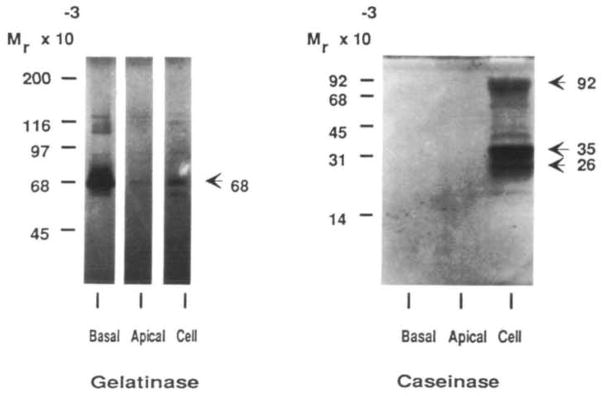
The 26K and 92K caseinase, seen in mammary tissue extracts, and a 35K were found in extracts of MMEC cultured on all three substrata. In contrast to gelatinases, however, the caseinases were only associated with the cells, and were not secreted (Fig. 6).
Fig. 6.
The distribution of the gelatinases and caseinases into the basal, apical and cellular compartments as resolved on SDS–gel zymograms. Conditioned medium (basal), EGTA-extractable fraction (apical), and cell fraction of MMEC cultured on EHS-matrix in serum-free medium were harvested on day 6 of culture and stored at −70°C. Lanes (basal and apical) were loaded with medium conditioned from 5.5×103 cells. The cell fraction was derived from the same number of cells (5.5×103 cells).
Vectorial secretion of proteinases in vitro
The formation of alveolar-like structures on EHS-matrix (Fig. 4) permitted us to address the question of whether the gelatin-degrading proteinases were secreted vectorially. Gelatinases were secreted mainly via the basal route with the exception of the 68K gelatinase, which was secreted into both apical and basal compartments (Fig. 6), but at a higher level basally. Only a small amount of gelatinolytic activity similar to the basally secreted gelatinases was found in the cells. Thus, while milk proteins are secreted apically into the lumen, the gelatinases were secreted in the direction of the basement membrane and stromal components.
Characterization of mammary proteinases Gelatin-degrading proteinases
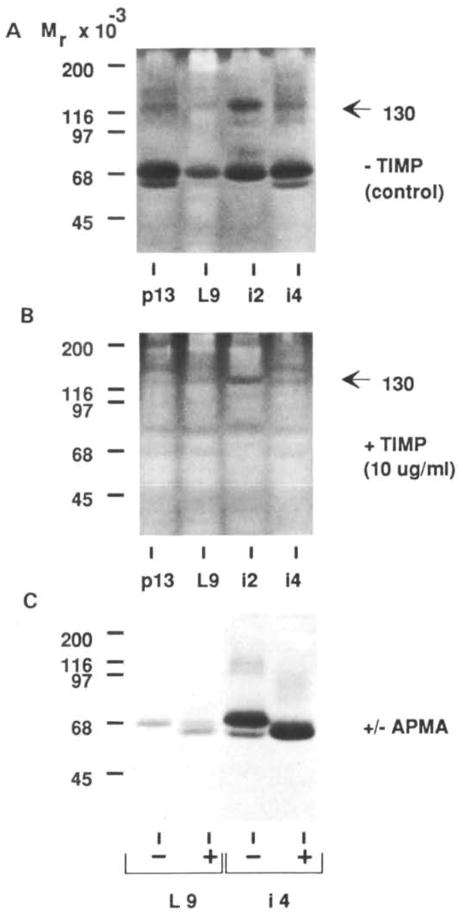
To classify the mammary proteinases, we incubated mammary tissue extracts, extracts of MMEC, conditioned medium from MMEC and mouse milk with proteinase inhibitors before and after electrophoretic separation. The 60K, 68K and 70K gelatinases were inhibited by TIMP (Fig. 7) indicating that these three gelatinases are metalloproteinases. In contrast, the 130K gelatinase observed during early involution was poorly inhibited by TIMP (Fig. 7A and 7B, arrowhead).
Fig. 7.
Characterization of the gelatinases. Mammary tissue extracts from 13-day pregnant (pl3), 9-day lactating (L9), and 2- and 4-day involuting (i2 and i4 respectively) mammary gland were separated on SDS–gelatin gels and then incubated without (A) or with (B) TIMP at 10 μg ml−1 in substrate buffer. (C) Activation of the 68K gelatinase after incubation of tissue extract with APMA. L9 and i4 tissue extracts were incubated for 16 h at ambient temperature in 1 mM APMA before loading on the gel. Lanes were loaded with 15 μg of protein.
The metalloproteinases are found in latent proen-zyme forms and cleaved active forms, both of which can be visualized by zymography because of the activating effect of SDS. Metalloproteinases are activated by a cysteine switch mechanism by organomercurials. Incubation of tissue extracts with 1 mM APMA converted the activity at 68K to 60K (Fig. 7C) indicating that the 60K is the activated form of the 68K gelatinase. Similar effects of TIMP and APMA were observed for all samples included in Fig. 1, as well as for milk and conditioned medium from cultured cells.
Casein-degrading proteinases
Analysis of the 26K cell-associated caseinase by SDS–casein substrate gels showed that it was not a metalloproteinase since it was not inhibited by TIMP, o-PHE, or EDTA (Table 1). Neither inhibitors of trypsin- and plasmin-like enzymes, TLCK, benzami-dine, Trasylol and EACA, nor an inhibitor of chymo-trypsin-like proteinase (TPCK) were effective. Further negative results were obtained with leupeptin (a thiol proteinase inhibitor), phosphoramidon (an inhibitor of clostridial collagenase, thermolysin, and metalloendo-proteinases) and pepstatin which acts against acid proteinases. DMSO was used as the solvent for several of the inhibitors and had no effect. However, inhibition of casein degradation by 2 mM PMSF and 0.1 to 0.2 mM NPGB indicated that the 26K caseinase was a serine arginine-esterase. This result was confirmed when mammary tissue extracts were assayed with the fluorogenic substrate BAAMC. This activity was inhibited >50% by NPGB and PMSF (Table 1). To determine whether this 26K caseinase was mammary specific, we analyzed tissue extracts from kidney, spleen, liver, heart and brain. None of the tissues had a casein-degrading activity at 26K (Fig. 8) while the 92K and 35K caseinases were widely distributed. Thus the 26K caseinase appears to be novel and mammary specific.
Table 1.
Effect of inhibitors on the activity of mammary tissue caseinases
| Inhibitor (Conc.) | Inhibition |
|
|---|---|---|
| A | B | |
| NPGB (0.1–0.2 mM) | + | 86–100% |
| PMSF(2mM) | + | 58% |
| TIMP (10 μml−1) | − | nd |
| o-PHE (5 mM) | − | ,, |
| EDTA (20 mM) | − | ,, |
| TLCK (1 mM) | − | ,, |
| Benzamidine (10 mM) | − | ,, |
| Trasylol (15 μml−1) | − | ,, |
| EACA (50 mM) | − | ,, |
| TPCK (1 mM) | − | ,, |
| Leupeptin (0.1 mM) | − | ,, |
| Phosphoramidon (100 μml−1) | − | ,, |
| Pepstatin A (100 μM) | − | ,, |
| DMSO (1%) | − | |
Mammary tissue extracts from virgin and pregnant CD-1 mice were preincubated with the proteinase inhibitors and then (A) separated on SDS–casein gels. The inhibitors were also added after protein separation with the substrate buffer. The ability (+) or inability (−) of the specific inhibitor to prevent the 26K cell-associated caseinase activity is shown. (B) The percent inhibition of mammary tissue caseinases as determined by the fluorescent assay described in methods (nd=not determined).
Fig. 8.
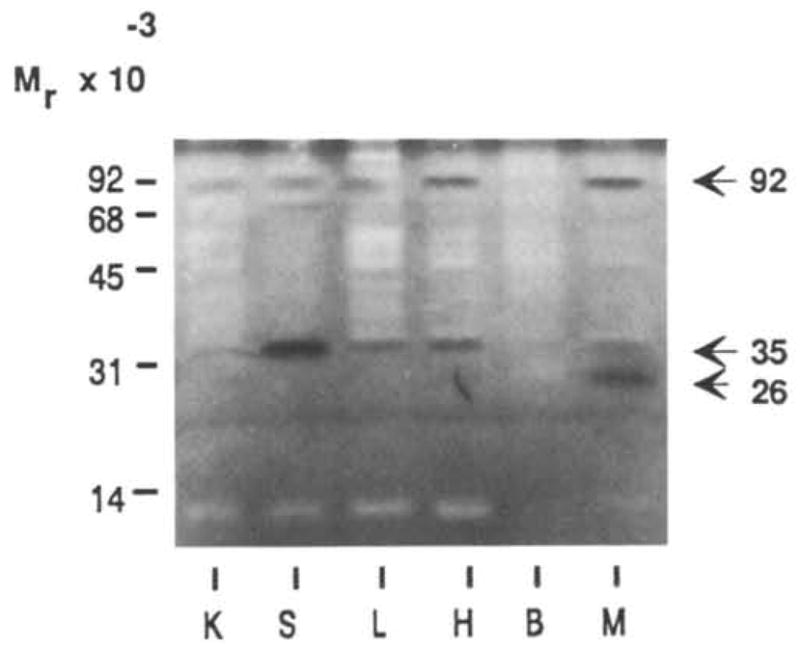
Caseinase activity in different tissues. Extracts of freshly isolated tissue from kidney (K), spleen (S), liver (L), heart (H), brain (B), and mammary gland (M) of 15 day pregnant CD-1 mice were assayed for casein-degrading activity by SDS–substrate gel zymography. Lanes were loaded with 15 μg of protein per lane.
Discussion
The involvement of matrix-degrading proteinases in metastasis and pathological conditions has been postulated and extensively investigated (Liotta, 1986; Sloane et al. 1986; Ballin et al. 1988; Baricos et al. 1988; Ossowski, 1988; Reich et al. 1988; Lyons et al. 1989). Studies documenting the role of matrix-degrading proteinases in the regulation of growth and morphogenesis in normal tissues, in contrast, are few (Dean et al. 1985; Brenner etal. 1989; Werb, 1989; Matrisian, 1990). In this study, we have demonstrated that normal mammary gland contains a number of secreted and cell-associated proteinases and that they are regulated during normal development of the gland in primiparous mice. These proteinases are produced, at least partly, by mammary epithelial cells themselves, and those secreted are routed basally in the direction of the basement membrane. We further show that there is at least one developmentally regulated, 26K cell-associated, proteinase that appears to be unique to the mammary gland.
Of the gelatinases found in tissue extracts (60/68K, 70K, and 130K), the one most consistently expressed during all stages of mammary gland development but down-regulated in lactation is a metalloproteinase with an apparent relative molecular mass of 68×103. Recent cloning of this metalloproteinase (Collier et al. 1988) showed that it is the proenzyme form of type IV collagenase with an actual relative molecular mass of 72×103 that migrates under non-reducing conditions at 68K. A similar 68K gelatin-degrading metalloproteinase is known to be secreted by endothelial cells (Herron et al. 1986a,b), and rabbit synovial fibroblasts (Unemori and Werb, 1988). Brenner et al. (1989) reported a 68K gelatinase/type IV collagenase that was implicated in the regulation of peri-implantation development and implantation of mouse embryos and is expressed in embryonal carcinoma cells differentiating to endoderm (Adler et al. 1990).
An intact basement membrane is essential to maintain the differentiated function of mammary epithelial cells in culture (Streuli and Bissell, 1990). Our results described here are consistent with this also being the case in vivo: the active form (60K) of type IV collagenase is abundant during pregnancy when mammary growth and ECM remodelling is prominent. The activity decreases three days before parturition and is absent during lactation and early involution when the gland is functionally differentiated (Forsyth, 1982; Knight and Peaker, 1982; Hurley, 1989), and there is minimal restructuring of the gland and remodelling of the ECM. The 60K gelatinase activity reappears 3 to 4 days after initiation of involution when massive restructuring of the ECM occurs. The function of the 130K gelatinase that is expressed during the early stages of involution, before overt degradation of the ECM remains to be determined.
Ossowski et al. (1979) showed a sharp peak of plasminogen activator produced by mammary cells at the fourth day of involution after a 2 day lactation. Similarly, Martinez-Hernandez et al. (1976) demonstrated that involuting mammary tissue extracts from Swiss-Webster mice hydrolyzed 125I-labeled insoluble basement membrane components. The ECM-degrading activity started 2 days postweaning and was maximal by day 4. In a separate study on the involution of the mammary gland, we have determined that stromelysin and tissue plasminogen activator expression reaches a maximum by day 4 of involution. However, the expression of TTMP is maximal during the early stages of involution (days 1 to 4) and decreases thereafter (Talhouk et al. 1990; and manuscript in preparation). These data lead us to postulate that a balance between ECM-degrading proteinases and their inhibitors during mammary gland development and involution may be a critical factor affecting function and morphology of the gland.
Of the caseinases detected on substrate gels, the cell-associated 26K activity is novel and most likely mammary specific, since it was not detected in tissue extracts from kidney, spleen, liver, heart and brain. Our inhibitor studies with NPGB and PMSF show that this caseinase is most likely a serine arginine-esterase. NPGB, a potent inhibitor of trypsin, plasminogen activator, plasmin and arginine esterases exerts its effect by complexing with the enzyme to form a stable acyl enzyme (Goldberg et al. 1975), thus making the enzyme unavailable for further activity. It is interesting to note that PMSF was effective against the mammary 26K caseinase, yet the other serine proteinase inhibitors were not. The lack of inhibition of this activity by Trasylol, benzamidine or TLCK suggests that this enzyme is not plasminogen activator, plasmin or one of the tissue kallikreins.
The 26K caseinase, like the gelatinases, was lowest in extracts from lactating and early involuting tissues. The relative difference between caseinolytic activity during various developmental stages as detected by [14C]casein degradation (Fig. 3) and those resolved on zymogram gels (Fig. 2) also suggests that proteinase inhibitors modulated the level of proteinase activities in vivo. The high level of casein-degrading activity in the pregnant gland is consistent with the fact that these cells produce substantial amounts of milk proteins which must be degraded before parturition. Studies by Wilde and Knight (1986) and Stewart et al. (1988) demonstrated intracellular degradation of newly synthesized caseins via a non-lysosomal pathway in goat mammary tissue explants. The casein-degrading activity was higher in mammary tissue explants from pregnant goats than from lactating goats. Whether the caseinases observed in our study are involved in ECM degradation or whether they only regulate casein levels in the gland cannot be answered at present. The fact that these caseinases are cell associated argues that they are involved in regulating intercellular casein levels as suggested by Wilde and Knight (1986) and Stewart et al. (1988). On the other hand, the presence of caseinase activity in tissue extracts from virgin and involuting mammary gland (Fig. 2), where lactogenesis does not occur, implies that caseinases could be involved in other processes such as matrix remodelling.
The presence of proteinases and proteinase inhibitors in milk has been recognized by others (reviewed by Lonnerdal, 1985), but it was hypothesized that this activity was derived from leukocytes in milk and not secreted by the mammary cells. However, mouse neutrophils and macrophages do not express the 68K gelatinase (Werb and Coworkers, unpublished observation) and the expression of gelatinases and caseinases in MMEC in culture is evidence that these proteinases can be synthesized by mammary epithelial cells. Furthermore the presence of the latent form of type IV collagenase in the lumenal fraction in cells on EHS-matrix indicate that there is some apical secretion explaining its existence in milk. Nevertheless, the fact that the majority of gelatinases are secreted in the direction of the basement membrane argues further that such gelatinases are involved in the degradation and remodelling of the stroma and basement membrane.
Both gelatinases and caseinases found in mammary gland extracts in vivo were also expressed by MMEC in culture. An analysis of the pattern of expression in culture and a comparison to in vivo situation unravelled two additionally interesting facts. First, MMEC under our culture conditions resemble the lactating tissue morphologically, ultrastructurally and in their ability to synthesize milk proteins and fat (Barcellos-Hoff et al. 1989; Chen and Bissell, 1989). However, the abundance of high relative molecular mass gelatinases (>70–200K) and the low levels of the 60K gelatinase relative to the 68K gelatinase by cells on EHS-matrix and floating collagen gel indicate that the differentiated epithelial cells in culture resemble those in early involution tissue, at a time when milk protein synthesis is still quite high (Knight and Peaker, 1982; Hurley, 1989). Second, the absence of discernable modulation of ECM-degrading proteinases by substrata indicate another interesting phenomenon. Streuli and Bissell (1990) have shown that deposition of a basement membrane by MMEC in culture positively correlated with the expression of differentiated functions, and Wicha et al. (1980) reported that inhibition of collagen deposition induced an involution-like process in the mammary gland. These data suggest an intimate relationship between basement membrane and differentiated function of mammary cells. In view of this, the separation of the differentiation events that encompass organization of a basement membrane (Streuli and Bissell, 1990) leading to a lactation-competent phenotype in vitro from the regulation of secretion of ECM-degrading proteinases was surprising. It appears from the data presented here and the fact that MMEC on plastic do not differentiate but express higher levels of TIMP mRNA than do differentiated cells on EHS-matrix or floating collagen gels (Bissell et al. 1990) that the major regulation of ECM remodelling may be due to the regulated production of proteinase inhibitors, which could affect both matrix deposition and remodelling. This hypothesis is being tested by introducing the TIMP gene into mammary cells. In conclusion, we have denned the nature and regulation of ECM-degrading proteinases during the normal development of the mammary gland. Of these proteinases, a novel, most likely mammary-specific, cell-associated 26K proteinase is characterized. Furthermore, we have demonstrated that ECM-degrading proteinases are produced by mammary epithelial cells and secreted basally. Studies are currently in progress to determine how these ECM-degrading enzymes and their inhibitors are involved in regulating the growth, differentiation and involution of the mammary gland.
Acknowledgments
This work was supported by the US Department of Energy, Office of Health and Environmental Research (contracts DE-AC03-76-SF00098 [for M.J.B.] and DE-AC03-76-SF01012 [for Z.W.]), a research grant from the National Institutes of Health (HD 23539), and a National Research Service Award (T32ES07106) from the National Institute of Environmental Health Sciences. A brief report of part of these data has been presented in abstract form (Unemori et al. 1987). We thank Drs A. Howlett, M. Martins-Green, R. Schwarz and C. Streuli, for critical reading of this manuscript.
References
- Adler RR, Brenner CA, Werb Z. Expression of extracellular matrix-degrading metalloproteinases and metalloproteinase inhibitors is developmentally regulated during endoderm differentiation of embryonal carcinoma cells. Development. 1990;110:211–220. doi: 10.1242/dev.110.1.211. [DOI] [PubMed] [Google Scholar]
- Alexander CM, Werb Z. Proteinases and extracellular matrix remodeling. Current Opinion In Cell Biology. 1989;1:974–982. doi: 10.1016/0955-0674(89)90068-9. [DOI] [PubMed] [Google Scholar]
- Anderson RR. Mammary gland. In: Larson BL, editor. Lactation. Ames, IA: Iowa State University Press; 1985. pp. 3–37. [Google Scholar]
- Ballin M, Gomez DE, Sinha CC, Thorgeirsson UP. Ras oncogene mediated induction of a 92 kDa metalloproteinase; strong correlation with the malignant phenotype. Biochem biophys Res Commun. 1988;154:832–838. doi: 10.1016/0006-291x(88)90215-x. [DOI] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989;105:223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baricos WH, Murphy G, Zhou YW, Nguyen HH, Shah SV. Degradation of glomerular basement membrane by purified mammalian metalloproteinases. Biochem J. 1988;254:609–612. doi: 10.1042/bj2540609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers WH, Strickland S, Reich E. Ovarian plasminogen activator, Relationship to ovulation and hormonal regulation. Cell. 1975;6:387–394. doi: 10.1016/0092-8674(75)90188-9. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Barcellos-Hoff MH. The influence of extracellular matrix on gene expression, Is structure the message? J Cell Sci Suppl. 1987;8:327–343. doi: 10.1242/jcs.1987.supplement_8.18. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Streuli CH, Chen L-H, Talhouk RS, Werb Z. Regulation of gene expression in mammary cells by extracellular matrix. Proc. of Eighty-First Annual Meeting of the American Association for Cancer Research.1990. [Google Scholar]
- Blum JL, Zeigler ME, Wicha MS. Regulation of rat mammary gene expression by extracellular matrix components. Expl Cell Res. 1987;173:322–340. doi: 10.1016/0014-4827(87)90274-6. [DOI] [PubMed] [Google Scholar]
- Brenner CA, Adler RR, Rappolee DA, Pederson RA, Werb Z. Genes for extracellular matrix-degrading metalloproteinases and their inhibitor, TIMP, are expressed during early mammalian development. Genes Dev. 1989;3:848–859. doi: 10.1101/gad.3.6.848. [DOI] [PubMed] [Google Scholar]
- Cawston TE, Galloway WA, Mercer E, Murphy G, Reynolds JJ. Purification of rabbit bone inhibitor of collagenase. Biochem J. 1981;195:159–165. doi: 10.1042/bj1950159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LH, Bissell MJ. A novel regulatory mechanism for whey acidic protein gene expression, Alveoli-like structures allow expression by sequestering or preventing production of diffusible inhibitor. J Cell Regul. 1989;1:45–54. doi: 10.1091/mbc.1.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin JR, Murphy G, Werb Z. Stromelysin, a connective tissue-degrading metalloendopeptidase secreted by stimulated rabbit synovial fibroblasts in parallel with collagenase. Biosynthesis, isolation, characterization, and substrates. J biol Chem. 1985;260:12367–12386. [PubMed] [Google Scholar]
- Collier IE, Wilhelm SM, Eisen AZ, Marmer BL, Grant GA, Seltzer JL, Kronberger A, He C, Bauer EA, Goldberg GI. H-ras oncogene-transformed human bronchial epithelial cells (TBE-1) secrete a single metalloprotease capable of degrading basement membrane collagen. J biol Chem. 1988;263:6579–6587. [PubMed] [Google Scholar]
- Daniel CW, Deome KB. Growth of mouse mammary glands in vivo after monolayer culture. Science. 1965;149:634–636. doi: 10.1126/science.149.3684.634. [DOI] [PubMed] [Google Scholar]
- Dean DD, Muniz OE, Berman I, Pita JC, Carreno MR, Woesnner JF, Jr, Howell DS. Localization of collagenase in the growth plate of rachitic rats. J din Invest. 1985;76:716–722. doi: 10.1172/JCI112026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembinski TC, Shiu RPC. Growth factors in mammary gland and function. In: Neville MC, Daniel CW, editors. The Mammary Gland, Development, Regulation and Function. New York-London: Plenum Publishing Corp; 1987. pp. 355–381. [Google Scholar]
- Emerman JT, Pttelka DR. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro. 1977;13:316–328. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- Forsyth IA. Growth and differentiation of mammary glands. Oxford Reviews of Reproductive Biology. 1982;4:47–85. [Google Scholar]
- Frisch SM, Werb Z. Molecular biology of collagen degradation. In: Olsen BR, Nimni ME, editors. Collagen, vol IV, Molecular Biology. Boca Raton, Fla: CRC Press; 1989. pp. 85–107. [Google Scholar]
- Fukuda Y, Masuda Y, Kishi J, Hashimoto Y, Hayakawa T, Nogawa H, Nakanishi Y. The role of interstitial collagens in cleft formation of mouse embryonic submandibular gland during initial branching. Development. 1988;103:259–267. doi: 10.1242/dev.103.2.259. [DOI] [PubMed] [Google Scholar]
- Goldberg AR, Wolf BA, Lefebvre PA. Proteases And Biological Control. Coldspring Harbor Laboratory; Coldspring Harbor: 1975. Plasminogen activator of transformed and normal cells; pp. 857–860. [Google Scholar]
- Grant GA, Eisen AZ, Marmer BL, Roswit WT, Goldberg GI. The activation of human skin fibroblast procollagenase: Sequence identification of the major conversion products. J biol Chem. 1987;262:5886–5889. [PubMed] [Google Scholar]
- Herron GS, Banda MJ, Clark EJ, Gavrilovic J, Werb Z. Secretion of metalloproteinases by stimulated capillary endothelial cells. II. Expression of collagenase and stromelysin activities is regulated by endogenous inhibitors. J biol Chem. 1986a;261:2814–2818. [PubMed] [Google Scholar]
- Herron GS, Werb Z, Dwyer K, Banda MJ. Secretion of metalloproteinases by stimulated capillary endothelial cells. I. Production of procollagenase and prostromelysin exceeds expression of proteolytic activity. J biol Chem. 1986b;261:2810–2813. [PubMed] [Google Scholar]
- Hurley WL. Mammary gland function during involution. J Dairy Sci. 1989;72:1637–1646. doi: 10.3168/jds.S0022-0302(89)79276-6. [DOI] [PubMed] [Google Scholar]
- Kleinman HK, Mcgarvey ML, Hassell JR, Star VL, Cannon FB, Laurie GW, Martin GR. Basement membrane complexes with biological activity. Biochemistry. 1986;25:312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- Knight CH, Peaker M. Development of the mammary gland. J Reprod Fert. 1982;65:521–536. doi: 10.1530/jrf.0.0650521. [DOI] [PubMed] [Google Scholar]
- Lascelles AK, Lee CS. Involution of the mammary gland. In: Larson BL, editor. Lactation: A Comprehensive Treatise. Vol. 4. New York, NY: Academic Press Inc; 1978. pp. 115–177. [Google Scholar]
- Lee EYH, Lee WH, Kaetzel CS, Parry G, Bissell MJ. Interaction of mouse mammary epithelial cells with collagen substrata, Regulation of casein gene expression and secretion. Proc natn Acad Sci USA. 1985;82:1419–1423. doi: 10.1073/pnas.82.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EYH, Parry G, Bissell MJ. Modulation of secreted proteins of mouse mammary epithelial cells by the collagenous substrata. J Cell Biol. 1984;98:146–155. doi: 10.1083/jcb.98.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ML, Aggeler J, Farson DA, Hatier C, Hassell J, Bissell MJ. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells. Proc natn Acad Sci USA. 1987;84:136–140. doi: 10.1073/pnas.84.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta L. Tumor invasion and metastases - role of the extracellular matrix, Rhoads Memorial Award lecture. Cancer Res. 1986;46:1–7. [PubMed] [Google Scholar]
- Lonnerdal B. Biochemistry and physiological function of human milk proteins. Am J clin Nutr. 1985;42:1299–1317. doi: 10.1093/ajcn/42.6.1299. [DOI] [PubMed] [Google Scholar]
- Lyons JG, Siew K, O’Grady RL. Cellular interactions determining the production of collagenase by a rat mammary carcinoma cell line. Int J Cancer. 1989;43:119–125. doi: 10.1002/ijc.2910430123. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A, Fink LM, Pierce GB. Removal of basement membrane in the involuting breast. Lab Invest. 1976;31:455–462. [PubMed] [Google Scholar]
- Matrisian LM. Metalloproteinases and their inhibitors in matrix remodelling. TIGS. 1990;6:121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- Ossowski L. Plasminogen activator dependant pathways in the dissemination of human tumor cells in the chick embryo. Cell. 1988;52:321–328. doi: 10.1016/s0092-8674(88)80025-4. [DOI] [PubMed] [Google Scholar]
- Ossowski L, Biegel D, Reich E. Mammary plasminogen activator, Correlation with involution, hormonal modulation and comparison between normal and neoplastic tissue. Cell. 1979;16:929–940. doi: 10.1016/0092-8674(79)90108-9. [DOI] [PubMed] [Google Scholar]
- Reich R, Thompson EW, Iwamoto Y, Martin GR, Deason JR, Fuller GC, Miskin R. Effects of inhibitors of plasminogen activator, serine proteinases, and collagenase IV on the invasion of basement membranes by metastatic cells. Cancer Res. 1988;48:3307–3312. [PubMed] [Google Scholar]
- Russo J, Russo IH. Development of the human mammary gland. In: Neville M, Daniel C, editors. The Mammary Gland, Development, Regulation and Function. New York-London: Plenum Publishing Corp; 1987. pp. 67–93. [Google Scholar]
- Sloane BF, Rozhin J, Johnson K, Taylor H, Crissman JD, Honn KV. Cathepsin B, Association with plasma membrane in metastatic tumors. Proc natn Acad Sci USA. 1986;83:2483–2487. doi: 10.1073/pnas.83.8.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler-Stevenson WG, Krutzsch HC, Wacher MP, Margulies IMK, Liotta LA. The activation of human type IV collagenase proenzyme. J biol Chem. 1989;264:1353–1356. [PubMed] [Google Scholar]
- Stewart GM, Addey CVP, Knight CH, Wilde CJ. Autocrine regulation of casein turnover in goat mammary explants. J Endocrinol. 1988;118:R1–R3. doi: 10.1677/joe.0.118r001. [DOI] [PubMed] [Google Scholar]
- Stoker AW, Streuli CH, Martins-Green M, Bissell MJ. Designer microenvironments for the analysis of cell and tissue function. Current Opinion in Cell Biol. 1990;2:864–874. doi: 10.1016/0955-0674(90)90085-s. [DOI] [PubMed] [Google Scholar]
- Streuli CH, Bissell MJ. Expression of extracellular matrix components is regulated by substratum. J Cell Biol. 1990;110:1405–1415. doi: 10.1083/jcb.110.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhouk RS, Bissell MJ, Werb Z. Expression of extracellular matrix-degrading proteinases and their inhibitors during involution of the mammary gland of CD-1 mice. J Cell Biol. 1990;111:14a. doi: 10.1083/jcb.118.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhouk RS, Streuli CH, Barcellos-Hoff MH, Bissell MJ. The extracellular matrix. In: Bittar EE, editor. Fundamentals of Medical Cell Biology. Greenwich, Connecticut: JAI Press Inc; 1991. (in press) [Google Scholar]
- Thiery JP, Duband J-L, Dufour S, Savanger P, Imhof BA. Roles of fibronectin in embryogenesis. In: Mosher DF, editor. Fibronectin. New York: Academic Press, Inc; 1988. pp. 181–212. [Google Scholar]
- Unemori EN, Chin JR, Werb Z, Bissell M. Expression and vectorial secretion of extracellular matrix-degrading enzymes by mammary epithelial cells. J Cell Biol. 1987;105:217a. [Google Scholar]
- Unemori EN, Werb Z. Reorganization of polymerized actin, A possible trigger for induction of procollagenase in fibroblasts cultured in and on collagen gels. J Cell Biol. 1986;103:1021–1031. doi: 10.1083/jcb.103.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unemori EN, Werb Z. Collagenase expression and endogenous activation in rabbit synovial fibroblasts stimulated by the calcium ionophore A23187. J biol Chem. 1988;263:16252–16259. [PubMed] [Google Scholar]
- Werb Z. Proteinases and matrix degradation. In: Kelley WN, Harris ED Jr, Ruddy S, Sledge CB, editors. Textbook of Rheumatology, W N. Philadelphia, PA: W. B. Saunders; 1989. pp. 300–321. [Google Scholar]
- Whitham SE, Murphy G, Angel P, Rahmsdorf HJ, Smith BJ, Lyons A, Harris TJR, Reynolds JJ, Herrlich P, Docherty AJP. Comparison of human stromelysin and collagenase by cloning and sequence analysis. Biochem J. 1986;240:913–916. doi: 10.1042/bj2400913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicha MS, Liotta LA, Vonderhaar BK, Kidwell WR. Effects of inhibition of basement membrane collagen deposition on rat mammary gland development. Devi Biol. 1980;80:253–266. doi: 10.1016/0012-1606(80)90402-9. [DOI] [PubMed] [Google Scholar]
- Wiens D, Park CS, Stockdale FE. Milk protein expression and ductal morphogenesis in the mammary gland in vitro, Hormone-dependant and -independent phases of adipocyte-mammary epithelial cell interaction. Devi Biol. 1987;120:245–258. doi: 10.1016/0012-1606(87)90122-9. [DOI] [PubMed] [Google Scholar]
- Wilde CJ, Knight CH. Degradation of newly synthesized casein in mammary explants from pregnant and lactating goats. Comp Biochem Physiol B: Comp Biochem. 1986;84:197–201. doi: 10.1016/0305-0491(86)90205-1. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Quigley JP, Ashe B, Dorn C, Goldfarb R, Troll W. Direct fluorescent assay of urokinase and plasminogen activators of normal and malignant cells: Kinetics and inhibitor profiles. Proc natn Acad Sci USA. 1978;75:750–753. doi: 10.1073/pnas.75.2.750. [DOI] [PMC free article] [PubMed] [Google Scholar]