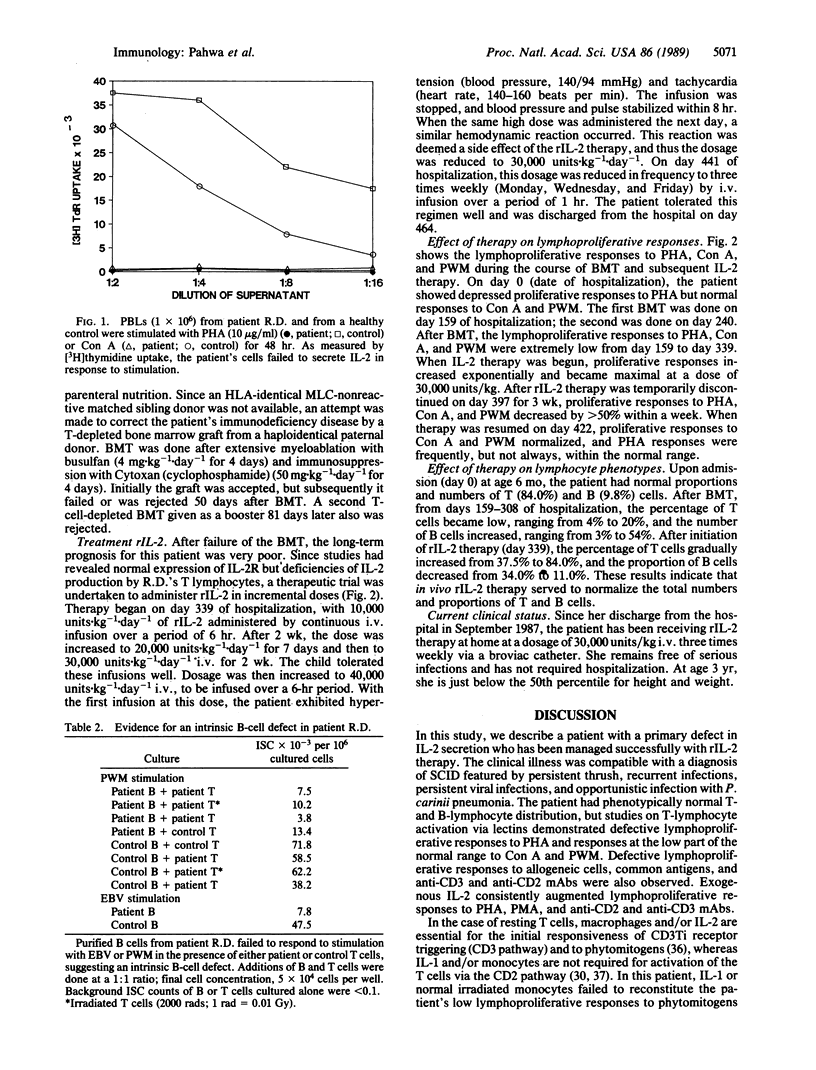
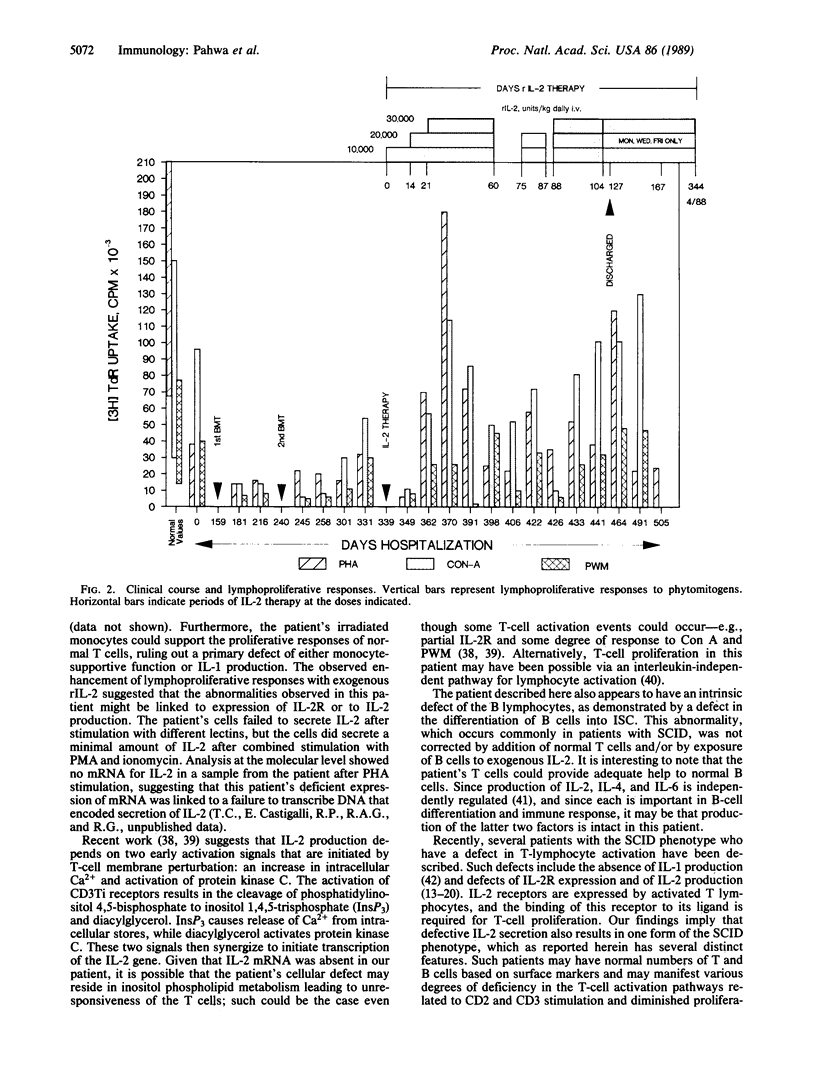
Abstract
Severe combined immunodeficiency disease (SCID) is a congenital disorder of severe B- and T-lymphocyte dysfunction in which several pathogenic mechanisms have been identified. The present study describes a female child with SCID who had a primary defect in the ability of T cells to secrete interleukin 2 (IL-2). B- and T-cell numbers were normal, but their functions were severely deficient. Mitogen and antigen-driven lymphoproliferative responses were diminished but were correctable in vitro with recombinant IL-2 (rIL-2). The patient's phytohemagglutinin-stimulated lymphocytes expressed IL-2 receptors normally. Despite the presence of the gene for IL-2, the patient's cells were grossly deficient in messenger RNA for IL-2 and endogenous IL-2 production. Pokeweed mitogen-driven B-cell differentiation was decreased and was not corrected by the addition of normal T cells to the B cells. Two attempts at immune reconstitution by haploidentical bone marrow transplantation failed. Therapy with rIL-2 (30,000 units/kg, given daily i.v.) resulted in marked clinical improvement as well in improved T-cell functions. The child, now 3 yr old, has been on rIL-2 therapy for 2 yr and receives rIL-2 (30,000 units/kg) three times weekly at home. This case study points to a new direction in the treatment of such disorders with rIL-2.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcover A., Ramarli D., Richardson N. E., Chang H. C., Reinherz E. L. Functional and molecular aspects of human T lymphocyte activation via T3-Ti and T11 pathways. Immunol Rev. 1987 Feb;95:5–36. doi: 10.1111/j.1600-065x.1987.tb00498.x. [DOI] [PubMed] [Google Scholar]
- Azogui O., Gluckman E., Fradelizi D. Inhibiton of IL 2 production after human allogeneic bone marrow transplantation. J Immunol. 1983 Sep;131(3):1205–1208. [PubMed] [Google Scholar]
- Chatila T. A., Schwartz D. H., Miller R., Geha R. S. Requirement for mitogen, T cell-accessory cell contact, and interleukin 1 in the induction of resting T-cell proliferation. Clin Immunol Immunopathol. 1987 Aug;44(2):235–247. doi: 10.1016/0090-1229(87)90068-7. [DOI] [PubMed] [Google Scholar]
- Chu E. T., Rosenwasser L. J., Dinarello C. A., Rosen F. S., Geha R. S. Immunodeficiency with defective T-cell response to interleukin 1. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4945–4949. doi: 10.1073/pnas.81.15.4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Mier J. W. Interleukins. Annu Rev Med. 1986;37:173–178. doi: 10.1146/annurev.me.37.020186.001133. [DOI] [PubMed] [Google Scholar]
- Doi S., Saiki O., Tanaka T., Ha-Kawa K., Igarashi T., Fujita T., Taniguchi T., Kishimoto S. Cellular and genetic analyses of IL-2 production and IL-2 receptor expression in a patient with familial T-cell-dominant immunodeficiency. Clin Immunol Immunopathol. 1988 Jan;46(1):24–36. doi: 10.1016/0090-1229(88)90003-7. [DOI] [PubMed] [Google Scholar]
- Flomenberg N., Welte K., Mertelsmann R., Kernan N., Ciobanu N., Venuta S., Feldman S., Kruger G., Kirkpatrick D., Dupont B. Immunologic effects of interleukin 2 in primary immunodeficiency diseases. J Immunol. 1983 Jun;130(6):2644–2650. [PubMed] [Google Scholar]
- Gillis S. Interleukin 2: biology and biochemistry. J Clin Immunol. 1983 Jan;3(1):1–13. doi: 10.1007/BF00919133. [DOI] [PubMed] [Google Scholar]
- Gillis S., Smith K. A. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977 Jul 14;268(5616):154–156. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- Hara T., Fu S. M., Hansen J. A. Human T cell activation. II. A new activation pathway used by a major T cell population via a disulfide-bonded dimer of a 44 kilodalton polypeptide (9.3 antigen). J Exp Med. 1985 Jun 1;161(6):1513–1524. doi: 10.1084/jem.161.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong R., Santosham M., Schulte-Wissermann H., Horowitz S., Hsu S. H., Winkelstein J. A. Reconstitution of B and T lymphocyte function in severe combined immunodeficiency disease after transplantation with thymic epithelium. Lancet. 1976 Dec 11;2(7998):1270–1272. doi: 10.1016/s0140-6736(76)92031-6. [DOI] [PubMed] [Google Scholar]
- Isakov N., Mally M. I., Scholz W., Altman A. T-lymphocyte activation: the role of protein kinase C and the bifurcating inositol phospholipid signal transduction pathway. Immunol Rev. 1987 Feb;95:89–111. doi: 10.1111/j.1600-065x.1987.tb00501.x. [DOI] [PubMed] [Google Scholar]
- June C. H., Ledbetter J. A., Rabinovitch P. S., Martin P. J., Beatty P. G., Hansen J. A. Distinct patterns of transmembrane calcium flux and intracellular calcium mobilization after differentiation antigen cluster 2 (E rosette receptor) or 3 (T3) stimulation of human lymphocytes. J Clin Invest. 1986 Apr;77(4):1224–1232. doi: 10.1172/JCI112425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koretzky G. A., Daniele R. P., Greene W. C., Nowell P. C. Evidence for an interleukin-independent pathway for human lymphocyte activation. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3444–3447. doi: 10.1073/pnas.80.11.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Crabtree G. R., Rudikoff S., Pumphrey J., Robb R. J., Krönke M., Svetlik P. B., Peffer N. J., Waldmann T. A. Molecular cloning and expression of cDNAs for the human interleukin-2 receptor. Nature. 1984 Oct 18;311(5987):626–631. doi: 10.1038/311626a0. [DOI] [PubMed] [Google Scholar]
- Linch D. C., Wallace D. L., O'Flynn K. Signal transduction in human T lymphocytes. Immunol Rev. 1987 Feb;95:137–159. doi: 10.1111/j.1600-065x.1987.tb00503.x. [DOI] [PubMed] [Google Scholar]
- López-Botet M., Fontán G., Garcia Rodriguez M. C., de Landázuri M. O. Relationship between IL 2 synthesis and the proliferative response to PHA in different primary immunodeficiencies. J Immunol. 1982 Feb;128(2):679–683. [PubMed] [Google Scholar]
- Martin P. J., Ledbetter J. A., Morishita Y., June C. H., Beatty P. G., Hansen J. A. A 44 kilodalton cell surface homodimer regulates interleukin 2 production by activated human T lymphocytes. J Immunol. 1986 May 1;136(9):3282–3287. [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Cantrell D. A., Hodgdon J. C., Schlossman S. F., Smith K. A., Reinherz E. L. Triggering of the T3-Ti antigen-receptor complex results in clonal T-cell proliferation through an interleukin 2-dependent autocrine pathway. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1509–1513. doi: 10.1073/pnas.81.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Fabbi M., Fox D., Acuto O., Fitzgerald K. A., Hodgdon J. C., Protentis J. P., Schlossman S. F., Reinherz E. L. An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell. 1984 Apr;36(4):897–906. doi: 10.1016/0092-8674(84)90039-4. [DOI] [PubMed] [Google Scholar]
- Moretta A., Pantaleo G., Lopez-Botet M., Moretta L. Involvement of T44 molecules in an antigen-independent pathway of T cell activation. Analysis of the correlations to the T cell antigen-receptor complex. J Exp Med. 1985 Sep 1;162(3):823–838. doi: 10.1084/jem.162.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Paganelli R., Aiuti F., Beverley P. C., Levinsky R. J. Impaired production of interleukins in patients with cell-mediated immunodeficiencies. Clin Exp Immunol. 1983 Feb;51(2):338–344. [PMC free article] [PubMed] [Google Scholar]
- Pahwa R. N., Pahwa S. G., Good R. A. T-lymphocyte differentiation in vitro in severe combined immunodeficiency. Defects of stem cells. J Clin Invest. 1979 Dec;64(6):1632–1641. doi: 10.1172/JCI109625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahwa S. G., Hoffman M. K., Pahwa R. N., Good R. A. Polyclonal and antigen-specific B-cell responses in patients with common variable immunodeficiency. J Clin Immunol. 1982 Jul;2(3):205–213. doi: 10.1007/BF00915223. [DOI] [PubMed] [Google Scholar]
- Pahwa S. G., Pahwa R. N., Good R. A. Heterogeneity of b lymphocyte differentiation in severe combined immunodeficiency disease. J Clin Invest. 1980 Sep;66(3):543–550. doi: 10.1172/JCI109886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahwa S. G., Quilop M. T., Lange M., Pahwa R. N., Grieco M. H. Defective B-lymphocyte function in homosexual men in relation to the acquired immunodeficiency syndrome. Ann Intern Med. 1984 Dec;101(6):757–763. doi: 10.7326/0003-4819-101-6-757. [DOI] [PubMed] [Google Scholar]
- Pahwa S., Sia C., Harper R., Pahwa R. T lymphocyte subpopulations in high-risk infants: influence of age and blood transfusions. Pediatrics. 1985 Dec;76(6):914–917. [PubMed] [Google Scholar]
- Pyke K. W., Dosch H., Ipp M. M., Gelfand E. W. Demonstration of an intrathymic defect in a case of severe combined immunodeficiency disease. N Engl J Med. 1975 Aug 28;293(9):424–428. doi: 10.1056/NEJM197508282930904. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Schwarz S., Spiess P. J. In vitro growth of murine T cells. II. Growth of in vitro sensitized cells cytotoxic for alloantigens. J Immunol. 1978 Nov;121(5):1951–1955. [PubMed] [Google Scholar]
- Saiki O., Shimizu M., Saeki Y., Kishimoto S., Kishimoto T. Dissociation in the production of B cell-stimulating factors (BCGF and BCDF) and interleukin 2 by T cells from a common variable immunodeficient patient. J Immunol. 1984 Oct;133(4):1920–1924. [PubMed] [Google Scholar]
- Smith K. A., Lachman L. B., Oppenheim J. J., Favata M. F. The functional relationship of the interleukins. J Exp Med. 1980 Jun 1;151(6):1551–1556. doi: 10.1084/jem.151.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. A. T-cell growth factor. Immunol Rev. 1980;51:337–357. doi: 10.1111/j.1600-065x.1980.tb00327.x. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., Matsui H., Fujita T., Takaoka C., Kashima N., Yoshimoto R., Hamuro J. Structure and expression of a cloned cDNA for human interleukin-2. Nature. 1983 Mar 24;302(5906):305–310. doi: 10.1038/302305a0. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A. The structure, function, and expression of interleukin-2 receptors on normal and malignant lymphocytes. Science. 1986 May 9;232(4751):727–732. doi: 10.1126/science.3008337. [DOI] [PubMed] [Google Scholar]
- Weiss A., Imboden J., Shoback D., Stobo J. Role of T3 surface molecules in human T-cell activation: T3-dependent activation results in an increase in cytoplasmic free calcium. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4169–4173. doi: 10.1073/pnas.81.13.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte K., Mertelsmann R. Human interleukin 2: biochemistry, physiology, and possible pathogenetic role in immunodeficiency syndromes. Cancer Invest. 1985;3(1):35–49. doi: 10.3109/07357908509040606. [DOI] [PubMed] [Google Scholar]
- Williams J. M., Deloria D., Hansen J. A., Dinarello C. A., Loertscher R., Shapiro H. M., Strom T. B. The events of primary T cell activation can be staged by use of Sepharose-bound anti-T3 (64.1) monoclonal antibody and purified interleukin 1. J Immunol. 1985 Oct;135(4):2249–2255. [PubMed] [Google Scholar]