Abstract
Inductively coupled plasma-mass spectrometry (ICP-MS) based assays lend themselves to multiplexing due to the high resolution between mass channels, the sensitivity, and the reliability of the technique. Here the potential of ICP-MS based protease assays is demonstrated with a quadruplex assay of cysteine proteases and metalloproteases. Four orthogonal peptide substrates were synthesized for the proteases calpain-1, caspase-3, MMP-9, and ADAM10. Each substrate carries a biotin tag at the C-terminus and a DTPA-based lanthanide complex at the N-terminus. The results demonstrate that this is simple and reproducible analysis technique with excellent correlation between the single and the multiplex assay formats.
Keywords: ICP-MS, Multiplex, Peptidase, Element-Tagging, Lanthanides
Proteases play crucial roles in all biological systems ranging from non-specific catabolism to specific signalling events. Disruption or acceleration of proteolysis of various protease substrates is observed in many diseases, making protease activity an important biomarker and a potential target for therapeutic intervention.1, 2 Proteases rarely act alone but function in a ‘protease web’ and thus, the multiplex analysis of many proteases in a single assay would aid in the diagnosis of diseases through the identification of characteristic patterns of proteolytic activity.3 Here we demonstrate a multiplex protease assay based upon lanthanide tagged protease substrates and ICP-MS analysis. There have been successful examples reporting multivariate bioassays for the detection of surface cell antigens4, glycoproteins5, and proteins6, 7 using ICP-MS. The potential of ICP-MS based assays for multiplexed enzyme assays has not yet been realized.
Recently, we demonstrated the use of a lanthanide-tagged protease substrate in a novel ICP-MS based protease assay.8 Here we demonstrate that this ICP-MS assay can be readily adapted to a multiplexed protease assay. A quadruplex assay of two cysteine proteases, calpain-1 and caspase-3, and two metalloproteinases, MMP-9 and ADAM10, was carried out to demonstrate the potential of the multiplexed assay for the simultaneous detection of four different families of protease activity each with different requirements for activity.
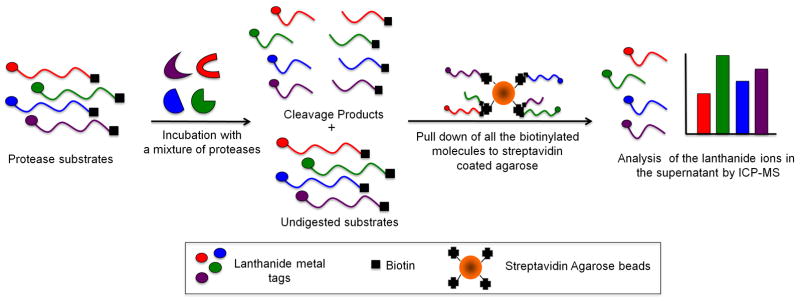
The design of the multiplex protease assay using ICP-MS is shown in Scheme 1. The assay is based on dual labeled peptide substrates that contain an N-terminal lanthanide chelate and a C-terminal biotin tag. All of the protease substrates were readily synthesized using standard solid phase peptide synthesis techniques. Each substrate was designed to hold a lanthanide ion by installing the general lanthanide chelator, diethylenetriaminepentaacetic acid (DTPA), at the N-terminus. Four β-alanine residues were introduced between the N-terminal DTPA chelator and the peptide substrate sequence. In our previous studies we observed that it was necessary to place a short repeat of β-alanine residues between the DTPA-Ln complex and the peptide substrate to ensure the DTPA ligand does not interfere with protease substrate recognition.8 Fmoc-Lys(biotin)-OH was used during the solid phase synthesis to introduce a biotin label at the C-terminus of the peptide substrates. The lanthanide metal ions were loaded into the DTPA labeled substrates after peptide purification.
Scheme 1.
Scheme of the multiplex protease assay. The protease substrates are incubated singly or as a mixture with either one protease or a mixture of four proteases. The undigested substrates were separated from the digestion products by adding streptavidin agarose beads followed by centrifugation. The supernatant was analyzed for lanthanide ion content by ICP-MS.
Significant knowledge about the substrate specificity of each of the four proteases investigated here is available, and has been adapted to generate four orthogonal peptide substrates (Table S1, supplementary material). The sequences of the peptide substrates were carefully selected to avoid cross reactivity between the proteases. Members of the caspase family are known to recognize the DEVD sequence according to the investigation of poly(ADP-ribose) polymerase (PARP), which is a natural substrate. A substrate for caspase-3 was synthesized based on this caspase's specific sequence.9 The synthesized calpain-1 substrate was based on the cleavage site within α-spectrin, one of its natural substrates.10 The MMP-9 substrate contained the Gly-Leu cleavable bond and is specific for MMPs.11 Finally, the ADAM10 substrate is based on the cleavage sequence of CD23. This substrate is known to be specific for the ADAMs, but can be cleaved by one of the MMPs (MMP-2).12 Each substrate was tagged with a different lanthanide ion (Tb3+, Ho3+, Gd3+, or Tm3+).
The multiplex assays were carried out in a buffer at pH 7.5 which offered a good compromise between the pH optima of the four proteases. The buffer also contained Ca2+, for calpain-1 activity, and Zn2+ for the metalloprotease activity. These divalent metals have significantly weaker affinity for the DTPA chelator than the lanthanides and thus do not affect the elemental tags on the protease substrates. The cysteine proteases, caspase-3 and calpain-1 require reducing conditions for activity. However, MMPs are multidomain proteases of which many contain disulfide bonds. Our initial studies showed that MMP-9 activity was dramatically reduced in the presence of low concentrations of the reducing agents dithiothreitol or β-mercaptoethanol. Similarly, all ADAMs contain an extracellular cysteine-rich domain before their transmembrane domains. Several observations suggest that the cysteine-rich domain is important for the biological activities of some ADAMs including cell adhesion, cell migration and proteolytic function.13
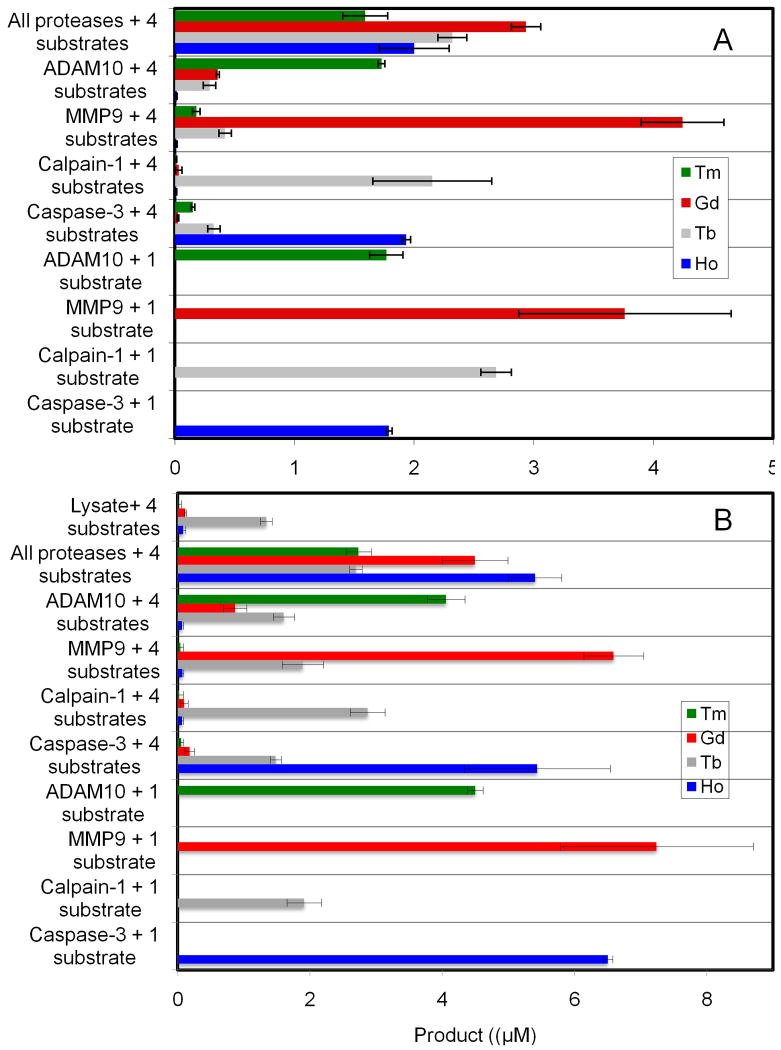
To address this issue, the assay protocol was adjusted to allow for incubations to be carried out under oxidizing and reducing conditions in a single multiplex assay. This was achieved by starting the assay incubation in the absence of reducing agent to allow for metalloproteinase activity, and then after a fixed time adding reducing agent followed by a second incubation period. 8 M urea proved ideal for quenching the assays as it denatures all proteases while not affecting the coordination chemistry of the DTPA ligand or denaturing streptavidin.14 After removing any undigested substrate, the supernatant was diluted and the lanthanide metal content was analyzed by ICP-MS. The type of lanthanide ion in the supernatant provides an indication of the type of protease activity and the concentration of the ion is a direct measure of the amount of protease activity present. All of the assays were run in triplicate. To estimate the ICP-MS instrumental error, three samples from each separate assay were analyzed. The instrumental error within an assay was generally within 3 %. The error bars (Fig. 1) from each assay represent the standard deviation of the average of the three independent assays.
Fig. 1.
Multiplex protease assays. A) Calpain-1 substrate (20 μM), caspase-3 substrate (10 μM), MMP-9 substrate (10 μM), and ADAM10 substrate (20 μM) were incubated with calpain-1 (200 nM), caspase-3 (100 nM), MMP9 (50 nM) or ADAM10 (200 nM), components in each assay are given on the y-axis. Reaction buffer used was, 50 mM Tris pH 7.5, 200 mM NaCl, 5 mM CaCl2, 20 μM ZnSO4, 0.05% Brij 35. Reactions were incubated for 1 hour at 37 °C followed by the addition of freshly prepared DTT (10 mM) to all the tubes. After another hour, the reaction was stopped by diluting the aliquots into 8 M urea (5 μL sample in 100 μL final volume). The samples were processed and analyzed by ICP-MS. Each bar represents the average of three separate assays analyzed in triplicate. Error bars represent ±SD of the three independent assays. B) Conditions were identical those in A except HeLa cell lysate was added to the buffer at a protein concentration of 100 μg/mL
To test the conversion of each substrate the assays were initially run in a single format using one protease and one substrate (lower four bars in each figure). Although a full kinetic evaluation of each substrate was not carried out, activity comparable to that expected based on literature fluorescence assays of a given peptide sequence was observed (Figure 1A). It should be noted that no additional optimization of these substrates was necessary, indicating the ready extension of known protease substrates to this assay format.
The orthogonality of the substrates was evaluated in a multiplex format including one protease with all the four elemental-tagged substrates (central four bar graphs in figure 1). Minimal cross-reactivity was observed between the substrates. Calpain-1 showed the least cross-reactivity with the other protease substrates. A small but significant hydrolysis of the calpain-1 substrate and to a lesser extent the ADAM10 substrate was observed with caspase-3 and MMP-9. Finally, a multiplex format with a mixture of all four proteases and all four substrates was run (top assays in each panel of Figure 1). Comparing the single and the multiplexed formats, very similar responses were observed, validating the multiplex approach. The similarity of the results between these assays clearly shows that there was no metal exchange between the element tagged substrates over the course of the two hour assay and further demonstrates the reproducibility of the assay.
To demonstrate the robustness of the assay to the presence of biological matrices, the assays were performed in HeLa cell lysates spiked with the proteases (Fig. 1B). In spite of a more complex medium, we observed reproducible results with a high correlation between the single and multiplex assays. This assay also demonstrates that background protease activity in the lysate can be detected, as a small amount of cleavage of the calpain-1 substrate was observed in the control assay (top four bars Figure 1B). This is consistent with our previous study showing that HeLa cell lysates have significant chymotrypsin-like activity.8 The calpain-1 substrate contains a Tyr∼Gly linkage which is susceptible to cleavage by chymotrypsin-like enzymes.
Previously, the highest homogeneous multiplexing protease assay report was a duplex assay based upon quantum dot systems.15 Impressive results have been achieved with using spatial resolution of the substrates on a chip, channel, or droplet for more highly multiplexed assays.16-18 Here we demonstrate that multiplexed assays can be achieved in a homogenous solution using an ICP-MS based approach and thus the method directly benefits from the high sensitivity, linearity and matrix robustness ICP-MS.19, 20 In addition to the obvious advantages of reducing the analyte consumption and the labour involved, this approach also provides an example of how multiplexed fingerprints of protease activity might be developed. In this work, we used concentrations of proteases that exceed the normal protease levels encountered in tissue and no attempts were made to reduce the protease levels. Future work will explore more highly mutiplexed assays for the detection of proteases activities found in biological samples of interest. In our work with antibodies and lectins, we have shown the advantage of using polymeric elemental tags able to carry many copies of a given metal to improve the sensitivity of the assay.4 Should greater sensitivity be required in this protease assay polymers could be adapted to increase the amount of a lanthanide label on a protease substrate.
Supplementary Material
Acknowledgments
We gratefully acknowledge financial support from NIH grant #GM076127-01A1 for the support of this work. We also thank Professor M. Winnik for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martinelli P, Rugarli EI. Emerging roles of mitochondrial proteases in neurodegeneration. Biochim Biophys Acta. 2010;1797:1–10. doi: 10.1016/j.bbabio.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Lopez-Otin C, Matrisian LM. Tumour micro environment - Opinion - Emerging roles of proteases in tumour suppression. Nat Rev Cancer. 2007;7:800–808. doi: 10.1038/nrc2228. [DOI] [PubMed] [Google Scholar]
- 3.Morrison CJ, Butler GS, Rodriguez D, Overall CM. Matrix metalloproteinase proteomics: substrates, targets, and therapy. Curr Opin Cell Biol. 2009;21:645–653. doi: 10.1016/j.ceb.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Lou XD, Zhang GH, Herrera I, Kinach R, Ornatsky O, Baranov V, Nitz M, Winnik MA. Polymer-based elemental tags for sensitive bioassays. Angew Chem Int Edit. 2007;46:6111–6114. doi: 10.1002/anie.200700796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leipold MD, Herrera I, Ornatsky O, Baranov V, N M. ICP-MS-Based Multiplex Profiling of Glycoproteins Using Lectins Conjugated to Lanthanide-Chelating Polymer. J Prot Res. 2008;8:443–449. doi: 10.1021/pr800645r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu SH, Zhang SC, Hu ZC, Xing Z, Zhang XR. Detection of multiple proteins on one spot by laser ablation inductively coupled plasma mass spectrometry and application to immuno-microarray with element-tagged antibodies. Anal Chem. 2007;79:923–929. doi: 10.1021/ac061269p. [DOI] [PubMed] [Google Scholar]
- 7.Terenghi M, Elviri L, Careri M, Mangia A, Lobinski R. Multiplexed Determination of Protein Biomarkers Using Metal-Tagged Antibodies and Size Exclusion Chromatography-Inductively Coupled Plasma Mass Spectrometry. Anal Chem. 2009;81:9440–9448. doi: 10.1021/ac901853g. [DOI] [PubMed] [Google Scholar]
- 8.Lathia US, Ornatsky O, Baranov V, Nitz M. Development of inductively coupled plasma-mass spectrometry-based protease assays. Anal Biochem. 2010;398:93–98. doi: 10.1016/j.ab.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Calvo M, Peterson EP, Rasper DM, Vaillancourt JP, Zamboni R, Nicholson DW, Thornberry NA. Purification and catalytic properties of human caspase family members. Cell Death Differ. 1999;6:362–369. doi: 10.1038/sj.cdd.4400497. [DOI] [PubMed] [Google Scholar]
- 10.Mittoo S, Sundstrom LE, Bradley M. Synthesis and evaluation of fluorescent probes for the detection of calpain activity. Anal Biochem. 2003;319:234–238. doi: 10.1016/s0003-2697(03)00324-5. [DOI] [PubMed] [Google Scholar]
- 11.Beekman B, Drijfhout JW, Bloemhoff W, Ronday HK, Tak PP, Koppele JMT. Convenient fluorometric assay for matrix metalloproteinase activity and its application in biological media. FEBS Lett. 1996;390:221–225. doi: 10.1016/0014-5793(96)00665-5. [DOI] [PubMed] [Google Scholar]
- 12.Moss ML, Rasmussen FH. Fluorescent substrates for the proteinases ADAM17, ADAM10, ADAM8, and ADAM12 useful for high-throughput inhibitor screening. Anal Biochem. 2007;366:144–148. doi: 10.1016/j.ab.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 13.Murphy G. Regulation of the proteolytic disintegrin metalloproteinases, the ‘Sheddases’. Sem Cell Devel Biol. 2009;20:138–145. doi: 10.1016/j.semcdb.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Kurzban GP, Bayer EA, Wilchek M, Horowitz PM. The Quaternary Structure of Streptavidin in Urea. J Biol Chem. 1991;266:14470–14477. [PubMed] [Google Scholar]
- 15.Xia ZY, Xing Y, So MK, Koh AL, Sinclair R, Rao JH. Multiplex Detection of Protease Activity with Quantum Dot Nanosensors Prepared by Intein-Mediated Specific Bioconjugation. Anal Chem. 2008;80:8649–8655. doi: 10.1021/ac801562f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim YP, Oh YH, Oh E, Ko S, Han MK, Kim HS. Energy transfer-based multiplexed assay of proteases by using gold nanoparticle and quantum dot conjugates on a surface. Anal Chem. 2008;80:4634–4641. doi: 10.1021/ac702416e. [DOI] [PubMed] [Google Scholar]
- 17.Henares TG, Mizutani F, Sekizawa R, Hisamoto H. Single-drop analysis of various proteases in a cancer cell lysate using a capillary-assembled microchip. Anal Bioanal Chem. 2008;391:2507–2512. doi: 10.1007/s00216-008-2105-x. [DOI] [PubMed] [Google Scholar]
- 18.Gosalina DN, Denney WS, Salisbury CM, Ellman JA, Diamond SL. Functional phenotyping of human plasma using a 361-fluorogenic substrate biosensing microarray. Biotech Bioeng. 2006;94:1099–1110. doi: 10.1002/bit.20927. [DOI] [PubMed] [Google Scholar]
- 19.Prange A, Pröfrock D. Chemical labels and natural element tags for the quantitative analysis of bio-molecules. J Anal At Spectrom. 2008;23:432–459. [Google Scholar]
- 20.Tanner M, Güntherb D. Short transient signals, a challenge for inductively coupled plasma mass spectrometry, a review. Anal Chim Acta. 2009;633:19–28. doi: 10.1016/j.aca.2008.11.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.