Abstract
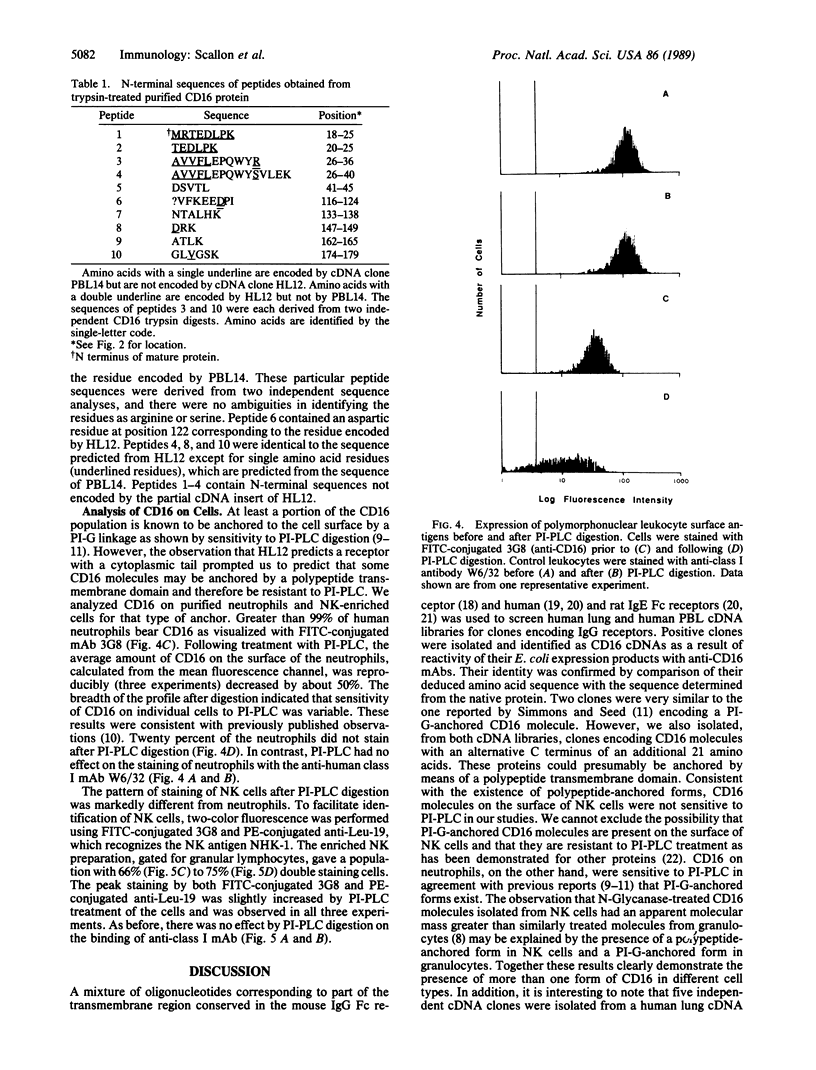
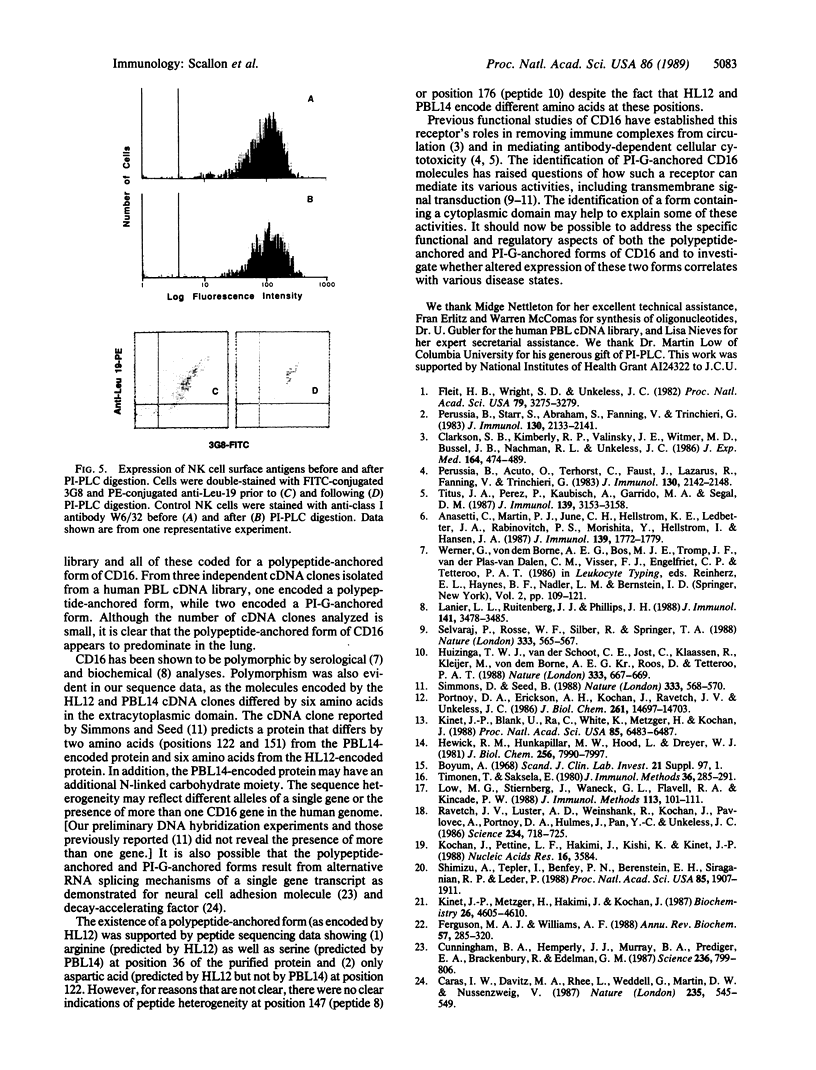
Several cDNA clones encoding the human immunoglobulin G receptor CD16 were isolated from human lung or peripheral blood leukocyte cDNA libraries. Nucleotide sequence comparisons revealed that the cDNAs could be divided into two groups. cDNA clones in one group encode a protein that terminates 4 amino acids after the putative transmembrane domain. Clones in the second group encode a protein with an extra 21 amino acids that could comprise a cytoplasmic domain. Direct peptide sequencing was used to determine the N terminus of the mature CD16 receptor protein and supported the existence of the two forms of the receptor. Treatment of neutrophils with phosphatidylinositol-specific phospholipase C resulted in the release of a large percentage of the CD16 molecules from the cell surface. In contrast, treatment of natural killer cells with phosphatidylinositol-specific phospholipase C did not release any CD16 from the cell surface. These data demonstrate that both polypeptide-anchored and phosphatidylinositol-glycan-anchored forms of the CD16 molecule exist and that they are differentially expressed on neutrophils and natural killer cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anasetti C., Martin P. J., June C. H., Hellstrom K. E., Ledbetter J. A., Rabinovitch P. S., Morishita Y., Hellstrom I., Hansen J. A. Induction of calcium flux and enhancement of cytolytic activity in natural killer cells by cross-linking of the sheep erythrocyte binding protein (CD2) and the Fc-receptor (CD16). J Immunol. 1987 Sep 15;139(6):1772–1779. [PubMed] [Google Scholar]
- Caras I. W., Davitz M. A., Rhee L., Weddell G., Martin D. W., Jr, Nussenzweig V. Cloning of decay-accelerating factor suggests novel use of splicing to generate two proteins. Nature. 1987 Feb 5;325(6104):545–549. doi: 10.1038/325545a0. [DOI] [PubMed] [Google Scholar]
- Clarkson S. B., Kimberly R. P., Valinsky J. E., Witmer M. D., Bussel J. B., Nachman R. L., Unkeless J. C. Blockade of clearance of immune complexes by an anti-Fc gamma receptor monoclonal antibody. J Exp Med. 1986 Aug 1;164(2):474–489. doi: 10.1084/jem.164.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham B. A., Hemperly J. J., Murray B. A., Prediger E. A., Brackenbury R., Edelman G. M. Neural cell adhesion molecule: structure, immunoglobulin-like domains, cell surface modulation, and alternative RNA splicing. Science. 1987 May 15;236(4803):799–806. doi: 10.1126/science.3576199. [DOI] [PubMed] [Google Scholar]
- Ferguson M. A., Williams A. F. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu Rev Biochem. 1988;57:285–320. doi: 10.1146/annurev.bi.57.070188.001441. [DOI] [PubMed] [Google Scholar]
- Fleit H. B., Wright S. D., Unkeless J. C. Human neutrophil Fc gamma receptor distribution and structure. Proc Natl Acad Sci U S A. 1982 May;79(10):3275–3279. doi: 10.1073/pnas.79.10.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Huizinga T. W., van der Schoot C. E., Jost C., Klaassen R., Kleijer M., von dem Borne A. E., Roos D., Tetteroo P. A. The PI-linked receptor FcRIII is released on stimulation of neutrophils. Nature. 1988 Jun 16;333(6174):667–669. doi: 10.1038/333667a0. [DOI] [PubMed] [Google Scholar]
- Kinet J. P., Blank U., Ra C., White K., Metzger H., Kochan J. Isolation and characterization of cDNAs coding for the beta subunit of the high-affinity receptor for immunoglobulin E. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6483–6487. doi: 10.1073/pnas.85.17.6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinet J. P., Metzger H., Hakimi J., Kochan J. A cDNA presumptively coding for the alpha subunit of the receptor with high affinity for immunoglobulin E. Biochemistry. 1987 Jul 28;26(15):4605–4610. doi: 10.1021/bi00389a002. [DOI] [PubMed] [Google Scholar]
- Kochan J., Pettine L. F., Hakimi J., Kishi K., Kinet J. P. Isolation of the gene coding for the alpha subunit of the human high affinity IgE receptor. Nucleic Acids Res. 1988 Apr 25;16(8):3584–3584. doi: 10.1093/nar/16.8.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L. L., Ruitenberg J. J., Phillips J. H. Functional and biochemical analysis of CD16 antigen on natural killer cells and granulocytes. J Immunol. 1988 Nov 15;141(10):3478–3485. [PubMed] [Google Scholar]
- Low M. G., Stiernberg J., Waneck G. L., Flavell R. A., Kincade P. W. Cell-specific heterogeneity in sensitivity of phosphatidylinositol-anchored membrane antigens to release by phospholipase C. J Immunol Methods. 1988 Oct 4;113(1):101–111. doi: 10.1016/0022-1759(88)90386-9. [DOI] [PubMed] [Google Scholar]
- Perussia B., Acuto O., Terhorst C., Faust J., Lazarus R., Fanning V., Trinchieri G. Human natural killer cells analyzed by B73.1, a monoclonal antibody blocking Fc receptor functions. II. Studies of B73.1 antibody-antigen interaction on the lymphocyte membrane. J Immunol. 1983 May;130(5):2142–2148. [PubMed] [Google Scholar]
- Perussia B., Starr S., Abraham S., Fanning V., Trinchieri G. Human natural killer cells analyzed by B73.1, a monoclonal antibody blocking Fc receptor functions. I. Characterization of the lymphocyte subset reactive with B73.1. J Immunol. 1983 May;130(5):2133–2141. [PubMed] [Google Scholar]
- Portnoy D. A., Erickson A. H., Kochan J., Ravetch J. V., Unkeless J. C. Cloning and characterization of a mouse cysteine proteinase. J Biol Chem. 1986 Nov 5;261(31):14697–14703. [PubMed] [Google Scholar]
- Ravetch J. V., Luster A. D., Weinshank R., Kochan J., Pavlovec A., Portnoy D. A., Hulmes J., Pan Y. C., Unkeless J. C. Structural heterogeneity and functional domains of murine immunoglobulin G Fc receptors. Science. 1986 Nov 7;234(4777):718–725. doi: 10.1126/science.2946078. [DOI] [PubMed] [Google Scholar]
- Selvaraj P., Rosse W. F., Silber R., Springer T. A. The major Fc receptor in blood has a phosphatidylinositol anchor and is deficient in paroxysmal nocturnal haemoglobinuria. Nature. 1988 Jun 9;333(6173):565–567. doi: 10.1038/333565a0. [DOI] [PubMed] [Google Scholar]
- Shimizu A., Tepler I., Benfey P. N., Berenstein E. H., Siraganian R. P., Leder P. Human and rat mast cell high-affinity immunoglobulin E receptors: characterization of putative alpha-chain gene products. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1907–1911. doi: 10.1073/pnas.85.6.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D., Seed B. The Fc gamma receptor of natural killer cells is a phospholipid-linked membrane protein. Nature. 1988 Jun 9;333(6173):568–570. doi: 10.1038/333568a0. [DOI] [PubMed] [Google Scholar]
- Timonen T., Saksela E. Isolation of human NK cells by density gradient centrifugation. J Immunol Methods. 1980;36(3-4):285–291. doi: 10.1016/0022-1759(80)90133-7. [DOI] [PubMed] [Google Scholar]
- Titus J. A., Perez P., Kaubisch A., Garrido M. A., Segal D. M. Human K/natural killer cells targeted with hetero-cross-linked antibodies specifically lyse tumor cells in vitro and prevent tumor growth in vivo. J Immunol. 1987 Nov 1;139(9):3153–3158. [PubMed] [Google Scholar]



