Abstract
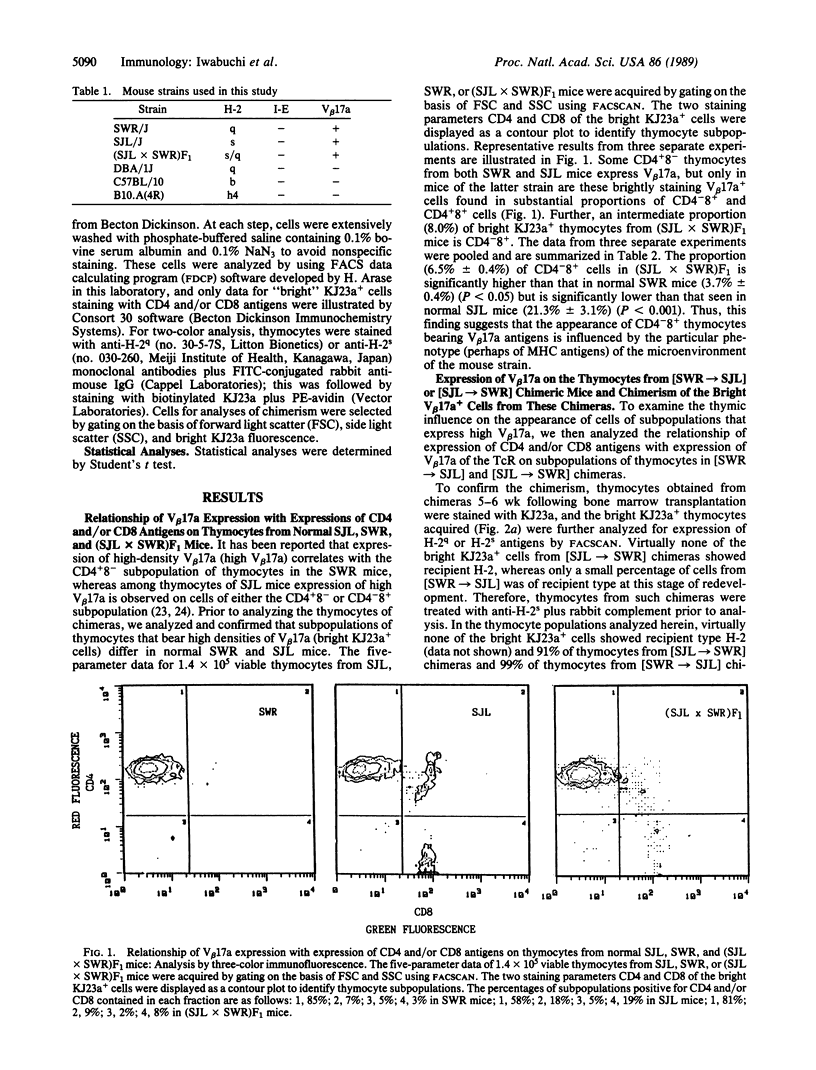
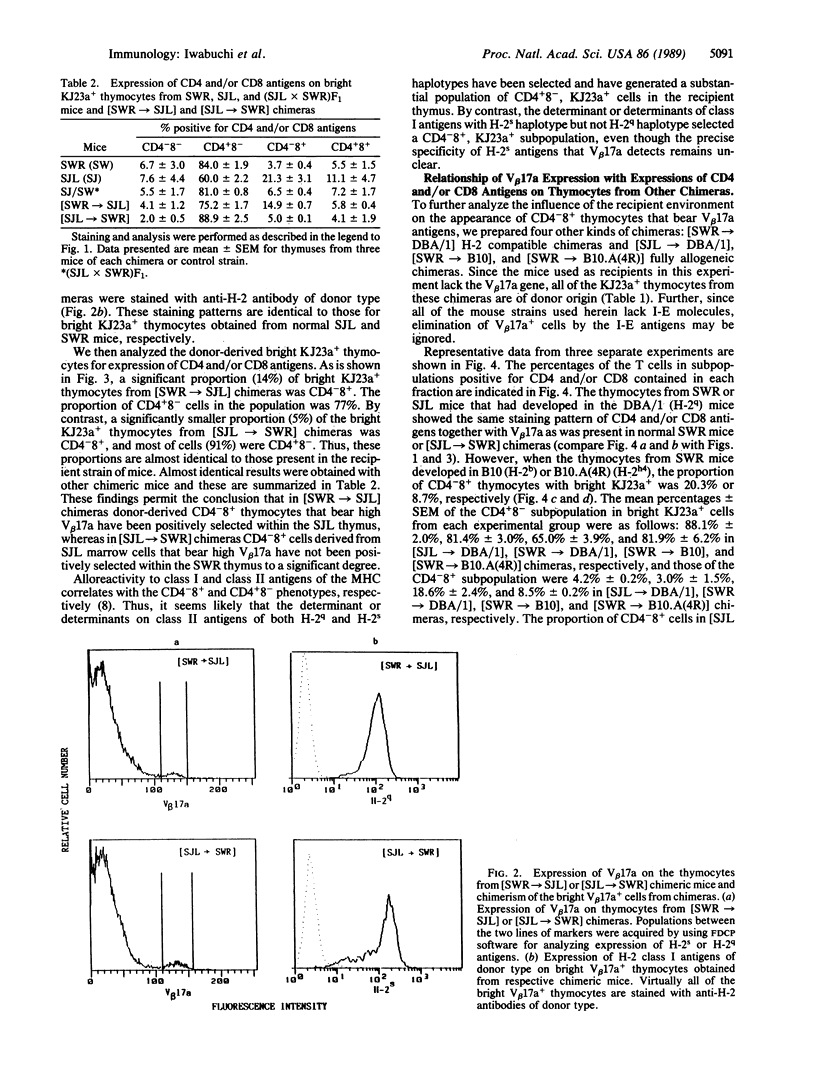
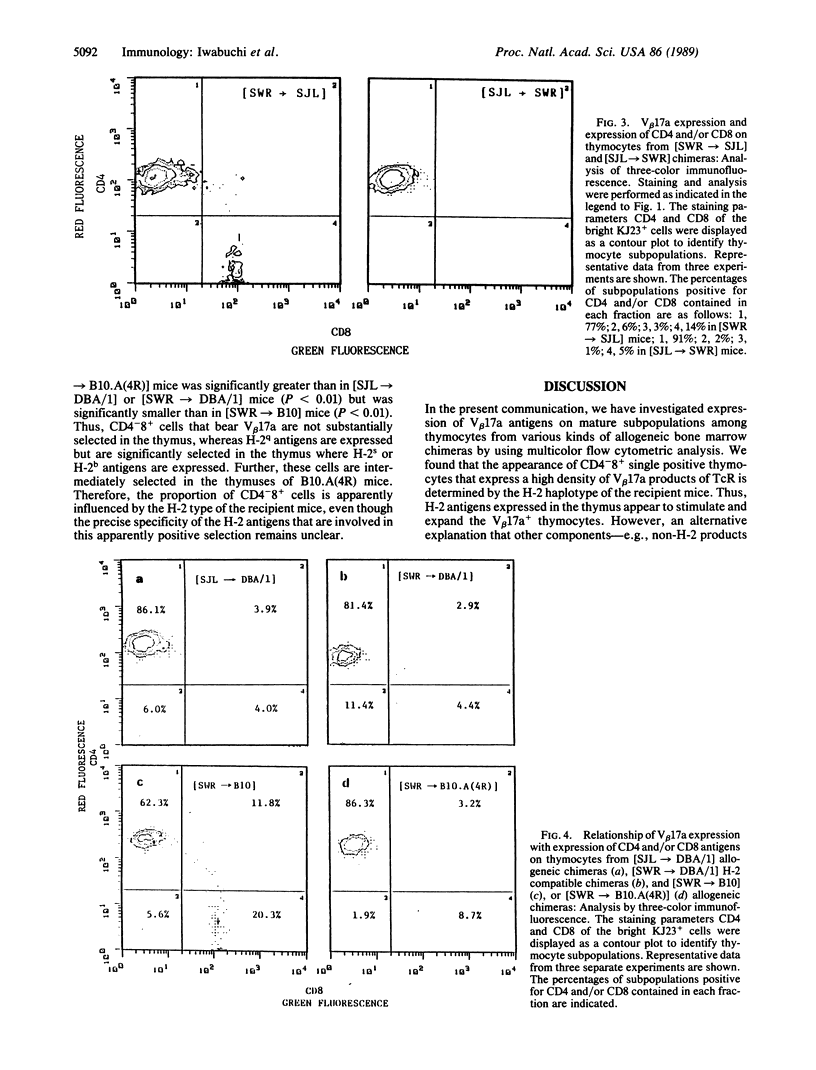
In SWR mice the expression with high-density V beta 17a (high V beta 17a) of the T-cell antigen receptors correlates with the CD4+8- subpopulation of thymocytes. By contrast, in thymocytes of SJL mice the expression of high V beta 17a is observed on the CD4+8- or CD4-8+ subpopulation. However, when the thymocytes from SWR mice have been developed in the SJL or B10 thymus but not in the H-2 compatible DBA/1 thymus, a greater proportion of thymocytes that express high V beta 17a was found to be CD4-8+. By contrast, only a small proportion of KJ23a+ thymocytes from SJL mice that had differentiated in the thymus of SWR or DBA/1 mice was CD4-8+, whereas a high proportion of CD4+8- cells expressed V beta 17a. Further, an intermediate proportion of KJ23a+ thymocytes that had derived from SJL donor mice was present on CD4-8+ thymocytes that had developed in B10.A(4R) thymus. These findings demonstrate that the appearance of a particular subpopulation of thymocytes (CD4-8+ with a beta chain of T-cell antigen receptor identified as V beta 17a) is determined by the histocompatibility complex products that are expressed in the thymic microenvironment in which the T cells develop.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe R., Vacchio M. S., Fox B., Hodes R. J. Preferential expression of the T-cell receptor V beta 3 gene by Mlsc reactive T cells. Nature. 1988 Oct 27;335(6193):827–830. doi: 10.1038/335827a0. [DOI] [PubMed] [Google Scholar]
- Boehmer H., Sprent J., Nabholz M. Tolerance to histocompatibility determinants in tetraparental bone marrow chimeras. J Exp Med. 1975 Feb 1;141(2):322–334. doi: 10.1084/jem.141.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb P., Meier B., Matsunaga T., Feldmann M. Nature of T-cell macrophage interaction in helper-cell induction in vitro. II. Two stages of T-helper-cell differentiation analyzed in irradiation and allophenic chimeras. J Exp Med. 1979 Mar 1;149(3):686–701. doi: 10.1084/jem.149.3.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink P. J., Bevan M. J. H-2 antigens of the thymus determine lymphocyte specificity. J Exp Med. 1978 Sep 1;148(3):766–775. doi: 10.1084/jem.148.3.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y., Onoe K., Aizawa M. Autologous or syngeneic mixed lymphocyte reaction in bone marrow and thymic chimera mice. Cell Immunol. 1982 Oct;73(1):141–150. doi: 10.1016/0008-8749(82)90442-7. [DOI] [PubMed] [Google Scholar]
- Iwabuchi K., Ogasawara K., Ogasawara M., Yasumizu R., Noguchi M., Geng L., Fujita M., Good R. A., Onoé K. A study on proliferative responses to host Ia antigens in allogeneic bone marrow chimera in mice: sequential analysis of the reactivity and characterization of the cells involved in the responses. J Immunol. 1987 Jan 1;138(1):18–25. [PubMed] [Google Scholar]
- Kappler J. W., Roehm N., Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987 Apr 24;49(2):273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Staerz U., White J., Marrack P. C. Self-tolerance eliminates T cells specific for Mls-modified products of the major histocompatibility complex. Nature. 1988 Mar 3;332(6159):35–40. doi: 10.1038/332035a0. [DOI] [PubMed] [Google Scholar]
- Kisielow P., Blüthmann H., Staerz U. D., Steinmetz M., von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988 Jun 23;333(6175):742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- Longo D. L., Schwartz R. H. T-cell specificity for H-2 and Ir gene phenotype correlates with the phenotype of thymic antigen-presenting cells. Nature. 1980 Sep 4;287(5777):44–46. doi: 10.1038/287044a0. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Hengartner H., Pedrazzini T. Intrathymic deletion of self-reactive cells prevented by neonatal anti-CD4 antibody treatment. Nature. 1988 Sep 8;335(6186):174–176. doi: 10.1038/335174a0. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Lees R. K., Schneider R., Zinkernagel R. M., Hengartner H. Positive selection of CD4+ thymocytes controlled by MHC class II gene products. Nature. 1988 Dec 1;336(6198):471–473. doi: 10.1038/336471a0. [DOI] [PubMed] [Google Scholar]
- Marrack P., Kappler J. The T cell receptor. Science. 1987 Nov 20;238(4830):1073–1079. doi: 10.1126/science.3317824. [DOI] [PubMed] [Google Scholar]
- Marrack P., Lo D., Brinster R., Palmiter R., Burkly L., Flavell R. H., Kappler J. The effect of thymus environment on T cell development and tolerance. Cell. 1988 May 20;53(4):627–634. doi: 10.1016/0092-8674(88)90578-8. [DOI] [PubMed] [Google Scholar]
- Onoé K., Fernandes G., Good R. A. Humoral and cell-mediated immune responses in fully allogeneic bone marrow chimera in mice. J Exp Med. 1980 Jan 1;151(1):115–132. doi: 10.1084/jem.151.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoé K., Iwabuchi K., Katsume C., Gotohda T., Arase A., Hatakeyama S., Mishima M., Good R. A., Ogasawara K. A study on graft-versus-host reaction (GVHR) by Simonsen's splenomegaly assay. Cells and antigen systems involved in induction of GVHR. Acta Pathol Jpn. 1989 Feb;39(2):101–110. doi: 10.1111/j.1440-1827.1989.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Onoé K., Yasumizu R., Noguchi M., Iwabuchi K., Ogasawara M., Kakinuma M., Okuyama H., Good R. A., Morikawa K. Analyses of H-2 restriction specificity of helper T cells in fully allogeneic bone marrow chimera in mice. Immunobiology. 1985 Feb;169(1):60–70. doi: 10.1016/S0171-2985(85)80054-1. [DOI] [PubMed] [Google Scholar]
- Onoé K., Yasumizu R., Oh-Ishi T., Kakinuma M., Good R. A., Morikawa K. Restricted antibody formation to sheep erythrocytes of allogeneic bone marrow chimeras histoincompatible at the K end of the H-2 complex. J Exp Med. 1981 Apr 1;153(4):1009–1014. doi: 10.1084/jem.153.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullen A. M., Marrack P., Kappler J. W. The T-cell repertoire is heavily influenced by tolerance to polymorphic self-antigens. Nature. 1988 Oct 27;335(6193):796–801. doi: 10.1038/335796a0. [DOI] [PubMed] [Google Scholar]
- Raulet D. H. Tolerance, and more. Cell. 1987 Apr 24;49(2):153–154. doi: 10.1016/0092-8674(87)90551-4. [DOI] [PubMed] [Google Scholar]
- Ron Y., Lo D., Sprent J. T cell specificity in twice-irradiated F1----parent bone marrow chimeras: failure to detect a role for immigrant marrow-derived cells in imprinting intrathymic H-2 restriction. J Immunol. 1986 Sep 15;137(6):1764–1771. [PubMed] [Google Scholar]
- Rosenthal A. S., Shevach E. M. Function of macrophages in antigen recognition by guinea pig T lymphocytes. I. Requirement for histocompatible macrophages and lymphocytes. J Exp Med. 1973 Nov 1;138(5):1194–1212. doi: 10.1084/jem.138.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilham M. W., Lang R., Acha-Orbea H., Benner R., Joho R., Hengartner H. Fine specificity and T-cell receptor beta-chain gene rearrangements of five H-2Db-specific cytotoxic T-cell clones. Immunogenetics. 1987;25(3):171–178. doi: 10.1007/BF00344031. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. T-lymphocyte recognition of antigen in association with gene products of the major histocompatibility complex. Annu Rev Immunol. 1985;3:237–261. doi: 10.1146/annurev.iy.03.040185.001321. [DOI] [PubMed] [Google Scholar]
- Sha W. C., Nelson C. A., Newberry R. D., Kranz D. M., Russell J. H., Loh D. Y. Positive and negative selection of an antigen receptor on T cells in transgenic mice. Nature. 1988 Nov 3;336(6194):73–76. doi: 10.1038/336073a0. [DOI] [PubMed] [Google Scholar]
- Singer A., Hathcock K. S., Hodes R. J. Self recognition in allogeneic radiation bone marrow chimeras. A radiation-resistant host element dictates the self specificity and immune response gene phenotype of T-helper cells. J Exp Med. 1981 May 1;153(5):1286–1301. doi: 10.1084/jem.153.5.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer A., Hathcock K. S., Hodes R. J. Self recognition in allogeneic thymic chimeras. Self recognition by T helper cells from thymus-engrafted nude mice is restricted to the thymic H-2 haplotype. J Exp Med. 1982 Jan 1;155(1):339–344. doi: 10.1084/jem.155.1.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T., Uracz W., Abe R., Asano Y. I-J as an inducible T cell receptor for self. Immunol Rev. 1985 Apr;83:105–124. doi: 10.1111/j.1600-065x.1985.tb00472.x. [DOI] [PubMed] [Google Scholar]
- Teh H. S., Kisielow P., Scott B., Kishi H., Uematsu Y., Blüthmann H., von Boehmer H. Thymic major histocompatibility complex antigens and the alpha beta T-cell receptor determine the CD4/CD8 phenotype of T cells. Nature. 1988 Sep 15;335(6187):229–233. doi: 10.1038/335229a0. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974 Apr 19;248(5450):701–702. doi: 10.1038/248701a0. [DOI] [PubMed] [Google Scholar]


