Abstract
In AIDS Clinical Trials Group A5095, 9% of participants who experienced an adverse event related to efavirenz substituted nevirapine. Most adverse events resolved; 15 participants ultimately discontinued nevirapine therapy. Grade 3/4 hepatotoxicity was observed in 14% of individuals who substituted nevirapine, compared with 6% who continued efavirenz therapy. Substitution of nevirapine because of efavirenz toxicity was generally safe and efficacious.
Data on the safety and efficacy of substitution of nevirapine (NVP) because of efavirenz (EFV)-related adverse events (AEs) are limited [1, 2]. Safety concerns are partially based on liver toxicity risks with NVP initiation at higher CD4+ T cell counts [3]; treatment-experienced patients may have a lower rate of overall AEs than expected, particularly with an HIV RNA level <400 copies/mL [4–6]. AIDS Clinical Trials Group (ACTG) A5095 was a prospective, randomized, blinded study comparing a triple nucleoside reverse-transcriptase inhibitor (NRTI) regimen (zidovudine, lamivudine, and abacavir) with EFV with either zidovudine and lamivudine or zidovudine, lamivudine, and abacavir that allowed substitution of NVP because of EFV-related AEs [7–9]. This post-hoc analysis of ACTG A5095 assesses the safety and efficacy of NVP substitution.
Methods
In ACTG A5095, 765 of 1147 treatment-naive individuals (enrolled from March 2001 through November 2002) were randomized to receive EFV. Participants who experienced a treatment-limiting AE related to EFV, in the opinion of the site investigator, could substitute NVP (200 mg once daily for the first 14 days, then 200 mg twice daily). All participants were followed up prospectively for 120 weeks after the last individual was enrolled. Visits were every 4 weeks for 24 weeks, then every 8 weeks. Additional study details are described elsewhere [8].
Cox proportional hazards models were used to evaluate baseline characteristics associated with substitution of NVP for EFV. Exploratory competing risks analyses investigated the same relationships according to the reason for switching (central nervous system [CNS], rash, or other AEs). Descriptive analyses of NVP substitution outcomes include all laboratory abnormalities grade ≥2 and their resolution after NVP substitution [10]. Hepatotoxicity rates (prevalence and incidence) among participants who substituted NVP and in a cohort of range-matched (baseline CD4+ T cell count, race/ethnicity, and sex), randomly selected nonsubstituting participants were obtained. Hepatotoxicity was defined as grade 3 (5.1–10.0 times the upper limit of normal) or 4 (>10.0 times the upper limit of normal) elevation in aspartate aminotransferase, alanine aminotransferase, total bilirubin, direct bilirubin, alkaline phosphatase, and/or gamma-glutamyl transpeptidase level. A bootstrapped 95% confidence interval (CI) of estimated rates in the non-switching group was obtained using 1000 bootstrapped sampling cohorts.
The proportion of patients with an HIV RNA level <50 copies/mL 16–32 weeks after NVP substitution was summarized using intent-to-treat (missing data was considered to be ignored), intent-to-treat (missing data and treatment discontinuation was considered to be treatment failure), and as-treated (including only on-treatment data) analyses. In the event of multiple evaluations in this period, a single value closest to 24 weeks after substitution was used.
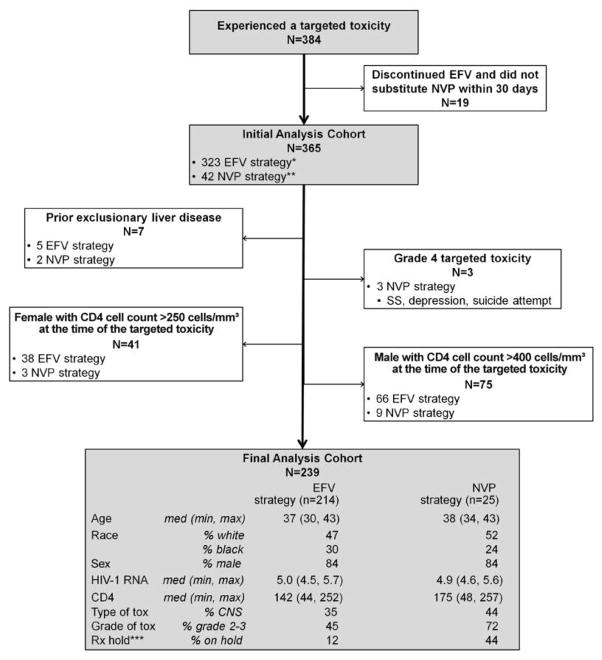
A controlled comparison of EFV-NVP switching strategy and not switching was performed for participants for whom continuing to receive EFV or substituting NVP were viable options. This cohort included only participants from the original ACTG A5095 cohort who experienced a non–life-threatening CNS or rash AE or received a CNS-related diagnosis during EFV-based therapy. Participants for whom NVP was contraindicated (ie, pre-existing ≥grade 3 liver enzyme or disease diagnosis, women with a CD4+ T cell count >250 cells/mm3, and men with CD4+ T cell count >400 cells/mm3 at the time of the targeted AE) and for whom continuing to receive EFV was not a realistic option (ie, a grade 4 AE) were excluded. Eligible participants were classified into either strategy group on the basis of whether they substituted NVP within 30 days after their first targeted AE or continued to receive EFV throughout this 30-day period; participants discontinuing EFV therapy without switching to NVP within the 30-day period were excluded. End points for this analysis included time to virologic failure (2 consecutive HIV RNA levels >200 copies/mL ≥12 weeks after the initial targeted AE), time to strategy discontinuation (discontinuation of NVP or EFV), and time to strategy failure (defined as first virologic failure or strategy discontinuation). Time zero was the date of the initial targeted AE. Analyses were adjusted for ACTG A5095 pretreatment covariates and covariates defined on the basis of ACTG A5095 follow-up before the targeted AE.
Results
Of 765 participants randomized to receive EFV (2 NRTIs [n = 382] or 3 NRTIs [n = 383]), 70 (9%; 15 female) with median pretherapy CD4+ T cell counts of 246 cells/mm3 substituted NVP for EFV because of CNS symptoms (n = 47), skin symptoms (n = 18), fatigue (n = 3), elevated transaminase levels (n = 1), or hypertriglyceridemia (n = 1) (Table 1). Most substitutions (78%) occurred within 24 weeks after initiation of treatment. NVP was started within 2 weeks after EFV therapy discontinuation in 71% of participants (53% within 1 week); participants who switched therapy because of skin symptoms had a longer duration between EFV discontinuation and NVP initiation (44% and 89% started NVP therapy within 2 and 4 weeks after EFV therapy discontinuation, respectively). Baseline characteristics and disposition of the cohort are reported elsewhere [7, 8].
Table 1.
Baseline Characteristics of 70 Participants Who Switched from Efavirenz (EFV) Therapy to Nevirapine (NVP) Therapy, by Reason for Switch
| Characteristic | Reason for switch (among those who switched) | Continued EFV therapy (n = 683) | ||
|---|---|---|---|---|
| CNS and/or neuropsychiatric symptoms (n = 47) | Rash and/or allergic reaction (n = 18) | Othera (n = 5) | ||
| Age, years | ||||
| Mean ± SD | 37 ± 9 | 39 ± 11 | 52 ± 13 | 38 ± 9 |
| Median (IQR) | 36 (31–41) | 42 (30–47) | 48 (45–63) | 37 (31–43) |
| Range | 22–77 | 20–59 | 37–67 | 18–75 |
| Sex | ||||
| Male | 38 (81) | 14 (78) | 3 (60) | 559 (82) |
| Female | 9 (19) | 4 (22) | 2 (40) | 124 (18) |
| Race/ethnicity | ||||
| White non-Hispanic | 26 (55) | 9 (50) | 1 (20) | 275 (40) |
| Black non-Hispanic | 10 (21) | 5 (28) | 3 (60) | 247 (36) |
| Hispanic | 11 (23) | 4 (22) | 1 (20) | 145 (21) |
| Other | 0 (0) | 0 (0) | 0 (0) | 16 (2) |
| HIV RNA level, log10 copies/mLb | ||||
| Mean ± SD | 4.64 ± 0.79 | 5.23 ± 0.73 | 5.06 ± 0.54 | 4.86 ± 0.72 |
| Median (IQR) | 4.61 (4.33–4.89) | 5.01 (4.78–5.64) | 4.70 (4.70–5.38) | 4.78 (4.38–5.41) |
| Range | 2.34–6.44 | 4.04–6.87 | 4.65–5.85 | 2.56–6.57 |
| CD4+ T cell count, cells/mm3b | ||||
| Mean ± SD | 298 ± 210 | 158 ± 164 | 251 ± 346 | 238 ± 190 |
| Median (IQR) | 276 (183–391) | 76 (33–257) | 182 (15–198) | 210 (77–333) |
| Range | 9–1051 | 5–588 | 9–849 | 0–1417 |
| Hepatitis B antigen positive | ||||
| Positive | 4 (9) | 1 (6) | 0 (0) | 22 (3) |
| Negative | 43 (91) | 15 (94) | 5 (100) | 609 (96) |
| Indeterminate | 0 (0) | 0 (0) | 0 (0) | 2 (0) |
| Hepatitis C antibody positive | ||||
| Positive | 6 (13) | 1 (6) | 0 (0) | 67 (11) |
| Negative | 41 (87) | 15 (94) | 5 (100) | 566 (89) |
| Indeterminate | 0 (0) | 0 (0) | 0 (0) | 1 (0) |
| History of psychiatric disease | ||||
| No | 24 (51) | 15 (83) | 4 (80) | 571 (84) |
| Yes | 23 (49) | 3 (17) | 1 (20) | 112 (16) |
| Treatment arm | ||||
| 2 NRTI plus EFV | 22 (47) | 8 (44) | 1 (20) | 349 (51) |
| 3 NRTI plus EFV | 25 (53) | 10 (56) | 4 (80) | 334 (49) |
NOTE. Data are no. (%) of participants, unless otherwise indicated. CNS, central nervous system; IQR, interquartile range; SD, standard deviation; NRTI, nucleoside reverse-transcriptase inhibitor.
Subject decision and lost to follow-up.
Baseline CD4+ T cell count and HIV RNA level were calculated as the mean (geometric mean) of the last 2 measurements obtained within 30 days after and not after the start of study medication.
The median CD4+ T cell count and HIV RNA level at the time of substitution were 323 cells/mm3 and 279 copies/mL, respectively. The only statistically significant predictor of substitution of NVP was a history of psychiatric disorder. Most participants experienced resolution of CNS symptoms after switching to NVP (46 of 47 patients); 5 reported new CNS symptoms, but none discontinued NVP because of CNS AEs. Fifteen participants discontinued NVP therapy within 32 weeks after substitution because of AEs (n = 8; skin symptoms [n = 4], hepatotoxicity [n = 3], and other [n = 1]); virologic failure (n = 3), and participant decision or loss to follow-up (n = 4). CD4+ T cell counts at time of substitution were 28, 316, 316, and 549 cells/mm3 in participants who discontinued NVP therapy because of skin symptoms and 72, 174 and 1003 cells/mm3 in participants who discontinued NVP therapy because of hepatotoxicity.
Three participants had persistent skin symptoms after substitution. Of fifteen participants (83%) who experienced resolution of skin symptoms, 5 experienced a recurrence while receiving NVP and 4 (1 woman) subsequently discontinued NVP therapy because of skin symptoms. CD4+ T cell counts at time of substitution were similar in the 4 participants who discontinued NVP therapy and in those who did not. Participants who switched because of skin symptoms discontinued EFV therapy earlier than did those who switched because of CNS AEs or other reasons. EFV-related fatigue resolved in all 3 participants.
The prevalence of grade 3/4 hepatotoxicity was 14% (10 of 70 participants) in participants who switched to NVP (incidence, 2.2 cases per 1000 patient-years; 95% CI, 1.5–3.1 cases per 1000 patient-years). The mean prevalence of grade 3/4 hepatotoxicity in bootstrapped nonsubstituting cohorts was 6% (incidence, 0.8 cases per 1000 patient-years; 95% CI, 0.06–1.0 cases per 1000 patient-years). The only grade 3/4 hepatic AEs in participants who substituted NVP occurred in men. Rates of grade 2–4 AEs after substitution were similar among participants with an HIV RNA level <200 copies/mL and among those with and HIV RNA level >200 copies/mL at time of substitution.
Of 70 NVP-substituting participants, 67 had their HIV RNA level measured 16–32 weeks after substitution. In the intent-to-treat analysis ignoring treatment status, 45 (67%) of 67 participants had suppressed viral load (HIV RNA level <50 copies/mL) 24 weeks after substitution. With missing and off-treatment evaluations considered as treatment failure (HIV RNA >50 copies/ml), 41 (59%) of 70 participants had a suppressed viral load. In the as-treated analysis (participants receiving treatment at the time of the 24-week postsubstitution evaluation), 41 (76%) of 54 participants had a suppressed viral load.
A total of 384 participants experienced a non–life-threatening CNS or rash AE while receiving EFV therapy; 239 of these patients were eligible for the switching strategy analysis (25 [10%] were classified in the NVP strategy group) (Figure 1). Baseline characteristics were well matched. Compared with the EFV strategy group, the NVP strategy group had a significantly greater hazard of strategy discontinuation (hazard ratio [HR], 2.2; 95% CI, 1.3–3.9; P = .005), a higher hazard of virologic failure (HR, 2.3; 95% CI, 1.2–4.4; P = .014), and greater risk of strategy failure (HR, 2.1; 95% CI, 1.2–3.6; P=.01). However, after adjusting for patient characteristics at ACTG A5095 baseline and time of the initial targeted AE, all of the associations were attenuated and were no longer statistically significant (strategy discontinuation: HR, 1.6 [95% CI, 0.9–2.9] P =.15; virologic failure: HR, 1.4 [95% CI, 0.6–3.0] P =.46; strategy failure: HR, 1.5 [95% CI, 0.8–2.7] P =.24). Key confounding factors associated with the shift in the estimated effect size were whether the subject temporarily discontinued anti-retroviral therapy within 4 weeks after the targeted AE and the grade of the targeted AE.
Figure 1.
Toxicity management strategy. CNS, central nervous system; EFV, efavirenz; NVP, nevirapine; SS, skin symptoms. *EFV strategy included participants who continued to receive EFV for 30 days after the initial targeted toxicity; participants may have made a subsequent switch to NVP after 30 days. **NVP strategy included participants who switched to NVP within 30 days after the initial targeted toxicity. ***Temporarily discontinued antiretroviral therapy within 4 weeks after targeted adverse event.
Discussion
Substitution of NVP because of EFV-related AEs was generally safe and maintained HIV suppression in most participants at 24 weeks after therapy substitution. Most neuropsychiatric symptoms resolved soon after the substitution. We did not observe a higher rate of AEs among women who substituted NVP for EFV.
The switch from EFV to NVP because of skin symptoms was generally safe and usually resulted in resolution. One-third of the participants who switched from EFV because of skin symptoms had mild-to-moderate skin hypersensitivity reactions after initiation of NVP therapy, with only 4 therapy discontinuations. We observed a higher rate of hepatotoxicity in the switch cohort than among randomly matched participants in ACTG A5095 who did not switch (14% vs 6%). However, only 3 participants discontinued NVP because of hepatotoxicity. AE rates were not different when stratified for HIV RNA suppression (<200 copies/mL at the time of substitution). There was not a correlation between CD4+ T cell count and hepatotoxicity at the time of substitution (data not shown).
This post-hoc analysis of prospective data was not powered specifically to examine the efficacy of NVP substitution. The case-control strategy to evaluate substituting versus not substituting was subject to substantial bias, as demonstrated by the loss of significant findings after correcting for confounders in the controlled comparison for the strategy of switching. Almost all participants who substituted NVP for EFV because of treatment-limiting toxicity experienced resolution of EFV-associated CNS symptoms, and most had resolution of EFV-associated skin symptoms, although liver levels need to be monitored. These results support the strategy of substituting NVP for EFV because of treatment-limiting toxicity.
Acknowledgments
We thank the many people who volunteered for this study and all of the AIDS Clinical Trials Group A5095 Protocol Team Members [8].
Financial support. Statistical and Data Management Center of the AIDS Clinical Trials Group (ACTG), National Institute of Allergy and Infectious Diseases (AI38858 and AI068636); National Institutes of Health (AI38858 and AI068636 ACTGCentral Grant, AI27659 to D.R.K., AI 51966 to R.M.G., AI 69419 to Cornell ACTU, UL1-RR024996 to Cornell CTSC); Bristol-Myers Squibb and GlaxoSmithKline supplied study medications and supportive funding for viral load and metabolic assays for A5095; Boehringer-Ingelheim also supplied nevirapine.
Footnotes
Clinical trials registration. NCT00013520.
Presented in part: 48th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy and the 46th Infectious Diseases Society of America Meeting, 28 September 2008, Washington, DC (abstract H-1236).
Potential conflicts of interest. D.R.K. has served as a consultant for and has received speaker’s fees and research support from Boehringer-Ingelheim, Bristol-Myers Squibb, and GlaxoSmithKline. R.M.G. has received research grants (awarded to Cornell University) from Merck and has served as an ad hoc consultant to Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead, GlaxoSmithKline, and Merck. All other authors: no conflicts.
References
- 1.Mehta U, Maartens G. Is it safe to switch between efavirenz and nevirapine in the event of toxicity? Lancet Infect Dis. 2007;7:733–738. doi: 10.1016/S1473-3099(07)70262-1. [DOI] [PubMed] [Google Scholar]
- 2.Ward DJ, Curtin JM. Switch from efavirenz to nevirapine associated with resolution of efavirenz-related neuropsychiatric adverse events and improvement in lipid profiles. AIDS Patient Care STDS. 2006;20(8):542–548. doi: 10.1089/apc.2006.20.542. [DOI] [PubMed] [Google Scholar]
- 3. [Accessed 12 October 2009];FDA public health advisory for nevirapine (Viramune) Last updated 30 April 2009. http://www.fda.gov/Drugs/DrugSafety/PublicHealthAdvisories/ucm051674.htm.
- 4.Ena J, Leach A, Nguyen P. Switching from suppressive protease inhibitor-based regimens to nevirapine-based regimens: a meta-analysis of randomized controlled trials. HIV Medicine. 2008;9:747–756. doi: 10.1111/j.1468-1293.2008.00627.x. [DOI] [PubMed] [Google Scholar]
- 5.Kesselring AM, Wit FW, Sabin CA, et al. Risk factors for treatment-limiting toxicities in patients starting nevirapine-containing antiretroviral therapy. AIDS. 2009;23(13):1689–1699. doi: 10.1097/QAD.0b013e32832d3b54. [DOI] [PubMed] [Google Scholar]
- 6.Mocroft A, Staszewski S, Weber R, et al. Risk of discontinuation of nevirapine due to toxicities in antiretroviral-naive and-experienced HIV-infected patients with high and low CD4+ T-cell counts. Antivir Ther. 2007;12(3):325–333. [PubMed] [Google Scholar]
- 7.Gulick RM, Ribaudo HJ, Shikuma CM, et al. Triple-nucleoside regimens versus efavirenz-containing regimens for the initial treatment of HIV-1 infection. N Engl J Med. 2004;350:1850–1861. doi: 10.1056/NEJMoa031772. [DOI] [PubMed] [Google Scholar]
- 8.Gulick RM, Ribaudo HJ, Shikuma CM, et al. Three- vs four-drug antiretroviral regimens for the initial treatment of HIV-1 infection: a randomized controlled trial. JAMA. 2006;296:769–781. doi: 10.1001/jama.296.7.769. [DOI] [PubMed] [Google Scholar]
- 9.Ribaudo HJ, Kuritzkes DR, Schackman BR, Acosta EP, Shikuma CM, Gulick RM. Design issues in initial HIV-treatment trials: focus on ACTG A5095. Antivir Ther. 2006;11(6):751–760. [PubMed] [Google Scholar]
- 10.Regulatory Compliance Center. [Accessed 14 October 2009];Division of AIDS table for grading severity of adult adverse experiences. 1992 August; http://rcc.tech-res.com/Document/safetyandpharmacovigilance/ToxicityTables_Adult_TRP_v01a.pdf.