Abstract
Bone morphogenetic proteins (BMPs), as well as the BMP-binding molecules Chordin (Chd), Crossveinless-2 (CV2) and Twisted Gastrulation (Tsg), are essential for axial skeletal development in the mouse embryo. We previously reported a strong genetic interaction between CV2 and Tsg and proposed a role for this interaction in the shaping of the BMP morphogenetic field during vertebral development. In the present study we investigated the roles of CV2 and Chd in the formation of the vertebral morphogenetic field. We performed immunostainings for CV2 and Chd protein on wild-type, CV2−/− or Chd−/− mouse embryo sections at the stage of onset of the vertebral phenotypes. By comparing mRNA and protein localizations we found that CV2 does not diffuse away from its place of synthesis, the vertebral body. The most interesting finding of this study was that Chd synthesized in the intervertebral disc accumulates in the vertebral body. This relocalization does not take place in CV2−/− mutants. Instead, Chd was found to accumulate at its site of synthesis in CV2−/− embryos. These results indicate a CV2-dependent flow of Chd protein from the intervertebral disc to the vertebral body. Smad1/5/8 phosphorylation was decreased in CV2−/−vertebral bodies. This impaired BMP signaling may result from the decreased levels of Chd/BMP complexes diffusing from the intervertebral region. The data indicate a role for CV2 and Chd in the establishment of the vertebral morphogenetic field through the long-range relocalization of Chd/BMP complexes. The results may have general implications for the formation of embryonic organ-forming morphogenetic fields.
Keywords: BMP signaling, CV2, Chd, Chdl-1, Chdl-2, long-range signaling, morphogenetic field, vertebral development, Tolloid, Twisted gastrulation
Introduction
Cells do not exist in isolation but rather develop in fields of hundreds or thousands of cells in which interactions with their neighbors determines differentiation, cell division or death. This is illustrated by the primary embryonic field of the Xenopus gastrula, which can be cut in half, so that each half can regenerate and form an identical twin (Spemann, 1938; De Robertis, 2009). This ability to self-regulate indicates that cells within the field must be in communication with each other over considerable distances. The early embryonic field is called the primary field. Later in development, secondary morphogenetic fields, such as eye, lens or the limb fields are formed (Huxley and De Beer, 1934; Eivers et al., 2008). This principle was discovered by Ross Harrison (1918), who transplanted half of the mesodermal forelimb field into the flank of a host salamander embryo and obtained the formation of a complete limb. Understanding how fields of cells communicate over long distances is a crucial question in developmental biology.
Positional information is provided to cells by the action of extracellular molecules designated morphogens by Türing (1952), which can form spatial gradients in tissues. The formation of a gradient and the local concentration and activity of the morphogen is regulated by a plethora of extracellular factors, which have been best studied in the Drosophila, Xenopus and zebrafish dorso-ventral (D-V) BMP primary embryonic field (Little and Mullins, 2006; O’Connor et al., 2006; De Robertis, 2009; Umulis et al., 2009). BMPs constitute a family of morphogens essential during embryogenesis and organogenesis, which are regulated by interactions with secreted BMP-binding molecules such as Chd, Chd-like-1 (Chdl-1), Chd-like-2 (Chdl-2), Noggin, Tsg and CV2 (Zakin and De Robertis, 2010).
It has been recently demonstrated that in the frog gastrula BMPs can flow from the dorsal Spemann’s organizer to the ventral-most regions of the embryo and that, importantly, this flux of BMPs requires Chd (Ben-Zvi et al., 2008; Plouhinec and De Robertis, 2009). Chd is a secreted protein containing four cysteine-rich (CR) domains, which mediate BMP binding and prevent interaction with BMP receptors (BMPRs) (Piccolo et al., 1996; Larrain et al., 2000), which mediate the anti-BMP activity of Chd. Chd itself is regulated by the Tolloid family of extracellular zinc metalloproteinases which can degrade Chd through cleavage at two specific sites (Piccolo et al., 1997). Chdl-1 (Coffinier et al., 2001; Nakayama et al., 2001; Sakuta et al., 2001) and Chdl-2 (Nakayama et al., 2004; Zhang et al., 2007) are structurally and functionally similar to Chd, but contain three CR BMP-binding domains instead of four. Tsg is a secreted protein that binds to BMP as well as to Chd, Chdl-1 and Chdl-2, acting as a BMP agonist or antagonist depending on the presence of Tolloid protease (Oelgeschläger et al., 2000; Larrain et al., 2001; Scott et al., 2001; Nosaka et al., 2003; Little and Mullins, 2004; Nakayama et al., 2004; Petryk et al., 2004; Zakin and De Robertis, 2004). Upon cleavage of Chd by Tolloid, BMPs are released from ternary BMP/Tsg/Chd complexes, allowing previously sequestered BMPs to signal (Piccolo et al., 1997; Larrain et al., 2001).
Another important player in the BMP network is CV2, which was first identified in Drosophila to be required for the formation of wing crossveins (Conley et al., 2000). These structures require high BMP levels (Blair, 2007), suggesting that CV2 functions to enhance BMP signaling. Homologs of CV2 have since been isolated in vertebrates (Coffinier et al., 2002) and both pro and anti-BMP effects have been described (Binnerts et al., 2004; Coles et al., 2004; Ikeya et al., 2006; O’Connor et al., 2006; Zhang et al., 2008). CV2 is a secreted molecule that contains five Chordin-like CR modules that bind BMPs, inhibiting signaling (Zhang et al., 2008). CV2 also displays a carboxy-terminal von Willebrand Factor type D (vWFd) domain containing a heparin binding site which binds to cell surface heparan sulfate proteoglycans, greatly limiting CV2 diffusion (Rentzsch et al., 2006; Serpe et al., 2008). Vertebrate CV2 is not degraded by Tolloid metalloproteinases (Ambrosio et al., 2008). Serpe et al. (2008) proposed a model to explain the dual activities of CV2. CV2 binds to BMPR Thickveins (Tkv), acting as a co-receptor, so that at high concentrations CV2 inhibits BMP signaling while at low concentrations it acts to facilitate BMP signaling (Umulis et al., 2009). CV2 is able to clear BMP4 from the extracellular space by triggering endocytosis (Kelley et al., 2009).
In the fly pupal wing, genetic mosaic studies have shown that CV2 is expressed in the crossvein where it serves to increase signaling by Dpp, a protein homologous to vertebrate BMP2/4, which is expressed in the longitudinal veins. In the intervein regions, pupal wing cells express large amounts of Short gastrulation (Sog), the homolog of Chd. Ralston and Blair (2005) have proposed that a signaling system based on extracellular flow of BMP, analogous to that of the early Drosophila embryo, may exist during pupal wing patterning. Other mutations that cause crossveinless phenotypes identical to CV2 include Tolloid-related metalloproteinase (Tlr, also known as Tolkin) (Ralston and Blair, 2005) and the Drosophila Twisted gastrulation 2 (Tsg-2) protein (Shimmi et al., 2005a; Vilmos et al., 2005), which was the first crossveinless mutation discovered (Bridges, 1920), and is called cv or cv-1. The hypothesis is that complexes of Dpp/dTsg-2/Sog could become concentrated in the crossvein region, in which active Dpp made in distant vein regions would be released by cleavage by Tlr (Blair, 2007).
We have proposed a molecular mechanism for the pro-BMP effects of CV2 in which this protein acts as a sink for the flow of Chd/Tsg/BMP complexes towards the ventral side of the Xenopus gastrula (Ambrosio et al., 2008). CV2 is expressed in the ventral side, where BMP signaling is highest. Biochemical studies showed that CV2 binds with high affinity (dissociation constant, KD, of 1 to 2 nM) to Chd, and with even higher affinity to Chd/BMP complexes or Chd fragments generated after cleavage by Tolloid (Ambrosio et al., 2008). In this model, CV2 concentrates Chd/Tsg/BMP complexes in the ventral side, where BMP can be released for signaling by Tolloid cleavage of Chd to achieve peak signaling levels (De Robertis, 2009, Lee et al., 2009).
Inactivation of CV2 in the mouse is lethal at birth and CV2−/− neonates present skeletal abnormalities, in particular the loss of cartilage and bone in the vertebral bodies (Ikeya et al., 2006; Zakin et al., 2008; Kelley et al., 2009). Chd−/− mice also have reduced vertebral bodies (Bachiller et al., 2003; Supplementary Fig. 1). CV2 mRNA is expressed in prospective vertebral body cartilage, while Tsg, Chd, Chdl-1, Chdl-2, Bmp4 and Tolloid-like1 (Tll-1/BMP1) mRNAs are transcribed in the prospective intervertebral discs (Zakin et al., 2008). An intriguing observation was that in CV2 mutants the C-terminal phosphorylation of Smad1/5/8, a measure of BMP signaling activity, was decreased in the intervertebral disc at a considerable distance from the prospective vertebral body in which CV2 is expressed (Zakin et al., 2008). Since CV2 was expected to have mostly local effects (Serpe et al., 2008), this long-range effect prompted us to investigate this phenomenon further.
In the present study, we analyzed the distribution of CV2 and Chd proteins, and compared them with that of their respective mRNAs. CV2 protein remained localized to the prospective vertebral body, where its mRNA is expressed. Surprisingly, Chd mRNA was transcribed in the prospective intervertebral disc, yet its protein accumulated in the prospective vertebral body. Knowing from biochemical studies that CV2 binds Chd (Ambrosio et al., 2008), we examined the distribution of Chd protein in CV2 mutant mice. We found that CV2 is required for the relocalization of Chd from the prospective intervertebral disc to the prospective vertebral body cartilage. We propose that the flow of growth factors and their antagonists may be a general feature of the organ-forming secondary morphogenetic fields described in the embryological literature.
Materials and methods
CV2 and Chd mutant mice and crosses
The CV2 and Chd mutant strains were previously described (Bachiller et al., 2000; Zakin et al., 2008) and maintained in the B6SJLF1/J hybrid background (Jackson Laboratories). The Chd strain used was the original isolate, which lacks the recently described secondary mutation in Tbx1 (Choi and Klingensmith, 2009) utilized in other studies (Bachiller et al., 2003; Delot et al., 2007). The CV2 and Chd mutant strains are publicly available from Jackson Laboratories through an agreement made possible by the Howard Hughes Medical Institute (http://jaxmice.jax.org; stock numbers are 007552 for Chd and 007554 for CV2). We generated wild-type, CV2−/−, and Chd−/− homozygous embryos by crossing CV2+/− and Chd+/− heterozygous animals. The embryos were collected at 12.5 d.p.c., fixed in 4% paraformaldehyde in PBS, dehydrated, embedded in paraffin, and serially sectioned at either 5 μm or 10 μm.
Genotyping
Genotyping of the CV2 mutant strain was performed by PCR using the following primers: pGN1 (5′ACC CTC TGT GTC CTC CTG TTA A3′), 3′CV2down (5′AGT CTC CTC CTA TGT TTC TTG C3′), CV2wtup (5′GCC AAG CCT GTG AGT CTT TC3′), and CV2wtdown (5′ACT TAC AGG GGT GGA TGC AG3′), as previously described (Zakin et al., 2008). Genotyping of the Chd mutant strain was performed by PCR using the following primers: Neo2 (5′GTT CCA CAT ACA CTT CAT TCT CAG3′), Null Low (5′GGT AGG AGA CAG AGA AGC GTA AAC T3′), Null Up (5′GAG TTA GGA GGT GGA GCT CTA CAC T3′), as previously described (Bachiller et al., 2000).
In situ hybridization and histology
Procedures for in situ hybridization on paraffin sections were as described (http://www.hhmi.ucla.edu/derobertis/), using the following probes: CV2 (Coffinier et al., 2002), Chd (Bachiller et al., 2000) and BMP4 (Winnier et al., 1995) on 10 μm sections. Hematoxylin and eosin (H&E) stainings were as described (Zakin and De Robertis, 2004) and performed on either 5 μm (Figs. 1 and 2) or 10 μm sections (Figs. 3 and 6). For H&E staining of previously immunostained sections, slides were placed obliquely in a Coplin jar containing Xylene to allow the coverslip to fall off. Subsequent procedures are as for standard H&E stainings.
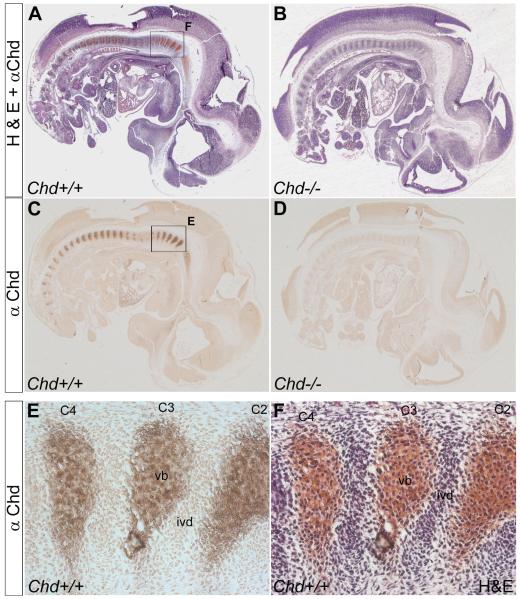
Fig. 1.
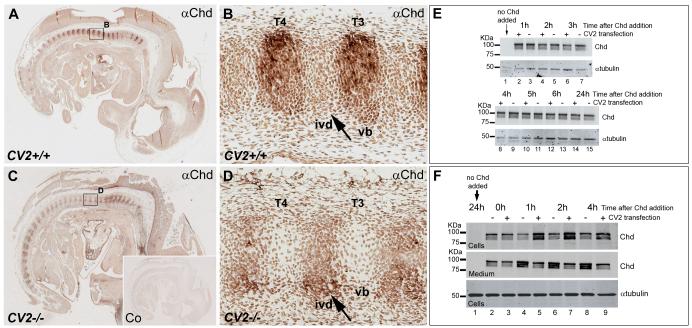
Chd protein concentrates in the prospective vertebral bodies. (A) Sagittal section of a Wild-type 12.5 d.p.c. mouse embryo stained with Chd antibody and then counterstained with H&E. (B) Sagittal section of a 12.5 d.p.c. Chd−/− mouse embryo similarly stained. (C) Chd antibody staining on wild-type embryo, showing Chd protein localized in prospective vertebral body. (D) No signal is detected on Chd−/− mouse embryo section, using Chd antibody thus showing that the Chd antibody is specific (n=2 Chd−/− embryos, with similar results). (E) Magnified view of boxed area in (C) showing Chd staining in vertebral bodies C2 through C4. (F) Same section as in (E) counterstained with H&E. C2-C4, cervical vertebrae; ivd, prospective intervertebral disc; vb, prospective vertebral body.
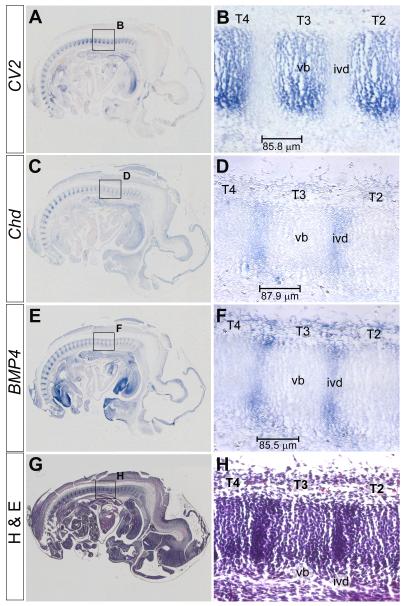
Fig. 2.
CV2 mRNA is transcribed in the prospective vertebral bodies, while Chd and BMP4 mRNAs are expressed in the prospective intervertebral discs. In situ hybridizations and H&E staining were performed on adjacent sagittal sections of the same 12.5 d.p.c. wild-type mouse embryo. (A) CV2 in situ hybridization, showing CV2 mRNA localized in the prospective vertebral bodies. (B) Magnified view of area boxed in (A) showing CV2 staining in thoracic vertebrae (T) 2 through 4. (C) Chd in situ hybridization showing Chd mRNA expressed in prospective intervertebral disc. (D) Magnified view of area boxed in (C) showing Chd mRNA expression. (E) BMP4 in situ hybridization showing BMP4 mRNA expression in prospective intervertebral discs. (F) Magnified view of area boxed in (E) showing BMP4 mRNA staining. (G) H&E staining on a section adjacent to the one shown in (E). (H) Magnified view of area boxed in (G) showing regions of low and high tissue density corresponding to the prospective vertebral body and intervertebral disc, respectively. The bars in B, D and F indicate the distance in μm between the intervertebral disc and the center of the vertebral body.
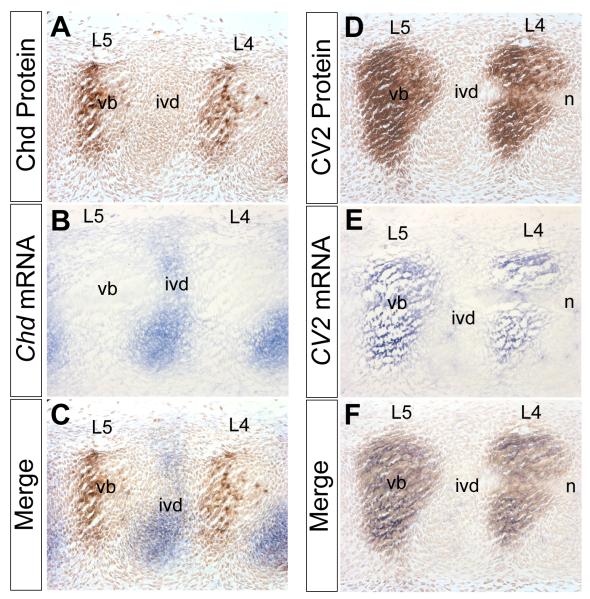
Fig. 3.
Chd mRNA is synthesized in the prospective intervertebral disc while Chd protein accumulates in the prospective vertebral bodies, in which CV2 mRNA and protein are also found. In situ hybridizations and immunostainings were performed on adjacent sagittal sections of a 12.5 d.p.c. wild-type mouse embryo. (A) Chd antibody staining showing Chd protein localized to the prospective lumbar (L) vertebral bodies. (B) Chd in situ hybridization in adjoining section showing Chd mRNA expressed in prospective intervertebral discs. (C) Merged image of panels (A) and (B), showing that Chd mRNA and Chd protein are localized in complementary regions. Chd protein accumulates at a considerable distance from where it is synthesized. Note also that Chd accumulates predominantly in the posterior half of the vertebral body at this lumbar level. (D) CV2 antibody staining showing CV2 protein localized in the prospective vertebral body. (E) CV2 in situ hybridization showing CV2 mRNA localized in the prospective vertebral body. (F) Merged images showing that CV2 mRNA and CV2 protein co-localize. The secreted CV2 protein does not diffuse far from its site of synthesis. n, notochord.
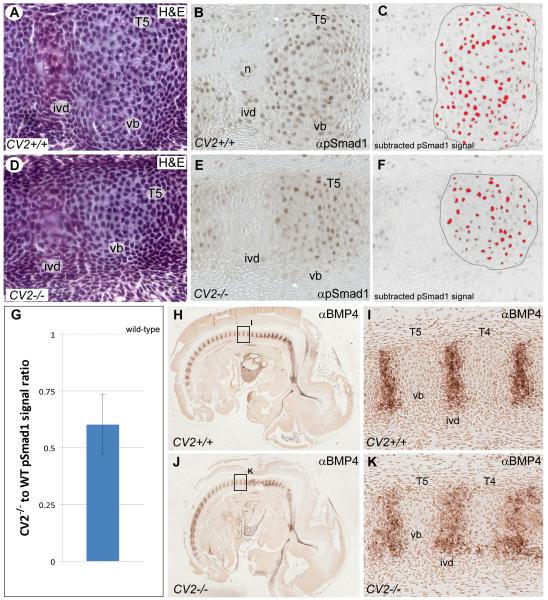
Fig. 6.
Localization and quantification of Smad1/5/8 phosphorylation and BMP4 expression in the vertebral column of wild-type and CV2−/− embryos. H&E staining, α–pSmad1/5/8 and α–BMP4 immunostainings were performed on sagittal sections of 12.5 d.p.c. mouse embryos. (A, D) H&E staining showing a similar cell density in the vertebral bodies (vb) of wild-type and CV2−/− embryos, even though the size of the vertebral bodies appears reduced and the intervertebral disc (ivd) domain is expanded in the mutant. (B, E) pSmad1/5/8 signal is nuclear in the vertebral bodies of wild-type and CV2−/− embryos. (C, F) Quantification of the nuclear pSmad1/5/8 signal. Pixels above background level in the outlined vertebral bodies are shown in red. Quantifications of 6 vertebral bodies indicated that CV2−/− cartilage precursors were less stained than in wild-type. (G) Quantification showing that the average ratio of pSmad1/5/8 signal between CV2−/− and wild-type was 60%. The error bar corresponds to the standard deviation of the ratio of pSmad1/5/8 signal between different individual vertebral bodies. The wild-type value is 1 as indicated. (H, J) BMP4 antibody staining on wild-type and CV2−/− embryos showing the presence of BMP4 protein in prospective intervertebral discs (ivd). (I, K) Higher power view of thoracic vertebrae 4 (T4) and 5 (T5) from sections shown in (H) and (J), respectively, showing a broadening of the BMP4 expression domain in the mutant reflecting the widening of the intervertebral disc region.
Immunohistochemistry
Commercial anti-CV2 (R&D AF2299), anti-Chd (R&D AF758) and anti-BMP4 (Chemicon Millipore MAB1049) antibodies were used at a 1:50 dilution and anti-pSmad1 (Cell Signaling 9511L) was used at 1:100 dilution. For antigen unmasking, slides were placed in a coplin jar containing boiling (i.e., previously brought to a boil in a microwave oven) 10 mM sodium citrate, pH 6 for a total of 40 minutes at room temperature. For Chd antibody staining, a reducing step was included to improve protein denaturation (sections are reduced with 4 mM DTT for 1 hour at room temperature, followed by 500 mM iodoacetate for 30 minutes at 37°C to block sulfhydryl groups). After incubation with biotinylated secondary antibody, two amplification steps were added to improve detection threshold using the ABC reagent (Vector Labs PK4001) and the TSA biotin system (Perkin Elmer NEL700A). A detailed protocol of this improved immunostaining procedure is available at http://www.hhmi.ucla.edu/derobertis.
Western blot analyses
Cos7 cells were transfected with 2 μg per 6-well plate of full-length mouse CV2 cDNA cloned into pCS2, or pCS2 empty vector using Fugene HD (Roche). Samples were solubilized in RIPA lysis buffer (50 mM Tris pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton X100, 0.5% Na Deoxycholate, 0.1% SDS) containing protease inhibitors (Complete tablets, Roche) or directly in electrophoresis loading buffer. Recombinant mouse Chd (R&D systems #758-CN-050) was diluted to 5 nM in serum-free medium. Anti-αTubulin (1:1000, Calbiochem #CP06), anti-mChd (1:250, R&D systems #AF758) and secondary antibodies anti-mouse IRDye 680CW (1:1000 Licor) and anti-goat IRDye 800CW (1:1000, Licor) were used for Western blot analyses, and signals detected using a Licor Odyssey infrared imager.
Quantification of pSmad1/5/8 signal in vertebral bodies
Images of immunostained sections were acquired using a Zeiss microscope and Axiovision software under identical exposure conditions. The brown pSmad1/5/8 signal was extracted using a deconvolution algorithm (Ruifrok et al., 2001;http://www.dentistry.bham.ac.uk/landinig/software/cdeconv/cdeconv.html) with the ImageJ software (http://rsbweb.nih.gov/ij/). For image histograms, background levels from sections immunostained with secondary antibody only were subtracted, and the region corresponding to the vertebral body was identified on an adjacent section stained with H&E. Pixels inside this area were selected as corresponding to pSmad1 signal when their values exceeded background levels. The normalized amount of pSmad1 signal was considered to be the number of these pixels divided by the number of nuclei in the selected region as counted on H&E sections. Calculations were performed on three vertebrae per histological section in two independent experiments. The result is presented as the ratio of nuclear pSmad1/5/8 in CV2−/− compared to wild-type.
Results
CV2 and Chd proteins are localized in the prospective vertebral bodies
Because both CV2 and Chd mutants had phenotypes in the axial skeleton, we decided to study the localization of CV2 and Chd proteins on sagittal paraffin sections of 12.5 d.p.c. wild-type embryos. At this stage, vertebral maturation is easily observed in a cranio-caudal sequence, and is the stage at which mutant phenotypes are first observed. Antibody specificity was assessed through staining of CV2 and Chd homozygous mutant embryo sections with anti-CV2 and anti-Chd antibodies, respectively. As expected, the anti-Chd (Figs. 1A-D) and anti-CV2 (Supplementary Fig. 2) antibodies did not show any cross-reaction on their respective mutants. Sections were counterstained with H&E to reveal morphology; the prospective vertebral body can be recognized by its lower cellular density compared to the prospective intervertebral disc.
Immunostaining with anti-CV2 antibody on wild-type embryo sections showed that CV2 protein is localized to the prospective vertebral body (Supplementary Fig. 2). To our surprise, anti-Chd antibody staining on wild-type embryo sections was also mainly localized in the prospective vertebral body (Figs. 1E, F).
The localization of CV2 protein to the prospective vertebral body is consistent with work in Drosophila (Serpe et al., 2008) showing that CV2 does not diffuse further than two cell diameters, as it is tethered to the cell membrane by the glypican Dally. In the mouse, it has been shown that BMP signaling is required in the prospective vertebral body cartilage (Yoon et al., 2005; Retting et al., 2009) for vertebral cartilage development. The observation that was unexpected was the detection of Chd protein accumulation in the prospective vertebral body. This is because Chd mRNA had been described to be transcribed in the intervertebral disc (Zakin et al., 2008). This finding was consistent with the idea that Chd protein facilitates the flow of BMPs to distant sites where their signaling is required (Piccolo et al., 1997; Eldar et al., 2002; Shimmi et al., 2005; Wang and Ferguson, 2005; Ben-Zvi et al., 2008), which in the case of the mouse vertebral column would correspond to the prospective vertebral body.
Localization of CV2, Chd, and BMP4 mRNA: a flow of Chd protein towards regions of high CV2 expression?
We first re-examined the localization of CV2, Chd and BMP4 mRNAs by in situ hybridization on adjacent serial paraffin sections of wild-type 12.5 d.p.c. embryos. We found CV2 mRNA was expressed in the prospective vertebral body (Figs. 2A, B), while Chd (Figs. 2C, D) and BMP4 (Figs. 2E, F) mRNAs were transcribed in the prospective intervertebral region of the developing vertebral column. Although the expression of all BMPs was not analyzed here, BMP2 and BMP7 mRNAs are also expressed in the prospective intervertebral region (Lyons et al., 1995, and data not shown). Within the detection levels of in situ hybridization, Chd and BMP4 were not found to be expressed in prospective vertebral bodies. Staining of an adjacent section with H&E (Figs. 2G, H), which better reveals the morphology of the vertebral column with the vertebral segments divided into condensed (intervertebral disc) and uncondensed regions (vertebral body), helped to unambiguously identify the regions where the mRNAs were expressed.
Because the differentiation of the vertebral column is dynamic, it was important to study the proteins compared to their mRNAs within the same embryo. Therefore, we compared the localization of Chd and CV2 proteins to that of their mRNAs in adjoining sections. As before, Chd protein was present in the prospective vertebral body, but we also noted a higher concentration of the protein in the posterior half of each prospective vertebral body (Fig. 3A), particularly in more immature lumbar vertebrae (see also posterior segments in Fig. 1C). In situ hybridization using a Chd probe on the same embryo showed the Chd mRNA localized to the prospective intervertebral disc (Fig. 3B). The merging of the two images clearly showed that Chd protein and Chd mRNA are found mainly in complementary regions (Fig. 3C). This finding is consistent with the proposed flow of Chd protein from the prospective intervertebral region, where it is synthesized, to the future vertebral body, where the Chd protein accumulates (Fig. 3C). Because Chd was found to be more concentrated in the posterior half of the vertebral body, this suggested that Chd may flow towards the vertebral body in a posterior to anterior direction, although this remains to be demonstrated. This preferential accumulation of Chd in the posterior vertebral body is seen mostly at lumbar levels at 12.5 d.p.c., but is not as marked at thoracic levels (Figure 5B below).
Fig. 5.
In the mouse embryo, Chd protein requires CV2 for its accumulation in the prospective vertebral bodies, and in cultured cells CV2 serves as a sink for Chd. Immunostainings with a Chd antibody and H&E staining performed on sagittal sections of 12.5 d.p.c. wild-type and CV2−/− embryos. (A) Chd antibody staining on a wild-type embryo, showing the accumulation of Chd in prospective vertebral bodies (vb). (B) Magnified view of area boxed in (A). (C) Chd antibody staining on CV2−/− embryo, showing that Chd protein accumulation is not detectable in vertebral bodies in the absence of CV2. (D) Magnified views of area boxed in (C). Note that mutation of CV2 prevents Chd accumulation in the prospective vertebral bodies; this result was observed in 4 different CV2 mutant embryos (8 independent experiments). (E) Western blot using an anti-Chd antibody showing that overexpression of a full-length CV2 construct in Cos7 cells does not stabilize added recombinant Chd over the course of 24 hours. Samples containing culture medium and cell extracts were collected at different time points; tubulin was used as a loading control. Note that CV2 does not affect Chd stability. (F) Western blot analysis of the cell fraction (containing cells and extracellular matrix) or culture medium from Cos7 cells minus or plus CV2 overexpression and treated with recombinant mouse Chd. Note that already after 1 hour of addition of recombinant Chd protein, CV2 is able to sequester Chd.
CV2 protein was found in the prospective vertebral body (Fig. 3D). In situ hybridization on an adjacent section of the same embryo indicated co-localization of CV2 mRNA and protein (Fig. 3E). Merged images confirmed that CV2 protein does not diffuse from its site of synthesis (Fig. 3F). Since physical binding between CV2 and Chd had been detected biochemically (Ambrosio et al., 2008), these results prompted the next question: does CV2 play a role in determining the localization of Chd protein in vivo?
CV2 is required for the localization of Chd in the prospective vertebral body
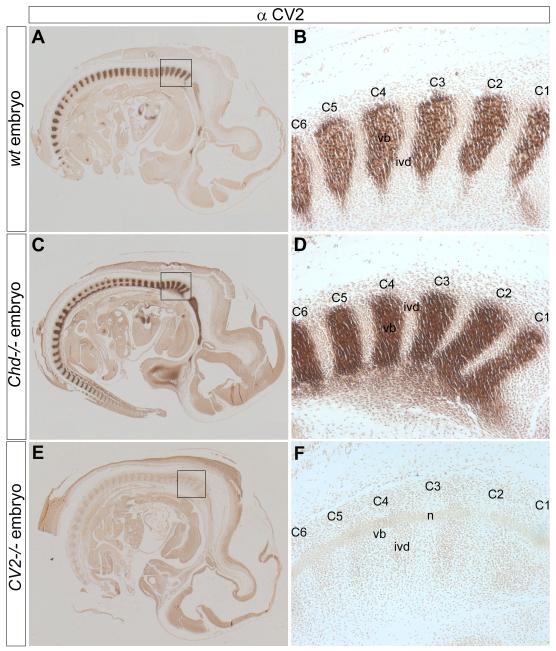
To test whether a possible interaction between CV2 and Chd was responsible for the relocalization of Chd from its site of synthesis in the prospective intervertebral disc towards the prospective vertebral body, we performed immunohistochemistry on paraffin sections of wild-type, CV2−/−, and Chd−/− mouse embryos. CV2 protein was found in the prospective vertebral body of wild-type (Figs. 4A, B) and Chd−/− mutant sections (Figs. 4C, D) but not in control CV2−/− sections (Figs. 4E, F). This control experiment demonstrated that CV2 does not require Chd for its proper localization.
Fig. 4.
The localization of CV2 is independent of Chd. Immunostainings with a CV2 antibody performed on sagittal sections of 12.5 d.p.c. wild-type, Chd−/− and CV2−/− mouse embryos. (A) CV2 antibody staining on a wild-type section, showing the presence of CV2 in the prospective vertebral body. (B) Magnified view of area boxed in (A) showing staining in the prospective vertebral bodies of cervical vertebrae C1 through C6. (C) CV2 antibody staining is normal in Chd−/− embryos. The basioccipital bone is also stained anterior to the vertebral column. (D) Higher-power view of area boxed area in (C) showing staining of CV2 in C1 through C6 similar to that of the wild-type embryo in panel (B). In this Chd−/− animal, CV2 staining was stronger than wild-type (compare panels A and C); however, this difference was not observed in two other mutants analyzed. (E) CV2 antibody staining was undetectable on a CV2−/− section, demonstrating the specificity of the antibody. (F) Magnified view of boxed area in (E).
Chd protein was localized to the prospective vertebral body in wild-type embryos (Figs. 5A, B). When sections of CV2−/− embryos were stained in parallel, no accumulation of Chd was found in the vertebral body region (Figs. 5C, D). Instead, Chd protein was detectable only in the intervertebral disc of CV2 mutants, where it accumulated at higher levels than in the wild-type (compare Fig. 5B to 5D, see arrows). The total levels of Chd accumulated did not reach the levels found in vertebral bodies in the wild-type, presumably because of degradation of Chd by Tolloid metalloproteinases. In situ hybridization experiments showed that Chd mRNA is synthesized in the intervertebral discs, but not in vertebral bodies, of both wild-type and CV2−/− mice (Supplementary Fig. 3). In wild-type embryos, Chd protein accumulates in the extracellular matrix of developing vertebral bodies (compare Fig. 1E and 5B to the histological images in Fig.1F and 6A). Similarly, CV2 protein was found in the extracellular matrix, rather than on the surface of vertebral body cells (Supplementary Figs. 2F, H). We conclude that the accumulation of Chd protein in vertebral bodies has an essential requirement for CV2 protein, presumably due to the binding of Chd to CV2 (Ambrosio et al., 2008).
It could be argued that the accumulation of Chd protein in the prospective vertebral body might be due to low level transcription of Chd in this region (which would have to be below detection levels in the in situ hybridization experiments in Figs. 2D and 3B). However, because Chd protein is no longer detected in CV2−/− vertebral bodies (Fig. 5B, D), implicit in this argument is that the presence of CV2 should have the ability to stabilize low levels of Chd protein. At least in tissue, culture this is not the case. In Fig. 5E, we tested whether CV2 stabilized Chd in Cos7 cells transfected with a full-length CV2 construct (cloned in pCS2) or empty vector. Chd protein was added to the cells (together with fresh culture medium) and samples (containing both the culture medium and the cells) analyzed by western blot at different time points. We found that CV2 overexpression did not affect Chd stability (Fig. 5E). Although the situation in tissue culture may differ from that in the vertebral body (since the translation and proteolysis rates might be different in Cos7 cells), this result does not support the hypothesis that low levels of Chd synthesized locally in the prospective vertebral body are stabilized by CV2. Instead, this experiment is consistent with the model that CV2 acts as a sink for the flux of Chd from the prospective intervertebral disc to the prospective vertebral body, in which it accumulates.
The results favor an enhanced sequestration model for the accumulation of Chd in prospective vertebral body cartilage. To confirm this, an in vitro experiment using Cos7 cells transfected with a full-length CV2 construct was carried out (Fig. 5F). Cells transfected with pCS2 vector or pCS2-CV2 were allowed to secrete CV2 overnight. The next day, the supernatant was replaced by serum-free medium containing commercial mouse Chd protein. The amount of Chd bound by the cell fraction (which included both cells and extracellular matrix), or remaining in the supernatant, was determined after one, two or four hours of incubation. As shown in Figure 5F, more Chd bound to cells in the presence of CV2, becoming partially depleted from the culture medium already after one hour of incubation (compare lanes 4 and 5). These results support the view that CV2 can sequester Chd, at least in cell culture.
Does the lack of CV2 cause a change in BMP signaling? Histological sections of wild-type and CV2 mutants were stained with a phospho-specific Smad1/5/8 antibody, which provides a measure of BMP signaling levels. As shown in Figs 6B and 6E, the size of the vertebral body of CV2 mutants was decreased, although the amount of extracellular matrix and the cellular density was not affected (Figs. 6A, D). Upon close inspection, phospho-Smad1 levels appeared lower in CV2 mutants. To quantify this, we used the imageJ software and a deconvolution algorithm (Ruifrok et al., 2001) to determine the average pSmad1/5/8 signal per cell nucleus in three vertebral bodies, in two different experiments (Fig. 6C, F). Using these methods, we estimate that the average ratio of pSmad1/5/8 signal between CV2−/− and wild-type vertebral bodies was 60%, indicating a 40% decrease in CV2−/− mutants (Fig. 6G). These analyses suggest that loss of CV2 causes a decrease in BMP signaling in prospective cartilage, as it does in the Drosophila wing (Ralston and Blair 2005).
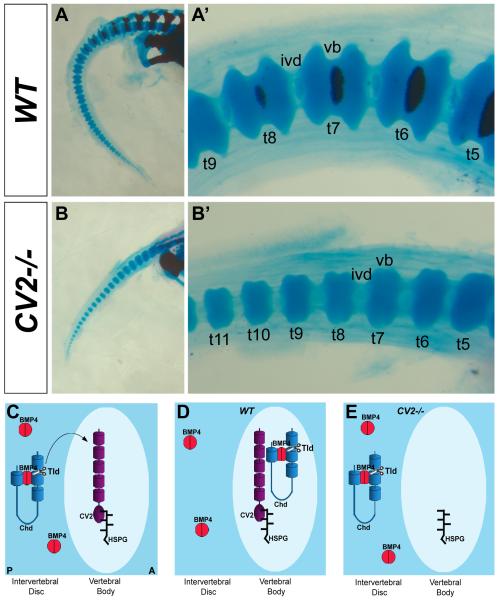
We also examined the localization of BMP4 protein. Immunostaining for BMP4 showed that this growth factor was expressed in CV2 mutants (Figs. 6E-H). BMP4 protein was detected in the intervertebral disc, even though phospho-Smad1/5/8 signaling takes place in the vertebral body. BMP4 could be relocalized to the vertebral body, but at levels below detection limits of histochemistry. Interestingly, BMP4 signaling levels are low in the intervertebral discs at this stage, perhaps because in this tissue BMP4 is coexpressed with its antagonists Chd, Chdl-1 and Chdl-2 (Figs. 6B, D). The region of BMP4 expression is somewhat wider (Fig. 6H), reflecting an expansion of the intervertebral disc domain in CV2−/− embryos (Figs. 6A, C). These results suggest that in the wild-type situation Chd/BMP4 complexes produced in the prospective intervertebral disc are relocalized to the vertebral body, presumably bound to Chd. Similar complexes could also be formed between Chd and BMP2 or Chd and BMP7, since BMP2 and BMP7 also bind Chd and their mRNAs are expressed in the prospective intervertebral region (Lyons et al., 1995). In this model, Chd/BMP inhibitory complexes would accumulate in the vertebral body extracellular matrix, from which complexed BMPs would be released for signaling by Tolloid proteolytic digestion of Chd. In the absence of CV2, Chd/BMP complexes would fail to concentrate in the vertebral bodies, resulting in the lowered BMP signaling. This flow of Chd/BMP complexes in the developing vertebral field could provide an explanation for the defects in vertebral body cartilage formation observed in CV2 mutant mice (Figs. 7A-B’; Zakin et al., 2008).
Fig. 7.
The CV2−/− phenotype in vertebral body cartilage and model of the role of CV2 in the proper localization of Chd in the mouse vertebral field. (A-B’) Skeletal preparations of wild-type (top panels) and CV2−/− (bottom panels) neonates stained with Alcian Blue (cartilage) and Alizarin Red (bone). Tail area is shown at low (left panels) and high (right panels) power magnifications. Note in the vertebral body cartilage of CV2−/− the reduction in size and loss of the spool-like shape compared to the wild-type. We propose that this phenotype is explained in part by the lack of facilitated diffusion of Chd/Tsg/BMP4 complexes from the intervertebral disc to the vertebral body. (C) Model depicting the localization of Chd, CV2 and BMP4 in wild-type and CV2−/− developing vertebrae. In the wild-type CV2 is retained in the vertebral body through its interaction with heparin sulfate proteoglycans (HSPGs), which are very abundant in developing cartilage matrix. (D) Chd/BMP4 complexes diffuse from the intervertebral disc, where they are synthesized, to the vertebral body where they bind to CV2. Once in the vertebral body, Chd is cleaved by a member of the Tolloid metalloproteinase family (Tld) allowing the release of BMP4 in the vertebral bodies so that the high BMP signaling levels required for cartilage development are reached. (E) In CV2−/− vertebrae, Chd/BMP4 complexes do not flow from the intervertebral disc to the vertebral body but instead remain in the intervertebral disc, where they are degraded by the action of Tolloid metalloproteinases on Chd. A, anterior; P, posterior; t5-t11 numbered tail vertebrae.
The results indicate that in the absence of CV2, Chd fails to relocate from its site of synthesis in the prospective intervertebral disc to the prospective vertebral body, helping explain the decrease in BMP signaling through a reduction in the accumulation of BMP4/Chd complexes. The accumulation of Chd protein at a distance requires the presence of CV2.
Discussion
In this study, we showed that both the CV2 and Chd proteins accumulate in the prospective vertebral body. In situ hybridizations revealed that the CV2 mRNA is expressed in this region, while BMP4 and Chd mRNAs are expressed in the prospective intervertebral disc. These results suggest that there is a flow of Chd from its site of synthesis in the intervertebral disc to the vertebral body, where the Chd protein accumulates (Figs 7C, D).
CV2 is required for the accumulation of Chd protein at a distance from its site of synthesis
CV2 protein is localized in the region where it is synthesized, the prospective vertebral body (Figs. 3D-F and Fig. 7C). This is in agreement with results in Drosophila in which, although CV2 is a secreted protein, it has been observed not to diffuse more than two cell diameters from its site of synthesis, remaining bound to the cell surface via its interaction with the glypican surface protein Dally (Serpe et al., 2008). In the mouse, CV2 was shown to be associated with the extracellular matrix of cap condensates and collecting ducts of the developing kidney (Ikeya et al., 2010). In zebrafish, it has also been shown that CV2 has a heparan sulfate proteoglycan binding site within its vWFd domain that limits diffusion (Rentzsch et al., 2006). This is particularly relevant in our case because the extracellular matrix of developing cartilage tissue, in which CV2 protein accumulates, contains large amounts of heparan sulfate proteoglycans.
The most remarkable finding reported in this paper is that Chd protein accumulates at a considerable distance from its site of synthesis. Indeed, at 12.5 d.p.c., the embryonic stage chosen for this study, the distance between the center of the intervertebral disc and the center of the vertebral body measured between 85.5 μm and 87.9 μm (Fig. 2). This suggests that a flow of Chd over considerable distances and many cell diameters takes place in vivo (Fig. 3). The molecular mechanism of this flow was not investigated here.
Because Chd binds to BMPs via its CR domains (Larrain et al., 2000), and the prospective intervertebral region co-expresses Chd, BMP4 and Tsg mRNAs (Fig. 2 and Zakin et al., 2008), one expects that the flow will be comprised not only of Chd but rather of the ternary complex formed by Chd/Tsg/BMP. The cartilage of the vertebral body, is the tissue in the vertebral column that requires the highest levels of BMP signaling in order to develop (Yoon et al., 2005; Retting et al., 2009). It remains to be determined which BMP member is responsible for BMP signaling in the vertebral body. We investigated the localization of BMP4 protein directly, and found that it is detected mostly in the intervertebral disc. Presumably the levels of BMP4 ligand required for BMP signaling are too low to be detectable by immunohistochemistry in the vertebral body. However, nuclear phospho-Smad1/5/8 signaling was detected mainly in the vertebral body at this stage, rather than the intervertebral disc where BMP4 was located. Presumably BMP4 was prevented from signaling because it was complexed to antagonists such as Chd, Chdl-1 and Chdl-2 (Zakin et al., 2008). In the absence of CV2, BMP4 was still expressed, but Smad1/5/8 signaling levels were reduced in the vertebral body (Fig. 6).
We propose that in vivo BMP signaling is enhanced by the flux of Chd/Tsg/BMP4 complexes from intervertebral regions into the developing vertebral cartilage where BMP4 would be released from Chd by the action of Tolloid enzymes (Figs. 7C, D). In order for this mechanism to work, at 12.5 d.p.c., preferential cleavage of Chd/BMP complexes should take place within the vertebral body, where the phospho-Smad1/5/8 signal is detected. Of the three Tolloid enzymes in the mouse, only Tll-1/BMP1, which is expressed in the intervertebral disc (Zakin et al., 2008), has a known expression pattern. The enzyme responsible for cleaving Chd in the future vertebral body might be one of the other two Tolloids, while Tll-1/BMP1 might be involved in the BMP signaling that takes place in the intervertebral body at earlier stages of development (Zakin et al., 2008). Alternatively, Tll-1/BMP1, a secreted protein, may diffuse together with Chd perhaps in an inactive state. Tolloids provide the rate limiting step in the Chd pathway, and their activity can be regulated by feedback inhibitors (Lee et al., 2009).
CV2 is required for the concentration of Chd in the vertebral body (Figs. 7D, E). Previously, we had determined biochemically that mouse CV2 can bind Chd and Chd/BMP4 complexes in vitro (Ambrosio et al., 2008). CV2 is required to trap Chd and Chd/Tsg/BMP4 complexes flowing towards the vertebral bodies in mouse embryos. As shown in Figure 5, in the absence of CV2, Chd and Chd complexes are no longer detected in the vertebral bodies, and remain at their site of synthesis These results show that CV2 is required for the localization of Chd protein in vivo during vertebral morphogenesis.
Flow of growth factor during patterning
During Drosophila D-V patterning, the diffusion of BMPs from lateral regions towards the dorsal amnioserosa in order to achieve peak BMP signaling has been very well documented. The flow of Sog carrying Dpp towards the dorsal midline was initially proposed by Holley and co-workers (1996). Since then, the flux of Sog/Dpp in Drosophila embryos has been directly demonstrated. Sog diffuses dorsally from the lateral neuroectoderm in which it is produced during gastrulation (Srinivasan et al., 2002). Later, it was directly demonstrated that Dpp itself can diffuse, in a Sog and Tsg dependent way, from ventral regions towards the dorsal side (Wang and Ferguson, 2005; Shimmi et al., 2005b). Mathematical modeling has shown that this flow of Sog/BMP makes the dorsal-ventral morphogen gradient much more robust in its response to perturbations (Eldar et al., 2002; Shimmi et al., 2005b).
The concept of morphogens was proposed by English mathematician Alan Türing (1952), who suggested that chemical substances diffusing through a tissue would be able to generate a pattern (Ummulis et al., 2009; Plouhinec and De Robertis, 2007, 2009). The key element is that morphogens must diffuse in vivo. Examples of morphogens include the Transforming Growth Factor-β family members (TGF-β) Activin and Nodal (Gurdon et al., 1994), and ligands of the Epidermal Growth Factor Receptor in Drosophila (Shilo, 2005). The physical flow of endogenous morphogens has been directly demonstrated for the Drosophila Sog/BMP patterning system in early embryos. Even though existing CV2 loss-of-function mutations are so far compatible with normal development in Drosophila, CV2 may play an auxiliary role in the formation of the Drosophila D-V gradient as CV2 is expressed in the dorsal side of the Drosophila early embryo (O’Connor et al., 2006; Serpe et al., 2008).
Flow of growth factors in vertebral column development
We have now demonstrated the accumulation of Chd at a distance from its site of transcription during the formation of a much later structure, the vertebral bodies. Vertebrate segmentation starts with the formation of somites, which then form sclerotomes, which in turn undergo resegmentation, so that the anterior half of one sclerotome fuses to the posterior half of the one preceding it to form a vertebral body (Bagnall et al., 1988). This elaborate developmental mechanism ensures that the tendons and muscles generated by each somite span adjoining vertebrae. The accumulation of Chd at a distance that has been reported here at embryonic day 12.5 takes place after somite resegmentation and is therefore a relatively late occurring event. This flow of Chd presumably has developmental consequences, since both the knockout of CV2 and Chd present vertebral column phenotypes (Bachiller et al., 2000; Zakin et al., 2008). Interestingly, Chd diffusion appears to be directional, as its accumulation is maximal in the posterior half of the vertebral body (Fig. 3C).
In the absence of CV2, Chd does not accumulate in vertebral body cartilage, remaining in the intervertebral disc region where it inhibits BMP signaling. CV2 and Chd mouse mutants do not have significant genetic interactions (Zakin et al., 2008). We proposed that this may be due to the expression in the same prospective intervertebral disc, of Chdl-1 (Nakayama et al., 2001) and Chdl-2 (Nakayama et al., 2004) in addition to Chd. These proteins are biochemically similar to Chd and may compensate for its loss. Furthermore, this redundancy could also explain why the vertebral column phenotypes are different in CV2 and Chd mutants. The defects are stronger in CV2−/− embryos than in Chd−/−embryos; the absence of CV2 may affect the localization of Chd, Chdl-1 and Chdl-2, while only the function of Chordin is affected in Chd mutants. It remains to be demonstrated whether Chdl-1 and Chdl-2 also bind CV2 and relocate to the vertebral body. In addition, Chd, Chdl-1, and Chdl-2 all require Tsg in order to form efficient complexes with BMP (Zhang et al., 2007). This may explain why Tsg−/− is epistatic over the CV2 mutant phenotype (Ikeya et al., 2008 and 2010; Zakin et al., 2008), as the function of all BMP antagonists of the Chd family would be impaired coordinately. In future, it would be interesting to determine the localization of Chd, Chdl-1, Chdl-2 and BMP4 in Tsg−/− and CV2−/−;Tsg−/− mutants. In the meantime, we would predict that the long-range inhibition of BMP signaling in early stage intervertebral discs observed previously in CV2 mutants (Zakin et al., 2008), is caused by the accumulation of all three Chd inhibitors which cannot relocalize to the vertebral body in the absence of CV2.
Morphogenetic fields
Since the discovery of morphogenetic fields by Ross Harrison (1918), understanding how groups of hundreds or thousands of cells communicate with each other to form complex anatomical structures has been a focus of interest for developmental biologists. The molecular mechanism of long-range communication of the primary D-V embryonic field requires the diffusion of BMPs complexed with Chd/Sog and Tsg (reviewed in Umulis et al., 2009; Zakin and De Robertis 2010). Both in Drosophila and Xenopus, this long-range flow has been directly demonstrated in early embryos (Srinivasan et al., 2002; Wang and Ferguson 2005; Shimmi et al., 2005; Ben-Zvi et al., 2008).
During organogenesis stages, many “secondary” cell differentiation fields are formed, but the molecular mechanism of their regulation is unclear. Current thinking is that pattern formation in diverse regenerating systems is mediated through “polar coordinate positional values” that can be explained in terms of strictly local cell-cell interactions (e.g., Bryant et al., 1981). However, in this study we showed that Chordin protein accumulates at a distance from its site of synthesis suggesting that Chordin diffuses extracellularly over considerable distances during the differentiation of vertebral cartilages. This tissue requires high BMP levels to develop, which are presumably relocalized as Chd/Tsg/BMP complexes from the intervertebral disc, where they are synthesized, to the vertebral body, in which they accumulate and are cleaved by Tolloid metalloproteinases. The Chd-binding protein CV2 is required for the accumulation of Chd in the extracellular matrix of developing vertebral body cartilage. Chd emanating from the intervertebral disc does not form a standard gradient with a high point in the site of origin. Instead, Chd antigen levels are highest at a distance, indicating that the concentration of CV2 in cartilage is high enough to act as a sink for Chd complexes. In future, it will be interesting to investigate whether other secondary organ-forming morphogenetic fields also involve the regulated flow of BMP growth factors and their antagonists across long distances.
Supplementary Material
Acknowledgements
The authors would like to thank Jack Greenan for technical assistance, and members of our laboratory for comments on the manuscript. This work was supported by the NIH (HD21502-24). E.M.D.R. is an investigator, and L.Z. a research specialist, of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Appendix A. Supplementary data
Supplementary Figures 1, 2 and 3.
References
- Ambrosio AL, Taelman VF, Lee HX, Metzinger CA, Coffinier C, De Robertis EM. Crossveinless-2 Is a BMP feedback inhibitor that binds Chordin/BMP to regulate Xenopus embryonic patterning. Dev. Cell. 2008;15:248–260. doi: 10.1016/j.devcel.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachiller D, Klingensmith J, Kemp C, Belo JA, Anderson RM, May SR, McMahon JA, McMahon AP, Harland RM, Rossant J, De Robertis EM. The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature. 2000;403:658–661. doi: 10.1038/35001072. [DOI] [PubMed] [Google Scholar]
- Bachiller D, Klingensmith J, Shneyder N, Tran U, Anderson R, Rossant J, De Robertis EM. The role of Chordin/BMP signals in mammalian pharyngeal development and DiGeorge syndrome. Development. 2003;130:3567–3578. doi: 10.1242/dev.00581. [DOI] [PubMed] [Google Scholar]
- Bagnall KM, Higgins SJ, Sanders EJ. The contribution made by a single somite to the vertebral column: experimental evidence in support of resegmentation using the chick-quail chimaera model. Development. 1988;103:69–85. doi: 10.1242/dev.103.1.69. [DOI] [PubMed] [Google Scholar]
- Ben-Zvi D, Shilo BZ, Fainsod A, Barkai N. Scaling of the BMP activation gradient in Xenopus embryos. Nature. 2008;453:1205–1211. doi: 10.1038/nature07059. [DOI] [PubMed] [Google Scholar]
- Binnerts ME, Wen X, Cante-Barrett K, Bright J, Chen HT, Asundi V, Sattari P, Tang T, Boyle B, Funk W, Rupp F. Human Crossveinless-2 is a novel inhibitor of bone morphogenetic proteins. Biochem. Biophys. Res. Commun. 2004;315:272–280. doi: 10.1016/j.bbrc.2004.01.048. [DOI] [PubMed] [Google Scholar]
- Blair SS. Wing vein patterning in Drosophila and the analysis of intercellular signaling. Annu. Rev. Cell Dev. Biol. 2007;23:293–319. doi: 10.1146/annurev.cellbio.23.090506.123606. [DOI] [PubMed] [Google Scholar]
- Bridges C. The mutant crossveinless in Drosophila Melanogaster. PNAS. 1920;6:660–663. doi: 10.1073/pnas.6.11.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant SV, French V, Bryant PJ. Distal Regeneration and Symmetry. Science. 1981;212:993–1002. doi: 10.1126/science.212.4498.993. [DOI] [PubMed] [Google Scholar]
- Choi M, Klingensmith J. Chordin is a modifier of tbx1 for the craniofacial malformations of 22q11 deletion syndrome phenotypes in mouse. PLoS Genet. 2009;5:e1000395. doi: 10.1371/journal.pgen.1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffinier C, Ketpura N, Tran U, Geissert D, De Robertis EM. Mouse Crossveinless-2 is the vertebrate homolog of a Drosophila extracellular regulator of BMP signaling. Mech. Dev. 2002;119(Suppl 1):S179–S184. doi: 10.1016/s0925-4773(03)00113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffinier C, Tran U, Larrain J, De Robertis EM. Neuralin-1 is a novel Chordin-related molecule expressed in the mouse neural plate. Mech. Dev. 2001;100:119–122. doi: 10.1016/s0925-4773(00)00507-4. [DOI] [PubMed] [Google Scholar]
- Coles E, Christiansen J, Economou A, Bronner-Fraser M, Wilkinson DG. A vertebrate crossveinless 2 homologue modulates BMP activity and neural crest cell migration. Development. 2004;131:5309–5317. doi: 10.1242/dev.01419. [DOI] [PubMed] [Google Scholar]
- Conley CA, Silburn R, Singer MA, Ralston A, Rohwer-Nutter D, Olson DJ, Gelbart W, Blair SS. Crossveinless 2 contains cysteine-rich domains and is required for high levels of BMP-like activity during the formation of the cross veins in Drosophila. Development. 2000;127:3947–3959. doi: 10.1242/dev.127.18.3947. [DOI] [PubMed] [Google Scholar]
- De Robertis EM. Spemann’s organizer and self-regulation in amphibian embryos. Nat. Rev. Mol. Cell Biol. 2006;7:296–302. doi: 10.1038/nrm1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis EM. Spemann’s organizer and the self-regulation of embryonic fields. Mech. Dev. 2009:925–941. doi: 10.1016/j.mod.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delot EC, Shneyder N, Zhang H, Bachiller D. Abnormal venous and arterial patterning in Chordin mutants. Dev. Dyn. 2007;236:2586–2593. doi: 10.1002/dvdy.21287. [DOI] [PubMed] [Google Scholar]
- Eivers E, Fuentealba LC, De Robertis EM. Integrating positional information at the level of Smad1/5/8. Curr. Opin. Genet. Dev. 2008;18:304–310. doi: 10.1016/j.gde.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar A, Dorfman R, Weiss D, Ashe H, Shilo BZ, Barkai N. Robustness of the BMP morphogen gradient in Drosophila embryonic patterning. Nature. 2002;419:304–308. doi: 10.1038/nature01061. [DOI] [PubMed] [Google Scholar]
- Gurdon JB, Harger P, Mitchell A, Lemaire P. Activin signalling and response to a morphogen gradient. Nature. 1994;371:487–492. doi: 10.1038/371487a0. [DOI] [PubMed] [Google Scholar]
- Harrison RG. Experiments on the development of the fore-limb of Amblystoma, a self-differentiating equipotential system. J. Exp. Zool. 1918:413–461. [Google Scholar]
- Holley SA, Neul JL, Attisano L, Wrana JL, Sasai Y, O’Connor MB, De Robertis EM, Ferguson EL. The Xenopus dorsalizing factor noggin ventralizes Drosophila embryos by preventing DPP from activating its receptor. Cell. 1996;86:607–617. doi: 10.1016/s0092-8674(00)80134-8. [DOI] [PubMed] [Google Scholar]
- Huxley JS, De Beer GR. The elements of experimental embryology. Cambridge University Press; Cambridge: 1934. [Google Scholar]
- Ikeya M, Kawada M, Kiyonari H, Sasai N, Nakao K, Furuta Y, Sasai Y. Essential pro-Bmp roles of crossveinless 2 in mouse organogenesis. Development. 2006;133:4463–4473. doi: 10.1242/dev.02647. [DOI] [PubMed] [Google Scholar]
- Ikeya M, Nosaka T, Fukushima K, Kawada M, Furuta Y, Kitamura T, Sasai Y. Twisted gastrulation mutation suppresses skeletal defect phenotypes in Crossveinless 2 mutant mice. Mech. Dev. 2008;125:832–842. doi: 10.1016/j.mod.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Ikeya M, Fukushima K, Kawada M, Onishi S, Furuta Y, Yonemura S, Kitamura T, Nosaka T, Sasai Y. Cv2, functioning as a pro-BMP factor via twisted gastrulation, is required for early development of nephron precursors. Dev. Biol. 2010;337:405–414. doi: 10.1016/j.ydbio.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Kelley R, Ren R, Pi X, Wu Y, Moreno I, Willis M, Moser M, Ross M, Podkowa M, Attisano L, Patterson C. A concentration-dependent endocytic trap and sink mechanism converts Bmper from an activator to an inhibitor of Bmp signaling. J. Cell Biol. 2009;184:597–609. doi: 10.1083/jcb.200808064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrain J, Bachiller D, Lu B, Agius E, Piccolo S, De Robertis EM. BMP-binding modules in chordin: a model for signalling regulation in the extracellular space. Development. 2000;127:821–830. doi: 10.1242/dev.127.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrain J, Oelgeschlager M, Ketpura NI, Reversade B, Zakin L, De Robertis EM. Proteolytic cleavage of Chordin as a switch for the dual activities of Twisted gastrulation in BMP signaling. Development. 2001;128:4439–4447. doi: 10.1242/dev.128.22.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HX, Mendes FA, Plouhinec JL, De Robertis EM. Enzymatic regulation of pattern: BMP4 binds CUB domains of Tolloids and inhibits proteinase activity. Genes Dev. 2009;23:2551–2562. doi: 10.1101/gad.1839309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little SC, Mullins MC. Twisted gastrulation promotes BMP signaling in zebrafish dorsal-ventral axial patterning. Development. 2004;131:5825–5835. doi: 10.1242/dev.01464. [DOI] [PubMed] [Google Scholar]
- Little SC, Mullins MC. Extracellular modulation of BMP activity in patterning the dorsoventral axis. Birth Defects Res. C. Embryo. Today. 2006;78:224–242. doi: 10.1002/bdrc.20079. [DOI] [PubMed] [Google Scholar]
- Lyons KM, Hogan BL, Robertson EJ. Colocalization of BMP 7 and BMP 2 RNAs suggests that these factors cooperatively mediate tissue interactions during murine development. Mech. Dev. 1995;50:71–83. doi: 10.1016/0925-4773(94)00326-i. [DOI] [PubMed] [Google Scholar]
- Nakayama N, Han CE, Scully S, Nishinakamura R, He C, Zeni L, Yamane H, Chang D, Yu D, Yokota T, Wen D. A novel chordin-like protein inhibitor for bone morphogenetic proteins expressed preferentially in mesenchymal cell lineages. Dev. Biol. 2001;232:372–387. doi: 10.1006/dbio.2001.0200. [DOI] [PubMed] [Google Scholar]
- Nakayama N, Han CY, Cam L, Lee JI, Pretorius J, Fisher S, Rosenfeld R, Scully S, Nishinakamura R, Duryea D, Van G, Bolon B, Yokota T, Zhang K. A novel chordin-like BMP inhibitor, CHL2, expressed preferentially in chondrocytes of developing cartilage and osteoarthritic joint cartilage. Development. 2004;131:229–240. doi: 10.1242/dev.00901. [DOI] [PubMed] [Google Scholar]
- Nosaka T, Morita S, Kitamura H, Nakajima H, Shibata F, Morikawa Y, Kataoka Y, Ebihara Y, Kawashima T, Itoh T, Ozaki K, Senba E, Tsuji K, Makishima F, Yoshida N, Kitamura T. Mammalian twisted gastrulation is essential for skeleto-lymphogenesis. Mol. Cell Biol. 2003;23:2969–2980. doi: 10.1128/MCB.23.8.2969-2980.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor MB, Umulis D, Othmer HG, Blair SS. Shaping BMP morphogen gradients in the Drosophila embryo and pupal wing. Development. 2006;133:183–193. doi: 10.1242/dev.02214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelgeschläger M, Larrain J, Geissert D, De Robertis EM. The evolutionarily conserved BMP-binding protein Twisted gastrulation promotes BMP signalling. Nature. 2000;405:757–763. doi: 10.1038/35015500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petryk A, Anderson RM, Jarcho MP, Leaf I, Carlson CS, Klingensmith J, Shawlot W, O’Connor MB. The mammalian twisted gastrulation gene functions in foregut and craniofacial development. Dev. Biol. 2004;267:374–386. doi: 10.1016/j.ydbio.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Piccolo S, Agius E, Lu B, Goodman S, Dale L, De Robertis EM. Cleavage of Chordin by Xolloid metalloprotease suggests a role for proteolytic processing in the regulation of Spemann organizer activity. Cell. 1997;91:407–416. doi: 10.1016/s0092-8674(00)80424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Sasai Y, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4. Cell. 1996;86:589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plouhinec JL, De Robertis EM. Systems biology of embryonic morphogens. Mol. Biosyst. 2007;3:454–457. doi: 10.1039/b701571b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plouhinec JL, De Robertis EM. Systems Biology of the Self-regulating Morphogenetic Gradient of the Xenopus Gastrula. Cold Spring Harbor Perspect Biol. 2009;1:a001701. doi: 10.1101/cshperspect.a001701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston A, Blair SS. Long-range Dpp signaling is regulated to restrict BMP signaling to a crossvein competent zone. Dev. Biol. 2005;280:187–200. doi: 10.1016/j.ydbio.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Rentzsch F, Zhang J, Kramer C, Sebald W, Hammerschmidt M. Crossveinless 2 is an essential positive feedback regulator of Bmp signaling during zebrafish gastrulation. Development. 2006;133:801–811. doi: 10.1242/dev.02250. [DOI] [PubMed] [Google Scholar]
- Retting KN, Song B, Yoon BS, Lyons KM. BMP canonical Smad signaling through Smad1 and Smad5 is required for endochondral bone formation. Development. 2009;136:1093–1104. doi: 10.1242/dev.029926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuta H, Suzuki R, Takahashi H, Kato A, Shintani T, Iemura S, Yamamoto TS, Ueno N, Noda M. Ventroptin: a BMP-4 antagonist expressed in a double-gradient pattern in the retina. Science. 2001;293:111–115. doi: 10.1126/science.1058379. [DOI] [PubMed] [Google Scholar]
- Scott IC, Blitz IL, Pappano WN, Maas SA, Cho KW, Greenspan DS. Homologues of Twisted gastrulation are extracellular cofactors in antagonism of BMP signalling. Nature. 2001;410:475–478. doi: 10.1038/35068572. [DOI] [PubMed] [Google Scholar]
- Serpe M, Umulis D, Ralston A, Chen J, Olson DJ, Avanesov A, Othmer H, O’Connor MB, Blair SS. The BMP-binding protein Crossveinless 2 is a short-range, concentration-dependent, biphasic modulator of BMP signaling in Drosophila. Dev. Cell. 2008;14:940–953. doi: 10.1016/j.devcel.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilo BZ. Regulating the dynamics of EGF receptor signaling in space and time. Development. 2005;132:4017–4027. doi: 10.1242/dev.02006. [DOI] [PubMed] [Google Scholar]
- Shimmi O, Ralston A, Blair SS, O’Connor MB. The crossveinless gene encodes a new member of the Twisted gastrulation family of BMP-binding proteins which, with Short gastrulation, promotes BMP signaling in the crossveins of the Drosophila wing. Dev. Biol. 2005a;282:70–83. doi: 10.1016/j.ydbio.2005.02.029. [DOI] [PubMed] [Google Scholar]
- Shimmi O, Umulis D, Othmer H, O’Connor MB. Facilitated transport of a Dpp/Scw heterodimer by Sog/Tsg leads to robust patterning of the Drosophila blastoderm embryo. Cell. 2005b;120:873–886. doi: 10.1016/j.cell.2005.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spemann H. Embryonic development and induction. Yale University Press; New Haven: 1938. [Google Scholar]
- Srinivasan S, Rashka KE, Bier E. Creation of a Sog morphogen gradient in the Drosophila embryo. Dev. Cell. 2002;2:91–101. doi: 10.1016/s1534-5807(01)00097-1. [DOI] [PubMed] [Google Scholar]
- Türing AM. The Chemical Basis of Morphogenesis. Philo. Trans. Royal Soc. London. 1952;237:37–72. [Google Scholar]
- Umulis D, O’Connor MB, Blair SS. The extracellular regulation of bone morphogenetic protein signaling. Development. 2009;136:3715–3728. doi: 10.1242/dev.031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilmos P, Sousa-Neves R, Lukacsovich T, Marsh JL. crossveinless defines a new family of Twisted-gastrulation-like modulators of bone morphogenetic protein signalling. EMBO Rep. 2005;6:262–267. doi: 10.1038/sj.embor.7400347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YC, Ferguson EL. Spatial bistability of Dpp-receptor interactions during Drosophila dorsal-ventral patterning. Nature. 2005;434:229–234. doi: 10.1038/nature03318. [DOI] [PubMed] [Google Scholar]
- Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- Yoon BS, Ovchinnikov DA, Yoshii I, Mishina Y, Behringer RR, Lyons KM. Bmpr1a and Bmpr1b have overlapping functions and are essential for chondrogenesis in vivo. Proc. Natl Acad. Sci. U S A. 2005;102:5062–5067. doi: 10.1073/pnas.0500031102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakin L, De Robertis EM. Inactivation of mouse Twisted gastrulation reveals its role in promoting Bmp4 activity during forebrain development. Development. 2004;131:413–424. doi: 10.1242/dev.00946. [DOI] [PubMed] [Google Scholar]
- Zakin L, Metzinger CA, Chang EY, Coffinier C, De Robertis EM. Development of the vertebral morphogenetic field in the mouse: interactions between Crossveinless-2 and Twisted Gastrulation. Dev. Biol. 2008;323:6–18. doi: 10.1016/j.ydbio.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakin L, De Robertis EM. Extracellular regulation of BMP signaling. Curr. Biol. 2010;20:R89–R92. doi: 10.1016/j.cub.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JL, Huang Y, Qiu LY, Nickel J, Sebald W. von Willebrand factor type C domain-containing proteins regulate bone morphogenetic protein signaling through different recognition mechanisms. J. Biol. Chem. 2007;282:20002–20014. doi: 10.1074/jbc.M700456200. [DOI] [PubMed] [Google Scholar]
- Zhang JL, Qiu LY, Kotzsch A, Weidauer S, Patterson L, Hammerschmidt M, Sebald W, Mueller TD. Crystal structure analysis reveals how the Chordin family member crossveinless 2 blocks BMP-2 receptor binding. Dev. Cell. 2008;14:739–750. doi: 10.1016/j.devcel.2008.02.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.