Abstract
To form epithelial organs cells must polarize and generate de novo an apical domain and lumen. Epithelial polarization is masterminded by polarity complexes, which are thought to direct downstream events such as polarized membrane traffic, though this interconnection is not well understood. We report that Rab11a regulates apical traffic and lumen formation via the Rab GEF Rabin8, and its target Rab8a. Rab8a/11a act via the exocyst to target Par3 to the apical surface, and control apical Cdc42 activation via the Cdc42 GEF, Tuba. These components assemble at a transient apical membrane initiation site to form the lumen. This Rab11a-directed network directs Cdc42-dependent apical exocytosis during lumen formation, revealing a novel interplay of the machineries of vesicular transport and polarization.
Most internal epithelial organs consist of a monolayer of polarized epithelial cells surrounding a central lumen. Polarization requires the interaction of the signaling complexes and scaffolds that define cortical domains with the polarized membrane sorting machinery1. In yeast, traffic from the trans-Golgi network to the surface is regulated by Ypt32p and Sec4p2, homologs of mammalian Rab11 and Rab8, respectively. These are linked by Sec2p (homolog of mammalian Rabin8), a guanine nucleotide exchange factor (GEF) for Sec4p, which is recruited by Ypt32p. Sec2p and Sec4p in turn interact with the exocyst, which docks vesicles to the surface3.
Definition of cortical domains in metazoa involves a complex of Par3, Par6, atypical PKC (aPKC), and the GTPase Cdc424. This complex is a master regulator of polarity, conventionally depicted upstream of membrane trafficking machinery. How this complex interfaces with membrane transport is poorly understood.
Here we show a molecular mechanism for lumen and apical surface formation, linking Rab8a/11a, exocyst, annexin2, Cdc42 and its GEF Tuba, and the Par3/aPKC complex. This novel pathway shows how the membrane traffic and cortical polarity machineries cooperate to generate the apical surface and lumen.
RESULTS
Apical polarization during lumen formation
Upon plating into 3D culture, individual MDCK cells proliferate and assemble into cyst structures - a polarized spherical monolayer surrounding a central lumen. Lumenogenesis requires the apical membrane determinant gp135/podocalyxin5 (PCX in figures). Initially, MDCK aggregates have podocalyxin at the ECM-contacting surface (Fig. 1a, 12 h; Fig. S1a), before polarity inversion occurs, with β-catenin and Na/K-ATPase at cell-cell junctions and podocalyxin now at the lumen (Fig. 1a, 24–48 h, arrows; Fig. S1d)6, 7. Early lumens occur at a site previously termed the “Pre-Apical Patch” (PAP), where opposing plasma membranes are separated, but the podocalyxin signal is non-resolvable by confocal microscopy7; expansion enables luminal space visualization (Fig. 1b). In contrast, apical proteins syntaxin-3 and GFP-CNT1 (Concentrative Nucleoside Transporter-1) label the entire surface before concentrating at the lumen (Fig. S1b–c, e–f)5.
Figure 1. Characterization of MDCK cyst lumen initiation.
(a–b) Development of polarity in MDCK cysts. Initially the apical marker podocalyxin (podocalyxin/gp135, red) is located at the extracellular matrix-contacting region of early aggregates (12 h after plating), while β-catenin (β-cat, green) is at cell-cell junctions. Polarity inversion then occurs (24 h) to form a preapical patch (PAP), with podocalyxin now at the cyst interior (arrow). The luminal space then opens (48 h). (b) Cartoon of cyst development from a. Black lines, plasma membrane; Red lines, apical surface; Blue, nuclei.
(c–e) Transient localization of polarity and trafficking machinery to an Apical Membrane Initiation Site (AMIS). Sec8 (red) and Par3 (white) relocalize from puncta at the lateral most part of cell-cell contacts (arrowheads) in early aggregates with peripheral GFP-podocalyxin (green) (c), to sites of vesicle coalescence at the developing lumen (AMIS, d, arrow). Sec8 and Par3 then relocalizes to tight junctions (e, arrowheads) in cysts with an open lumen. Occludin initially localizes to the entire cell-cell contact (f), while aPKC localizes to the periphery with podocalyxin in early aggregates (i, arrowheads). Both Occludin and aPKC also converge at the AMIS (f, i), before redistributing to the edges of the lumen as the PAP forms (g, j, arrowheads). Both partially concentrate at the tight junctions in cysts with open lumens (h, k, arrowheads), though aPKC retains some luminal labeling. Blue, nuclei.
Smaller panels in this and subsequent figures are higher magnifications of regions indicated. Bar, 20 μm.
Binding of antibodies to GFP-VSVG-podocalyxin at the periphery of cysts, then incubating further to allow lumenogenesis to occur, revealed that podocalyxin at the PAP (arrowheads) and in vesicles (arrows) is at least partially derived from transcytosed peripheral podocalyxin (Fig. S1g).
Transcytosis of podocalyxin to the surface to establish the PAP represents formation of apical-basal polarization. We thus examined localization of select polarity (Par3/aPKC), trafficking (exocyst complex; Sec8/Sec10/Sec15A), and junctional (occludin) proteins during lumen initiation. Strikingly, although these proteins showed differing localizations before lumen formation, all converged transiently during lumen initiation (Fig. 1c–k; Table 1). When GFP-podocalyxin was peripheral, Par3 and Sec8 colocalized in puncta at the edge of cell-cell contacts (Fig. 1c, arrowheads). When GFP-podocalyxin was internalized and transcytosed some Par3 and Sec8 concentrated at the first detectable site of GFP-podocalyxin delivery to the nascent apical surface (Fig. 1d, arrow). We term this the Apical Membrane Initiation Site (AMIS). Later, Par3 and Sec8 instead enriched at the tight junction (Fig. 1e, arrowheads). We define early apical structures where several tight junction markers have become distinctly localized from podocalyxin as the PAP (see below)7, rather than the earlier AMIS, where tight junction markers are not resolved from podocalyxin by confocal.
Table 1.
Distribution of trafficking and polarity proteins during lumen initiation and expansion.
| Lumen Stage | |||
|---|---|---|---|
| Protein | AMIS | PAP | Open Lumen |
| Occludin | Cell-cell contact | Cell-cell contact | Cell-cell contact/TJ |
| Par3 | AMIS | PAP | TJ |
| aPKC | AMIS/peripheral | PAP | TJ/luminal |
| Podocalyxin | Vesicles/peripheral | PAP | Lumen |
| Rab8a | Vesicles | Subapical vesicles | Subapical vesicles |
| Rab11a | Vesicles | Subapical vesicles | Subapical vesicles |
| Sec8 | AMIS | PAP | TJ |
| Sec10 | Cell-cell contact | PAP | TJ |
| Sec15A | Vesicles | Subapical vesicles | Subapical vesicles |
AMIS, Apical Membrane Initiation Site; PAP, Pre-Apical Patch; TJ, Tight Junction.
Sec10 and occludin, in contrast, initially localized along the entire cell-cell contact in early aggregates with peripheral podocalyxin (Fig. S2a; data not shown). Podocalyxin delivered to the AMIS partially overlapped with Sec10 and occludin (Fig. 1f; Fig. S2b; arrows). Though occludin remained along the entire contact, Sec10 condensed toward the AMIS. As the lumen expanded, Sec10 and occludin enriched at the tight junction (Fig. 1g–h; Fig. S2c–f), though some occludin remained along cell-cell contacts (Fig. 1h).
aPKC follows yet a different pattern, with distinct pools initially localized with peripheral podocalyxin (arrowheads) and the AMIS (Fig. 1i, arrows), before enriching at PAP edges and finally the tight junction and lumen (Fig. 1j–k, arrowheads). Together, these data show the complex movement of trafficking and cortical polarity proteins, converging transiently at the AMIS.
The Rab8 and Rab11 GTPase families direct lumen initiation
We examined potential control of AMIS and lumen formation upon perturbation of select Rab GTPases involved in apical, basolateral, or junctional trafficking (Fig. 2a–f; Fig. S3f–l). In contrast to control cysts with a single lumen, apical podocalyxin and basolateral β-catenin, knockdown or dominant negatives of Rab8 (Rab8a/b) and Rab11 (Rab11a/25, but not Rab11b) family members significantly decreased single lumenogenesis (Fig 2f), instead displaying multi-lumens and accumulating podocalyxin in vesicles (Fig. 2b–e, arrowheads; data not shown) close to the cell surface (marked by β-catenin). For single lumen-perturbing knockdowns, phenotypes were confirmed using additional shRNAs (Fig. S3l), and additional cargos (Fig. S3a–c). Rab10, Rab11b, Rab13, and Rab14 perturbation did not overtly perturb lumenogenesis and were not pursued (Fig. 2f).
Figure 2. The Rab8 and Rab11 GTPase families direct lumen initiation.
Knockdown of select members of the Rab8 and Rab11 GTPase families (b, Rab8a; c, Rab8b; d, Rab11a; e, Rab25) revealed that in contrast to control RNAi cysts (a) with apical podocalyxin (red) and basolateral β-catenin (green), Rab knockdown resulted in the appearance of multi-lumens and vesicular podocalyxin accumulation (arrowheads).
(f) Quantiation of single lumenogenesis at 48 h in cysts with either stable knockdown or overexpression of dominant-negative alleles (DN) of indicated Rab GTPases. Note requirement for Rab8 family (Rab8a, Rab8b), and select Rab11 family (Rab11a, Rab25), members in lumenogenesis. Line represents 0.75-fold single lumenogenesis, normalized to control levels. For all single lumenogenesis quantitation, values represent the average of ≥ three different experiments ± S.D., where *p <0.05, ***p<0.0001. Control, n = 868; Rab8a, n = 302; Rab8b, n = 312; Rab10, n = 336; Rab11a, n = 307; Rab11b, n = 1,528; Rab13, n = 315; Rab14, n = 331; Rab25, n = 310.
(g–h) GFP-Rab11a was present on vesicles below the surface, initially very close to the AMIS marked by Par3 (g, arrow) in early aggregates, then underneath the apical surface, once lumens open and Par3 is at tight junctions (h, arrowheads).
(i–m) Podocalyxin (red) and GFP-Rab11a (green) localization during cyst development. Initially podocalyxin (i, arrows) and a pool of GFP-Rab11a (i, yellow arrowheads) localized to the periphery, then internalized into GFP-Rab11a vesicles (j, k, white arrowheads), transiting to the nascent lumen (k–m, white arrows). The lumen then expands and GFP-Rab11a concentrates underneath the lumen (m, yellow arrowheads). Blue, nuclei.
Smaller panels are higher magnifications of regions indicated. Bar, 20 μm.
The Rab11 family regulates transcytosis8 and lumenogenesis in diverse systems9–11. GFP-Rab11a localized to vesicles underlying the AMIS (marked by Par3; arrow in Fig. 2g), then remained on subapical vesicles once lumens expanded (Fig. 2h; Table 1). Podocalyxin transcytosed to the AMIS via Rab11a vesicles. When podocalyxin was peripheral (arrow), GFP-Rab11a localized to juxtanuclear and peripheral vesicles (Fig. 2i, white and yellow arrowheads, respectively). Upon internalization, podocalyxin localized to GFP-Rab11a vesicles (Fig. 2j, arrowheads), then both were delivered to the cyst interior (Fig. 2k, arrowheads). Here, regions of podocalyxin devoid of GFP-Rab11a began to emerge (Fig. 2k–m), representing podocalyxin surface delivery (arrows). Similarly, IgA transcytosed to the PAP (Fig. S1h). As the lumen expanded, GFP-Rab11a clustered underneath the apical surface (Fig. 2l–m). Notably, overexpression of GFP-Rab11a (WT or activated Q70L) increased single lumenogenesis, while dominant negative GFP-Rab11aS25N attenuated single lumens and accumulated podocalyxin intracellularly (Fig. S4a; data not shown). Thus, Rab11a promotes transcytosis to the AMIS and single lumenogenesis.
Rab8 family GTPases were also required for single lumen formation (Fig. 2b–c, f), and Rab8a localized to transcytosing podocalyxin vesicles and the AMIS7 (Table 1; data not shown). Knockdown of Rab11a caused upregulation of Rab8a, and vice versa (Fig. S3f–g), suggesting compensation or co-operation between Rab11 and Rab8 families. To this end, we knocked down Rab8 and Rab11 family members, alone or in combination (Fig. S4b). Of tested combinations, co-knockdown of Rab8a/b, with or without Rab11a knockdown, most severely reduced single lumenogenesis. This suggests that the Rab8 family may act downstream of Rab11a. Accordingly, Rab8a knockdown blocked GFP-Rab11aQ70L-induced increased single lumenogenesis (Fig. S4c). These data are consistent with hypothesis that the Rab8 family acts, at least in part, downstream of Rab11a during AP transport and lumenogenesis, though the precise interaction between these Rabs may be more complex.
Regulation of Rab8 during lumenogenesis
Rab11 binds the Rab GEF Rabin8 and stimulates its activity towards Rab812. We reasoned that Rab11a may control subapical Rabin8/Rab8 targeting. In control cysts, a small pool of Rabin8, and to a lesser extent Rab8a localized to dispersed puncta, with some clustered subapically (Fig. 3a, arrows). Expression of GFP-Rab11aWT, but not GFP-Rab11aS25N, strongly enhanced recruitment of Rabin8 and Rab8a to Rab11a-positive subapical vesicles (Fig. 3a, arrowheads; see also Fig. 5k for colocalization). Like endogenous Rab8a (Fig. 3a), GFP-Rab8aWT was cytoplasmic and in subapical vesicles, the latter of which was enhanced upon activated GFP-Rab8aQ67L expression (Fig. S4f). Thus, active Rab11a recruits active Rab8a to subapical vesicles, likely through Rabin8.
Figure 3. A Rab11-Rabin8-Rab8 module governs apical transport and single lumenogenesis.
(a) Rab11a recruits Rabin8 and Rab8a to subapical vesicles. Control MDCK, GFP-Rab11a (WT or S25N; green) cysts grown for 48 h were stained for either endogenous Rabin8 or Rab8a (both in red). Note diffuse vesicular labeling, with low-level subapical accumulation of Rabin8 and Rab8a (arrows) in control cysts. Arrowheads indicate strong co-recruitment and clustering of Rabin8 and Rab8a vesicles, co-labelled for GFP-Rab11aWT, to the subapical region, but not with the S25N mutant.
(b) Rabin8 expression occurred as two bands, at the predicted MW of its α and β isoforms. Knockdown of the α isoform with two shRNAs (Rabin8_4, Rabin8_5) revealed reduction of Rabin8α was accompanied by upregulation of the β isoform. GAPDH was used as a loading control.
(c–e) Lumogenesis and apical podocalyxin transport require Rabin8α. Rabin8α knockdown caused the appearance of multi-lumens, the intracellular accumulation of a pool of podocalyxin (c, red, arrows), and a significant decrease in single lumenogenesis (e) in cysts at 48 h. Expression of RNAi-resistant human GFP-Rabin8α WT (green) rescued luminal targeting of podocalyxin and GFP-Rabin8α (d, arrows), and restored single lumenogenesis (e). Two different GEF domain mutants (L196A, F201A) were unable to rescue single lumenogenesis (e) or podocalyxin surface targeting (d), instead coaccumulating with podocalyxin on vesicles (d, F201A, arrowheads) below the cell surface marked by F-actin (blue). Single lumenogenesis quantitation values represent the average of ≥ three different experiments ± S.D., where *p <0.05, **p<0.001. Control, n = 312; Rabin8α KD, n = 340; Rabin8α KD + GFP-hRabin8α WT, n = 316; Rabin8α KD + GFP-hRabin8α L196A, n = 317; Rabin8α KD + GFP-hRabin8α F201A, n = 328.
Smaller panels are higher magnifications of regions indicated. Bar, 20 μm.
Figure 5. Tuba and Cdc42 regulate transport from Rab8a/11a vesicles.
(a–g) Cdc42 associates with Rab11a vesicles. In cysts with an open lumen (a), whilst the majority of GFP-Cdc42 was cytoplasmic, a pool of GFP-Cdc42 (green) localized to Apple-Rab11a-positive (red) subapical vesicles (yellow, see arrowheads). Expression of activated Cdc42 (GFP-Cdc42Q61L; b, green) in cysts with an expanded lumen revealed GFP-Cdc42Q61L localization at cell-cell contacts, and the luminal region marked by podocalyxin (red, arrowheads). In early cysts with peripheral podocalyxin (red) (c), GFP-Cdc42Q61L localized all over the surface. When podocalyxin was internalized into Rab11a vesicles (d, vesicular podocalyxin) and concentrated at the AMIS (e), GFP-Cdc42Q61L now extensively overlapped with these vesicles (arrowheads). As the PAP (f) and lumen (g) formed, podocalyxin and Rab11a no longer overlapped, whilst GFP-Cdc42Q61L maintained some overlap with both (arrows).
(h–n) Tuba and Cdc42 are required for transport from Rab8a/11a vesicles. In contrast to control cysts (h) with apical podocalyxin (red) and either basolateral β-catenin (h–j, green), or subapical GFP-Rab11a and Rab8a (k–m, green and blue, respectively), Cdc42 (i,l) or Tuba (j,m) knockdown resulted in the appearance of multi-lumens and the accumulation of podocalyxin in Rab8a/Rab11a-positive vesicles (l–m, arrowheads). Quantitation of Cdc42 and Tuba knockdown (n; verified by two different shRNAs each) revealed strongly perturbed single lumenogenesis both in MDCK or MDCK stably overexpressing GFP-Rab11a cysts at 48 h. Single lumenogenesis quantitation values represent the average of ≥ three different experiments ± S.D., where **p<0.001, ***p <0.0001. Control, n = 311; Cdc42_2, n = 321; Cdc42_3, n = 307; Tuba_1, n = 319; Tuba_2, n = 319; GFP-Rab11a Control, n = 600; GFP-Rab11a + Cdc42_2, n = 316; GFP-Rab11a + Tuba_1, n = 308.
Blue in a–b, h–j, nuclei (nuc); c–g, Rab11a; k–m, Rab8a.
Smaller panels are higher magnifications of regions indicated. Bar, 20 μm.
MDCK Rabin8 appeared as two bands, corresponding to its α and β isoforms: both possess the Rab11-binding region12 (Fig. 3b; Fig. S5a). Rabin8α knockdown accumulated some podocalyxin in vesicles (Fig. 3b–c, arrowheads), and although only to a modest level, significantly decreased single lumenogenesis (Fig. 3e). This modest effect is likely due to compensatory up-regulation of the Rabin8β isoform observed upon Rabin8α knockdown (Fig. 3b). Dual α/β knockdown caused severe cell death, precluding further analysis (not shown). Expression of RNAi-resistant GFP-hRabin8αWT, which localized to the luminal region (arrows), in endogenous Rabin8α knockdown cysts restored single lumenogenesis and podocalyxin localization (Fig. 3d–e). In contrast Rabin8α GEF domain mutants (Fig. S5a–c) further decreased single lumenogenesis and co-accumulated with podocalyxin on vesicles (Fig. 3d–e, arrowheads) beneath the surface marked by F-actin. Similarly, overexpression of GFP-TBC1D30WT, a GAP specific to the Rab8 family13, but not GAP-deficient GFP-TBC1D30R140A, perturbed single lumenogenesis (Fig. S5d–e). These data suggest that a Rab11a-Rabin8α-Rab8a cascade, experimentally opposed by TBC1D30, is part of a regulatory module governing apical transport and lumenogenesis.
The exocyst and Par3/aPKC complexes regulate apical polarization
Rab8a and Rab11a associate with the Sec15 exocyst subunit14, in turn linking to Sec10 and other subunits as part of a chain tethering vesicles to the basolateral3 and apical membranes15. The exocyst also interacts with the Par3/aPKC complex16, 17. As these factors converge at the AMIS, we examined their requirement in apical traffic and single lumenogenesis.
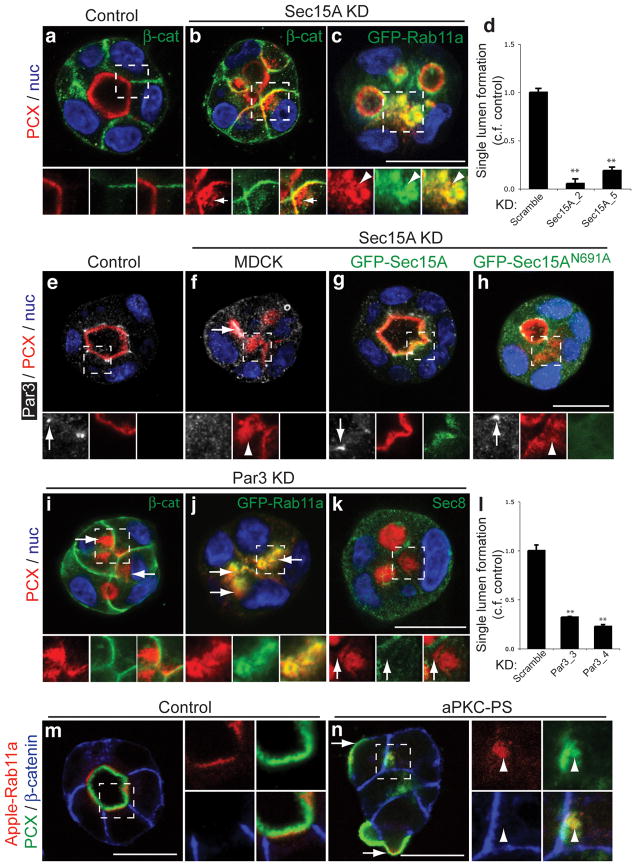
In contrast to control cysts with apical podocalyxin and basolateral β-catenin (Fig. 4a), Sec15A knockdown (Fig. S3m) accumulated podocalyxin in prominent, GFP-Rab11a positive vesicles close to the surface (Figs. 4b, arrows; 4c arrowheads); this Rab11a compartment seemed expanded relative to control cysts (compare Fig. 2m). Additionally, cysts were defective in apical polarization, mistargeting apical cargo to regions of cell-cell contact (Fig. 4b; Fig. S3d). Accordingly, Sec15A knockdown caused almost complete loss of single lumenogenesis (Fig. 4d). Similarly, Sec10 knockdown decreased single lumenogenesis and caused vesicular accumulation of podocalyxin (Fig. S2g–i). Thus, the exocyst regulates podocalyxin transport from Rab11a vesicles to the forming apical surface.
Figure 4. The exocyst and Par3/aPKC regulate lumenogenesis.
(a–d) Sec15A is required for AMIS formation. In contrast to control cysts (a) with apical podocalyxin and basolateral β-catenin, knockdown of Sec15A via two separate shRNAs (Sec15A_2, Sec15A_5) revealed a marked decreased in single lumenogenesis at 48 h (d), and the striking intracellular accumulation of a pool of podocalyxin (b, arrows) in GFP-Rab11a vesicles (c, arrowheads), and mistargeting of podocalyxin to β-catenin-positive membranes (b, Sec15A_2 is depicted). Single lumenogenesis quantitation values represent the average of ≥ three different experiments ± S.D., where **p<0.001. Scramble, n = 334; Sec15A_2, n = 355; Sec15A_5, n = 345.
(e–h) Par3 targeting to the AMIS requires the exocyst. Par3 is targeted to tight junctions in control cysts with open lumens (e), but is mostly lost at regions of podocalyxin vesicle coalescence upon Sec15A knockdown (f, arrowheads), though some Par3 can be recruited to abnormal lumens. Note that expression of RNAi-resistant GFP-Sec15AWT (g), but not a Rab-uncoupled mutant (h, N691A), in cysts with endogenous Sec15A knockdown rescued both single lumenogenesis and surface targeting of podocalyxin and Par3 (arrows).
(i–n) The Par3/aPKC complex is required for lumen initiation. Knockdown of Par3 using two different shRNAs (l, Par3_3, Par3_4) resulted in the accumulation of podocalyxin in vesicles (i, red, arrows), co-labeled for GFP-Rab11a (j, green), beneath the surface (outlined by β-catenin in i, green), and a marked reduction in single lumenogenesis (l). Also upon Par3 knockdown, GFP-podocalyxin (k, pseudo-colored red) is at multi-lumens and intracellular vesicles near the surface (arrows), whereat Sec8 (green) failed to be recruited to form the AMIS. Similarly, inhibition of aPKCs (n, aPKC-PS) resulted in the accumulation of podocalyxin in Apple-Rab11a vesicles (arrowheads), beneath the surface marked by β-catenin. In contrast, control cysts (m) displayed apical podocalyxin, basolateral β-catenin and subapical Apple-Rab11a. In addition, aPKC-PS (n) also blocked internalization of peripheral podocalyxin in some cells (arrows).
Single lumenogenesis quantitation values represent the average of ≥ three different experiments ± S.D., where *p <0.05, **p<0.001, and n ≥ 100 cysts/replicate. Scramble, n = 326; Par3_3, n = 348; Par3_4, n = 369.
Smaller panels are higher magnifications of regions indicated. Bar, 20 μm.
We also examined the role of the exocyst on Par3 transport and AMIS formation. In control cysts, Par3 localized to tight junctions (Fig. 4e, arrows). In Sec15A knockdown cysts, Par3 showed varying, though always abnormal, localization. In regions where a PAP formed, Par3 was recruited to the surface (Fig. 4f). However, in regions of vesicular podocalyxin accumulation, Par3 failed to be recruited to the surface and an AMIS was undetectable (Fig. 4f, arrowhead). Expression of RNAi-resistant GFP-Sec15AWT, which localized to subapical vesicles (Fig. 4g), in cysts with endogenous Sec15A knockdown rescued single lumenogenesis, and surface delivery of podocalyxin and Par3 localization (i.e. at tight junctions once lumens had formed).
To test the role of exocyst coupling to Rabs, we used GFP-Sec15AN691A, a Rab11-uncoupled mutant15. This mutant was completely unable to rescue the trafficking and single lumenogenesis defects caused by knockdown of endogenous Sec15A (Fig. 4h). Thus, coupling of exocyst to Rab8/11 is required for surface targeting of podocalyxin and Par3 to the AMIS.
Similar to exocyst knockdown, Par3 knockdown also resulted in intracellular podocalyxin accumulation close to the surface marked by β-catenin (Fig. 4i, arrows), in vesicles co-labeled for GFP-Rab11a (Fig. 4j, arrows), and a strong disruption of single lumenogenesis (Fig 4l). Par3 knockdown also mistargeted some GFP-CNT1 to cell-cell contacts (Fig. S3e). Moreover, upon Par3 knockdown, Sec8 was not recruited to surface regions adjacent to vesicular podocalyxin (Fig. 4k), representing a failure to form the AMIS.
Inhibition of aPKC, using its pseudosubstrate inhibitor (aPKC-PS; Fig 4m–n) similarly perturbed AMIS and single lumenogenesis, causing accumulation of podocalyxin in Rab11a-positive vesicles close to the surface marked by β-catenin (Fig. 4n, arrowheads). In addition, aPKC inhibition caused lack of podocalyxin internalization from the periphery in some cells (Fig. 4n, arrows), likely representing an additional function of aPKC at this locale (see Fig. 1i). Together, these data demonstrate a crucial role for the exocyst/Par3/aPKC complex in podocalyxin delivery from Rab11a vesicles to form the lumen.
Annexin2-Cdc42 associate with Rab11a vesicles during lumeogenesis
Luminal targeting of aPKC in MDCK cysts requires interaction of GTP-Cdc42 with the PI(4,5)P2-binding protein Annexin2 (Anx2)6. Anx2 both transits to the surface via, and regulates the function of, Rab11a recycling vesicles18, 19. We thus examined interplay between Anx2, Cdc42 and the Rab11a-Rab8a module.
In early cysts with peripheral podocalyxin (arrow), and subperipheral Apple-Rab11a, GFP-Anx2 localized to the surface (Fig. S6a). When podocalyxin was in condensed Rab11a vesicles beneath the AMIS, some GFP-Anx2 now also localized to these vesicles (Fig. S6b, arrowheads). Once podocalyxin was at the open lumen (Fig. S6c, arrow), GFP-Anx2 localized to both apical and basolateral surfaces, but no longer to subapical Rab11a vesicles. Thus, GFP-Anx2 transiently associates with Rab11a vesicles during lumen initiation.
In contrast to controls expressing Anx2 WT with luminal podocalyxin, subapical Apple-Rab11a, and apical and basolateral GFP-Anx2 (Fig. S6d, f), expression of dominant negative Anx2 (Anx2 XM) perturbed lumenogenesis and caused the accumulation of GFP-Anx2 with podocalyxin in Rab11a-positive vesicles (Fig. S6e, arrowheads). Conversely, knockdown of Rab8a or Rab11a caused intracellular accumulation of podocalyxin in structures co-labeled with GFP-Anx2 (Fig. S6g–h, arrowheads). Thus, Anx2 and Rab8a/11a co-operate in the delivery of podocalyxin to the surface.
We next examined whether Cdc42 associated with Rab11a vesicles. Unlike Anx2, GFP-Cdc42, though possessing a large cytoplasmic pool, strongly overlapped with subapical Apple-Rab11a in cysts with open lumens (Fig. 5a, arrowheads). Activated Cdc42 (GFP-Cdc42Q61L) localized to cell-cell contacts and the luminal region, marked by podocalyxin (Fig. 5b; arrowheads). As GFP-Cdc42Q61L removed cytoplasmic background labeling, and expression did not perturb single lumens (Fig. 6i), we used this allele to further examine Cdc42 localization. In early cysts with peripheral podocalyxin (arrows), GFP-Cdc42Q61L localized to the surface (Fig. 5c). When podocalyxin was internalized into Rab11a vesicles and subsequently concentrated at the AMIS, GFP-Cdc42Q61L now extensively overlapped with these vesicles (Fig. 5d–e, arrowheads). As the PAP (Fig. 5f) and open lumen (Fig. 5g) formed, podocalyxin and Rab11a largely no longer overlapped, whilst GFP-Cdc42Q61L maintained some overlap with both (arrows). Thus, active Cdc42 associates with Rab11a vesicles during lumenogenesis.
Figure 6. Rab8a/11a regulate Cdc42 during apical transport.
(a–e) Rab8a-Rab11a control Cdc42 activation and targeting. Global Cdc42-GTP levels upon either Rab knockdown (a) or stable expression of activated Rabs (b) were determined as described in Methods. Values represent the average of three different experiments ± S.D., where *p<0.05. Notably knockdown of Rab8a, but not Rab11a, decreased global GTP levels of Cdc42 (a), while Rab8aQ67L, but not Rab11aQ70L, strongly increased GTP-Cdc42 levels (b). Thus Rab8a modulates global Cdc42 activation. Examination of localization of a PBD-YFP probe to detect activated Cdc42 (and possibly also Rac) revealed that PBD-YFP targeted to surface domains in control cells (c), but particularly the luminal surface (marked by podocalyxin in red; luminal localization denoted by yellow arrowhead). Rab8a knockdown abrogated global PBD-YFP surface labeling (d). Notably, Rab11a depletion blocked luminal, but not lateral, targeting of PBD-YFP (e), suggesting that Rab11a targets luminal Cdc42 via Rab8a.
(f–i) Correction of Rab8a/Rab11a knockdown by active Cdc42 overexpression. In MDCK cysts, Rab8a or Rab11a knockdown decreased single lumenogenesis (i). In contrast, over-expression of active Cdc42 (GFP-Cdc42Q61L), which localized to the plasma membrane and was enriched at the lumen (f), in Rab8a (g) or Rab11a (h) knockdown cysts restored apical targeting of podocalyxin (f–g) and single lumenogenesis to levels resembling control cells (f, i). Blue, nuclei (nuc). Lumenogenesis values represent the average of ≥ three different experiments ± S.D., where *p<0.05. Control, n = 868; Rab8a KD, n = 302; Rab11a KD, n = 307; GFP-Cdc42Q61L Control, n = 343; GFP-Cdc42Q61L + Rab8a KD, n = 319; GFP-Cdc42Q61L + Rab11a KD, n = 308.
Smaller panels are higher magnifications of regions indicated. Bar, 20 μm.
Tuba-Cdc42 function in apical transport from Rab8a/Rab11a vesicles
We next determined whether Cdc42 is required for transport from Rab11a vesicles. As shown previously, Cdc42 knockdown perturbed lumenogenesis (Fig. 5n), causing accumulation of podocalyxin in VACS (arrows) or vesicles (arrowheads) close to the surface marked by β-catenin (Fig. 5i)6. Notably, intracellular podocalyxin observed upon Cdc42 knockdown was localized to Rab8a/11a vesicles, suggesting Cdc42 regulates transport from these vesicles (Fig. 5l, arrowheads).
We20 and Qin et al21 identified Intersectin-2 and Tuba as the only Cdc42-specific GEFs essential for MDCK lumenogenesis. As Intersectin-2 knockdown did not disrupt transport of podocalyxin in cysts20, we examined if Tuba regulates Cdc42-dependent podocalyxin transport. Tuba knockdown phenocopied Cdc42 knockdown, disrupted single lumenogenesis, and accumulated podocalyxin in Rab8a/11a vesicles (Fig. 5j,m–n, arrowheads). Notably, Tuba, and to greater extent Cdc42, knockdown blocked GFP-Rab11a-induced increased single lumenogenesis (Fig. 5n), suggesting that Rab11a operates upstream of both Tuba and Cdc42. Thus, Tuba-dependent Cdc42 activation is required for podocalyxin apical transport.
Tuba is required for Cdc42 apical targeting21. We examined whether Rab8a/11a also influenced Cdc42 activation. Rab8a, but not Rab11a, knockdown strikingly decreased global GTP-Cdc42 levels (Fig. 6a). Similarly, overexpression of GFP-Rab8aQ67L, but not GFP-Rab11aQ70L, robustly activated Cdc42 (Fig. 6b), suggesting that Rab8a influences global Cdc42 activation.
We examined whether Rab8a/Rab11a regulate apical Cdc42 targeting. A PBD-YFP probe of activated Cdc426 labeled the luminal surface, along with podocalyxin (Fig. 6c, arrowheads), and to a lesser extent cell-cell contacts, mirroring activated Cdc42 localization (Fig. 5b). Rab8a knockdown abrogated PBD-YFP membrane association, despite retaining luminal podocalyxin labeling (Fig. 6d, arrowhead). Strikingly, Rab11a knockdown resulted in a loss of apical (arrowhead), but not basolateral, PBD-YFP (arrows) (Fig. 6c). Thus, Rab8a is required for global activation and surface targeting of Cdc42, while Rab11a controls apical Rab8a, and consequently, active Cdc42 targeting.
We reasoned that as Rab8a/11a influenced apical targeting of active Cdc42, overexpression of active Cdc42 may rescue single lumenogenesis upon Rab8a/11a knockdown. Indeed expression of active Cdc42 (GFP-Cdc42Q61L) rescued apical targeting of podocalyxin (Fig. 6g–h) and single lumenogenesis in cysts with Rab8a or Rab11a knockdown (Fig. 6i). These data support the conclusion that Cdc42, regulated by Rab8a/11a, is required for apical transport of podocalyxin. Taken together, Rab11a regulates a molecular network directing the apical polarity and trafficking machineries to initiate de novo lumen formation.
DISCUSSION
How membrane trafficking and polarity complex machineries work together to form the apical surface and lumen is a fundamental issue22. We describe a molecular chain linking membrane trafficking machinery with delivery of the Par3/aPKC/Cdc42 complex to the nascent apical surface, from early cell aggregates, to apical membrane initiation (AMIS), formation of a PAP, then to cysts with open lumens (Fig. 7a). This emphasizes the complex spatiotemporal orchestration needed to construct a new membrane (see Table 1).
Figure 7. A molecular network for de novo lumen generation.
(a) Cartoon diagram of the different stages of lumenogenesis and apical polarization in MDCK cysts. Initially podocalyxin is localized to the periphery of cysts (Early Aggregate), before internalization into Rab8a/11a-positive vesicles, and delivery to the AMIS (Apical Membrane Initiation). As podocalyxin at the apical domain and tight junctions become separately localized, the AMIS progresses to a PAP (Pre-Apical Patch), representing the early stages of apical-basal polarization. Expansion then allows opening of the luminal space (Open Lumen). Note the co-accumulation of polarization and trafficking machinery at the AMIS, despite varying localization during other stages of lumenogenesis. Red lines, podocalyxin; black lines, plasma membrane; grey ovals, nuclei; brown ovals, tight junctions; brown rectangle, AMIS; L, lumen.
(b) A model cartoon diagram of the molecular network involved in delivery of apical vesicles (podocalyxin) to the AMIS during apical membrane initiation.
Podocalyxin is initially at the ECM-contacting periphery (Fig. 7a), before internalization into Rab11a vesicles, to which Rab8a is recruited via the GEF Rabin8 (and opposed by the GAP, TBC1D30) (Fig. 7b). Apical vesicle delivery and lumenogenesis is regulated by both Rab proteins. The exocyst, a Rab effector, docks vesicles with the surface to create the AMIS, in cooperation with Par3/aPKC (Fig. 7b). Anx2 and Cdc42 associate with Rab8a/11a vesicles, regulating apical transport and single lumenogenesis, dependent on the Cdc42 GEF Tuba. Par6 likely bridges Cdc42 to the aPKC/Par3/exocyst complex at the AMIS. Thus, apical polarity and membrane domain identity is initiated de novo by membrane delivery from Rab8a/11a vesicles. Finally, the luminal space is expanded by pumps and channels (Fig. 7k)7, 23.
Apical trafficking and polarization
Podocalyxin is the earliest marker of apical polarization that we have studied, and its initial appearance at the nascent apical surface marks the AMIS. Notably, the Rab11a-Rabin8α-Rab8a cascade also regulates mammalian ciliogenesis, and the homologous yeast pathway regulates budding24, suggesting it is an ancient polarity-generating module.
The exocyst is a Rab effector for transport of podocalyxin from Rab11 endosomes. This transport also required the Par3/aPKC polarity complex, revealing a mutual interdependence of these complexes for localization to the AMIS. Similarly, in developing neurites, the exocyst and Par3/aPKC associate17 with the exocyst required for Par3 localization to growing neurite tips. Interaction of the exocyst with Par3/aPKC may focus exocyst-dependent vesicle docking events transiently to the AMIS, allowing initiation of apical polarity, before both complexes relocalize to the tight junction for subsequent transport events. That the exocyst was also required for Par3 localization demonstrates that vesicle trafficking operates both upstream and downstream of cortical polarity proteins, suggesting a feedback loop.
Rab11 vesicles also deliver the Crumbs3-Pals1-PatJ complex to early lumens25. In Drosophila Crumbs acts to dissociate Par3 from Par6 and aPKC at the apical surface, restricting Par3 to apicolateral borders26, 27. Similarly, Crumbs3 delivered to the AMIS may exclude a pool of Par3 from the nascent apical surface, allowing it to concentrate to the sides of the developing lumens, such as in the transition from AMIS to PAP reported here.
Regulation of Cdc42 during lumen formation
aPKC/Par6 form a complex with Cdc4228. Cdc42, in conjunction with Anx26, regulates vesicular transport to the apical surface from Rab8a/11a vesicles. Cdc42, however, also regulates the orientation of cell division in conjunction with Par6/aPKC20, 21, 29. Indeed, the Cdc42 GEFs Intersectin-2 and Tuba regulate Cdc42 activation and division orientation during MDCK lumenogenesis20, 21. Intersectin-2 knockdown causes multi-lumens without vesicular podocalyxin accumulation20, suggesting it functions in cell division but not vesicle transport. In contrast, Tuba regulates apical targeting of Cdc4221 and, as demonstrated here, podocalyxin transport from Rab8a/11a vesicles.
Active Cdc42 localized to Rab11a vesicles. In yeast, Cdc42 is also delivered to the bud site30, suggesting that vesicular transport of Cdc42 to membrane being generated de novo is a conserved polarity-generating event. Notably, Rab8a/11a were required for Cdc42 activation at the lumen. Rab8a regulated global Cdc42 activation; Rab11a regulated apical Cdc42 targeting. That global Cdc42 activation was greatly decreased upon Rab8a but not Rab11a knockdown, suggests that while Tuba acts downstream of Rab11 and regulates a pool of Cdc42 activation on Rab11a vesicles, Rab8a may influence additional GEF proteins, such as Intersectin-2. How Rab8a/11a influence Cdc42 GEFs remains to be elucidated. Similarly, we demonstrate a role for aPKC in apical transport from Rab11 vesicles, suggesting that the Cdc42-Par6-aPKC complex may function, in addition to in cell division, in membrane transport31, 32. These data reveal a novel role for Anx2-Cdc42-aPKC-Par3 in conjunction with the exocyst and Tuba, in apical transport to the AMIS.
Mechanisms of central lumen formation
Our perturbations yielded multi-lumens and accumulation of apical proteins in subapical vesicles. Perturbation of apical traffic could cause multiple lumen formation via several overlapping mechanisms. Reduced apical delivery may prevent initially small lumens from enlarging and consolidating into one central lumen, which occurs in several mammalian organs33. Multiple small lumens also require ion pumping to hydrostatically enlarge and coalesce lumens. Apical trafficking defects could cause defective junctions and/or mislocalization of pumps and channels, preventing enlargement23.
Orientation of mitosis also regulates lumen formation. In Caco-2 cysts, Cdc42 knockdown correlates with disrupted spindle orientation, without notable apical transport defects29. Caco-2 lumen formation requires artificially increasing cAMP, which strongly promotes apical exocytosis34, potentially masking detection of apical transport defects. As Rab8/11, exocyst, and Par3/aPKC also have roles in spindle orientation and/or cytokinesis35–37, the extent to which their function in cell division is separate from apical trafficking is unknown. Apical membrane traffic might be needed to localize proteins that orient mitosis, such as Cdc42, aPKC and LGN20, 21, 38, 39. Notably, multi-lumens can occur without disruption to apical transport20, 39, suggesting that the two processes can be uncoupled. For example, in MDCK and other systems, lumens can form in the absence of cell division or apoptosis7, 40–43, instead requiring vesicular transport of podocalyxin to the cyst interior. Thus, coordination of division orientation and apical transport mechanisms are likely to be key to generating a single lumen.
Recently, the Par proteins, as well as aPKC and Cdc42, have been demonstrated as regulators of polarity, via endocytosis32. We demonstrate that the membrane traffic, especially exocytosis, is both upstream and downstream of the Par complex. Our data buttress an emerging view of the Par complex as a multifunctional platform modulating membrane traffic31, 32, and suggest Cdc42/aPKC/Par3 as a convergence between the machineries of cortical polarization and vesicular transport.
METHODS
Cyst culture
MDCK cysts were subcultured in 5% FBS (Gibco, Carlsbad, CA) MEM or grown in 3D Matrigel cultures (BD, San Jose, CA), as described6. Cells were trypsinized to a single cell suspension at 1.5 × 104 cells/ml in complete medium containing 2% Matrigel. Suspensions (250 μl) were plated into 8-well coverglass chambers (Nunc, Rochester, NY), pre-coated with 5 μl of 100% Matrigel. Cells were grown for 24–48 h before fixation in 4% PFA. In some experiments, cells were treated with aPKC-PS (40 μM; Invitrogen, Carlsbad, CA) from the time of plating to inhibit aPKCs6. In most instances, exogenous proteins were from stably expressing cell lines. For Anx2 XM experiments, cells in 2D were transiently transfected with Anx2 XM (Lipofectamine 2000, Invitrogen, Carlsbad, CA) 24 h prior to plating into 3D.
RNAi
Stable RNAi was achieved by viral shRNA. For Sec10 knockdown, cells were transfected with siRNA oligonucleotides as described6. In all instances knockdown was verified by standard western blot or Q-RT PCR procedures (Brilliant-II SYBR Green Kit, Agilent, Santa Clara, CA), normalizing to GAPDH expression. Q-RT PCR Primers are presented in Supplementary Table 1. RNAi target sequences are presented in Supplementary Table 2. Rab8a_1, Rab10, Rab11a_1, Rab11b, in pRVH1-puro or –hygro retroviruses were previously published44. All other shRNAs were generated in pLKO.1-puro45, or pLKO.1-blast, which was constructed by exchanging the puromycin resistance gene for blasticidin. Cdc42, Par3 and Tuba shRNAs were adapted for pLKO.1 from published sequences21, 46. pLKO.1 lentiviruses were constructed according to the Addgene pLKO.1 protocol (www.addgene.org) using iRNAi (www.mekentosj.com), and target sequences were based on an (AA)N19 algorithm. RNAi sequences were submitted to BLAST (NCBI) to verify target specificity. For isoform-specific RNAi to Rabin8, shRNAs predicted to target the α isoform (Rabin8_4, Rabin8_5) or to both α and β (Rabin8_2) isoforms of canine Rabin8 were extrapolated from sequence alignment with human Rabin8 splice forms (mined from NCBI). For knockdown and rescue experiments, GFP-tagged plasmids of transcripts from human or rat, which are not targeted by anti-canine shRNAs, were used.
Virus production and transduction
Retrovirus production was performed essentially as previously described 44, except pRVH1 plasmids were transfected into 293-GPG cells (O. Weiner, UCSF)47. 48 h post transfection, viral supernatants were collected daily for 7 d. For lentivirus production, pLKO.1 plasmids were co-transfected with ViraPower packaging mix into 293-FT cells according to manufacturer’s protocols (Invitrogen, Carlsbad, CA). All supernatants were centrifuged to remove cell debris and frozen in liquid nitrogen for further use.
For retrovirus transduction (pRVH1), subconfluent cultures of MDCK cells, 16 h after plating, were incubated with virus-containing supernatants supplemented with 10 μg/ml Polybrene (Millipore, Billerica, MA) for 24 h at 32°C. Upon changing to fresh medium, cells were incubated for a further 24 h at 37°C, before passage into appropriate antibiotic-containing medium. For lentivirus transduction (pLKO.1), subconfluent MDCK cultures, 1–4 h after plating, were infected with virus-containing supernatants for 12–16 h at 37°C. Viral supernatants were then diluted 1:1 with growth medium, cultured for a further 48 h, then passaged into appropriate antibiotic-containing medium. Hygromycin (0.5 mg/ml), puromycin (5 μg/ml), and blasticidin (12.5 μg/ml) were used.
Plasmids
Plasmids kindly provided were: GFP-Cdc42 (Q61L; Addgene, Cambridge, MA), GFP-Rab11a (WT, S22N; E. Brown, UCSF), GFP-Rab8a (WT, Q67L), GFP-Sec15A (J. Stow, U. Queensland, Australia), GFP-VSVG-podocalyxin, pRVH1-puro/hygro (control, Rab8a, Rab10, Rab11a and Rab11b; K. Simons, EMBL), GFP-TBC1D30 (WT, R140A; F. Barr, U. Liverpool). GFP-Cdc42, YFP-PBD, GFP-Annexin2 (WT, XM)6, GFP-Rab13 (T22N; A. Zharaoui, CEA CNRS, France), GFP-Rab14 (S25N; J. Wilson, U. Arizona), GFP-Rabin8α48, Syntaxin3-2xmyc (T Weimbs, UCSD)49, GFP-CNT150 cell lines/plasmids as described. All additional plasmids were constructed through site-directed mutagenesis (Quikchange, Agilent, Santa Clara, CA) or standard subcloning. For generation of stable lines, transfected cells underwent FACS after selection to obtain appropriate expression levels.
Antibodies
Primary mouse antibodies used were: Cdc42 (BD Biosciences, San Jose, CA) [Dilution: Western Blot (WB), 1:1000]; GAPDH (Millipore, Billerica, MA) [Dilution: WB, 1:10,000]; gp135/podocalyxin (G. Ojakian) [Dilution: IF, 1: 1000]; myc (9E10, Santa Cruz Biotechnology, Santa Cruz, CA) [Dilution: IF, 1:200]; p58/Na/K-ATPase (K. Matlin) [Dilution: IF, 1:200]; Tuba (Abnova, Taipei City, Taiwan) [Dilution: WB, 1:500]; VSVG (P5D4) [Dilution: Transcytosis assay, 1:1000] 51. Primary rabbit antibodies used were: aPKCζ (C-20) [Dilution: IF, 1:200]; β-catenin (Santa Cruz Biotechnology, Santa Cruz, CA) [Dilution: IF, 1:200]; GFP (Invitrogen, Carlsbad, CA) [Dilution: Transcytosis assay, 1:1000]; Occludin (Invitrogen, Carlsbad, CA) [Dilution: IF, 1:200]; Par3 (Millipore, Billerica, MA) [Dilution: IF, 1:100; WB, 1:1000]; Rabin8 [Dilution: IF, 1:50; WB, 1:500]; and Rab8a [Dilution: IF, 1:50; WB, 1:500]; 48; Rab8b (ProteinTech, Chicago, IL) [Dilution: WB, 1:500]; Rab10 (Sigma, St. Louis, MO) [Dilution: WB, 1:500]; Rab11a (Millipore, Billerica, MA) [Dilution: IF, 1:100; WB, 1:1000]; Rab25 (Cell Signaling Technology, Danvers, MA) [Dilution: WB, 1:500]; Sec8 (Enzo Life Sciences, Plymouth Meeting, PA) [Dilution: IF, 1:100]; Sec10 [Dilution: IF, 1:250 (fix in MeOH:acetic acid; WB, 1:250] (W. Guo). Rat anti-ZO-1 (R40.76; B. Stevenson) [Dilution: IF, 1:200]; was also used. Alexa Fluorophore-conjugated secondary antibodies [Dilution: IF, 1:250 for all secondary antibodies] or Phalloidin (Invitrogen, Carlsbad, CA) [Dilution: IF, 1:400], and Hoescht to label nuclei [Dilution: 10 μg/ml], were utilized. Cyst were stained essentially as previously described6.
Statistics
Single lumen formation was quantified as described6. The percentage of cysts with a single lumen was determined, and normalized to control cysts as 1.0. Values are mean ± SD from 3 replicate experiments, with n ≥100 cysts/replicate. Significance was calculated using a paired, two-tailed Student’s t-test. *p < 0.05, ** < 0.001, *** < 0.0001.
Transcytosis and IgA uptake
For PCX transcytosis, cells stably expressing GFP-VSVG-podocalyxin were plated into 8-well chamber slides and grown 12 h at 37°C. Slides were placed on ice and washed twice with serum-free medium at 4°C before incubation at 4°C for 30 min with serum-free medium containing indicated antibodies. Cysts were then washed twice with cold serum-free medium, before being grown for 24 h at 37°C in fresh serum-containing medium supplemented with 2% Matrigel.
pIgA, kindly provided by Professor J.P. Vaerman (Catholic University of Louvain, Brussels, Belgium), was biotinylated using sulfo-NHS-LC-biotin (Pierce, Rockford, IL). For IgA uptake, MDCK PTR-9 cells stably expressing hTfR and rabbit pIgR52 were grown for 36 h in 3D to induce cyst formation, then incubated with 100 μg/ml biotinylated IgA in complete medium for 40 min. Biotinylated IgA was detected using fluorescently labeled streptavidin (Invitrogen, Carlsbad, CA).
GTPase activation
GTP loading of Cdc42 was by GST-PAK pull down (Cytoskeleton, Denver, CO), according to manufacturer’s protocol, with modifications. Cells were lysed in Mg Lysis Buffer (Millipore, Billerica, MA) containing 1 mM PMSF and protease inhibitor cocktail, lysates were passed through a 27 gauge needle (BD, Franklin Lakes, NJ), and cleared of debris by centrifugation. A sample of lysate was taken for protein concentration determination (BCA, BioRad, Hercules, CA), and lysates were snap-frozen. 3 μg protein from appropriate condition was incubated with 20 μg of GST-PAK bead for 30 min at 4°C. Beads were collected by centrifugation and washed thrice in buffer before SDS-PAGE. Total and GTP-Cdc42 levels were detected by blotting using a Cdc42 antibody (BD Biosciences, San Jose, CA).
Rabin8 mutagenesis
GEF mutants of Rabin8 were modeled on critical, conserved residues in the Rab GEF domain governing Sec2p-Sec4p interactions53. L196A and F201A mutants were generated from pEGFP-C1-Rabin8α and pGEX-2T-Rabin8α48 (Quikchange, Agilent, Santa Clara, CA). Decreased, direct association of Rab8a with Rabin8 mutants was verified by GST pulldown. Briefly, GST-Rabin8, GST-Rabin8-L196A, GST-Rabin8-F201A protein (15°C overnight; 200 μM IPTG) or GST protein expression (37°C for 3 h) was induced in bacteria. Lysates containing GST fusion proteins were incubated with glutathione-agarosebeads (Sigma, St. Louis, MO) at 4°C for 2 h, then washed thrice during30 min with binding-buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 2 mM MgCl2,1% Triton X-100). Rab8A-T22N and Rab8A-Q67L were translated in vitro using a TNT Quick kit (Promega, Madison, WI) accordingto the manufacturer’s instructions. The in vitro translation productswere incubated with GST proteins coupled to glutathione agarose beads in binding buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 2 mM MgCl2,1% Triton X-100) on a wheel at 4°C for 1 h. The beads were washed four times with binding-buffer over 30 min. Bound material was eluted from the beads with Laemmli sample buffer and loaded onto a 12% SDS-polyacrylamide gel. 1/10 of the in vitro translation reactions was used asa control. Bands were visualized by autoradiographyof the driedgels. Densitometry revealed that the L196A and F201A mutants decreased association with Rab8a by 39% and 52%, respectively, confirming their reduction-of-function characteristics.
Supplementary Material
Acknowledgments
We thank F. Barr, E. Brown, J. Stow, W. Guo, I. Macara, K. Simons, J. Wilson, T. Weimbs, and A. Zahraoui for generous gifts of reagents and unpublished data, and the Mostov lab for kind assistance. Supported by a Susan G Komen Foundation Fellowship (DMB), a DOD Breast Cancer Concept Award (AD), and NIH R01DK074398, R01AI25144 and P01AI53194 (KM).
Footnotes
The authors declare that they have no competing financial interests
AUTHOR CONTRIBUTIONS
D.M.B., A.D., A.R.F, F.M.B., and J.P. designed the experiments; D.M.B., A.D., A.R.F, and J.P. did the experimental work; D.M.B. and K.E.M. analysed the experiments; D.M.B. and K.E.M wrote the manuscript.
References
- 1.Bryant DM, Mostov KE. From cells to organs: building polarized tissue. Nat Rev Mol Cell Biol. 2008;9:887–901. doi: 10.1038/nrm2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009 doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 3.He B, Guo W. The exocyst complex in polarized exocytosis. Curr Opin Cell Biol. 2009 doi: 10.1016/j.ceb.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein B, Macara IG. The PAR proteins: fundamental players in animal cell polarization. Dev Cell. 2007;13:609–622. doi: 10.1016/j.devcel.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meder D, Shevchenko A, Simons K, Fullekrug J. Gp135/podocalyxin and NHERF-2 participate in the formation of a preapical domain during polarization of MDCK cells. J Cell Biol. 2005;168:303–313. doi: 10.1083/jcb.200407072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin-Belmonte F, et al. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell. 2007;128:383–397. doi: 10.1016/j.cell.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrari A, Veligodskiy A, Berge U, Lucas MS, Kroschewski R. ROCK-mediated contractility, tight junctions and channels contribute to the conversion of a preapical patch into apical surface during isochoric lumen initiation. J Cell Sci. 2008;121:3649–3663. doi: 10.1242/jcs.018648. [DOI] [PubMed] [Google Scholar]
- 8.Casanova JE, et al. Association of Rab25 and Rab11a with the apical recycling system of polarized Madin-Darby canine kidney cells. Mol Biol Cell. 1999;10:47–61. doi: 10.1091/mbc.10.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaye DD, Casanova J, Llimargas M. Modulation of intracellular trafficking regulates cell intercalation in the Drosophila trachea. Nat Cell Biol. 2008;10:964–970. doi: 10.1038/ncb1756. [DOI] [PubMed] [Google Scholar]
- 10.Li BX, Satoh AK, Ready DF. Myosin V, Rab11, and dRip11 direct apical secretion and cellular morphogenesis in developing Drosophila photoreceptors. J Cell Biol. 2007;177:659–669. doi: 10.1083/jcb.200610157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desclozeaux M, et al. Active Rab11 and functional recycling endosome are required for E-cadherin trafficking and lumen formation during epithelial morphogenesis. Am J Physiol Cell Physiol. 2008;295:C545–556. doi: 10.1152/ajpcell.00097.2008. [DOI] [PubMed] [Google Scholar]
- 12.Knodler A, et al. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc Natl Acad Sci U S A. 2010;107:6346–6351. doi: 10.1073/pnas.1002401107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshimura S, Egerer J, Fuchs E, Haas AK, Barr FA. Functional dissection of Rab GTPases involved in primary cilium formation. J Cell Biol. 2007;178:363–369. doi: 10.1083/jcb.200703047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu S, Mehta SQ, Pichaud F, Bellen HJ, Quiocho FA. Sec15 interacts with Rab11 via a novel domain and affects Rab11 localization in vivo. Nat Struct Mol Biol. 2005;12:879–885. doi: 10.1038/nsmb987. [DOI] [PubMed] [Google Scholar]
- 15.Oztan A, et al. Exocyst requirement for endocytic traffic directed toward the apical and basolateral poles of polarized MDCK cells. Mol Biol Cell. 2007;18:3978–3992. doi: 10.1091/mbc.E07-02-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zuo X, Guo W, Lipschutz JH. The exocyst protein Sec10 is necessary for primary ciliogenesis and cystogenesis in vitro. Mol Biol Cell. 2009;20:2522–2529. doi: 10.1091/mbc.E08-07-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lalli G. RalA and the exocyst complex influence neuronal polarity through PAR-3 and aPKC. J Cell Sci. 2009;122:1499–1506. doi: 10.1242/jcs.044339. [DOI] [PubMed] [Google Scholar]
- 18.Hayes MJ, Moss SE. Annexin 2 has a dual role as regulator and effector of v-Src in cell transformation. J Biol Chem. 2009;284:10202–10210. doi: 10.1074/jbc.M807043200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zobiack N, Rescher U, Ludwig C, Zeuschner D, Gerke V. The annexin 2/S100A10 complex controls the distribution of transferrin receptor-containing recycling endosomes. Mol Biol Cell. 2003;14:4896–4908. doi: 10.1091/mbc.E03-06-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez-Fraticelli AE, et al. The Cdc42 GEF Intersectin 2 controls mitotic spindle orientation to form the lumen during epithelial morphogenesis. J Cell Biol. 2010;189:725–738. doi: 10.1083/jcb.201002047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qin Y, Meisen WH, Hao Y, Macara IG. Tuba, a Cdc42 GEF, is required for polarized spindle orientation during epithelial cyst formation. J Cell Biol. 2010;189:661–669. doi: 10.1083/jcb.201002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lubarsky B, Krasnow MA. Tube morphogenesis. Making and shaping biological tubes. Cell. 2003;112:19–28. doi: 10.1016/s0092-8674(02)01283-7. [DOI] [PubMed] [Google Scholar]
- 23.Bagnat M, Cheung ID, Mostov KE, Stainier DY. Genetic control of single lumen formation in the zebrafish gut. Nat Cell Biol. 2007;9:954–960. doi: 10.1038/ncb1621. [DOI] [PubMed] [Google Scholar]
- 24.Ortiz D, Medkova M, Walch-Solimena C, Novick P. Ypt32 recruits the Sec4p guanine nucleotide exchange factor, Sec2p, to secretory vesicles; evidence for a Rab cascade in yeast. J Cell Biol. 2002;157:1005–1015. doi: 10.1083/jcb.200201003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schluter MA, et al. Trafficking of Crumbs3 during Cytokinesis Is Crucial for Lumen Formation. Mol Biol Cell. 2009 doi: 10.1091/mbc.E09-02-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morais-de-Sa E, Mirouse V, St Johnston D. aPKC phosphorylation of Bazooka defines the apical/lateral border in Drosophila epithelial cells. Cell. 2010;141:509–523. doi: 10.1016/j.cell.2010.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walther RF, Pichaud F. Crumbs/DaPKC-dependent apical exclusion of Bazooka promotes photoreceptor polarity remodeling. Curr Biol. 2010;20:1065–1074. doi: 10.1016/j.cub.2010.04.049. [DOI] [PubMed] [Google Scholar]
- 28.Joberty G, Petersen C, Gao L, Macara IG. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol. 2000;2:531–539. doi: 10.1038/35019573. [DOI] [PubMed] [Google Scholar]
- 29.Jaffe AB, Kaji N, Durgan J, Hall A. Cdc42 controls spindle orientation to position the apical surface during epithelial morphogenesis. J Cell Biol. 2008;183:625–633. doi: 10.1083/jcb.200807121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris KP, Tepass U. Cdc42 and vesicle trafficking in polarized cells. Traffic. 2010 doi: 10.1111/j.1600-0854.2010.01102.x. [DOI] [PubMed] [Google Scholar]
- 31.Balklava Z, Pant S, Fares H, Grant BD. Genome-wide analysis identifies a general requirement for polarity proteins in endocytic traffic. Nat Cell Biol. 2007;9:1066–1073. doi: 10.1038/ncb1627. [DOI] [PubMed] [Google Scholar]
- 32.Shivas JM, Morrison HA, Bilder D, Skop AR. Polarity and endocytosis: reciprocal regulation. Trends Cell Biol. 2010 doi: 10.1016/j.tcb.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hogan BL, Kolodziej PA. Organogenesis: molecular mechanisms of tubulogenesis. Nat Rev Genet. 2002;3:513–523. doi: 10.1038/nrg840. [DOI] [PubMed] [Google Scholar]
- 34.Brignoni M, et al. Exocytosis of vacuolar apical compartment (VAC) in Madin-Darby canine kidney epithelial cells: cAMP is involved as second messenger. Exp Cell Res. 1993;205:171–178. doi: 10.1006/excr.1993.1072. [DOI] [PubMed] [Google Scholar]
- 35.Matheson J, Yu X, Fielding AB, Gould GW. Membrane traffic in cytokinesis. Biochem Soc Trans. 2005;33:1290–1294. doi: 10.1042/BST0331290. [DOI] [PubMed] [Google Scholar]
- 36.Zhang H, Squirrell JM, White JG. RAB-11 permissively regulates spindle alignment by modulating metaphase microtubule dynamics in Caenorhabditis elegans early embryos. Mol Biol Cell. 2008;19:2553–2565. doi: 10.1091/mbc.E07-09-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pohl C, Jentsch S. Final stages of cytokinesis and midbody ring formation are controlled by BRUCE. Cell. 2008;132:832–845. doi: 10.1016/j.cell.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 38.Horne-Badovinac S, et al. Positional cloning of heart and soul reveals multiple roles for PKC lambda in zebrafish organogenesis. Curr Biol. 2001;11:1492–1502. doi: 10.1016/s0960-9822(01)00458-4. [DOI] [PubMed] [Google Scholar]
- 39.Zheng Z, et al. LGN regulates mitotic spindle orientation during epithelial morphogenesis. J Cell Biol. 2010;189:275–288. doi: 10.1083/jcb.200910021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu W, et al. Formation of cysts by alveolar type II cells in three-dimensional culture reveals a novel mechanism for epithelial morphogenesis. Mol Biol Cell. 2007;18:1693–1700. doi: 10.1091/mbc.E06-11-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu KD, et al. Rac1 is required for reorientation of polarity and lumen formation through a PI 3-kinase-dependent pathway. Am J Physiol Renal Physiol. 2007;293:F1633–1640. doi: 10.1152/ajprenal.00053.2007. [DOI] [PubMed] [Google Scholar]
- 42.Tanimizu N, Miyajima A, Mostov KE. Liver progenitor cells fold up a cell monolayer into a double-layered structure during tubular morphogenesis. Mol Biol Cell. 2009;20:2486–2494. doi: 10.1091/mbc.E08-02-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang AZ, Wang JC, Ojakian GK, Nelson WJ. Determinants of apical membrane formation and distribution in multicellular epithelial MDCK cysts. Am J Physiol. 1994;267:C473–481. doi: 10.1152/ajpcell.1994.267.2.C473. [DOI] [PubMed] [Google Scholar]
- 44.Schuck S, Manninen A, Honsho M, Fullekrug J, Simons K. Generation of single and double knockdowns in polarized epithelial cells by retrovirus-mediated RNA interference. Proc Natl Acad Sci U S A. 2004;101:4912–4917. doi: 10.1073/pnas.0401285101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moffat J, et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell. 2006;124:1283–1298. doi: 10.1016/j.cell.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 46.Sfakianos J, et al. Par3 functions in the biogenesis of the primary cilium in polarized epithelial cells. J Cell Biol. 2007;179:1133–1140. doi: 10.1083/jcb.200709111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ory DS, Neugeboren BA, Mulligan RC. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc Natl Acad Sci U S A. 1996;93:11400–11406. doi: 10.1073/pnas.93.21.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hattula K, Furuhjelm J, Arffman A, Peranen J. A Rab8-specific GDP/GTP exchange factor is involved in actin remodeling and polarized membrane transport. Mol Biol Cell. 2002;13:3268–3280. doi: 10.1091/mbc.E02-03-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kreitzer G, et al. Three-dimensional analysis of post-Golgi carrier exocytosis in epithelial cells. Nat Cell Biol. 2003;5:126–136. doi: 10.1038/ncb917. [DOI] [PubMed] [Google Scholar]
- 50.Mangravite LM, Lipschutz JH, Mostov KE, Giacomini KM. Localization of GFP-tagged concentrative nucleoside transporters in a renal polarized epithelial cell line. Am J Physiol Renal Physiol. 2001;280:F879–F885. doi: 10.1152/ajprenal.2001.280.5.F879. [DOI] [PubMed] [Google Scholar]
- 51.Kreis TE. Microinjected antibodies against the cytoplasmic domain of vesicular stomatitis virus glycoprotein block its transport to the cell surface. Embo J. 1986;5:931–941. doi: 10.1002/j.1460-2075.1986.tb04306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown PS, et al. Definition of distinct compartments in polarized Madin-Darby canine kidney (MDCK) cells for membrane-volume sorting, polarized sorting and apical recycling. Traffic. 2000;1:124–140. doi: 10.1034/j.1600-0854.2000.010205.x. [DOI] [PubMed] [Google Scholar]
- 53.Sato Y, et al. Asymmetric coiled-coil structure with Guanine nucleotide exchange activity. Structure. 2007;15:245–252. doi: 10.1016/j.str.2007.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.