Abstract
Background
Mycophenolate mofetil (MMF), a prodrug of mycophenolic acid (MPA), is used during nonmyeloablative and reduced-intensity conditioning haematopoetic cell transplantation (HCT) to improve engraftment and reduce graft versus host disease (GVHD). However, information about MPA pharmacokinetics is sparse in this context and its use is still empirical.
Objectives
To perform a pilot pharmacokinetic study and to develop maximum a posteriori Bayesian estimators (MAP-BEs) for the estimation of MPA exposure in HCT.
Patients and methods
Fourteen patients given orally MMF 15 mg/kg 3 times daily were included. Two consecutive 8-hour PK profiles were performed the same day respectively three days before and 4 days after the HCT. One 8-hour PK profile was performed on day 27 after transplantation. For these 8-hours pharmacokinetic profiles, blood samples were collected pre-dose and 20, 40, 60, 90 min, 2, 4, 6 and 8 h post-dose after administration of the drug.
Using the iterative two-stage method (ITS), two different one-compartment open PK models with first-order elimination were developed to describe the data: one with two gamma laws and one with three gamma laws to describe the absorption phase. For each PK profile, the Akaike criterion was calculated to evaluate model fitting. On the basis of the population PK parameters, MAP-BEs were developed for the estimation of MPA pharmacokinetics and area under the concentration-time curves (AUC0-8h) at the different studied periods using a limited sampling strategy (LSS). These MAP-BEs were then validated using a data-splitting method.
Results
The ITS approach allowed the development of MAP-BEs based either on “double-gamma” or “triple-gamma” models, the combination of which allowed correct estimation of MPA pharmacokinetics and AUC on the basis of a C20min-C90min-C240min sampling schedule. The mean bias of the Bayesian versus reference (trapezoidal) AUCs was <5% with less than 16% of the patients with absolute bias on AUC > 20%. Akaike criterion was systematically calculated for the choice of the most appropriate model fitting the data.
Conclusion
PK models and MAP-BEs for MMF when administered to HCT patients have been developed. In the studied population, they allowed the estimation of MPA exposure based on three blood samples, which could be helpful in conducting clinical trials for the optimization of MPA in reduced-intensity HCT. However, prior studies will be needed to validate them in larger populations.
Keywords: Adult; Anti-Inflammatory Agents, Non-Steroidal; pharmacokinetics; Area Under Curve; Bayes Theorem; Drug Monitoring; methods; Female; Hematopoietic Stem Cell Transplantation; methods; Humans; Male; Middle Aged; Models, Theoretical; Mycophenolic Acid; administration & dosage; analogs & derivatives; blood; pharmacokinetics; Pharmacokinetics; Pilot Projects; Prodrugs; pharmacokinetics; Young Adult
Keywords: Mycophenolate mofetil, clinical pharmacokinetics; Bayesian estimation; haematopoietic cell transplantation
Introduction
Mycophenolate mofetil (MMF) is a prodrug of mycophenolic acid (MPA) largely used as an immunosuppressive drug to prevent acute rejection following solid organ transplantation [1, 2]. MMF is also used in the prophylaxis and treatment of acute and chronic graft-versus-host disease (GVHD) after hematopoietic stem cell transplantation (HCT), or to improve the engraftment in nonmyeloablative and reduced-intensity conditioning HCT [3–15].
On the one hand, the therapeutic drug monitoring (TDM) of MMF when used in solid organ transplantation is debated [16] but could be recommended as it has been demonstrated that the control of exposure to MPA helps to maximize its immunosuppressive effects [2]. More precisely, an area under the twelve-hour concentration-time curve (AUC0-12) of MPA between 30 and 60 mg.h/L is recommended for at least the first six months post-renal or heart transplantation, when MMF is used in combination with cyclosporine and steroids [1, 2]. Bayesian estimators, based on pharmacokinetic models and using sparse individual data have been developed to estimate the dose providing a target AUC value for each patient [17, 18]. Some of these Bayesian estimators, derived from PK models built from large populations of transplanted patients, have been successfully used in a prospective “concentration-controlled” clinical trial using a limited sampling strategy (20 min, 1 h, 3 h) [19]. These tools are also routinely used by transplantation centres via an expert system available on a website (https://pharmaco.chu-limoges.fr/abis.htm) [20].
On the other hand, very little information is available about the TDM of MMF when used in HCT patients. The previous pharmacokinetic studies have reported a wide interpatient variability with a mean total MPA exposure below that recommended in solid organ transplantation [3, 5, 7–9, 14]. Interestingly, mean dose-normalized MPA AUC values observed in HCT patients are almost 50% lower than in renal transplant patients [7, 14]. These observations advocate for MMF administration tid instead of bid as in solid organ transplantation [10, 21]. This also implies dose adjustments in HCT patients, but also strongly suggests that the pharmacokinetic parameters and their associated tools developed for solid organ transplant recipients would not be suitable for HCT. Furthermore, a recent study underlines the interest of TDM of MPA in terms of reduction of inter-patient variability [22].
This pilot study aimed at modelling MPA pharmacokinetics and developing maximum a posteriori Bayesian estimators (MAP-BEs) for the estimation of MPA exposure in reduced-intensity HCT patients given MMF as part of their immunosuppressive regimen.
Patients and methods
Patients
Fourteen patients were enrolled in the study. The inclusion criteria were: patient likely to receive a reduced-intensity HCT as determined by the French Haematological Society, age ranging from 18 to 70 years, signing of informed consent, absence of intercurrent disease which could interfere with the short term survival of the patient or graft. Non inclusion criteria were hypersensitivity to MPA, patients who present contraindications to MPA, patients with severe gastro-intestinal disorders, drug addictions or psychiatric disorders which mean they might not be able to understand the protocol. This clinical trial was designed in accordance with the legal requirements and approved by a local ethics committee.
The reduced-intensity regimen was a combination of fludarabine and melphalan (in 8 cases), with total body irradiation (2 Gy in 2 cases) or with both cyclophosphamide and total body irradiation (2 Gy in 4 cases. The immunosuppressive regimen for the prevention of GVHD was a combination of cyclosporine administered orally twice daily (start dose: 5mg/kg/day; targeted through concentration: 250 ng/mL) and MMF administered at the dose of 15 mg/kg three times daily. However, because of the galenic formulation of MMF (tablets of 500 and capsules of 250 mg), the actual dose administered was rounded off 1g of MMF tid. The two treatments were started 3 days before the HCT.
The supportive care medication was as previously described [23]. Briefly, it consisted in bacterial prophylaxis using phenoxymethylpenicilline (3 MU/day) or spiramycine (3 MU/day). Valacyclovir p.o. was administered to prevent CMV prophylaxis: 500 mg twice daily if donor and recipient was serologically CMV negative; 1g ×3 daily when donor and/or recipient was CMV positive. Oral itraconazole (400 mg daily) or posaconazole (200 mg tid) were administered if fungal prophylaxis was needed.
Pharmacokinetic profiles
Three profiles were performed during the first month of HCT: one at the beginning of the treatment three days before the HCT and one 7 (± 1) days later, i.e. 4 days after HCT. Finally a PK profile was collected after the morning on day 30 (± 5) after the first PK profile. The two first PK profiles correspond with a two 8-hour PK profiles performed consecutively at the initiation of the MMF treatment. The sequence of sampling was: pre-dose, 20, 40 and 60 minutes after the first administration in the morning, thereafter 2, 4, 6 and 8 hours. Then samplings were performed at 20, 40 and 60 minutes and 2, 4, 6 and 8 hours after the administration of the second dose of MMF. Finally, the last PK profile corresponds with a 8-hour PK profile collected after the morning on day 30.
Assay
The measurement of total MPA was performed using an HPLC method with UV detection derived from [24]. Briefly, a mixture of 1 mL whole blood and 4 μg of internal standard (thiopental) were acidified with chlorhydric acid and extracted with 4 mL of dichloromethane. The organic fraction was then evaporated to dryness under a stream of nitrogen. The drug residue was reconstituted with the elution solvent (NaH2PO4 buffer/acetonitrile (58/42 v/v) at pH = 2.7). Thirty μL of sample were injected into the HPLC system with a X-Terra column and precolumn (Waters, Saint Quentin en Yvelines, France) and with a UV detection at 254 nm. This method exhibits a lower limit of quantification of 0.1 mg/L and a coefficient of variation of 6.0% for interday accuracy at the 1 mg/L concentration.
PK modelling
The pharmacokinetic profiles were described by one-compartment open models with first-order elimination combined with a Gamma model of absorption with either two or three parallel absorption routes. These PK models were previously published to describe PK profiles of MPA in renal transplant patients [17, 18] and in patients suffering from lupus [25]. Briefly, in these models, the absorption rate at time t was described by a sum of Gamma distributions:
| (1) |
with:
| (2) |
where F is the bioavailability coefficient, D is the administered dose, fi represents the absorption rate through the i-th route (normalized by FD), Γ is the Gamma function, (ai, bi) the parameters of the Gamma distributions, ri is the fraction of drug absorbed through the i-th route. No independent determination of the bioavailability factor F was possible, since no intravenous data was available for these patients.
The disposition kinetics, i. e. the impulse response I(t) of the system, was described by a one-compartment model, according to the equation:
| (3) |
Where I(t) is the drug concentration at time t following an intravenous bolus of a unit dose D0, AIV is the initial concentration following an intravenous bolus administration of a unit dose, and κ is the apparent elimination rate following the same intravenous bolus administration.
The convolution product of the absorption rate and disposition function was computed analytically as previously described [26, 27]:
| (4) |
Where C is the concentration at time t, C0 the trough concentration, ai and bi the parameters of the Gamma distributions, ri is the fraction of drug absorbed through the i-th route, and P denotes the incomplete Gamma function:
| (5) |
Where n is the exponent of the incomplete Gamma function, x is the independent variable (argument of the incomplete Gamma function) and z is the integration variable.
Bayesian estimation of individual pharmacokinetic parameters
The individual parameters (vector θ) for each patient were determined by minimizing the following objective function:
| (6) |
Where the symbols have the following meanings:
Φ: objective function corresponding to the Bayesian posterior distribution
n: number of experimental points
Ci: concentration measured at time ti
f(ti, θ): concentration computed at time ti
vi: variance of measured concentration
θ: vector of model parameters
μ: mean parameter values in the reference population
Ω: variance-covariance matrix of parameters in the reference population
the symbol T denotes matrix transposition
The variance-covariance matrix V of the posterior distribution of the parameter estimates for each patient was computed by the classical approximation:
| (7) |
Where J denotes the Jacobian matrix (Jij = ∂f(ti, θ)/∂ θj) and W the diagonal matrix of weights (Wii = 1/vi). The determinant of V (detV) was used as a measure of the precision of the determined parameters with which parameters were determined (the lower the determinant, the higher the precision).
Population pharmacokinetics
The population pharmacokinetic parameters for were determined by the iterative two-stage (ITS) method [28, 29], using our own program. At each iteration of the method, the following two steps were performed:
Individual estimates θk and Vk (k = 1..N) for the N patients were obtained by Bayesian estimation as described above
- New estimates of the population mean vector μ and population variance-covariance matrix Ω were computed by:
(8) (9)
The interindividual variability of the pharmacokinetic parameters was assessed by computing the medians and 50% dispersion factors (DF50). DF50 is defined as (Q75 - Q25)/1.32, whereQ75 and Q25 are the 25% and 75% quartiles, respectively, and 1.32 represents the width of the interval covering 50% of the observations in the case of a normal distribution. DF50 is equal to the standard deviation for normally distributed parameters and provides a reasonable estimate of the dispersion of a non-Gaussian distribution [26].
The calibration experiments showed that the standard deviation Si of a measured concentration Ci could be expressed as a polynomial function Si = 0.02 + 0.033 Ci, where Ci and Si are in mg/L. Si was used as concentration weighting factor.
For each PK profile, the two PK model were applied and the best one was selected based on the lowest Akaike Information Criterion [30], calculated as follows:
Where Φmin is the value of the objective function at minimum, and p the number of parameters in the model.
Determination of a Limited Sampling Strategy for the estimation of the AUC
For each pre- and post-transplantation period, limited sampling strategies were tested for Bayesian forecasting with the aim of estimating the AUC0-8h. As previously done with renal [17, 18], combinations of a maximum of three sampling times within 4 hours post-dose were tested for Bayesian forecasting. Indeed, such a sampling scheme seemed to be a good compromise between the precision of parameter estimates and a possible implementation in clinical practice.
The D-optimality criterion [30] applied to Bayesian estimation was used to compare these candidate LSS as follows:
For given sampling times t1, t2, … the corresponding concentrations C1, C2, …were computed by eq. (4), using the population mean vector μ.
The variance-covariance matrix was then computed with eq. (7). The sampling times giving the lowest value of det V were selected as the ‘best’ sampling times.
The Bayesian estimators corresponding to each period were evaluated separately, by comparison of observed and estimated AUC0–8 h estimates obtained using the selected limited-sampling strategy and BE and the linear trapezoidal rule applied to the full profiles (reference values). Additionally bias and Root Mean Squared Error (RMSE) were computed according to Sheiner and Beal’s recommendations [31].
In a second step, a data-splitting procedure was performed to validate these MAP-BEs, in accordance with previous reports [32, 33]. For each period, patients were randomly divided into subsets containing approximately 80% of the patients. To test the robustness of the model, new population parameters were obtained in each “80% subset” and were then compared with those resulting from the entire population. To test the predictive performance of the MAP-BEs, the parameter estimates from each of the subsets were used to estimate the PK parameters and the AUCs of the remaining 20% patients. The predicted AUCs were compared with those obtained using the linear trapezoidal rule by means of the mean bias and RMSE.
Results
The characteristics of the patients are summarized in Table 1. All PK profiles could be achieved except in two cases: one on D30 due to missing data and one on D7 because the MMF treatment was administered intravenously after the development of an oral mucositis. The MMF dose was of 1000 mg tid for all the patients for the first PK profile. On D7, the MMF dose was of 1000 mg tid except in three patients (two at 750 mg tid and 1 at 500 mg tid). The reducing of these doses was maintained for the last PK period (D30) and extended to one additional patient (750 mg tid). On D30, the MMF dose was increased for one patient (1500 mg tid).
Table 1.
Patient characteristics at the time of the first pharmacokinetics
| Patients Characteristics | Number of patients or median value (range) |
|---|---|
| Age (years) | 50.5 (20 – 60) |
| Ratio female/male | 6/8 |
| Diagnosis | |
| Hodgkin lymphoma stage IV | 1 |
| Myelodysplastic syndrome - refractory anemia with excess of blasts | 1 |
| Chronic myeloid leukemia | 1 |
| Acute myeloid leukemia (whatever the type) | 7 |
| Chronic lymphoblastic leukemia | 1 |
| agnogenic myeloid metaplasia | 1 |
The observed concentration-time profiles obtained before transplantation and on D7 and D30 are presented in figure 1. Whatever the period, these individual profiles were satisfactorily described using a one-compartment model with either a double or a triple gamma absorption. Some typical examples illustrating the good-of-fit are given on figure 2. The performance of the models are depicted in table x (published as only online supplementary material). Interestingly, for each parameter of the models, the precision of the estimation gave satisfactory results (from 5.2 to 32.6%, depending on both period or PK model). Also, the medians were very close to the means, the DF50 values very close to the standard deviations and the Kolmogorov-Smirnov test did not find significant deviations from the normal distribution, as required. Thus, population PK parameters of the two models were used as priors for Bayesian estimation. The Akaike criterion was pertinent in discriminating which model best fitted each patient’s data. A typical example of the difference in data modelling using a “double gamma” or a “triple gamma” model and the corresponding Akaike criterion values is presented in figure 3.
Figure 1.
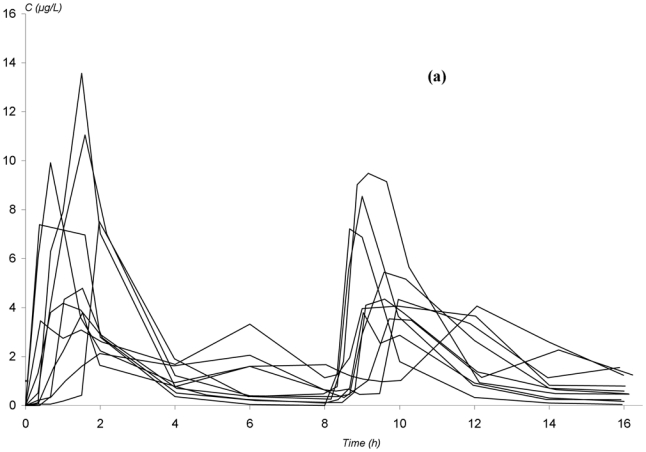
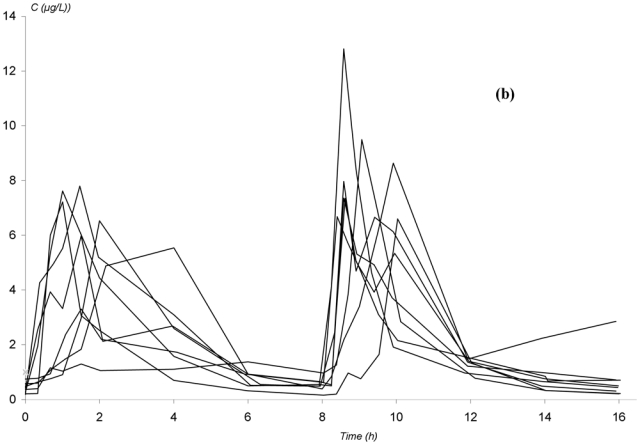
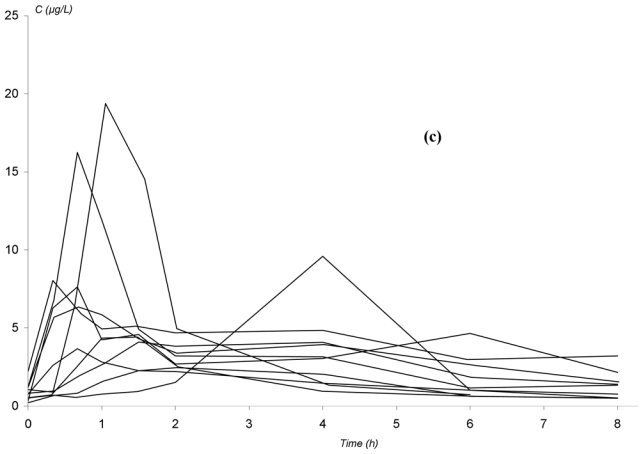
Observed concentration-time profiles of mycophenolic acid in patients given mycophenolate mofetil every 8 hours in non myeloablative haematopoietic cell transplantation. (a) before transplantation; (b) 4 days after the initiation of MMF treatment; (c) 27 days after the initiation of MMF treatment.
Figure 2.
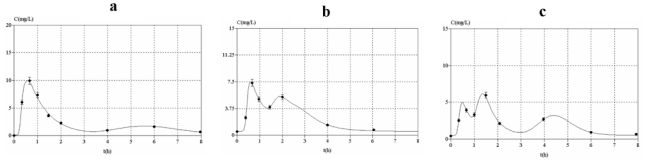
Individual mycophenolic acid plasma concentration versus time curves at different periods of the study fitted using the developed PK models. (a): after the first administration of MMF; (b): 4 days after the HCT; (c): 27 days after the HCT
Figure 3.
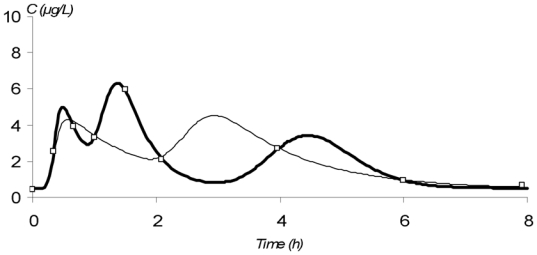
Modeling of the concentration-time profile of mycophenolic acid obtained from a patient given mycophenolate mofetil every 8 hours on day 7 after the initiation of MMF treatment, using either a “triple gamma” model (_) or a “double gamma” model (_). (□) represent the observed concentrations. AIC values where 20 and 32 for a “triple gamma” and a “double gamma” model, respectively
With respect to both the AUC0–8 h estimation performance and the D-optimality criterion, the combination of sampling times at 20min, 90min and 240min post-dose was found to be the best compromise when considering all the pre- and post-transplantation periods. Figure 3 gives four typical examples of MPA PK profiles estimated using Bayesian fitting. The predictive performance of the different MAP-BEs is summarized in table 2. For each period, mean bias between “calculated” and “trapezoidal” AUC was less than 2.5% and the percentage of patients with a bias greater than 20% was less than 16%. Precisely, on D7, 2 out of 12 patients were estimated with a bias of −23% and +28%, respectively. These 2 last cases are presented in figure 3.
Table 2.
Parameters of the different one-compartment open models with first-order elimination combined with a Gamma model of absorption with either two or three parallel absorption routes. 3 gamma laws. Estimation (%) represents the mean is the considered sub-group.
| Parameter |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st administration | 3 gamma laws | n = 14 | C0 | a1 | b1 | a2 | b2 | a3 | b3 | r1 | r2 | F*A_IV |
| Mean | 0.013 | 28.054 | 56.930 | 51.157 | 43.887 | 38.284 | 8.576 | 0.277 | 0.511 | 1.704 | ||
| SD | 0.018 | 8.423 | 13.474 | 15.572 | 6.736 | 15.202 | 3.228 | 0.150 | 0.223 | 0.851 | ||
| Min | 0.003 | 17.893 | 38.413 | 18.629 | 35.783 | 8.609 | 3.525 | 0.011 | 0.139 | 0.799 | ||
| Max | 0.073 | 51.524 | 75.000 | 74.771 | 55.571 | 61.549 | 13.719 | 0.558 | 0.915 | 3.363 | ||
| estimation (%) | 15.3 | 21.8 | 21.4 | 17.0 | 16.8 | 22.0 | 21.1 | 21.4 | 10.2 | 7.9 | ||
| 2 gamma laws | n = 14 | C0 | a1 | b1 | a2 | b2 | r | F*A_IV | Alpha | |||
| Mean | 0.014 | 22.280 | 38.991 | 35.409 | 22.829 | 0.430 | 1.450 | 0.856 | ||||
| SD | 0.005 | 10.310 | 11.498 | 10.009 | 8.199 | 0.282 | 0.808 | 0.337 | ||||
| Min | 0.010 | 9.843 | 9.665 | 22.591 | 7.272 | 0.010 | 0.326 | 0.182 | ||||
| Max | 0.026 | 46.537 | 50.000 | 50.000 | 35.990 | 1.000 | 3.057 | 1.627 | ||||
| estimation (%) | 10.7 | 27.4 | 28.0 | 25.6 | 27.7 | 16.1 | 6.6 | 5.1 | ||||
| 2nd administration | 3 gamma laws | n =14 | C0 | a1 | b1 | a2 | b2 | a3 | b3 | r1 | r2 | F*A_IV |
| Mean | 0.053 | 27.376 | 53.236 | 44.184 | 30.205 | 44.285 | 14.739 | 0.293 | 0.461 | 1.700 | ||
| SD | 0.037 | 14.206 | 13.356 | 7.935 | 10.495 | 18.293 | 4.414 | 0.181 | 0.115 | 0.612 | ||
| Min | 0.002 | 10.290 | 26.712 | 29.258 | 12.028 | 7.553 | 8.539 | 0.010 | 0.266 | 0.887 | ||
| Max | 0.120 | 61.550 | 74.991 | 55.238 | 45.385 | 74.976 | 24.838 | 0.535 | 0.608 | 2.951 | ||
| estimation (%) | 11.1 | 28.3 | 25.5 | 18.4 | 17.9 | 25.0 | 20.8 | 20.3 | 21.4 | 13.9 | ||
| 2 gamma laws | n = 14 | C0 | a1 | b1 | a2 | b2 | r | F*A_IV | Alpha | |||
| Mean | 0.056 | 26.124 | 37.081 | 33.192 | 19.462 | 0.502 | 1.897 | 1.582 | ||||
| SD | 0.037 | 12.211 | 9.330 | 13.502 | 8.703 | 0.288 | 1.014 | 0.954 | ||||
| Min | 0.010 | 10.458 | 21.105 | 9.847 | 3.071 | 0.010 | 0.400 | 0.274 | ||||
| Max | 0.120 | 49.828 | 50.000 | 49.999 | 34.870 | 1.000 | 4.233 | 3.609 | ||||
| estimation (%) | 5.2 | 19.2 | 17.6 | 24.6 | 25.8 | 11.9 | 11.0 | 9.8 | ||||
| 4 days after HCT | 3 gamma laws | n = 13 | C0 | a1 | b1 | a2 | b2 | a3 | b3 | r1 | r2 | F*A_IV |
| Mean | 0.087 | 30.478 | 55.532 | 40.862 | 31.617 | 46.226 | 14.321 | 0.229 | 0.375 | 2.264 | ||
| SD | 0.098 | 7.468 | 11.380 | 9.919 | 7.452 | 12.421 | 4.084 | 0.125 | 0.118 | 0.727 | ||
| Min | 0.021 | 16.411 | 32.574 | 21.406 | 14.999 | 18.313 | 5.416 | 0.033 | 0.103 | 0.843 | ||
| Max | 0.414 | 44.680 | 75.000 | 59.891 | 44.362 | 68.189 | 22.779 | 0.483 | 0.580 | 4.189 | ||
| estimation (%) | 4.5 | 20.0 | 22.5 | 19.6 | 20.0 | 20.5 | 20.1 | 15.4 | 15.8 | 17.1 | ||
| 2 gamma laws | n = 13 | C0 | a1 | b1 | a2 | b2 | r | F*A_IV | Alpha | |||
| Mean | 0.089 | 20.780 | 33.694 | 33.914 | 17.332 | 0.481 | 1.768 | 1.242 | ||||
| SD | 0.087 | 6.804 | 13.136 | 10.766 | 6.297 | 0.256 | 0.968 | 0.501 | ||||
| Min | 0.019 | 9.713 | 12.880 | 13.883 | 5.771 | 0.067 | 0.374 | 0.340 | ||||
| Max | 0.390 | 32.030 | 50.000 | 50.000 | 34.355 | 1.000 | 4.552 | 2.186 | ||||
| estimation (%) | 4.4 | 23.0 | 25.8 | 26.1 | 27.8 | 14.8 | 13.7 | 12.1 | ||||
| 27 days after HCT | 3 gamma laws | n = 13 | C0 | a1 | b1 | a2 | b2 | a3 | b3 | r1 | r2 | F*A_IV |
| Mean | 0.0873 | 32.958 | 62.467 | 38.9 | 29.932 | 43.49 | 18.46 | 0.2478 | 0.222 | 2.6028 | ||
| SD | 0.0276 | 7.784 | 7.6511 | 8.8215 | 8.2111 | 14.719 | 2.7245 | 0.1352 | 0.109 | 1.0434 | ||
| min | 0.0556 | 19.049 | 50.559 | 26.763 | 23.294 | 16.66 | 14.12 | 0.0118 | 0.063 | 1.0866 | ||
| max | 0.1115 | 44.872 | 75 | 57.247 | 38.734 | 64.809 | 22.554 | 0.4426 | 0.412 | 4.1407 | ||
| estimation (%) | 11.8 | 16.0 | 16.1 | 15.9 | 22.0 | 19.9 | 15.0 | 29.7 | 32.6 | 28.3 | ||
| 2 gamma laws | n = 13 | C0 | a1 | b1 | a2 | b2 | r | F*A_IV | Alpha | |||
| mean | 0.074 | 15.410 | 21.477 | 31.423 | 16.071 | 0.341 | 3.042 | 1.762 | ||||
| SD | 0.022 | 3.796 | 4.553 | 8.571 | 2.628 | 0.172 | 1.925 | 0.819 | ||||
| min | 0.047 | 8.779 | 13.629 | 19.625 | 12.164 | 0.031 | 0.577 | 0.821 | ||||
| max | 0.119 | 24.147 | 26.726 | 45.680 | 20.742 | 0.611 | 6.644 | 3.254 | ||||
| estimation (%) | 9.6 | 21.5 | 20.1 | 17.2 | 14.2 | 29.7 | 23.1 | 23.6 | ||||
The MAP-BEs were then validated using a data-splitting strategy. After randomly dividing the patients of the full dataset into subsets containing approximately 20% of the patients, the individual PK parameters of each patient in the remaining “80% subset” was obtained using the final model developed from the whole dataset. In this first step the mean parameter estimates obtained in each “80% subset” were not statistically different from those resulting from the entire dataset, which showed the robustness of the model. The mean ± SD of the parameter estimates from each “80% subset” were then used to predict the individual AUC values in the remaining “20% subset”. In this second step, the mean bias between individual AUC values estimated using the 20min-90min-240min sampling schedule and the trapezoidal rule was less than 9%. Whatever the period, less than 16% of the AUC were estimated with a relative difference of more than 20% (2 out of 14 patients on day 7 and 2 out of 14 patients on the 1st administration).
Discussion
We report the development of pharmacokinetic models that adequately describe full pharmacokinetic profiles of MPA obtained in patients receiving MMF three times daily in the context of a reduced-intensity HCT. We also developed Bayesian estimators allowing the determination of the MPA AUC0-8h on the basis of a three-point limited sampling strategy within the first 4 hours post-dose.
Patients enrolled in the present study provided full MPA PK profiles before transplantation, then on D4 and D27 post-transplantation. When looking at the raw data, we observed that the patients usually exhibited profiles with more than one peak. For this reason, we decided to develop a model able to describe a secondary peak, similar to that previously reported for solid organ transplantation [17, 18]. Due to the complexity of some profiles, a model allowing the description of an additional peak was also constructed. As this leads to an increase in the number of independent estimated parameters, we proposed the use of the Akaike criterion (AIC) to prevent overfitting and to determine the model that best explains each individual data with a minimum of PK parameters.
Indeed, AIC reflects not only the goodness of fit, but also includes a penalty which is an increasing function of the number of estimated parameters. For each patient, Bayesian fitting was thus automatically performed using the two PK models, and only the results corresponding to the lowest AIC value were considered and reported. Of note, such a strategy is routinely performed on the ISBA website (https://pharmaco.chu-limoges.fr/abis.htm) where PK modelling is performed systematically using a one- and a two-gamma absorption model, and the AIC criterion is used to choose which results will be used for dose adjustment of MMF when used in solid organ transplantation.
Only a few pharmacokinetic studies have been published describing the MPA PK parameters or exposures when MMF is administered to HCT patients in prevention of GVHD, especially after reduced-intensity regimen [7, 8, 12, 34]. These studies have reported that the mean exposure to MPA (in its total or unbound form) is significantly lower than that observed in solid organ transplantation. The causes of this phenomenon are still controversial. However, to the best of our knowledge, only two MPA PK studies have been performed in HCT patients with the aim of building a tool for improving the therapeutic drug monitoring of MMF [35, 36]. Using a noncompartmental approach, Ng et al. have developed different equations derived from multilinear regression models allowing the estimation of total or unbound MPA AUC in HCT patients given MMF orally or intravenously. In the second study, Huang et al aimed at developing a PK model able to estimate unbound MPA concentrations from unbound MPA concentrations. However, using their multilinear regression equation failed to provide a satisfactory prediction for clinical assessment. In the present study, we developed Bayesian estimators able to accurately estimate AUC as well as the relevant pharmacokinetic parameters or exposure indices to MPA using three sampling times. As compared to algorithms using multilinear regression models, the combination of the patient’s information and the PK model leads to an accurate estimation of “unusual” pharmacokinetic profiles and is less sensitive to imprecision in sampling time.
A data-splitting strategy was performed here to validate both the PK models and MAPB-BEs. This approach was recommended by the U.S. Department of Health and Human Services of the U.S. Food and Drug Administration (FDA) [37]. Precisely, although external validation is the “gold standard” for testing the accuracy of a population model for predictive purposes in clinical practice, this method is hardly usable when the population studied is too small to be divided into a building and a validation group. This was the case in the present study. In fact, the diminution of the building group’s size leads to a decrease in the accuracy of the parameters and variability estimates. However, an external validation of the developed MAP-BEs is required before they can either be proposed to the physicians in charge of HCT patients through the ISBA website, or used to conduct a concentration-controlled trial such as the one which recently showed the significant improvement for renal transplant patients using such a strategy [19] and a couple of other on-going studies in liver transplantation.
Conclusion
Currently mycophenolate mofetil is widely used in solid organ transplantation. Numerous pharmacokinetic studies and some “controlled-concentration” trials have led, in the last few years, to a significant improvement of its monitoring in these patients. MMF is also prescribed in HCT, but prescribed doses are still empirical as target exposure indices still have to be defined. In this study, we developed PK that satisfactory described MPA absorption profiles when given to HCT patients. Additionally, Bayesian estimators able to determine the AUC on the basis of a routinely usable limited sampling strategy. However, further studies are needed to validate these tools in independent groups of patients.
Figure 4.
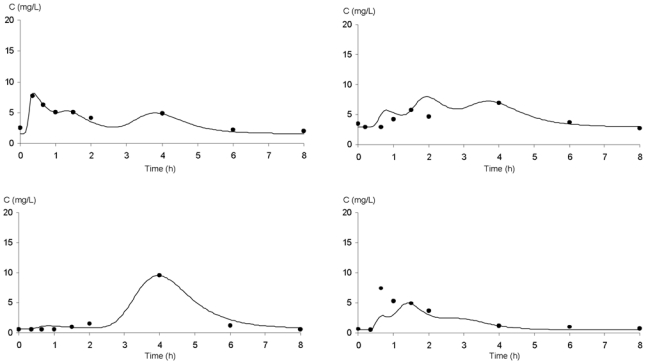
Bayesian fitting of four MPA pharmacokinetic profiles using a limited sampling strategy (t20min; t90min; t240min) in patients receiving MMF every 8 hours after an HCT.
Table 2.
Performance of the PK models and Bayesian estimators
| Model | ||||
|---|---|---|---|---|
| Before transplantation | After transplantation | |||
| 1st administration | 2nd administration | D4 | D27 | |
| Bias between AUCmod and AUCtrap | −0.014 ± 0.068 | −0.010 ± 0.049 | −0.035 ± 0.049 | 0.022 ± 0.057 |
| (m ± sd) [range] | [−0.082; 0.156] | [−0.081; 0.086] | [−0.108; 0.058] | [−0.092; 0.136] |
| RMSE | 0.067 | 0.048 | 0.058 | 0.040 |
| Bayesian estimators based on a t20min–t90min – t240min sampling | ||||
| schedule | ||||
| Before transplantation | After transplantation | |||
| 1st administration | 2nd administration | D4 | D27 | |
| Bias between AUCmod and AUCtrap | −0.008 ± 0.068 | −0.060 ± 0.081 | −0.025 ± 0.121 | 0.022 ± 0.06 |
| (m ± sd) [range] | [−0.179; 0.195] | [−0.153; 0.078] | [−0.230; 0.281] | [−0.092; 0.136] |
| RMSE | 0.010 | 0.098 | 0.112 | 0.040 |
References
- 1.Arns W, Cibrik DM, Walker RG, et al. Therapeutic drug monitoring of mycophenolic acid in solid organ transplant patients treated with mycophenolate mofetil: review of the literature. Transplantation. 2006 Oct 27;82( 8):1004–12. doi: 10.1097/01.tp.0000232697.38021.9a. [DOI] [PubMed] [Google Scholar]
- 2.van Gelder T, Le Meur Y, Shaw LM, et al. Therapeutic drug monitoring of mycophenolate mofetil in transplantation. Ther Drug Monit. 2006 Apr;28( 2):145–54. doi: 10.1097/01.ftd.0000199358.80013.bd. [DOI] [PubMed] [Google Scholar]
- 3.Basara N, Blau WI, Kiehl MG, et al. Efficacy and safety of mycophenolate mofetil for the treatment of acute and chronic GVHD in bone marrow transplant recipient. Transplant Proc. 1998 Dec;30( 8):4087–9. doi: 10.1016/s0041-1345(98)01351-7. [DOI] [PubMed] [Google Scholar]
- 4.Basara N, Blau WI, Kiehl MG, et al. Mycophenolate mofetil for the prophylaxis of acute GVHD in HLA-mismatched bone marrow transplant patients. Clin Transplant. 2000 Apr;14( 2):121–6. doi: 10.1034/j.1399-0012.2000.140204.x. [DOI] [PubMed] [Google Scholar]
- 5.Baudard M, Vincent A, Moreau P, et al. Mycophenolate mofetil for the treatment of acute and chronic GVHD is effective and well tolerated but induces a high risk of infectious complications: a series of 21 BM or PBSC transplant patients. Bone Marrow Transplant. 2002 Sep;30( 5):287–95. doi: 10.1038/sj.bmt.1703633. [DOI] [PubMed] [Google Scholar]
- 6.Bolwell B, Sobecks R, Pohlman B, et al. A prospective randomized trial comparing cyclosporine and short course methotrexate with cyclosporine and mycophenolate mofetil for GVHD prophylaxis in myeloablative allogeneic bone marrow transplantation. Bone Marrow Transplant. 2004 Oct;34( 7):621–5. doi: 10.1038/sj.bmt.1704647. [DOI] [PubMed] [Google Scholar]
- 7.Bornhauser M, Schuler U, Porksen G, et al. Mycophenolate mofetil and cyclosporine as graft-versus-host disease prophylaxis after allogeneic blood stem cell transplantation. Transplantation. 1999 Feb 27;67( 4):499–504. doi: 10.1097/00007890-199902270-00001. [DOI] [PubMed] [Google Scholar]
- 8.Giaccone L, McCune JS, Maris MB, et al. Pharmacodynamics of mycophenolate mofetil after nonmyeloablative conditioning and unrelated donor hematopoietic cell transplantation. Blood. 2005 Dec 15;106( 13):4381–8. doi: 10.1182/blood-2005-06-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiehl MG, Schafer-Eckart K, Kroger M, et al. Mycophenolate mofetil for the prophylaxis of acute graft-versus-host disease in stem cell transplant recipients. Transplant Proc. 2002 Nov;34( 7):2922–4. doi: 10.1016/s0041-1345(02)03489-9. [DOI] [PubMed] [Google Scholar]
- 10.Maris MB, Niederwieser D, Sandmaier BM, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood. 2003 Sep 15;102( 6):2021–30. doi: 10.1182/blood-2003-02-0482. [DOI] [PubMed] [Google Scholar]
- 11.Mohty M, de Lavallade H, Faucher C, et al. Mycophenolate mofetil and cyclosporine for graft-versus-host disease prophylaxis following reduced intensity conditioning allogeneic stem cell transplantation. Bone Marrow Transplant. 2004 Sep;34( 6):527–30. doi: 10.1038/sj.bmt.1704640. [DOI] [PubMed] [Google Scholar]
- 12.Nash RA, Johnston L, Parker P, et al. A phase I/II study of mycophenolate mofetil in combination with cyclosporine for prophylaxis of acute graft-versus-host disease after myeloablative conditioning and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2005 Jul;11( 7):495–505. doi: 10.1016/j.bbmt.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Neumann F, Graef T, Tapprich C, et al. Cyclosporine A and mycophenolate mofetil vs cyclosporine A and methotrexate for graft-versus-host disease prophylaxis after stem cell transplantation from HLA-identical siblings. Bone Marrow Transplant. 2005 Jun;35( 11):1089–93. doi: 10.1038/sj.bmt.1704956. [DOI] [PubMed] [Google Scholar]
- 14.van Hest RM, Doorduijn JK, de Winter BC, et al. Pharmacokinetics of mycophenolate mofetil in hematopoietic stem cell transplant recipients. Ther Drug Monit. 2007 Jun;29( 3):353–60. doi: 10.1097/FTD.0b013e31805d8816. [DOI] [PubMed] [Google Scholar]
- 15.Vogelsang GB, Arai S. Mycophenolate mofetil for the prevention and treatment of graft-versus-host disease following stem cell transplantation: preliminary findings. Bone Marrow Transplant. 2001 Jun;27( 12):1255–62. doi: 10.1038/sj.bmt.1703076. [DOI] [PubMed] [Google Scholar]
- 16.Knight SR, Morris PJ. Does the evidence support the use of mycophenolate mofetil therapeutic drug monitoring in clinical practice? A systematic review. Transplantation. 2008 Jun 27;85( 12):1675–85. doi: 10.1097/TP.0b013e3181744199. [DOI] [PubMed] [Google Scholar]
- 17.Premaud A, Debord J, Rousseau A, et al. A double absorption-phase model adequately describes mycophenolic acid plasma profiles in de novo renal transplant recipients given oral mycophenolate mofetil. Clin Pharmacokinet. 2005;44( 8):837–47. doi: 10.2165/00003088-200544080-00005. [DOI] [PubMed] [Google Scholar]
- 18.Premaud A, Le Meur Y, Debord J, et al. Maximum a posteriori bayesian estimation of mycophenolic acid pharmacokinetics in renal transplant recipients at different postgrafting periods. Ther Drug Monit. 2005 Jun;27( 3):354–61. doi: 10.1097/01.ftd.0000162231.90811.38. [DOI] [PubMed] [Google Scholar]
- 19.Le Meur Y, Buchler M, Thierry A, et al. Individualized mycophenolate mofetil dosing based on drug exposure significantly improves patient outcomes after renal transplantation. Am J Transplant. 2007 Nov;7( 11):2496–503. doi: 10.1111/j.1600-6143.2007.01983.x. [DOI] [PubMed] [Google Scholar]
- 20.Saint-Marcoux F, Debord J, Hoizey G, et al. Statistics of MMF dose adjustment on ISBA, a free website for dose adjustment of immunosuppressive drugs [abstract] Ther Drug Monit. 2007;29( 4):241. [Google Scholar]
- 21.Maris MB, Sandmaier BM, Storer BE, et al. Unrelated donor granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cell transplantation after nonmyeloablative conditioning: the effect of postgrafting mycophenolate mofetil dosing. Biol Blood Marrow Transplant. 2006 Apr;12( 4):454–65. doi: 10.1016/j.bbmt.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 22.Haentzschel I, Freiberg-Richter J, Platzbecker U, et al. Targeting mycophenolate mofetil for graft-versus-host disease prophylaxis after allogeneic blood stem cell transplantation. Bone Marrow Transplant. 2008 Jul;42( 2):113–20. doi: 10.1038/bmt.2008.85. [DOI] [PubMed] [Google Scholar]
- 23.Larosa F, Marmier C, Robinet E, et al. Peripheral T-cell expansion and low infection rate after reduced-intensity conditioning and allogeneic blood stem cell transplantation. Bone Marrow Transplant. 2005 May;35( 9):859–68. doi: 10.1038/sj.bmt.1704889. [DOI] [PubMed] [Google Scholar]
- 24.Na-Bangchang K, Supasyndh O, Supaporn T, et al. Simple and sensitive high-performance liquid chromatographic. J Chromatogr B Biomed Sci Appl. 2000 Jan 28;738( 1):169–73. doi: 10.1016/s0378-4347(99)00487-9. [DOI] [PubMed] [Google Scholar]
- 25.Zahr N, Amoura Z, Debord J, et al. Pharmacokinetic study of mycophenolate mofetil in patients with systemic lupus erythematosus and design of Bayesian estimator using limited sampling strategies. Clin Pharmacokinet. 2008;47( 4):277–84. doi: 10.2165/00003088-200847040-00005. [DOI] [PubMed] [Google Scholar]
- 26.Debord J, Risco E, Harel M, et al. Application of a gamma model of absorption to oral cyclosporin. Clin Pharmacokinet. 2001;40( 5):375–82. doi: 10.2165/00003088-200140050-00004. [DOI] [PubMed] [Google Scholar]
- 27.Saint-Marcoux F, Knoop C, Debord J, et al. Pharmacokinetic study of tacrolimus in cystic fibrosis and non-cystic fibrosis lung transplant patients and design of Bayesian estimators using limited sampling strategies. Clin Pharmacokinet. 2005;44( 12):1317–28. doi: 10.2165/00003088-200544120-00010. [DOI] [PubMed] [Google Scholar]
- 28.Proost JH, Eleveld DJ. Performance of an iterative two-stage bayesian technique for population pharmacokinetic analysis of rich data sets. Pharm Res. 2006 Dec;23( 12):2748–59. doi: 10.1007/s11095-006-9116-0. [DOI] [PubMed] [Google Scholar]
- 29.Steimer JL, Mallet A, Golmard JL, et al. Alternative approaches to estimation of population pharmacokinetic parameters: comparison with the nonlinear mixed-effect model. Drug Metab Rev. 1984;15( 1–2):265–92. doi: 10.3109/03602538409015066. [DOI] [PubMed] [Google Scholar]
- 30.D’Argenio DZ. Incorporating prior parameter uncertainty in the design of sampling schedules for pharmacokinetic parameter estimation experiments. Math Biosci. 1990 Apr;99( 1):105–18. doi: 10.1016/0025-5564(90)90141-k. [DOI] [PubMed] [Google Scholar]
- 31.Sheiner LB, Beal SL. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm. 1981 Aug;9( 4):503–12. doi: 10.1007/BF01060893. [DOI] [PubMed] [Google Scholar]
- 32.Ishibashi T, Yano Y, Oguma T. Population pharmacokinetics of platinum after nedaplatin administration and model validation in adult patients. Br J Clin Pharmacol. 2003 Aug;56( 2):205–13. doi: 10.1046/j.1365-2125.2003.01871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irtan S, Saint-Marcoux F, Rousseau A, et al. Population pharmacokinetics and bayesian estimator of cyclosporine in pediatric renal transplant patients. Ther Drug Monit. 2007 Feb;29( 1):96–102. doi: 10.1097/FTD.0b013e3180310f9d. [DOI] [PubMed] [Google Scholar]
- 34.Jacobson P, Rogosheske J, Barker JN, et al. Relationship of mycophenolic acid exposure to clinical outcome after hematopoietic cell transplantation. Clin Pharmacol Ther. 2005 Nov;78( 5):486–500. doi: 10.1016/j.clpt.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 35.Huang J, Jacobson P, Brundage R. Prediction of unbound mycophenolic acid concentrations in patients after hematopoietic cell transplantation. Ther Drug Monit. 2007 Aug;29( 4):385–90. doi: 10.1097/FTD.0b013e318074d979. [DOI] [PubMed] [Google Scholar]
- 36.Ng J, Rogosheske J, Barker J, et al. A limited sampling model for estimation of total and unbound mycophenolic acid (MPA) area under the curve (AUC) in hematopoietic cell transplantation (HCT) Ther Drug Monit. 2006 Jun;28( 3):394–401. doi: 10.1097/01.ftd.0000211821.73231.8a. [DOI] [PubMed] [Google Scholar]
- 37.Guidance for industry on population pharmacokinetics. U.S. Department of Health Services, Food and Drug Administration; 1999. [cited; Available from: http://www.fda.gov/Cder/guidance/1852fnl.pdf. [Google Scholar]


