Abstract
In this study, we assessed the ability of a highly tumor-selective oncolytic vaccinia virus armed with a yeast cytosine deaminase gene to infect and lyse human and murine ovarian tumors both in vitro and in vivo. The virus vvDD-CD could infect, replicate in and effectively lyse both human and mouse ovarian cancer cells in vitro. In two different treatment schedules involving either murine MOSEC or human A2780 ovarian carcinomatosis models, regional delivery of vvDD-CD selectively targeted tumor cells and ovarian tissue, effectively delaying the development of either tumor or ascites and leading to significant survival advantages. Oncolytic virotherapy using vvDD-CD in combination with the prodrug 5-fluorocytosine (5-FC) conferred an additional long-term survival advantage upon tumor-bearing immunocompetent mice. These findings demonstrate that a tumor-selective oncolytic vaccinia combined with gene-directed enzyme prodrug therapy (GDEPT) is a highly effective strategy for treating advanced ovarian cancers in both syngeneic mouse and human xenograft models. Given the biological safety, tumor selectivity and oncolytic potency of this armed oncolytic virus, this dual therapy merits further investigation as a promising new treatment for metastatic ovarian cancer.
Keywords: poxvirus, ovarian cancer, peritoneal carcinomatosis, suicide gene, cytosine deaminase
INTRODUCTION
Ovarian cancer is the second most common gynecological malignancy, afflicting 22,430 women in the U. S. and 190,000 women worldwide annually. The majority of women present with FIGO stage 3 or 4 disease and approximately 15,280 women will die of the advanced disease in the U.S. in 2007.1 Current therapy for advanced ovarian cancer consists of surgical cytoreduction, intraperitoneal chemotherapy and systemic administration of paclitaxel and platinum containing chemotherapy regimens.2,3 Metastatic disease is frequently isolated to the peritoneal cavity; this offers a unique opportunity for localized therapy such as intraperitoneal chemotherapy. Unfortunately the majority of patients will develop recurrent disease despite aggressive therapy. The prevalence and mortality associated with epithelial ovarian malignancies has led to the development of alternative treatment strategies with the aim to reduce recurrence and improve survival.
Oncolytic viruses and gene therapy have been actively investigated as a new way for treating cancer. 4–8 Early clinical trials with the oncolytic adenovirus Onyx-015 in human cancer patients have shown that single agent efficacy was relatively limited. However, when combined with cisplatin and 5-fluorouracil (5-FU) chemotherapy, effective antitumoral activity has been demonstrated.9 A phase III study of oncolytic adenovirus H101 plus cisplatin and 5-FU for the treatment of head and neck squamous cell carcinoma and esophageal cancer showed significant improvements in clinical outcomes;10 this paved the way for China to approve H101 as the first oncolytic virus in the world for clinical use. Another recent study has shown that a suicide gene-armed oncolytic adenovirus along with 5-fluorocytosine (5-FC) plus gancyclovir prodrug therapy may provide a potential long-term benefit to patients of prostate cancer.11 Together, these results suggest that combination therapies with oncolytic viruses may prove beneficial to some cancer patients and are worth further investigation.
A few oncolytic viruses have been investigated for the treatment of ovarian cancer in mouse models. The recombinant viruses used in ovarian cancer models have included herpes simplex virus, adenovirus, measles virus, reovirus, vesicular stomatitis virus, and sindbis virus.12–17 While some promising results have been obtained in these and later studies, additional improvements will be required before the clinical promises of such therapies can be realized.
Vaccinia virus (VV) has many attributes that make it an attractive vector for tumor directed gene therapy and oncolytic virotherapy.18–21 Systemic delivery of wild type and tumor-selective VV in animal models have shown that the highest level of infectious virus is isolated from the tumor and ovary, with little to no viral recovery from other normal organs when a tumor-selective VV was used.22–25 We have shown that a number of tumor-selective VV can inhibit cancer models after systemic delivery in both nude mice and immunocompetent mice.25–27 Toxicity studies indicate that the highly tumor-selective vvDD is safe in nonhuman primates.28 Most importantly, preliminary results from phase I/II clinical trials suggest that oncolytic VV are effective in human cancer patients.8 Taken together, these results encouraged us to further investigate the oncolytic virus vvDD and its derivatives in additional tumor models.
There are multiple ways to enhance the potency of an oncolytic VV. One way is to arm the virus with an immunostimulatory factor such as GM-CSF.29 Another method is to arm it with a gene-directed enzyme prodrug therapy (GDEPT).7,30,31 Other investigators have shown that combined virus/prodrug treatment may enhance the replication/spread of oncolytic adenovirus in mouse tumor models.32,33 As for VV, we have previously combined tk gene deleted VV with bacterial CD/5-FC, or purine nucleoside phosphorylase gene and 6-methyl purine deoxyribonucleoside.34,35 Even though positive results on tumor regression using these viruses were obtained, complex interactions between the viral oncolytic effect and the enzyme/prodrug effect occurred in the context of oncolytic VV.36 Among a list of enzyme/prodrug systems,30,31 we selected the yeast CD/5-FC system over other potential GDEPT systems for our new virus used in this study, based on the superior properties of yeast CD/5-FC combination,37 as well as the extensive experience associated with clinical use of 5-FU in cancer chemotherapy. A major limitation for the use of bacterial CD is that it is inefficient in the conversion of 5-FC into 5-FU. Kievit et al. have shown that the yeast CD has a much higher 5-FC/5-FU conversion rate compared to bacterial CD.37 In addition to the proven reliability and effectiveness of 5-FU in gastrointestinal cancers, the yeast CD/5-FC system is a compelling choice for ovarian peritoneal tumors because of the excellent diffusibility of the prodrug 5-FC, and the likelihood that the tumor has not been previously exposed to the active chemotherapeutic agent 5-FU.
In this study, our aim was to first demonstrate the effectiveness of the highly tumor-selective vvDD-CD, based on the combined results of the oncolytic effects from the virus per se and the chemotherapeutic effect of yeast CD with 5-FC on ovarian cancer cells in vitro. Next we applied this oncolytic virus and CD/5-FC enzyme/prodrug system to treat models of early and late ovarian carcinomatosis of both human and mouse origins. We demonstrated for the first time that an oncolytic virus combined with GDEPT worked effectively in ovarian carcinomatosis models, representing the treatment of early and late stage ovarian carcinomatosis.
MATERIALS AND METHODS
Cell culture
Human ovarian cancer cell lines A2780, OVCAR-3, and IGROV-1 were obtained from the American Type Culture Collection (Manassas, VA). Human cells derived from tissue specimens of ovarian cancer patients (HGOP-1-680 and TPO2-738R) were kind gifts from Dr. DeLoia at the University of Pittsburgh. The MOSEC cancer cell line (clone ID8) was a kind gift from Dr. K. Roby of the University of Kansas.38 Other cell lines HeLa, MC38, CV-1 cells and human primary fibroblast cells MRC-5 (ATCC) have been frequently used in our laboratory. All cell lines were maintained in DMEM supplemented with 10% heat-inactivated bovine calf serum, 2 mM glutamine, and penicillin/streptomycin (100 units/ml and 100 μg/ml, respectively) in an incubator at 37°C with 5% CO2, and serially passaged every 3 to 4 days.
Recombinant VV
The tumor-selective vaccinia, vvDD (or called vvDD-GFP), containing the deletion of dual viral genes encoding thymidine kinase (TK) and vaccinia growth factor (VGF), has been created and characterized previously.24 To make a vvDD-CD which expresses the FCY1 gene encoding cytosine deaminase from Saccharomyces cerevisiae (GenBank accession no. U55193),39 the full coding sequence of the gene was amplified from genomic DNA of S. cerevisiae via PCR using pfu DNA polymerase (Stratagene, La Jolla, CA), and the correct gene sequence was confirmed by DNA sequencing. The FCY1 gene was ligated into pCB02311, resulting in pMP-CD, in which the fcy1 gene is driven by the strong synthetic early late promoter for vaccinia.40 To create the new virus vvDD-CD, CV-1 cells were transfected with the shuttle plasmid pMP-CD, and then infected with virus vSC20 that contains a genetic deletion in the vgf locus.25 Recombinant VV was subsequently isolated from the cells by mycophenolic acid selection, and the final viral construct was confirmed with DNA sequencing of viral genomic DNA.
Assay for CD enzymatic activity
An enzymatic assay for CD activity was performed as described by Hirschowitz et al,41 with the exception that the incubation time for the enzymatic conversion of 5-FC to 5-FU was shortened from 24 h to 0.5 h. The enzymatic activity was determined by spectrometry.
In vitro viral growth assay
IGROV-1, OVCAR-3, A2780 and MC38 cells were infected with 200 pfu (MOI ~ 0.0005) of vvDD-CD in 1 ml of MEM-2.5% FCS for 2 h at 37°C. MOSEC cells and normal MRC-5 cells were infected with either vvDD or vvDD-CD at an MOI = 0.1 for 2 hours. Subsequently, cells were washed with 1 × PBS. DMEM with 10% FCS was then added and cells were incubated until harvesting at various time points after infection ranging from 6 to 96 hours post infection. After three freeze-thaw cycles, virus was quantified by plaquetitering on CV-1 cells as described previously.25, 26
In vitro cytotoxicity assays
The viability of cells infected with various amounts of vvDD or vvDD-CD alone or in combination with 5-FC was assessed using an MTS assay per the manufacturer’s protocol (Promega Corp, Madison, WI). Briefly, 5.0 × 103 cells were plated in triplicate in 96 well flat bottom tissue culture plates overnight (Costar, Corning, NY). Varying amounts of virus was then added to the cells followed by incubation for 6, 18, 24, 48 and 72 hr at 37°C. Subsequently, the dye solution was added to each well and allowed to incubate for another 0.5 to 4 hr at 37°C. The optical absorbance of the samples was then read at the wavelength of 490 nm.
Mice
Female athymic nude mice and immunocompetent C57BL/6 mice of 5–6 weeks old, were obtained from the Taconic Corporation (Germantown, NY). They were housed in standard conditions and given food and water ad libitum. All animal studies were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh.
Biodistribution of the viruses in mice
C57BL/6 mice (female; 6–7 weeks old) were injected intraperitoneally (i.p) with 7.5 × 106 MOSEC cancer cells in 0.5 ml HBSS. Fifteen days after injection of MOSEC cells, the mice were injected i.p. with 1.0 × 108 pfu of vvDD-CD in 0.5 ml HBSS. At various time points after viral administration (4, 8 and 12 days), mice were sacrificed and whole sections of brain, liver, spleen, lung, ovary, and tumor were taken and homogenized in HBSS and kept at −70°C until use. Dilutions of the homogenate were incubated on CV-1 cells in 6 well plates at 37°C in 5% CO2, and titers were determinedas described previously. Viral titers were standardized to totalprotein. Data is expressed as pfu virus per mg total protein.
Xenograft model of human ovarian carcinomatosis
In the xenograft model of ovarian carcinomatosis, nude mice were injected i.p. with 1.0 × 106 A2780 ovarian cancer cells suspended in 100 μl of PBS. Mice were then treated with 1.0 × 108 pfu of vvDD suspended in 500 μl of PBS (n = 10) either immediately after or 10 days following tumor cell injection. Mice in the early treatment group were euthanized at 41 days when the majority of control animals had met IACUC criteria for euthanasia. Animals were necropsied and examined for the presence of peritoneal carcinomatosis. The incidence of tumor formation between groups was compared using the x2 test. Survival data for the delayed treatment group was plotted using the method of Kaplan-Meier with results compared using log-rank test.
Immunocompetent model of ovarian carcinomatosis
Immunocompetent C57BL/6 mice were injected i.p. with 7.5 × 106 MOSEC cells suspended in 500 μl of PBS. Mice were then treated either immediately or at 30 days following tumor cell inoculation with 1.0 × 109 pfu vvDD-CD suspended in 500 μl PBS (n = 10) or PBS alone (n = 10) and monitored for survival. Survival data was then plotted on a Kaplan-Meier curve with results compared using log-rank test.
In a separate study, tumor bearing C57BL/6 mice (n = 10) treated with vvDD-CD 30 days following MOSEC cell inoculation were given i.p. injections of 5-FC (Sigma, St Louis, MO) at a concentration of 1180 kg/mg/day for 10 days following treatment with 1.0 × 109 pfu of vvDD-CD i.p. Additional groups of mice (n = 10) were treated with vvDD-CD only, 5-FC only, or vehicle (PBS) Animals were followed for survival, and the results plotted on a Kaplan-Meier curve.
Statistical analysis
Survival analysis was performed using the method of Kaplan-Meier and differences between curves were assessed using the log-rank test. P values of < 0.05 were considered statisticallysignificant in all analyses.
RESULTS
Construction of vvDD-CD
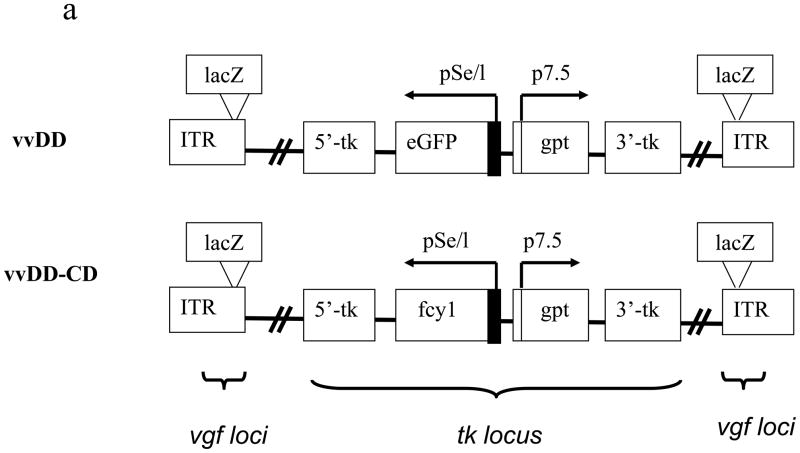
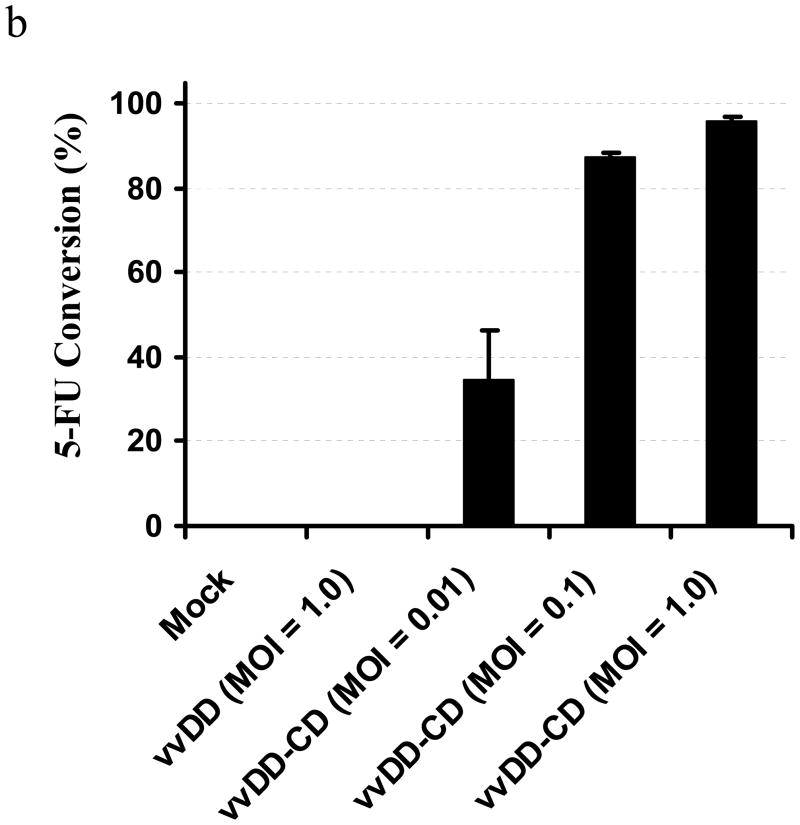
We have previously demonstrated that vvDD, a recombinant VV deleted in viral genes encoding TK and VGF, is a highly tumor-selective replicating oncolytic virus and is effective in a murine colon cancer model.25 Our goal was to enhance the oncolytic potency of the virus by arming it with an efficient and safe suicide gene/prodrug system. We selected yeast CD/5-FC based on the superior properties of yeast CD/5-FC combination.37 Therefore, we constructed a new vvDD derivative armed with the yeast gene fcy1 encoding CD. The virus, containing deletion of dual viral genes of tk and vgf and insertion of the expression cassette for the yeast fcy1 in the tk locus, is designated vvDD-CD (Figure 1a). The main difference between vvDD and vvDD-CD is that the gene encoding EGFP in vvDD is replaced with the yeast fcy1 gene encoding CD in the new virus vvDD-CD. The structure of the new virus has been confirmed by both PCR assays and DNA sequencing of the tk and vgf loci. The expression of functional CD enzyme in vvDD-CD infected cancer cells was demonstrated (Figure 1b). Only MOSEC cancer cells infected with vvDD-CD, but not mock-infected cells or cells infected with a control virus (vvDD), expressed CD enzymatic activity. The CD enzymatic activity was viral dose-dependent.
Figure 1. The new virus vvDD-CD and its expression of functional cytosine deaminase.
(a) Schematic representation of the two oncolytic VV, vvDD and vvDD-CD, utilized in the present study. Presented is the schematic representation of deletions of two viral genes for TK and VGF and the insertion of the transgenes for enhanced green fluorescent protein (eGFP) or Saccharomyces cerevisiae fcy1 gene encoding the cytosine deaminase (CD) at the tk locus in the viral genome. In the virus vvDD-CD, the lacZ genes at the vgf loci have been inactivated due to the negative selection. (b). The new virus vvDD-CD expresses high CD activity in the virus-infected MOSEC cells. MOSEC cancer cells grown in 6-well plates were mock-infected or infected with vvDD or vvDD-CD at the indicated MOI. At 24 hr postinfection, cells were harvested and extracts were made with 250 ul of lysis buffer. CD enzymatic activity in the cell extracts was measured. The values are the mean +/− SD from the triplicates.
The virus vvDD-CD replicates efficiently in ovarian cancer cells but poorly in human normal cells
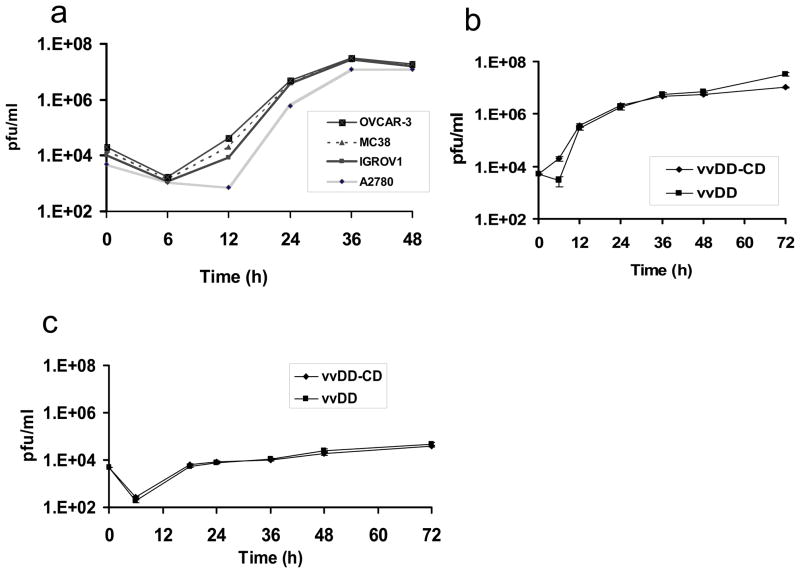
Previously, we have shown that vvDD retains full potency to replicate in cancer cells in vitro and in MC38 colon cancer in vivo. However, vvDD is attenuated in confluent NIH-3T3 cells due to lack of nucleotide TTP from non-dividing cells.25 In this study, we examined vvDD-CD viral replication in both human (A2780, OVCAR-3 and IGROV-1) and murine MC38 cancer cells (Figure 2a). Viral recovery peaked at 36 h in all 4 cancer cell lines under these conditions. We then compared vvDD-CD to its parental virus vvDD for replication in murine MOSEC ovarian cancer cells. The two viruses replicated with similar kinetics and displayed comparable total high viral yields (Figure 2b). In contrast, both viruses replicated poorly in human primary fibroblast MRC-5 cells, with yields 3–4 log lower than those from cancer cells (Figure 2c). Together, these results showed that addition of the CD gene to the virus did not change the tumor-targeting ability of the virus, and that vvDD-CD can replicate at high efficiency in both human and murine ovarian cancer cells in vitro.
Figure 2. In vitro viral growth curves of vvDD-CD and vvDD in cancer cells and normal human cells.
The replication of vvDD-CD in human ovarian cancer cells (a), and a comparison of vvDD and vvDD-CD in murine MOSEC cancer cells (b) and human primary normal MRC-5 cells (c) are presented. The viral dose was MOI = 0.1 for all cells. Viral yields were determined by plaque assays in CV-1 cells. The values are the mean +/− SD from the triplicates.
vvDD-CD exhibits and addition of 5-FC enhances the cytotoxicity against ovarian cancer cells
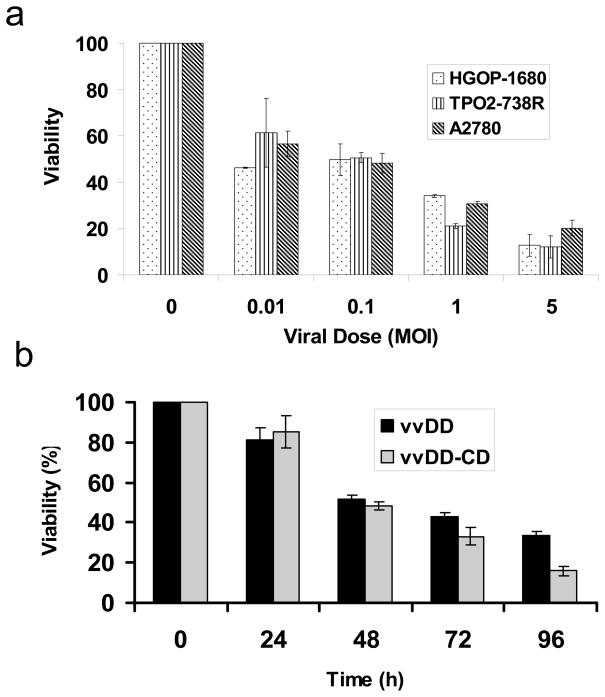
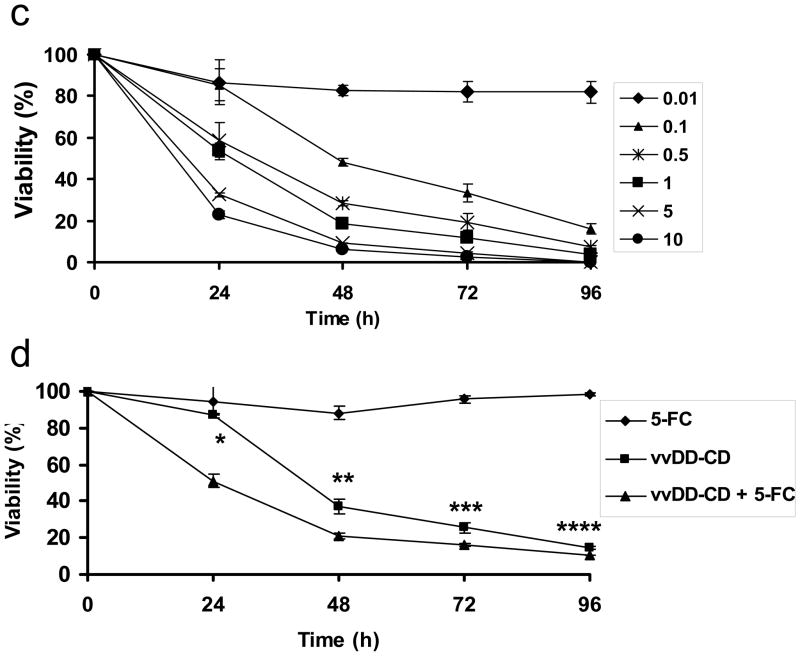
We have analyzed the selective cytotoxicity against both human and murine ovarian tumor cells in the absence or presence of the prodrug 5-FC. Primary human ovarian cancer cells (HGOP-680 and TP02-738R) and a human ovarian cancer cell line (A2780) were plated in 96-well plates overnight and then infected with vvDD-CD at different MOI as indicated. Cell viability at various times after infection was determined by an MTS assay. As shown in Figure 3a, ovarian cancer cells died in response to the dose of viral infection. When infected with vvDD-CD at an MOI of 0.1, about 50% tumor cells remained viable at 48 hours following infection. However, when the viral dose increased to MOI of 5, then the cell viability reduced to 10–20% at 48 h post-infection, depending on the particular cells. The cytotoxicity of the virus is also time dependent, as demonstrated with MOSEC cells infected with either vvDD or vvDD-CD at an MOI of 0.1 (Figure 3b). vvDD-CD and vvDD showed similar kinetics of cancer cell killing at an MOI of 0.1 in addition to MOIs ranging from 0.01–10 (data not shown). We then explored the viral doses and combination treatment with 5-FC on mouse ovarian cancer cells (Figure 3c and 3d). Mouse MOSEC cancer cells were infected with various doses of vvDD-CD in the absence of 5-FC, and the cytotoxicity at different time points after infection was determined by MTS assay. Data in Figure 3c illustrated that cytotoxicity of the virus vvDD-CD to MOSEC cells was both viral dose and incubation-time dependent. Finally we have examined the effect of combination treatment (Figure 3d). MOSEC cells were not sensitive to 5-FC at the dose tested [0.5mM]; MOSEC cells displayed little cytotoxicity at 5-FC doses up to 5mM (data not shown). Treatment with vvDD-CD at MOI = 0.1 alone reduced cell viability to 40% at 48 h postinfection, and gradually to ~18% at 96 h. The addition of 5-FC to the medium enhanced the cytotoxicity at time points of 24 (p<0.01), 48 (p<0.02), 72 (p<0.04) and 96 hr (p<0.05).
Figure 3. Cytotoxicity of the oncolytic virus vvDD-CD and vvDD against ovarian tumor cells.
(a). Human primary ovarian tumor cells (HGOP-1680 and TPO2-738R) were compared with a human ovarian tumor cell line (A2780) following infection with increasing doses of vvDD-CD. The cell viability at 48 h post infection was determined by MTS assays. (b). MOSEC murine ovarian cancer cells were treated with vvDD-CD and vvDD at MOI = 0.1 in the absence of 5-FC and cell viability was measured with a MTS assay. The data showed infected cell viability was time-dependent. (c). A viral dose- and time-dependent cytotoxicity by vvDD-CD on MOSEC cells. (d). Synergistic cytotoxic effect of vvDD-CD and 5-FC on MOSEC cancer cells. MOSEC cells treated with none, 5-FC (0.5 mM), vvDD-CD (MOI = 0.1) or the dual treatment over time. Combination treatment showed increased cytotoxicity compared to vvDD-CD alone. The values are the mean +/−SEM. Data are representative of three independent experiments. (* p<0.01, ** p<0.02, *** p<0.04, **** p<0.05)
These results demonstrated viral dose- and time-dependent cell cytotoxicity in human and murine ovarian cancer cells. At low viral doses (below an MOI of 0.5) the addition of 5-FC to the medium enhanced the cytotoxicity of the virus to the cancer cells. However, due to the fact that VV is a very rapidly growing and efficient virus, its oncolytic potency by itself is quite high. As a result, any additive or synergistic effects with 5-FC are less pronounced compared to those of combination viral/prodrug therapies with other viral systems such as adenovirus.
Viral pathogenecity and biodistribution in mice
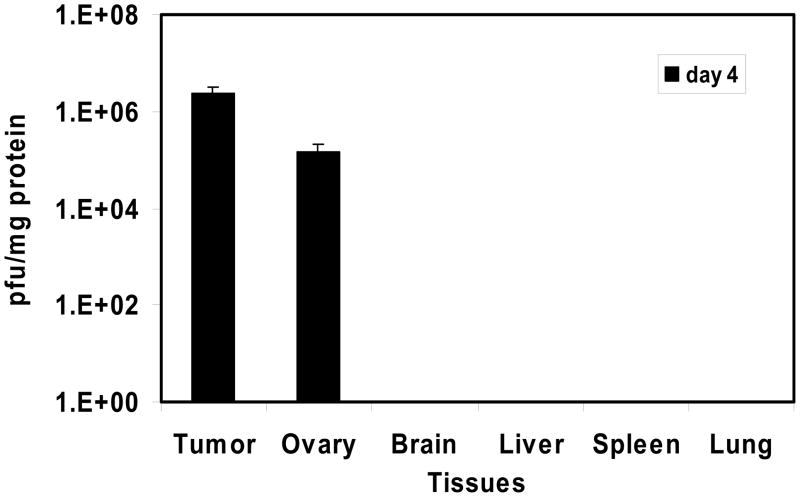
The pathogenecity and biodistribution of VV in naïve and tumor-bearing nude mice were investigated. We have previously addressed this with vvDD in immunodeficient mice only.25 In this study represents the first time we have examined biodistribution of an oncolytic VV in tumor-bearing immunocompetent mice. The tissue biodistribution of vvDD-CD in tumor-bearing mice was examined utilizing MOSEC-tumor-bearing C57BL/6 mice injected i.p. with 1.0 × 108 pfu of vvDD-CD at day 15 following tumor cell inoculation. At 4, 8 and 15 days after virus injection, mice were sacrificed, samples of normal tissues and tumor were harvested, and infectious viruses were titered on CV-1 cells, with viral yield (pfu/mg) calculated based on protein levels in tissue samples (Figure 4). Remarkably, vvDD-CD was recovered only from tumor and ovarian tissues at high levels on day 4, but not detectable in any other normal tissues including brain, liver, spleen and lung. No virus was recovered from any tissues on days 8 and 16. We wondered if the virus would replicate in other normal tissues, thus we have performed biodistribution assays with intravenous injection of 1.0 × 108 pfu of vvDD-CD in naïve C57BL/6 mice. Tissues were harvested on day 5, and the presence of viruses in tissues was examined by plaque assay on CV-1 cells. No virus was detected in any of the normal tissues we have examined, including brain, blood, bone marrow, testis (male), small intestine, spleen, liver and muscle (data not show). We were initially surprised by the lack of viral recovery from bone marrow. However, in a previous study, we did not recover any virus after intravenous delivery of 1.0 × 108 pfu vvDD in immunocompetent Rhesus macques,28 even though we recovered 5.0 × 102 pfu/mg protein in bone marrow cells in immunodeficient mice.25 In summary, we showed that vvDD-CD was highly tumor-targeted in naïve immunocompetent mice. It may be that the innate and adaptive immunity of the host restricts VV replication in normal tissues, thus further enhancing its tumor specificity.
Figure 4. Viral biodistribution following intraperitoneal inoculation of 1.0 × 108 pfu of vvDD-CD in MOSEC ovarian carcinomatosis model.
The experiment was performed as described in Materials and Methods. Infectious viral particles were detected in tumor cells following peritoneal lavage on day 4 following viral administration. Virus was also recovered from ovary but was not found in any other normal tissues examined. No virus was recovered in any tissues on days 8 and 15. The data are the median values +/− SD of each treatment (n = 5/timepoint).
Therapeutic effects on ovarian carcinomatosis models
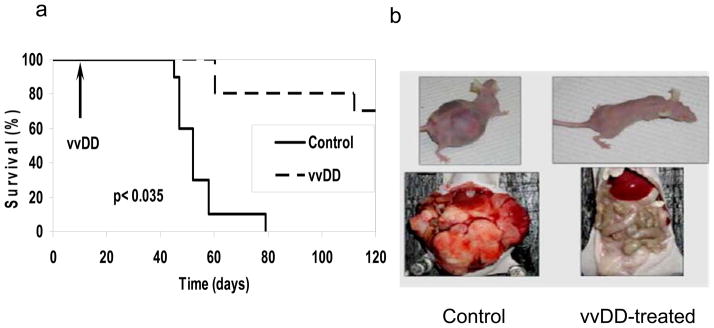
The effect of the vvDD-CD virus on both human and mouse ovarian carcinomatosis models was examined. In a xenograft model, nude mice with either incipient or established peritoneal carcinomatosis of A2780 human ovarian tumor cells were treated with vehicle alone (saline) or with 1.0 × 108 pfu of vvDD i.p. Initially we conducted a pilot experiment in which vvDD was administered immediately following tumor cell inoculation. At the time of sacrifice on day 50, 9 of the 15 animals in the control group developed tumor while only 2 of the 15 treated nude mice developed visible evidence of disease. The median tumor weight in the control group was 54 mgs compared to 0 mg in the vvDD-treated group (data not shown). This preliminary experiment suggested that this tumor-selective virus may be efficacious for ovarian cancer. Next we carefully evaluated the efficacy and safety of the oncolytic VV in a delayed treatment model. Nude mice treated with 1.0 × 108 pfu of vvDD (n = 10) at 10 days following tumor cell inoculation had a significantly better survival (p < 0.0002) when compared with PBS treated controls (n = 10; Figure 5a). Grossly, untreated mice demonstrated significant abdominal tumors as compared with treated mice (Figure 5b). Although all control (untreated) mice died by day 79 following tumor inoculation, 70% of the animals in the treated group survived until completion of the experiment (120 days post tumor cell inoculation). Necropsies performed at day 120 indicated that none of the surviving animals (7/10) in the vvDD treatment group displayed any gross evidence of tumor formation.
Figure 5. Delayed treatment with vvDD resulted in both decreased tumor burden and prolonged survival in a xenograft model of peritoneal carcinomatosis.
(a) Nude mice were injected with 1.0 × 106 human ovarian cancer A2780 cells to allow for growth of peritoneal ovarian tumors. On day 10 following inoculation of tumor cells, 1.0 × 108 pfu of vvDD was administered i.p. The toxicity of the virus and the growth of tumor and survival of mice were monitored daily. The survival data were analyzed by log rank test (p < 2.0 × 10e-5; control vs. vvDD). (b). The appearance and tumor growth of representative mice in both control and treated groups. Shown are one mouse in control group on day 79 and a vvDD virus-treated mouse on day 120.
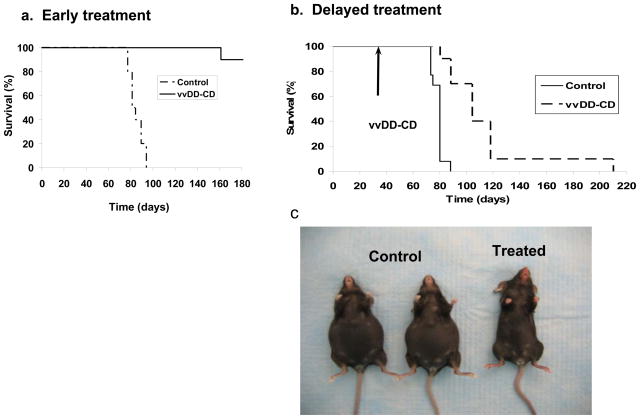
In human patients, ovarian cancer tends to present in late stages due to its asymptomatic presentation until peritoneal metastases have occurred. Current therapy includes tumor debulking followed by intraperitoneal chemotherapy.2,3 We wished to simulate treatment of the peritoneal cavity after debulking to prove that instillation of vaccinia at the time of surgery could prevent the formation of caricinomatosis by eradicating free tumor cells. Therefore, we have designed an early treatment mode in the MOSEC ovarian carcinomatosis model that would mimic the situation in human cancer patients. The MOSEC cancer line, derived from a C57BL/6 mouse, leads to a highly malignant neoplasm containing both carcinomatous and sarcomatous components as well as production of hemorrhagic ascitic fluid following intraperitoneal inoculation. These characteristics make this model particularly attractive as it mimics the disease seen in women with late stage ovarian cancer.38 Thus, we performed experiments utilizing this murine ovarian carcinomatosis model in syngeneic C57BL/6 mice (Figure 6). The MOSEC tumor-bearing C57BL/6 mice treated with vvDD-CD (1.0 × 109 pfu/mouse) on the day of tumor cell inoculation displayed a highly significant survival advantage compared to controls (p< 1.0 × 10e-6) (Figure 6a). In a delayed treatment model, viral treatment was not performed until day 30 following inoculation of MOSEC cells to allow for establishment of this slow-growing tumor in vivo. This delayed mode of vvDD-CD treatment also resulted in a significantly improved survival when compared with untreated controls (p< 0.000008). Despite the delay in treatment, 70% virus-treated mice were alive at day 100 while all control mice had died by day 86. One vvDD-CD treated mouse survived for greater than 200 days and displayed no signs of disease at time of sacrifice (210 days) (Figure 6b). In this case, the dramatic difference in appearance of the mice between control and treated group is evident (Figure 6c).
Figure 6. Early or delayed treatment (day 30) with vvDD in the absence of 5-FC resulted in reduced tumor burden and improved survival in an immunocompetent murine ovarian tumor model.
(a). C57BL/6 mice were injected with 7.5 × 106 MOSEC cells to allow for growth of peritoneal ovarian tumors. Early treatment (day 0) consisted of 1.0 × 109 pfu of vvDD-CD in order to overcome the immunocompetent nature of the mice (p < 1.0 × 10e-5; control vs. vvDD-CD). (b). The delayed treatment mode (virus administration on day 30) revealed that vvDD-CD was also effective (p< 8.0 × 10e-6; control vs. vvDD-CD). (c). The physical appearance of representative control and vvDD-CD treated mice (day 80) in the delayed treatment model.
VV-mediated oncolysis is further enhanced by GDEPT with 5-FC treatment
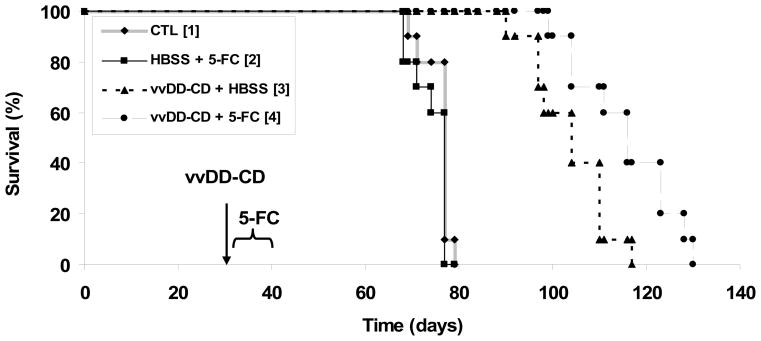
The virus vvDD-CD expresses the potent yeast enzyme cytosine deaminase from a strong viral promoter. The CD enzyme converts non-toxic prodrug 5-FC to a cytotoxic drug 5-FU in virus-infected cancer cells. One of the major advantages of a suicide gene therapy is the bystander effect. We have shown earlier in this study that the virus produced a functional CD enzyme (Figure 1b) and that the addition of 5-FC to the medium enhanced the cytotoxicity of the virus in infected cancer cells in vitro (Figure 3c). In order to investigate if the two therapeutic modalities could be combined for better efficacy, MOSEC tumor-bearing mice were treated with vvDD-CD (1.0 × 109 pfu/mouse) on day 30 and then followed by 5-FC administration for 10 days. Identical control groups of tumor bearing mice were treated with either vehicle (HBSS), 5-FC alone, or vvDD-CD alone. All animals were monitored for survival, and the resulting data was analyzed by log-rank test. These data presented in Figure 7 clearly reveal that dual therapy conferred a further improvement in survival compared with the vvDD-CD montherapy (p<0.0075). As expected, vvDD-CD monotherapy showed a significant inhibitory effect on tumor growth and animal survival (p<0.000008 vs. vehicle [HBSS] control), while 5-FC treatment alone showed no improvement over the control group (p < 0.22).
Figure 7. Actuarial survival of C57BL/6 mice after intraperitoneal administration of vvDD-CD and subsequent treatment with 5-FC.
MOSEC tumor-bearing mice were treated with HBSS or vvDD-CD at 1.0 × 109 pfu per mouse on day 30 after tumor cell inoculation. On day 4 following vvDD-CD administration, animals were treated with 5-FC or vehicle as described in Materials and Methods. The groups of mice are, [1], control; [2], HBSS + 5-FC; [3]: vvDD-CD + HBSS; and [4], vvDD-CD + 5-FC. Survival data is displayed on a Kaplan-Meier plot, and differences in survival were determined by log rank test. The p values between different groups are, [1] vs. [2], p< 0.224; [1] vs. [3], p < 8.0 × 10e-6; [1] vs. [4], p < 8.0 × 10e-6; [2] vs. [3], p < 1.9 × 10e-5; [2] vs. [4], p < 1.9 × 10e-5; and [3] vs. [4], p < 0.0075.
DISCUSSION
In this work, we have studied the highly tumor-selective oncolytic vvDD derivative armed with GDEPT for treating advanced ovarian cancer of both human and mouse origins in murine models. The selectivity of vvDD for both tumor and ovary prompted our investigation of this virus for the treatment of ovarian tumors. Indeed, we have shown that vvDD-CD is highly targeted and selectively replicated in both tumor and ovarian tissue after i.p. regional delivery. Our previous results with vvDD in nude mice showed that about 5 × 102 pfu/mg of virus was recovered from bone marrow, while new results with vvDD-CD showed no viral recovery from bone marrow in immunocompetent mice. Our toxicity study in monkeys recovered no vvDD virus from bone marrow after systemic delivery.28 Together, these data suggest that host innate and adaptive immunity against the virus helps in the clearance of the virus in normal tissue, thus further enhancing the tumor targeting index of the virus.
This oncolytic virus is efficacious in treating both human and murine ovarian tumor models. In the first treatment mode, we wanted to simulate treatment of the peritoneal cavity after debulking to prove that instillation of vaccinia at the time of surgery could prevent the formation of caricinomatosis by eradicating free tumor cells. We obtained excellent results in a syngeneic MOSEC model in C57BL/6 mice with oncolytic virotherapy in early treatment mode. In the late treatment mode, we have also observed excellent oncolytic effect from the virus, and additional survival advantage was demonstrated with combination therapy utilizing the prodrug 5-FC. vvDD-CD was also shown to be efficacious in the A2780 human ovarian carcinomatosis model.
The combination of oncolytic virus and suicide gene therapy has been an attractive strategy.7,30 Addition of a suicide gene will allow more diffuse tumor cell killing based on a bystander effect, either directly or through cytokine cascade in vivo.42,43 vvDD-CD displayed high infectivity and significant cytotoxicity to cancer cells both in vitro and in vivo. Addition of 5-FC to cancer cell cultures demonstrated efficient CD enzyme expression and high efficiency conversion of 5-FC to 5-FU. In the MOSEC ovarian carcinomatosis model, the animals treated with vvDD-CD, but no 5-FC, had significantly less tumor burden and a significant increase in survival. The dual therapy further enhanced survival. We have observed a modest synergistic effect of oncolytic virus and GDEPT in the MOSEC carcinomatosis model, perhaps less than would have been expected. This may result from a number of reasons. First, the most predominant feature of the MOSEC model in our hands is the formation of large quantities of ascites in the intraperitoneal cavity in syngeneic mice. At the time of virus administration (30 days after tumor cell inoculation) and prodrug treatment, the tumor cells are likely widely distributed in the cavity, thus it making it more difficult to obtain bystander effects. Secondly, unlike adenovirus, oncolytic VV replicated extremely quickly with the entire life cycle complete in 6 hours; the progeny virus are then available to infect neighboring cancer cells. With such rapid kinetics, the virus itself is functionally overlaps with the CD/5-FC prodrug system to kill the neighboring tumor cells. Even though there are many excellent GDEPT systems developed and available,7,30 it is hard for us to imagine that any GDEPT system would provide exceptional synergism within the context of an oncolytic VV. Third, it is very possible that administration of 5-FC inhibited replication of vvDD-CD, thus reducing the anti-tumoral cytotoxicity induced by the oncolytic virus itself. Our previous experience with a VV expressing bacterial CD indicated that 5-FC inhibited VV replication. It is ideal to engineer a system so that the oncolytic virus and prodrug therapy would work synergistically. Some studies have shown that combined virus/prodrug treatment may enhance the replication/spread of oncolytic adenoviruses in mouse tumor models.32,33 A recent study indicated that 5-FU potentiated HSV cytotoxic effects primarily through increasing viral replication by 19-fold.44
While our manuscript was in preparation, a relevant study on utilizing VV for controlling ovarian cancer models in mice was published. The study by Hung et al. has applied a wild type and a tk gene deleted VV and showed good efficacy for treating the tumor models.45 Our current work has three significant improvements over the other study. First, our oncolytic vvDD-CD is a genetically engineered tumor-targeting virus, with significantly increased tumor selectivity and thus enhanced safety. Second, vvDD-CD treatment on a well established syngeneic tumor model in immunocompetent mice 30 days after tumor cell incubation has proven to be effective in our study. This treatment modality closely mimics those of cancer patients who present with advanced stage ovarian cancer, and is perhaps more clinically relevant and could be translated into clinical applications. Third, we have used an oncolytic virus armed with a superior suicide gene, the yeast cytosine deaminase gene, thus combining the oncolytic effects of the tumor-selective virus with GDEPT for this type of cancer. We demonstrate that the combination therapy is better than the monotherapy alone. Based on the results of the present study and those of Hung et al.,45 we feel that vaccinia, due to its favorable attributes and proven safety profile, will prove to be an effective agent in the treatment of ovarian carcinomatosis. In the future, we may explore the incorporation of immunotherapy components into this VV for triple modality therapy of cancer.
Acknowledgments
We thank Drs. Ta-Chiang Liu, David Kirn and Bingliang Fang for critical reading of the manuscript, Dr. Katherine Roby for MOSEC ovarian cancer cells, and Dr. Julie A. DeLoia for primary human ovarian cancer cells. We also thank Ms. Eve Blasko for excellent administrative assistance. This work was supported in part by the NIH R01 grant CA100415, the Intramural Research Program of the NIH, and by David C. Koch Regional Cancer Therapy Center.
Footnotes
Part of this work was presented at an oral session at the 9th Annual Meeting of the American Society of Gene Therapy in Baltimore, Maryland, June 2006.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu JQ, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Alberts DS, Delforge A. Maximizing the delivery of intraperitoneal therapy while minimizing drug toxicity and maintaining quality of life. Semin Oncol. 2006;33(6 Suppl 12):S8–17. doi: 10.1053/j.seminoncol.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Wenzel LB, Huang HQ, Armstrong DK, Walker JL, Cella D. Health-related quality of life during and after intraperitoneal versus intravenous chemotherapy for optimally debulked ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2007;25:437–443. doi: 10.1200/JCO.2006.07.3494. [DOI] [PubMed] [Google Scholar]
- 4.Kanerva A, Raki M, Hemminki A. Gene therapy of gynaecological diseases. Expert Opin Biol Ther. 2007;7:1347–1361. doi: 10.1517/14712598.7.9.1347. [DOI] [PubMed] [Google Scholar]
- 5.Davis JJ, Fang B. Oncolytic virotherapy for cancer treatment: challenges and solutions. J Gene Med. 2005;7:1380–1389. doi: 10.1002/jgm.800. [DOI] [PubMed] [Google Scholar]
- 6.Parato KA, Senger D, Forsyth PA, Bell JC. Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer. 2005;5:965–976. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- 7.Schepelmann S, Springer CJ. Viral vectors for gene-directed enzyme prodrug therapy. Curr Gene Ther. 2006;6:647–670. doi: 10.2174/156652306779010679. [DOI] [PubMed] [Google Scholar]
- 8.Liu TC, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract Oncol. 2007;4:101–17. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- 9.Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L, et al. A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879–885. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- 10.Xia ZJ, Chang JH, Zhang L, Jiang WQ, Guan ZZ, Liu JW, et al. Phase III randomized clinical trial of intratumoral injection of E1B gene-deleted adenovirus (H101) combined with cisplatin-based chemotherapy in treating squamous cell cancer of head and neck or esophagus. Ai Zheng. 2004;23:1666–1670. [PubMed] [Google Scholar]
- 11.Freytag SO, Stricker H, Peabody J, Pegg J, Paielli D, Movsas B, et al. Five-year follow-up of trial of replication-competent adenovirus-mediated suicide gene therapy for treatment of prostate cancer. Mol Ther. 2007;15:636–642. doi: 10.1038/sj.mt.6300068. [DOI] [PubMed] [Google Scholar]
- 12.Coukos G, Makrigiannakis A, Kang EH, Rubin SC, Albelda SM, Molnar-Kimber KL. Oncolytic herpes simplex virus-1 lacking ICP34.5 induces p53-independent death and is efficacious against chemotherapy-resistant ovarian cancer. Clin Cancer Res. 2000;6:3342–3353. [PubMed] [Google Scholar]
- 13.Bauerschmitz GJ, Lam JT, Kanerva A, Suzuki K, Nettelbeck DM, Dmitriev I, et al. Treatment of ovarian cancer with a tropism modified oncolytic adenovirus. Cancer Res. 2002;62:1266–1270. [PubMed] [Google Scholar]
- 14.Peng KW, TenEyck CJ, Galanis E, Kalli KR, Hartmann LC, Russell SJ. Intraperitoneal therapy of ovarian cancer using an engineered measles virus. Cancer Res. 2002;62:4656–4662. [PubMed] [Google Scholar]
- 15.Hirasawa K, Nishikawa SG, Norman KL, Alain T, Kossakowska A, Lee PW. Oncolytic reovirus against ovarian and colon cancer. Cancer Res. 2002;62:1696–1701. [PubMed] [Google Scholar]
- 16.Stojdl DF, Lichty BD, tenOever BR, Paterson JM, Power AT, Knowles S, et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4:263–75. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- 17.Tseng JC, Hurtado A, Yee H, Levin B, Boivin C, Benet M, et al. Using sindbis viral vectors for specific detection and suppression of advanced ovarian cancer in animal models. Cancer Res. 2004;64:6684–6692. doi: 10.1158/0008-5472.CAN-04-1924. [DOI] [PubMed] [Google Scholar]
- 18.Zeh HJ, Bartlett DL. Development of a replication-selective, oncolytic poxvirus for the treatment of human cancers. Cancer Gene Ther. 2002;9:1001–1012. doi: 10.1038/sj.cgt.7700549. [DOI] [PubMed] [Google Scholar]
- 19.Guo ZS, Bartlett DL. Vaccinia as a vector for gene delivery. Expert Opin Biol Ther. 2004;4:901–917. doi: 10.1517/14712598.4.6.901. [DOI] [PubMed] [Google Scholar]
- 20.Shen Y, Nemunaitis J. Fighting cancer with vaccinia virus: teaching new tricks to an old dog. Mol Ther. 2005;11:180–195. doi: 10.1016/j.ymthe.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 21.Thorne SH, Bartlett DL, Kirn DH. The use of oncolytic vaccinia viruses in the treatment of cancer: a new role for an old ally? Curr Gene Ther. 2005;5:429–443. doi: 10.2174/1566523054546215. [DOI] [PubMed] [Google Scholar]
- 22.Karupiah G, Coupar B, Ramshaw I, Boyle D, Blanden R, Andrew M. Vaccinia virus-mediated damage of murine ovaries and protection by virus-expressed interleukin-2. Immunol Cell Biol. 1990;68:325–333. doi: 10.1038/icb.1990.44. [DOI] [PubMed] [Google Scholar]
- 23.Puhlmann M, Brown CK, Gnant M, Huang J, Libutti SK, Alexander HR, et al. Vaccinia as a vector for tumor-directed gene therapy: biodistribution of a thymidine kinase-deleted mutant. Cancer Gene Ther. 2000;7:66–73. doi: 10.1038/sj.cgt.7700075. [DOI] [PubMed] [Google Scholar]
- 24.McCart JA, Mehta N, Scollard D, Reilly RM, Carrasquillo JA, Tang N, et al. Oncolytic vaccinia virus expressing the human somatostatin receptor SSTR2: molecular imaging after systemic delivery using 111In-pentetreotide. Mol Ther. 2004;10:553–561. doi: 10.1016/j.ymthe.2004.06.158. [DOI] [PubMed] [Google Scholar]
- 25.McCart JA, Ward JM, Lee J, Hu Y, Alexander HR, Libutti SK, et al. Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes. Cancer Res. 2001;61:8751–8757. [PubMed] [Google Scholar]
- 26.Guo ZS, Naik A, O’Malley ME, Popovic P, DeMarco R, Hu Y, et al. The enhanced tumor selectivity of an oncolytic vaccinia lacking the host range and anti-apoptosis genes SPI-1 and SPI-2. Cancer Res. 2005;65:9991–9998. doi: 10.1158/0008-5472.CAN-05-1630. [DOI] [PubMed] [Google Scholar]
- 27.Yang ST, Guo ZS, O’Malley ME, Yin XY, Zeh HJ, Bartlett DL. A new recombinant vaccinia with targeted deletion of three viral genes: its safety and efficacy as an oncolytic virus. Gene Ther. 2007;14:638–647. doi: 10.1038/sj.gt.3302914. [DOI] [PubMed] [Google Scholar]
- 28.Naik AM, Chalikonda S, McCart JA, Xu H, Guo ZS, Langham G, et al. Intravenous and isolated limb perfusion delivery of wild type and a tumor-selective replicating mutant vaccinia virus in nonhuman primates. Hum Gene Ther. 2006;17:31–45. doi: 10.1089/hum.2006.17.31. [DOI] [PubMed] [Google Scholar]
- 29.Mastrangelo MJ, Maguire HC, Eisenlohr LC, Laughlin CE, Monken CE, McCue PA, et al. Intratumoral recombinant GM-CSF-encoding virus as gene therapy in patients with cutaneous melanoma. Cancer Gene Ther. 1999;6:409–422. doi: 10.1038/sj.cgt.7700066. [DOI] [PubMed] [Google Scholar]
- 30.Kirn D, Niculescu-Duvaz I, Hallden G, Springer CJ. The emerging fields of suicide gene therapy and virotherapy. Trends Mol Med. 2002;8:S68–73. doi: 10.1016/s1471-4914(02)02318-3. [DOI] [PubMed] [Google Scholar]
- 31.Niculescu-Duvaz I, Springer CJ. Introduction to the background, principles, and state of the art in suicide gene therapy. Mol Biotechnol. 2005;30:71–88. doi: 10.1385/MB:30:1:071. [DOI] [PubMed] [Google Scholar]
- 32.Bernt KM, Steinwaerder DS, NS, Li ZY, Roffler SR, Lieber A. Enzyme-activated prodrug therapy enhances tumor-specific replication of adenovirus vectors. Cancer Res. 2002;62:6089–6098. [PubMed] [Google Scholar]
- 33.Schepelmann S, Ogilvie LM, Hedley D, Friedlos F, Martin J, Scanlon I, et al. Suicide gene therapy of human colon carcinoma xenografts using an armed oncolytic adenovirus expressing carboxypeptidase G2. Cancer Res. 2007;67:4949–4955. doi: 10.1158/0008-5472.CAN-07-0297. [DOI] [PubMed] [Google Scholar]
- 34.Gnant M, Puhlmann M, Alexander HR, Bartlett DL. Systemic administration of a recombinant vaccinia virus expressing the cytosine deaminase gene and subsequent treatment with 5-flurocytosine leads to tumor-specific gene expression and prolongation of survival in mice. Cancer Res. 1999;59:3396–3403. [PubMed] [Google Scholar]
- 35.Puhlmann M, Gnant M, Brown CK, Alexander HR, Bartlett DL. Thymidine kinase-deleted vaccinia virus expressing purine nucleoside phosphorylase as a vector for tumor-directed gene therapy. Hum Gene Ther. 1999;10:649–657. doi: 10.1089/10430349950018724. [DOI] [PubMed] [Google Scholar]
- 36.McCart JA, Puhlmann M, Lee J, Hu Y, Libutti SK, Alexander HR, et al. Complex interactions between the replicating oncolytic effect and the enzyme/prodrug effect of vaccinia-mediated tumor regression. Gene Ther. 2000;7:1217–1223. doi: 10.1038/sj.gt.3301237. [DOI] [PubMed] [Google Scholar]
- 37.Kievit E, Bershad E, Ng E, Sethna P, Dev I, Lawrence TS, et al. Superiority of yeast over bacterial cytosine deaminase for enzyme/prodrug gene therapy in colon cancer xenografts. Cancer Res. 1999;59:1417–1421. [PubMed] [Google Scholar]
- 38.Roby KF, Taylor CC, Sweetwood JP, Cheng Y, Pace JL, Tawfik O, et al. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis. 2000;21:585–591. doi: 10.1093/carcin/21.4.585. [DOI] [PubMed] [Google Scholar]
- 39.Erbs P, Exinger F, Jund R. Characterization of the Saccharomyces cerevisiae FCY1 gene encoding cytosine deaminase and its homologue FCA1 of Candida albicans. Curr Genet. 1997;31:1–6. doi: 10.1007/s002940050169. [DOI] [PubMed] [Google Scholar]
- 40.Chakrabarti S, Sisler JR, Moss B. Compact, synthetic, vaccinia virus early/late promoter for protein expression. BioTechniques. 1997;23:1094–1097. doi: 10.2144/97236st07. [DOI] [PubMed] [Google Scholar]
- 41.Hirschowitz EA, Ohwada A, Pascal WR, Russi TJ, Crystal RG. In vivo adenovirus-mediated gene transfer of the Escherichia coli cytosine deaminase gene to human colon carcinoma-derived tumors induces chemosensitivity to 5-fluorocytosine. Hum Gene Ther. 1995;6:1055–1063. doi: 10.1089/hum.1995.6.8-1055. [DOI] [PubMed] [Google Scholar]
- 42.Freeman SM, Abboud CN, Whartenby KA, Packman CH, Koeplin DS, Moolten FL, et al. The “bystander effect”: tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res. 1993;53:5274–5283. [PubMed] [Google Scholar]
- 43.Ramesh R, Marrogi AJ, Munshi A, Abboud CN, Freeman SM. In vivo analysis of the ‘bystander effect’: a cytokine cascade. Exp Hematol. 1996;24:829–838. [PubMed] [Google Scholar]
- 44.Eisenberg DP, Adusumilli PS, Hendershott KJ, Yu Z, Mullerad M, Chan MK, et al. 5-fluorouracil and gemcitabine potentiate the efficacy of oncolytic herpes viral gene therapy in the treatment of pancreatic cancer. J Gastrointest Surg. 2005;9:1068–1077. doi: 10.1016/j.gassur.2005.06.024. discussion 1077–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hung CF, Tsai YC, He L, Coukos G, Fodor I, Qin L, et al. Vaccinia virus preferentially infects and controls human and murine ovarian tumors in mice. Gene Ther. 2007;14:20–29. doi: 10.1038/sj.gt.3302840. [DOI] [PubMed] [Google Scholar]