Abstract
The vertebrate retina has multiple demands for utilization of cholesterol and must meet those demands either by synthesizing its own supply of cholesterol or by importing cholesterol from extraretinal sources, or both. Unlike the blood-brain barrier, the blood-retina barrier allows uptake of cholesterol from the circulation via a lipoprotein-based/receptor-mediated mechanism. Under normal conditions, cholesterol homeostasis is tightly regulated; also, cholesterol exists in the neural retina overwhelmingly in unesterified form, and sterol intermediates are present in minimal to negligible quantities. However, under certain pathological conditions, either due to an inborn error in cholesterol biosynthesis or as a consequence of exposure to selective inhibitors of enzymes in the cholesterol pathway, the ratio of sterol intermediates to cholesterol in the retina can rise dramatically and persist, in some cases resulting in progressive degeneration that significantly compromises the structure and function of the retina. Although the relative contributions of de novo synthesis versus extraretinal uptake are not yet known, herein we review what is known about these processes and the dynamics of cholesterol in the vertebrate retina and indicate some future avenues of research in this area.
Keywords: cholesterol/biosynthesis, eye/retina, Smith-Lemli-Opitz syndrome
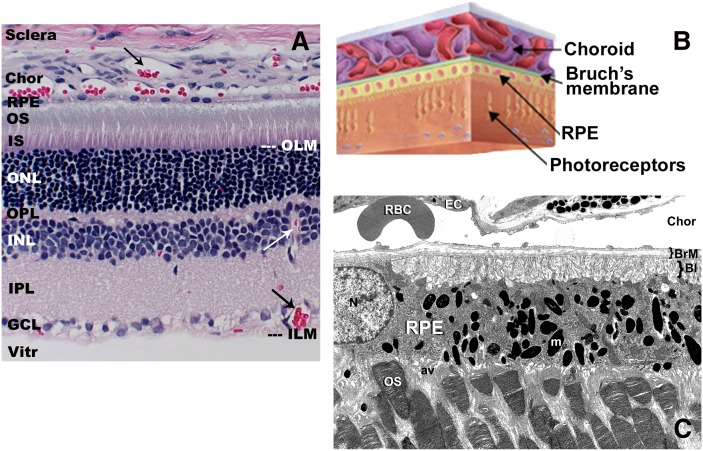
The mature vertebrate retina is a highly stratified, multicellular tissue classically described as consisting of six fundamental neuronal cell types (photoreceptor, bipolar, ganglion, horizontal, amacrine, and interplexiform cells) plus glial cells (Müller cells, astrocytes, and microglia). [For an excellent overview of retinal structure and cytoarchitecture, see (1) and http://webvision.med.utah.edu/]. The neuronal cells are distributed into discrete histological layers in the fully differentiated retina (Fig. 1), with the ganglion cell layer arranged proximal to the vitreous and the photoreceptor cells (rods and cones) residing at the opposite side of the neural retina, just underneath the retinal pigment epithelium (RPE) layer. The RPE is a monolayer of polarized epithelial cells that serves as the interface between the neural retina and the choroid, the blood supply to the outer cell layers of the retina. The apical tips of the photoreceptor outer segments (OS) are adjacent to and invaginate into the apical face of the RPE (one of the few examples of apical-apical cellular contact in the body) (Fig. 1C). Bruch's membrane (BrM; Fig. 1B, C) is not a membrane, per se, but rather a pentalaminar structure that consists of the basement membranes of the RPE cells and the endothelial cells of the choriocapillaris (Fig. 1C), plus the extracellular matrix that fills the space between these two membranes [reviewed in (2, 3)].
Fig. 1.
Histology and ultrastructure of the vertebrate retina. A: Light microscopic view of an adult rat retina (paraffin embedment, hematoxylin/eosin stain) illustrating the histological layers of the retina and the anatomical relationships between the neural retina, the RPE, and the choroid (Chor). Arrows (→) indicate blood vessels (filled with red-stained blood cells) in the choroid and in the inner retinal layers. B: Schematic illustration depicting the location of BrM in relation to the choroid, RPE, and neural retina elements. C: Electron micrograph illustrating key ultrastructural features of the photoreceptor-RPE interface and choroid. Note the invagination of photoreceptor outer segment (OS) tips into the apical villi (av) of the RPE, the basal infoldings (BI) of the RPE plasma membrane proximal to BrM, the numerous melanin granules (m) filling the RPE cytoplasm, and the presence of a large red blood cell (RBC) adjacent to a choroidal capillary endothelial cell (EC). ILM, inner limiting membrane; IPL, inner plexiform layer; IS, inner segment; N, nucleus; OLM, outer limiting membrane; ONL, outer nuclear layer; OPL, outer plexiform layer; Vitr, vitreous.
The relatively modest compendium of cell types mentioned above, however, belies the amazing, higher order complexity of this sensory tissue. It has been estimated that there are actually about 55 anatomically distinct neuronal cell types in the adult mammalian retina, of which about 22 have been thoroughly characterized with regard to their fine structure and functional repertoire [reviewed in (4)]. For example, photoreceptors consist of both rods and cones, with cones being subdivided into two (three in primates) spectral types according to their wavelength maxima. There are 10 different types of bipolar cells, including both ON and OFF subclasses that serve either rod- or cone-driven circuits. There are >20 types of amacrine cells and at least 10 distinct subtypes of ganglion cells. Each of these cell types contains membrane-bound intracellular compartments as well as a plasma membrane plus (for neurons) a dynamic population of synaptic vesicles that is actively engaged in neurotransmitter release. In addition, the photoreceptor cells engage each day in a rhythmic process of synthesis and turnover of their outer segment membranes: in mammals, approximately 10% of the membrane mass of the photoreceptor outer segment is turned over each day via shedding of packets of so-called “disk” membranes at the distal tip (proximal to the RPE), with a compensatory amount of new membranes being added at the base of the outer segment (proximal to the inner segment of the cell) [reviewed in (5, 6)]. In the retina of Xenopus laevis, the African clawed frog (a favored species for studies of photoreceptor membrane renewal and intracellular membrane trafficking), this equates to an estimated 4,500 μm2 of disk membrane surface area per day per rod cell (6). Hence, the demands on the retina for cholesterol to achieve and maintain normal structure and function are prodigious.
A number of questions immediately come to mind: Where does the retina get its supply of cholesterol (e.g., what are the relative contributions of de novo synthesis vs. uptake from extraretinal sources)? What cell types synthesize cholesterol in the retina? How is cholesterol homeostasis in the retina maintained? Is cholesterol required for normal retinal development or to achieve and maintain the normal structure and function of the retina? Herein, we will review what is known about both the “supply side” as well as the turnover and export of cholesterol in the retina and identify remaining deficiencies in our knowledge in this area. To address the question of whether or not the retina requires cholesterol, per se, for achieving and maintaining normal retinal structure and function, we will focus on cholesterol biosynthesis defects, particularly studies that have employed an animal model of the Smith-Lemli-Opitz syndrome (SLOS). Finally, we will close with a perspective of future avenues of research in this field. Throughout this review, because there are both parallels as well as distinct differences between the brain and the retina [both of which are integral parts of the central nervous system (CNS)], we will compare and contrast these tissues with regard to various aspects of cholesterol biology.
SOME INITIAL CONSIDERATIONS
Unesterified (free) cholesterol is the predominant form of cholesterol in the CNS, of which the retina is a part. The brain has the highest cholesterol content (∼14 mg/g wet weight) of all organs in the body compared with only ∼2–3 mg/g in most tissues (7, 8), including the neural retina and RPE (9). When compared with the major cholesterogenic tissues (i.e., liver and intestine), the rate of cholesterol synthesis in the mature brain and retina is relatively slow (10, 11). Despite this, however, essentially all of the cholesterol in the brain is derived from endogenous de novo synthesis, most of which is utilized to support the production of myelin (8). In contrast, the neural retina of most vertebrate species is not myelinated. The need of the brain to synthesize cholesterol endogenously is driven, at least in part, by the fact that the blood-brain barrier differentially excludes the entry of exogenous cholesterol (e.g., as carried by blood-borne lipoproteins) (7, 8). The retina, however, is different; recent studies using the naturally fluorescent cholesterol analog cholestatrienol [cholesta-5,7,9(11)-trien-3β-ol] have shown that sterols can readily pass from blood-borne lipoproteins into the RPE and all layers of the neural retina (12). Also, feeding rats a high-cholesterol diet when their endogenous cholesterol biosynthetic capacity has been pharmacologically compromised can dramatically alter the steady-state cholesterol content of the retina (13) (discussed below). Hence, although a blood-retina barrier does exist anatomically (14, 15), it does not exclude extraretinal cholesterol from entering the retina. The slow de novo synthesis rate of cholesterol in the brain is nearly balanced by the rather long half-life of cholesterol (approximately 4–6 months) (16, 17). In contrast, the turnover time for cholesterol in the neural retina is comparatively short. From the results of a recent study (12) that evaluated the uptake dynamics of intravenously injected, deuterium-labeled LDL cholesterol particles by the rat retina, an estimate of retinal cholesterol turnover in the range of 6–7 days was obtained, assuming a linear process. In another study (S. J. Fliesler, unpublished observations), based upon measurements of retinal cholesterol specific activity as a function of time following intravitreal [3H]acetate injection, the half-time for turnover of de novo synthesized cholesterol in the rat retina was estimated to be 17–18 days, which is comparable to the turnover time measured for the major phospholipid classes in the retina (18, 19).
CHOLESTEROL IN THE RETINA: CONTENT AND DISTRIBUTION
In the neural retina, similar to the brain, cholesterol exists almost exclusively in the unesterified form (19, 20). In a detailed analysis of the sterol composition of bovine neural retina and rod outer segment (ROS) membranes derived therefrom, Fliesler and Schroepfer (21) reported that cholesterol accounts for at least 98% of the total sterols of whole retina and >99% of the total sterols of ROS membranes. Hence, the presence of appreciable steady-state levels of biogenic sterol precursors of cholesterol in the retina is vanishingly small. Cholesterol accounts for ∼2%, by weight, of the retina and ROS membranes or ∼10 mol% of the total lipid in ROS membranes (19, 21). Other neutral lipids, such as triglycerides, are extremely minimal if not completely absent in the retina (19, 20). In contrast, the RPE and it closely adherent neighboring anatomical elements, BrM and choroid (which are often harvested together for biochemical analyses), are notably different, with the proportion of cholesteryl esters (CE) exceeding that of free cholesterol (20). [For a more extensive discussion of sterols and steryl ester components of the RPE, BrM, and choroid, see recent reviews in this thematic series by Curcio et al. (22) and Rodriguez and Larrayoz (23).] As indicated above, contrary to the brain and with the exception of the optic nerve, neurons from the neural retina are not myelinated. Cholesterol appears to be broadly distributed in all layers of the neural retina (24), although the inner plexiform layer is found to be relatively enriched in cholesterol compared with the outer segment layer (25–28). However, in the outer segments of retinal rod photoreceptors, a gradient of cholesterol exists, with more cholesterol being present at the base (proximal to the inner segment) of the ROS than at the distal tip (proximal to the RPE) (27, 29, 30). When normalized to total phospholipid content, the cholesterol content from base to tip of the ROS can vary by nearly 6-fold. The ROS consists of a stack of so-called disk membranes that are assembled at the base of the outer segment and shed at the distal tip (5, 6). The outer segment houses the components of the phototransduction cascade, which converts the energy of photons (absorbed by the visual pigment, rhodopsin) into an electrochemical signal in the first steps of the visual process. A high-cholesterol environment, such as that present in the basal disks, has been found to reduce the efficiency of the phototransduction cascade by hindering activation of rhodopsin and impairing the cyclic nucleotide phosphodiesterase activity that hydrolyzes cGMP [for a comprehensive review on the role of cholesterol in ROS membranes, see (30)].
DE NOVO SYNTHESIS OF CHOLESTEROL IN THE RETINA
The history of studies aimed at elucidating the biosynthesis of cholesterol and related isoprenoid molecules in the vertebrate retina has been reviewed previously by Fliesler and Keller (31). Early attempts to examine the de novo synthesis of cholesterol and related isoprenoid molecules in the retina employed either short-term bovine retinal explants (32) or cell-free homogenates (the 10,000 g supernatant fraction) prepared therefrom (33), using [3H]mevalonate as a radiolabeled precursor. Although these studies demonstrated that the mammalian retina has the capacity to synthesize a range of isoprenoid products, including cholesterol and its biogenic precursors, the synthesis of cholesterol, per se, was minimal. Instead, squalene and various sterol biogenic precursors of cholesterol were the most abundant radiolabeled products observed. Subsequently, related studies were performed using frog (Rana pipiens) retinal explant organ cultures, with both [3H]acetate and, separately, [2H]water as radiolabeled precursors (34). Again, radiolabeled squalene, rather than cholesterol, was the dominant product detected. One unusual feature about the frog retina, however, that came out in the course of these studies was that both the whole retina and ROS membranes contain significant steady-state levels of squalene. Hence, the unlabeled pool of squalene acted like a “metabolic trap”, diluting out the radiolabeled products derived via the mevalonate pathway. These latter experiments, using [3H]water, also allowed an estimate of the absolute rate of de novo synthesis via the isoprenoid pathway. When correlative in vivo studies were performed (35), using intravitreal injection of either [3H]mevalonate or [3H]acetate in frog eyes, similar results were obtained; in fact, little radiolabeled cholesterol appeared until a day or so postinjection. In total, the results of these studies lead (in retrospect) to the erroneous conclusion that the retina has a very limited capacity to synthesize its own cholesterol.
Subsequently, in vivo experiments were performed using intravitreal injection of [3H]actetate in rats. In striking contrast to the results described above, the radiolabeled precursor was taken up and incorporated efficiently into cholesterol within a few hours, with relatively minimal accumulation of radiolabeled intermediates (10). In those same studies, it was demonstrated, using intravitreal [3H]actetate as a radiolabeled precursor, that a single intravitreal injection of lovastatin could quantitatively shut down de novo cholesterol biosynthesis in the retina. Additional experiments showed that when [3H]farnesol was intravitreally injected in rat eyes, [3H]cholesterol and biogenically related sterols were recovered from the retina (36). In that same study, when the squalene epoxidase inhibitor, NB-598, was coinjected with the radiolabeled precursor, not only was formation of radiolabeled sterols blocked, but squalene mass also accumulated in measurable amounts in the retina (unlike in control rat retinas, which exhibit no appreciable steady-state levels of squalene). These findings demonstrated that the mammalian retina has the capacity to synthesize its own cholesterol de novo.
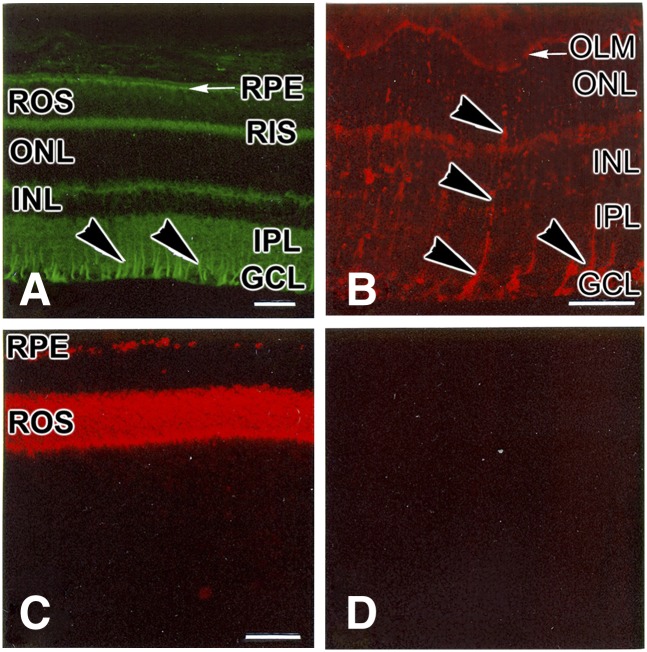
The question arises: Which cells in the retina are capable of synthesizing cholesterol de novo? One way to answer this question is to ascertain which cells express the major rate-limiting enzyme of the cholesterol biosynthetic pathway, namely HMG-CoA reductase [reviewed in (37, 38)]. Using immunohistochemistry on frozen sections of rat retina, one of us (S.J.F.) has demonstrated that Müller cells, rod inner segments, and RPE cells exhibit strong immunoreactivity against monospecific antibodies to HMG-CoA reductase (39) (Fig. 2). Correlative in situ hybridization studies (S.J. Fliesler, unpublished observations) have confirmed this result. These findings support the hypothesis that (rod) photoreceptor cells, Müller cells, and RPE cells have the capacity to synthesize isoprenoids, such as cholesterol. However, none of the above evidence is adequate to unambiguously determine whether or not de novo cholesterol synthesis can account for the steady-state levels of cholesterol found in the retina or whether de novo synthesis is both necessary and sufficient to meet the multiple needs of the retina for cholesterol.
Fig. 2.
Immunohistochemical localization of proteins in the rat retina. A: HMG-CoA reductase. B: Cellular retinaldehyde-binding protein, a Müller glia marker protein. C: Opsin, the apoprotein of the rod cell visual pigment, rhodopsin. D: Negative control (preimmune primary serum). Arrowheads (B, C) indicate the radial labeling pattern typical of Müller glial cells. Abbreviations as in Fig. 1. Scale bars (all panels) = 25 μm. [Reproduced with permission from (39).]
UPTAKE AND INTRARETINAL EXCHANGE OF CHOLESTEROL BY THE RETINA
The human diet provides approximately 400 mg of cholesterol and the liver secretes about 1 g daily (40, 41). Approximately 50% of the cholesterol in the intestine is absorbed, while the remainder is excreted in feces (42, 43). Most dietary cholesterol exists in the free form; CE accounts for only 10–15% of total dietary cholesterol intake. Only free cholesterol can be absorbed by enterocytes, meaning that CE must be hydrolyzed to release free cholesterol for absorption. Several cholesterol transporters have been identified in the brush border of the enterocyte, including Niemann-Pick C1-like 1, the ATP-binding cassette transporters (ABC) G5 and ABCG8, and the “scavenger receptors” scavenger receptor B (SR-B) I and CD36 [reviewed in (44)]. After entering the enterocyte, cholesterol is reesterified by acyl-CoA:cholesterol acyltransferases and packed into chylomicrons. This pathway is important, because 70–80% of cholesterol entering the lymphatic duct as chylomicrons is esterified. Nevertheless, triacylglycerols represent the predominant lipid class in chylomicrons (Table 1), and their composition resembles the dietary intake in fatty acids. Chylomicrons and VLDL both have apolipoprotein (Apo) B as their main Apo [reviewed in (45)]. ApoB is expressed in the liver as a full-length form called ApoB100, whereas the enterocyte secretes ApoB48, which is a truncated form representing 48% of the N-terminal portion of ApoB100. Chylomicrons and VLDL are rapidly metabolized in the circulation by lipases and especially the plasma lipoprotein lipase that is bound at the surface of capillary endothelial cells in many tissues [reviewed in (46)]. Hydrolysis of triacylglycerols is accompanied by the relative enrichment of the lipoproteins in CE. Thus, CE account for the main class of lipids in LDL (Table 1). VLDL, LDL, and HDL deliver their content to the cells thanks to the recognition of the Apos by specific receptors and nonspecific scavenger receptors present at the external side of cell membranes [reviewed in (42)].
TABLE 1.
Composition of the major plasma lipoproteins fractions [adapted from (149)] and LLPs purified from RPE/choroid collected in humans (62, 64)
| Lipoprotein Fraction | Density Range (g/ml) | Free Cholesterol | CE | Phospholipid | Triacylglycerol | Major Apo |
|---|---|---|---|---|---|---|
| % mass of lipids | ||||||
| Chylomicrons | d<1.00 | Traces | 3 | 5 | 92 | B48, C-II, C-III |
| VLDL | d<1.006 | 7 | 16 | 17 | 60 | B100, C-I, C-II, C-III, E |
| IDL | 1.006<d<1.019 | 8 | 27 | 26 | 38 | |
| LDL | 1.019<d<1.063 | 11 | 56 | 28 | 5 | B100 |
| HDL | 1.063<d<1.21 | 7 | 36 | 53 | 4 | A-I, A-II, A-IV, B100, C-II, C-III, D, E |
| VHDL | 1.21<d<1.25 | 1 | 9 | 78 | 13 | |
| LLP | d<1.24 | 23 | 32 | 33 | 3 | A-I, B48, B100 |
As mentioned in the introduction to this article, the RPE is the cellular and metabolic interface between the neural retina and the choroid (Fig. 1). Similar to most epithelial cells, RPE cells exhibit a distinct polarity, having basolateral and apical surfaces [reviewed in (2, 3)]. The RPE exhibits a three-dimensional network of tight junctions along its basolateral sides that helps to maintain a physical barrier that restricts the movement of water, small organic and inorganic molecules, and larger cargo between the neurosensory retina and choroid. BrM forms an interface between the basal surface of the RPE and the choriocapillaris. The apical plasmalemma of the RPE contains numerous villi (Fig. 1C), which extend into the subretinal space; the tips of the photoreceptor outer segments invaginate into and are enveloped by these apical villi. This close anatomical association between the RPE and photoreceptors is one of the factors that promotes the ability of the RPE to, on the one hand, provide nutrients and oxygen to the photoreceptor cells and, on the other hand, eliminate metabolic wastes generated by the neural retina, including those resulting from the shedding of outer segments tips and their subsequent phagocytosis and degradation by the RPE. The RPE cells express a variety of lipoprotein-specific and scavenger receptors: VLDL receptor (47), LDL receptor (LDL-R) (12, 48, 49), and the so-called scavenger receptors CD36 (48, 50–54), SR-BI, and BII (50, 52, 55, 56). Interestingly, LDL-R was also localized to the endothelial cells of the choriocapillaris, the photoreceptor inner segments, retinal ganglion cells, and Müller cells (12). The LDL-R-related proteins, such as LDL receptor-like protein (LRP-1), constitute a related family of high-molecular weight apoE receptors of the LDL-R family [reviewed in (57, 58)]. LRP-1 has been identified in retinal ganglion cells (59) and cells extending from the inner to the outer limiting membranes in the course of oxygen-induced retinal neovascularization (60). These data somewhat unexpectedly suggest first that those receptors may be involved in processes other than or in addition to the recognition of lipoproteins. A variety of specific roles for LRPs in neurons have been considered, including involvement in the trafficking of β-amyloid precursor protein and Aβ production (61). Meanwhile, the mechanism of lipoprotein recognition by LDL-R would be applicable both to exogenous (blood-borne) and endogenous (intra-retinal) sources.
The ability of the retina to synthesize lipoprotein-like particles (LLP) has been demonstrated (62, 63) [see also recent review by Curcio et al. (22), this Thematic Series]. LLP are rich in lipids and particularly in cholesterol and CE (62, 64). Those lipids accumulate with age proximal to the RPE basalamina within BrM in both normal eyes (62, 65–69) and eyes with age-related maculopathy (28, 66, 70). It is postulated that accumulation of such material participates in creating a physical barrier, the so-called lipid wall, that may limit the exchanges between the choriocapillaris and the RPE (71–73). One of the consequences of accumulation of CE at the basal face of the RPE would be a decreased nutrient intake by the neural retina, resulting in compromised retinal function (24). Until now, the relative contribution of circulating lipoproteins and endogenous LLP synthesis to the age-dependent deposition of cholesterol-rich material within BrM remains unresolved. The simplistic assumption that comparing the lipid composition of LLP, circulating lipoproteins, and neural retina would elucidate their origin has been disappointing. Indeed, LLP are highly enriched in free cholesterol, like the neural retina, but also in CE, similarly to LDL (Table 1). As in CE from lipoproteins, linoleic acid is the major fatty acid in CE from RPE/BrM/choroid (20, 62, 64). Docosahexaenoic acid (DHA, or 22:6n-3) is the predominant polyunsaturated fatty acid in the neural retina and in retinal photoreceptor outer segment membranes [reviewed in (19)]. It accounts for nearly 15% of the fatty acids in human retina (20) to more than 30% in the retinas of rats and mice (24, 74). DHA is thought to be essential in visual function, largely by virtue of its ability to provide a membrane environment conducive to supporting the phototransduction cascade (30, 75–78). In striking contrast, DHA is found in relatively low levels in the membrane phospholipids of the RPE/BrM/choroid complex: 4.4% in humans and very little in CE (from 0.3 to 0.5%) (20, 62). Interestingly, the DHA content in CE and in the neural retina strongly correlate in humans (20), suggesting that CE may be carriers of circulating DHA to the neural retina. Twenty years ago, Bazan et al. (79) reported that retinal DHA originates in the liver, with subsequent transport in the blood and uptake by the retina via the RPE; in situ synthesis and recycling of DHA within the retina also contribute to the steady-state levels of DHA in the retina. Circulating DHA originates mainly from dietary DHA and to a very minor extent from biosynthesis in the liver. For instance, the conversion of α-linolenic acid into DHA was not found in rat hepatocytes (80). Increasing α-linolenic acid intake in humans is not associated with a rise in DHA levels in plasma phospholipids [reviewed in (81, 82)]. However, supplementation trials with long-chain n-3 fatty acids have reported a positive association between intake and circulating DHA (83–86). In a recent study in humans, circulating DHA was shown to account for only a minor part of the retinal levels of DHA. Indeed, the amount of DHA in the neural retina did not correlate with DHA in adipose tissue, which was considered a surrogate for circulating DHA (20). These data suggest the pivotal importance of recycling DHA for the maintenance of retinal DHA levels rather than uptake of circulating DHA. Whether or not a similar “recycling” of cholesterol occurs in the retina remains to be evaluated.
Nevertheless, as indicated above, the retina can take up circulating LDL-borne cholesterol (12) in notable contrast to exclusion of such uptake by the brain (5, 87, 88). Using labeled LDL particles, it was shown that LDL can cross BrM and reach the RPE (12, 48, 89, 90). The role of LDL as a carrier of cholesterol for the retina is further supported by data showing accumulation within BrM of lipid-rich material in LDL-R knockout mouse models (91, 92), including CE in the ApoB100,LDLR−/− mouse, a relevant murine model of aging of the human retina (24). Nevertheless, the biological relevance of LDL-derived cholesterol in the maintenance of cholesterol steady-state levels in the retina remains an open question. Indeed, even after abrogating the transfer of lipids from LDL (i.e., in ApoB100,LDLR−/− mice), the fatty acid profile and cholesterol content (the latter measured by filipin binding) in the neural retina appeared comparable to that of wild-type animals. Alternatively, processes other than apoB100-LDL-R recognition may also participate in the transfer of circulating lipids to the retina, e.g., via CD36, albumin, or other proteins involved in lipid transport (50, 64, 93). Finally, it is possible that the retina also may respond to decreased uptake of exogenous sterol by upregulating the de novo synthesis of cholesterol.
In fact, although LDL was found to be the preferred vehicle for delivery of lipoprotein-bound cholesterol to the retina, HDL is also able to perform this function, albeit far less efficiently (50). Partners of the HDL pathway include ABCA1, ApoA1, ApoE, and the scavenger receptors SR-BI and SR-BII. Those proteins participate in the reverse efflux of cholesterol from the cells to nascent HDL particles. Although there are some differences between SR-BI and SR-BII, both proteins have been localized to the retinal ganglion cells and Müller cells as well as to photoreceptors (50, 55). ApoA1 and ABCA1 were both detected in retinal ganglion cells and the outer plexiform layer (50, 56). ApoA1 immunoreactivity was also observed to be associated with photoreceptor outer segments (most likely present in the inter-photoreceptor matrix (IPM), the extracellular matrix fills the subretinal space) and the inner segment of rods, but not cones, as well as with the apical face of RPE cells (50).
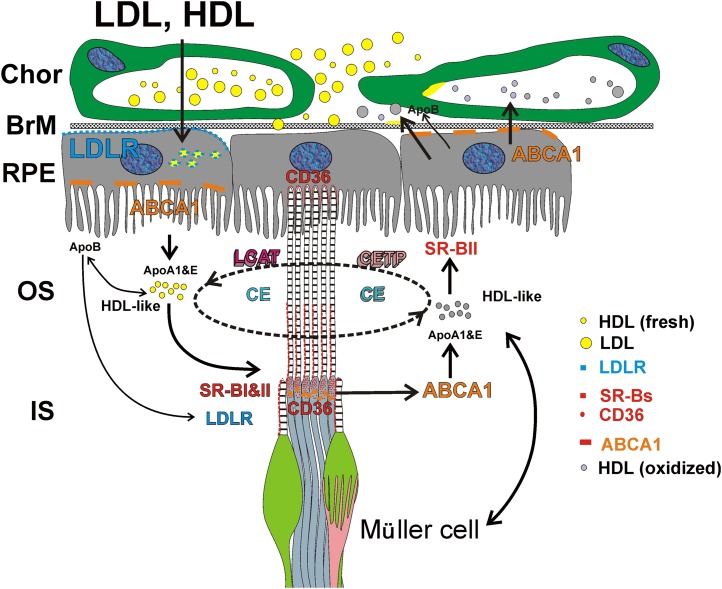
The presence of these known lipid transport proteins in various cell types within the neural retina and RPE supports the existence of an HDL-based intraretinal lipid transport mechanism, as proposed by Tserentsoodol et al. (50) (Fig. 3). According to this proposed mechanism, which remains speculative and is based upon the immunohistochemical localization of proteins involved in lipid remodeling, the RPE (and also likely the Müller cells; see below) engages an avid uptake of exogenous lipoproteins and reassembles the lipids derived therefrom, along with de novo synthesized lipids, into their own endogenous HDL-like particles, which contain ApoA1 and ApoE. These HDL-like particles are then exported from RPE cells by ABCA1. The scavenger receptors SR-BI and SR-BII present on or near the photoreceptor outer segments may then facilitate uptake of these HDL-like particles by the photoreceptor cells. Photoreceptors also may engage in lipid particle export, as evidenced by localization of both ABCA1 and ApoA1 in association with the rod inner segments. LDL (or LDL-like) particles apparently also have important roles in the shuttling of lipids (especially CE) into and within the retina. This is further supported by the finding of ApoB localization to the apical membranes of the RPE as well as in close association with ApoB, lecithin:cholesterol acyltransferase, and CETP with the photoreceptor outer segments, indicating that photoreceptor cells may be capable of HDL particle maturation.
Fig. 3.
Hypothetical schematic of uptake and intraretinal transport of cholesterol and related lipids by the vertebrate retina. HDL and LDL enter the retina from the choroidal (Chor) blood supply, traversing BrM and utilizing scavenger receptors (SR-BI, SR-BII, and CD36) and LDL-R localized to the RPE. LDL particles are degraded in the RPE and reassembled into lipoprotein-like (e.g., HDL-like) particles by recruiting ApoA1 and ApoE. These lipoprotein particles are then secreted from the apical face of the RPE into the IPM via the ABCA1 transporter. HDL-like particles mature further with the assistance of lecithin:cholesterol acyltransferase (LCAT) and cholesteryl ester transfer protein (CETP) in the IPM. Cholesterol, CEs, and other lipids shuttle between the RPE and photoreceptor cells [both inner (IS) and outer (OS) segments], and possibly between the Müller cells and photoreceptors, as cargo on the HDL-like particles. The RPE also may export cholesterol (or its oxysterol derivatives) in the form of LLPs into the choroidal circulation, thereby maintaining cholesterol homeostasis. [Reproduced with permission from (50).]
In addition to lipoprotein receptor-mediated mechanisms, transcytosis of LDL particles from retinal capillaries would also likely contribute to the supply of lipids, including sterols, to the retina. Although endothelial cells possess tight junctions that, a priori, limit the entry of macromolecules into the neural retina, ApoB (e.g., associated with LDL) is able to reach Müller cells by transcytosis. [For a more complete discussion, see review by Rodriguez and Larrayoz (23), this Thematic Series.] Similar to the brain, where astrocytes surround endothelial cells and maintain the blood-brain barrier, Müller cells may represent cellular partners that contribute to the integrity of the blood-retinal barrier. Alternatively or in addition, Müller cells may participate in remodelling the lipid core of lipoproteins and delivery of lipids and nutrients to retinal neurons. The relative contribution of such a mechanism (speculative at this point) compared with others, as well as the ability of cholesterol to enter the retina through this pathway, remains to be determined.
Pfrieger et al. [reviewed in (94, 95)] have proposed a role for glial-derived cholesterol in supporting neuronal synaptogenesis in the CNS. Early studies by Pfrieger and Barres (96) demonstrated that CNS neurons in culture were able to form ultrastructurally normal synapses, but they were not as active or as functionally competent as when the neurons were cocultured in the presence of glia. As alluded to in the beginning of this review article, the needs of neurons for cholesterol and other membrane lipids are manifold, not only to support synapse formation, but to form and maintain intracellular membrane compartments as well as the plasma membrane, the axon, and dendritic arbors and to generate a dynamic supply of synaptic vesicles for neurotransmitter release. Also as discussed above, the retina has a unique and extremely important additional need, i.e., the generation and turnover of photoreceptor outer segment membranes. More recent experiments (97) utilizing rat postnatal CNS neurons and glia cultured in serum-free media have demonstrated that neurons produce cholesterol less efficiently than do glia. In the model put forward by Pfrieger (94) in 2003, it is hypothesized that astrocytes (or glia) release lipoproteins that are subsequently taken up by neighboring neurons via a receptor-mediated process. Although the original model specified that the location for such uptake would be in the synaptic zone, involving the presynaptic endings and postsynaptic spines, there is no a priori reason why this zone of activity could not be more expansive, e.g., involving the cell body. The lipoprotein receptor density may be upregulated via a sterol-sensing pathway in the neurons and also positively stimulate additional release of lipoproteins from astrocytes/glia. It is further speculated that electrical activity may increase neuronal lipoprotein receptor density and possibly also induce cholesterol biosynthesis and lipoprotein release in adjacent astrocytes/glia, thereby supporting the need for additional synaptogenesis. The system also exhibits homeostatic feedback control; above some threshold level of cholesterol, which may be different for different types of neurons, the neurons respond by secreting their own lipoprotein particles or oxysterols, which then downregulate cholesterol synthesis and lipoprotein release by proximal astrocytes/glia. The ability of Müller cells to supply cholesterol, either de novo synthesized or repackaged by those glia, to neighboring photoreceptors or other retinal neurons and for those neurons to then utilize that specific pool of cholesterol for membrane biogenesis and other functions has yet to be demonstrated. However, it appears that the requisite components of such an interactive system are at least present in retinal neurons and glia.
DOES THE RETINA REQUIRE CHOLESTEROL TO ACHIEVE NORMAL STRUCTURE AND FUNCTION?
Clues to the roles of cholesterol in the retina might be obtained by modulating the levels of cholesterol and measuring the impact on some quantifiable physiological or structural parameter. The best way to find out whether something is necessary is to remove it and examine the consequences of that action. Nearly 20 years ago, attempts to deplete the retina of its endogenous cholesterol were performed by intravitreal injection of lovastatin (98, 99), an inhibitor of HMG-CoA reductase, the rate-limiting enzyme of sterol and other isoprenoid biosynthesis (37, 38). At the time, it was thought that the retina, like the brain, could not take up cholesterol from the blood; the premise, then, was that turnover of endogenous retinal cholesterol stores in the absence of de novo synthesis would eventually deplete the retina of its cholesterol. This turned out not to be the case. In fact, there was no significant decrease in the total retinal cholesterol content at anytime within 3 weeks after lovastatin injection under conditions where incorporation of [3H]acetate into retinal nonsaponifiable lipids was almost totally blocked. Intravitreal lovastatin caused a rather unusual, progressive retinal degeneration, but this turned out to be due to abrogation of protein prenylation rather than disruption of the cholesterol pathway per se (98, 99).
Nature has provided a number of examples of human cholesterol deficiency syndromes, all of which have a severe impact on the nervous system in addition to a variety of other phenotypic abnormalities [reviewed in (100–102)]. SLOS was the first of this class of multiple congenital anomaly metabolic diseases to be discovered (103) and is the most common such disease. It took nearly 30 years from the initial report describing affected patients to discover the primary biochemical defect involved, which is at the level of the conversion of 7-dehydrocholesterol [(7DHC) cholesta-5,7-dien-3β-ol] to cholesterol (104, 105). Hence, the key biochemical hallmarks of this disease, most often, are abnormally high levels of 7DHC and extremely low levels of cholesterol in all bodily tissues. The affected enzyme, 3β-hydroxysterol-Δ7-reductase (DHCR7; EC1.3.1.21), is encoded by the DHCR7 gene, and >100 mutations in this gene have been associated with SLOS [reviewed in (106–108)]. A rodent model of SLOS was developed (109) by treating normal rats with AY9944 [(trans-1,4-bis(2-dichlorobenzylamino-ethyl) cyclohexane dihydrochloride], a relatively selective inhibitor of DHCR7 (110, 111), replicating the biochemical hallmarks and, under certain conditions, at least some of the phenotypic features of the human disease. This animal has been modified more recently to extend postnatal viability for up to 3 months to examine the effects of disrupted cholesterol biosynthesis on the development of retinal structure and function (112, 113). Rats that had been exposed in utero to sublethal doses of AY9944 were viable at birth and exhibited 7DHC-cholesterol mole ratios that were typically >2:1 in serum, liver, brain, and retina, whereas 7DHC levels were below detection limits in the tissues of age-matched control rats (112). At this stage, the neural retina in rodents is mostly neuroblastic (undifferentiated), and the retinas of treated rats did not exhibit morphological features appreciably different from those of age-matched controls. Even by postnatal month 1, although the retinal 7DHC-cholesterol mole ratio was nearly 4:1, the neural retina appeared histologically and ultrastructurally normal and exhibited robust electrophysiological responses to light stimulation.
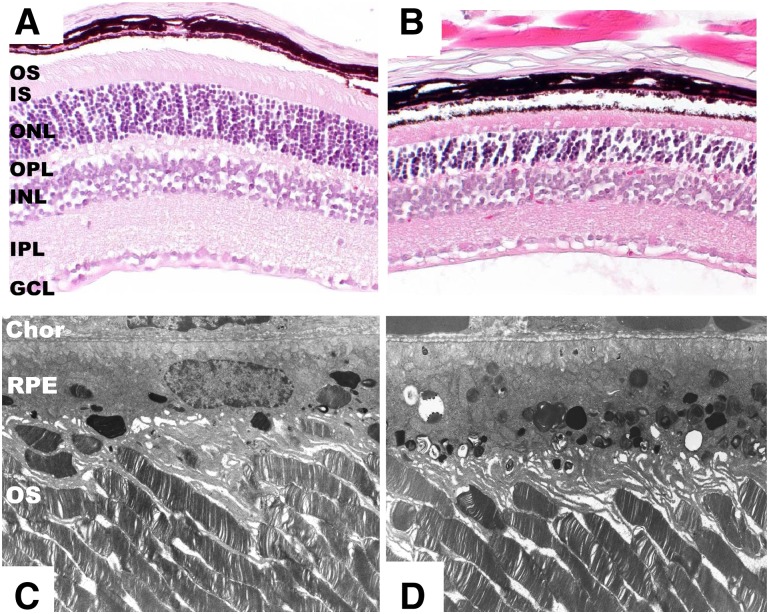
However, when treated for an additional 2–3 months with AY9944, a progressive and irreversible retinal degeneration ensued (113) (Fig. 4); the 7DHC-cholesterol ratio of the retina and ROS membranes was >5:1, photoreceptors degenerated, died, and dropped out, the RPE became markedly congested with membranous inclusions and lipid droplets, and the responsiveness to photic stimulation under both dark-adapted (rod-driven) and light-adapted (cone-driven) conditions was markedly compromised. Although the ROS were >30% reduced in length by postnatal month 3, compared with controls, the overall ultrastructure of the remaining outer segments was comparable to that of controls (e.g., the disk membranes were not distended, elongated, truncated, whorled, or otherwise abnormal in appearance). Further electrophysiological analysis revealed that the sensitivity of rod photoreceptors to light and their maximal response amplitudes were decreased nearly 2-fold compared with age-matched controls, and the timing of both rod and cone responses to photic stimulation was significantly delayed (113). This indicated that the phototransduction cascade machinery in the ROS was rendered sluggish and inefficient. However, it was also determined that there was no net change in the total sterol content of the retina or ROS membranes; instead, there was a one-for-one molecular replacement of about 80% of the cholesterol with 7DHC. In contrast, total serum sterol levels dropped by 80–90% in the AY9944-treated animals compared with controls, and the 7DHC-cholesterol ratio was >10:1 in many of the treated animals by postnatal month 3, consistent with the well-known serum cholesterol-lowering and systemic sterol metabolism-altering effects of AY9944 (110, 111).
Fig. 4.
Histological and ultrastructural features of retinal degeneration in the AY9944-induced SLOS rat model. A, B: Histological sections (paraffin embedment; hematoxylin/eosin stained) of retina from 10-wk-old control (A) and age-matched AY9944-treated (B) Sprague-Dawley rats. Note marked thinning of the photoreceptor layer, particularly the outer nuclear layer (ONL) and outer segment (OS) layer, in the AY9944-treated retina compared with the control (C, D). Corresponding electron micrographs of companion retinas (Epon resin embedment; OsO4 stained, lead citrate counterstained) from 10-wk-old control (C) and AY9944-treated (D) rats. Note hypertrophy and congestion of RPE with membranous inclusions in panel D. Abbreviations as in Fig. 1. [EM micrographs adapted from (113) with permission.]
So why does the retina undergo progressive degeneration in the AY9944-induced rat model of SLOS? The simple and most honest answer is, we do not really know yet. Is it because there is some requisite threshold level of cholesterol necessary for achieving and maintaining normal retinal structure and function? Is it because 7DHC, even though replacing cholesterol mole for mole, cannot fulfill the structural or functional roles that cholesterol plays in retinal cells? Is it because 7DHC, or molecules derived therefrom (e.g., oxysterols) that progressively form and accumulate in the retina and RPE, are toxic to retinal cells? Any or all of these answers may be correct and certainly have been considered by others in the context of understanding the fundamental pathophysiological mechanisms that underlie the SLOS (100–102, 114). One of us (SJF) has proposed that, although disruption of cholesterol biosynthesis and accumulation of 7DHC is the initial insult, this predisposes affected cells and tissues to a series of sequellae that involves other metabolic pathways (e.g., fatty acid and phospholipid metabolism) and other cellular processes (e.g., oxidative stress, protein and lipid oxidation, etc.), synergistically acting in concert to undermine cellular viability, integrity, and function (115).
The literature to date is fairly sparse concerning the histopathology and electrophysiology of the retina in patients affected with SLOS. Kretzer et al. (116) provided a case report of a 1-month-old male Caucasian child diagnosed with clinical features consistent with SLOS; they reported “extensive dropout of peripheral ganglion axons with incipient optic nerve demyelination,” as well as “mitochondrial disintegration” in the RPE and “unusual amorphous cytoplasmic masses…continuous with photoreceptor discs” within the subretinal space of the peripheral retina. Notably, the retinal layers were fully stratified, and differentiated rods and cones were evident. However, considerable time had elapsed before the eyes were placed into fixative, so postmortem artifacts are quite likely. Also, this case was presented more than a decade before the biochemical and associated gene defects in SLOS were discovered, so there was no confirmation of the SLOS diagnosis by biochemical or molecular genetics testing. Also, one cannot draw firm conclusions regarding the representative features of a disease based upon a single histopathological specimen. Atchaneeyasakul et al. (117) reported on ocular findings in eight children and a spontaneously aborted fetus affected with SLOS; these included pale optic discs, optic atrophy, and optic nerve hypoplasia, again signifying ganglion cell death and dropout. They also reported markedly elevated 7- and 8-dehydrocholesterol levels and decreased cholesterol levels (compared with normal patients) in several ocular tissues, consistent with the biochemical hallmarks of SLOS. There are only two publications, to date, regarding the electroretinographic findings in SLOS patients, one focused on rod function (118) and a very recent follow-up paper regarding cone function in the same patient cohort (119). In these patients (median age, 4 years old), rod sensitivity was decreased nearly 2-fold and the kinetics of both activation and deactivation of the rod photoresponse were far below normal limits in nearly all SLOS patients examined; however, there were no significant abnormalities noted in cone function. Regarding their rod findings, the authors stated “this is likely a consequence of altered sterol composition in the cell membranes of the rod photoreceptors. To our knowledge, this is the first demonstration of altered kinetics of a membrane-bound signaling system in SLOS” (118). Importantly, however, all of these patients had received cholesterol supplementation therapy; hence, these findings are not interpretable within the context of the electrophysiological features of the disease per se (i.e., in the absence of therapeutic intervention). Based upon the results obtained with the AY9944-induced rat model of SLOS cited above, it is highly likely that these patients would have experienced significant rod and cone visual defects without therapeutic intervention.
Other studies have been done to see whether a sterol other than cholesterol could support normal retinal structure and function. Treatment of neonatal rats systemically by subcutaneous or intraperitoneal injection with U18666A [3-β(2-diethylaminoethoxy)androst-5-en-17-one hydrochloride], an inhibitor of 3β–hydroxysterol-Δ24-reductase (desmosterol reductase) (120), results in accumulation of desmosterol (cholesta-5,24-dien-3β-ol) and depletion of cholesterol in all bodily tissues, but, unlike AY9944, it also causes cataract formation (121). This has been used as an animal model of desmosterolosis, another rare hereditary human disease in the constellation of postsqualene defects in the cholesterol biosynthetic pathway (122, 123). When rats were treated systemically for 21 days, starting from birth, with U18666A, the desmosterol-cholesterol mole ratio in the retina was nearly 1.0; following intravitreal injection of [3H]acetate, >88% of the nonsaponifiable radioactivity was found in desmosterol (124). In contrast, the retinas from age- and sex-matched control rats had desmosterol-cholesterol mole ratios <0.006, and >80% of the [3H]acetate-derived radiolabel in nonsaponifiable lipids was found in cholesterol. Up to postnatal week 4, retinal histology and ultrastructure, ROS renewal rates, electrophysiological function, and rhodopsin synthesis and intracellular trafficking were comparable in both treated and control animals (124). These results would suggest that desmosterol either is able to replace cholesterol, structurally and functionally, in the retina or, because there were still substantial levels of cholesterol present, the residual cholesterol was sufficient, perhaps in conjunction with desmosterol, to support normal retinal structure and function. However, the same was true for 7DHC in the AY9944 rat model of SLOS up to the first postnatal month (112); it has yet to be assessed whether a longer time course with U18666A treatment would result in a progressive retinal degeneration in this rat model of desmosterolosis. Also, and perhaps more importantly, more recent studies with U18666A have revealed that it has pleiotropic effects not merely restricted to inhibition of desmosterol reductase [reviewed by Cenedella (125)]. Hence, it is difficult based upon the above cited studies to make unambiguous conclusions about the ability of desmosterol to support normal retinal structure and function in the retina.
The above discussion has considered the effects of either depleting cholesterol or replacing it with an alternate, biogenically and structurally related sterol in the retina. What about trying to substantially elevate the levels of cholesterol in the brain or retina? As indicated above, while an as-yet-to-be-determined percentage of the total cholesterol in the retina is synthesized in situ, the brain synthesizes virtually all of its cholesterol de novo and the blood-brain barrier excludes uptake of circulating cholesterol. Therefore, dietary cholesterol supplementation is ineffective in altering the steady-state levels of brain cholesterol. However, the same is not true for the retina, at least under conditions where normal cholesterol metabolism has been perturbed. In fact, in an aforementioned rat model of SLOS, feeding a high-cholesterol diet (2%, by weight) was effective in nearly normalizing the cholesterol content of the retina, with a concomitant normalizing trend in the electrophysiological competence of the retina, particularly as involves cone-mediated visual transduction (13). Attempts to “load” the retina of normal rats by similar dietary cholesterol supplementation have not proved successful (S.J. Fliesler, unpublished observations). However, Niemann-Pick type C disease is characterized by cholesterol accumulation in neurons of the brain (126) and retina and is associated with age-dependent altered electroretinographic properties and neurodegeneration (127) [cf. recent study by Claudepierre et al. (128)].
In sum, these findings regarding the effects of either cholesterol excess or deficiency suggest that altering cholesterol metabolism can have profoundly deleterious effects in the CNS. Hence, maintaining normal steady-state levels of cholesterol in the brain and retina appears to be critical.
EXPORT OF CHOLESTEROL FROM THE RETINA
Although the brain cannot take up blood-borne cholesterol, it has achieved an exquisite mechanism for divesting itself of excess cholesterol. Among proteins involved in cholesterol export from the brain, ApoE seems to be one of the most prevalent and active, being responsible for eliminating about 1 mg/d of cholesterol in humans (129). However, a more prodigious means of exporting cholesterol from the brain involves side-chain oxidation of cholesterol by the enzyme cholesterol-24S-hydroxylase (CYP46A1), which can facilitate the removal of about 6 mg/d of cholesterol from the brain (7, 130, 131). CYP46A1 is a microsomal cytochrome-P450 enzyme that is exclusively expressed in neurons within the brain (132) and the retina (9, 133). CYP46A1-dependent hydroxylation of cholesterol generates 24S-hydroxycholesterol, which can readily traverse cellular membranes and the blood-brain barrier (presumably also the blood-retina barrier) and then be picked up by circulating lipoproteins (e.g., HDL) and transported to the liver, where it is converted to bile acids and excreted. In parallel to profiling lipids and fatty acids in human retina (20), 24S-hydroxycholesterol has been found in levels ranging from 33 to 162 ng/retina (79 ± 37 ng, mean ± SD), corresponding to 0.11−0.47 ng of 24S-hydroxycholesterol per microgram of cholesterol (n = 17; L. Bretillon, unpublished observations; cf. data from Mast et al. (134), discussed below), a value similar to that observed in human cerebellum (0.27−0.58 ng of 24S-hydroxycholesterol per microgram of cholesterol) (131). In rat brain, the rate for the production of 24S-hydroxycholesterol was found to be nearly equal to that of cholesterol synthesis (135), supporting a role of CYP46A1 in maintaining cholesterol homeostasis in the brain. Because CYP46A1 is exclusively expressed in brain neurons, the circulating level of 24S-hydroxycholesterol has been suggested to be a potential marker for neurodegeneration in the brain. For example, the early phases of neuronal cell loss in the course of Alzheimer's disease are characterized by increased plasma 24S-hydroxycholesterol levels (136). Conversely, patients in late stages of the disease have reduced levels of this oxysterol (137) due to fewer neurons and lower concentrations of both cholesterol and 24S-hydroxycholesterol, as well as CYP46A1 activity, in the brain (138). Meanwhile, glial expression of CYP46A1 in the brain of Alzheimer's patients has been reported (139, 140). Therefore, glial cells may substitute for neurons with regard to the conversion of cholesterol to 24S-hydroxycholesterol. However, it remains unknown if the amount of 24S-hydroxycholesterol formed in glia would compensate for that normally derived from neurons. Activation of glial cells supports the survival of neurons but on the other hand may accelerate the progression of neuronal loss (141). The neurotoxic effects of 24-hydroxycholesterol in neuroblastoma cells have been reported (142). Mild induction of oxidation and inflammation in RPE cells incubated with 24-hydroxycholesterol has been observed (143). Considering the low concentration of 24S-hydroxycholesterol in retina (9), it is actually unlikely that 24S-hydroxycholesterol would trigger neurodegeneration in the retina. As reviewed by Björkhem (7), at least in the brain, upregulation of CYP46A1 may prevent formation of β-amyloid by reducing cholesterol in neurons and by direct inhibition of the formation of β-amyloid by 24S-hydroxycholesterol. Recently, in a transgenic mouse model for Alzheimer's disease, β-amyloid deposits in the retina were found to be associated with retinal degeneration (144). These data suggest that the mechanism that links CYP46A1, 24S-hydroxycholesterol, and neurodegeneration involves β-amyloid. It is tempting to speculate that, under pathological conditions, a decline in CYP46A1 due to the death of retinal neurons abrogates the inhibition of β-amyloid formation by 24S-hydroxycholesterol, thereby exacerbating β-amyloid deposition and provoking (or contributing to) retinal degeneration.
Hydroxylation at the C24 position of cholesterol is not the only means by which cholesterol is rendered more hydrophilic in preparation for being eliminated from tissues via reverse cholesterol transport. Indeed, another P450-dependent sterol hydroxylase, cholesterol-27S-hydroxylase, hydroxylates cholesterol at the C27 position [reviewed in (145)]. This enzyme has been detected by immunohistochemistry in monkey retina by Lee et al. (146), where it reportedly is localized primarily to photoreceptor inner segments (cell bodies), with minimal expression in Müller cells, RPE cells, ganglion cells, the nerve fiber layer, and the endothelial cells of the choriocapillaris. Based upon these results, one would expect to detect appreciable levels of 27-hydroxycholesterol in the retina. However, those authors did not report quantification of the steady-state levels of 27-hydroxycholesterol in monkey retina. Intriguingly, Lee et al. (146) reported that when they mixed 27-hydroxycholesterol with 7-ketocholesterol (the latter a highly cytotoxic oxysterol), it blocked the ability of 7-ketocholesterol to kill cultured ARPE-19 cells, whereas the same molar amount of cholesterol failed to block 7-ketocholesterol cytotoxicity. [See Rodriguez and Larroyoz (23), this Thematic Review series, for a comprehensive overview of the cytotoxicity of 7-ketocholesterol and its effects on retinal cells.] By contrast, in a very recent report by Mast et al. (134), 27-hydroxycholesterol was below the level of detection (using a highly sensitive isotope dilution GC-MS method) in bovine and human retinas and in RPE cells. Rather, 3β-hydroxy-5-cholestenoic acid (27-COOH), an oxidation product derived from 27-hydroxycholesterol, was found in human and bovine retina and RPE in amounts that far exceeded those of 24-hydroxycholesterol. This suggests that 27-hydroxycholesterol formed in the retina is immediately converted to the corresponding 27-COOH product. These authors reported that the level of 24-hydroxycholesterol in bovine choroid (∼18 pmol/mg protein) was one-half that found in the bovine retina (∼36 pmol/mg protein), whereas 27-COOH was undetectable in choroid. In human retina, 27-COOH was present at an average concentration of ∼79 pmol/mg protein (range, 37–125 pmol/mg protein, N = 3), whereas 24-hydroxycholesterol was detected at only 1–4 pmol/mg protein. The levels of 27-COOH were much lower in human RPE (range, 1–3 pmol/mg protein, N = 3) than in the retina. By way of comparison, the levels of these types of oxysterols are >1,000-fold lower than the levels of cholesterol in retina and RPE cells. In total, these findings not only exclude the choroid as a source of contamination for the 24-hydroxycholesterol and 27-COOH found in the retina but also are consistent with reverse cholesterol transport from the retina to the RPE and into the choroidal circulation. Importantly, they also indicate a significant difference between the retina and brain with regard to the method of elimination of cholesterol from these two neural tissues.
REMAINING QUESTIONS AND FUTURE DIRECTIONS
From the foregoing, although we currently have a fundamental understanding about the general features of cholesterol content, biosynthesis, and turnover in the vertebrate retina, there are many questions remaining. For example, we still do not know the relative contributions of endogenous de novo synthesis versus uptake of cholesterol from extraretinal sources to the steady-state content of cholesterol in the retina. We do not yet know if the photoreceptor can make all of its own cholesterol to satisfy its multiple needs for sterols, e.g., in support of outer segment membrane assembly, if it relies upon extraretinal sources or neighboring glia (Müller cells) for this. We do not know to what extent cholesterol is recycled in the retina, either within the neurons themselves (e.g., photoreceptor cells) or via exchange between the adjacent RPE cells or glia and the photoreceptor cells. One way of answering this question would be to selectively knock out cholesterol synthesis in rod (or cone) photoreceptors, Müller cells, or RPE cells, and to then examine the ability of the photoreceptors to incorporate cholesterol into their outer segment membranes. One of us (SJF) currently is pursuing this line of inquiry. In fact, it is not yet known how each of the multiple cell types in the retina obtains its supply of cholesterol or exactly what the turnover rates for cholesterol are in those cells. Because deposition of free cholesterol and esters is one of the crucial features of aging of the retina and is relevant to the development of AMD, determining whether such deposits originate exclusively from retina and/or RPE/choroid merits further elucidation. [For a more complete discussion, see review articles by Curcio et al. (22) and Rodriguez and Larrayoz (23), this Thematic Review series.]
In the rat model of SLOS discussed herein, it also is not yet known why the retina degenerates or if treatments are available to prevent the degeneration once it starts. Because there still is very little information regarding progressive changes in the retina in SLOS or any of the other known human genetic diseases involving defective cholesterol biosynthesis, particularly in the absence of cholesterol supplementation therapy, it also is not known if (as in the SLOS rat model) the retina undergoes degeneration or not. Oxysterol dynamics in the retina also are not well understood, nor is much known about the relative toxicities of physiologically relevant oxysterols that may be involved in the various human pathologies where retinal degeneration is a factor. With regard to SLOS, a library of 7DHC-derived oxysterols recently has been established (147) that should afford one the ability to examine the relative toxicities of each oxysterol on various cells of interest in culture as well as to perform intravitreal injections of such compounds in animal models. In fact, studies of this kind have been reported recently (148). Sterols other than cholesterol appear to have a limited ability to support normal retinal structure and function, but it is not really clear whether this is due to certain structural features of those sterols or whether the formation of cytotoxic products derived from those sterols underlie the subsequent degeneration and demise of retinal cells. Clearly, there is much work remaining to be done in this area of research, and the answers to these questions may provide essential clues for the development of more effective therapeutic interventions into retinal degenerations associated with inborn errors in cholesterol biosynthesis.
Acknowledgments
We thank our many collaborators who contributed to the studies cited herein over the years, including: R. Kennedy Keller, Neal S. Peachey, Ignacio R. Rodriguez, Richard Cenedella, Michael J. Richards, Barbara A. Nagel, Dana K. Vaughan, Steven J. Pittler, Lawrence M. Rapp, Robert E. Anderson, Forbes D. Porter, Ingemar Björkhem, Niyazi Acar, Corinne Joffre, Alain M. Bron, Catherine P. Creuzot-Garcher, Olivier Berdeaux, Lucy Martine, Stéphane Grégoire, Gilles Thuret, and Philippe Gain.
Footnotes
Abbreviations:
- ABC
- ATP-binding cassette transporter
- Apo
- apolipoprotein
- BrM
- Bruch's membrane
- CE
- cholesteryl ester
- CNS
- central nervous system
- 27-COOH
- 3β-hydroxy-5-cholestenoic acid
- DHA
- docosahexaenoic acid
- DHCR7
- 3β-hydroxysterol-Δ7-reductase
- 7DHC
- 7-dehydrocholesterol
- IPM
- inter-photoreceptor matrix
- LDL-R
- LDL receptor
- LLP
- lipoprotein-like particle
- RPE
- retinal pigment epithelium
- IS
- inner segment
- OS
- outer segment
- ROS
- rod outer segment
- SLOS
- Smith-Lemli-Opitz syndrome
- SR-B
- scavenger receptor B
- VHDL
- very high-density lipoprotein
Research in our labs was supported, in part, by United States Public Health Service (National Institutes of Health/National Eye Institute) Grant EY007361 (S.J.F.), the March of Dimes Foundation (S.J.F.), an Unrestricted Grant and a Senior Scientific Investigator Award from Research to Prevent Blindness (S.J.F.), grants from the INRA AlimH Department (L.B.), and grants from the French Regional Council of Burgundy (L.B.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
REFERENCES
- 1.Hogan M. J., Alvarado J. A., Weddell J. E. 1971. Retina. Histology of the Human Eye: An Atlas and Textbook. W. B. Saunders Co, Philadelphia, PA: 393–522. [Google Scholar]
- 2.Nguyen-Legros J. 1978. Fine structure of the pigment epithelium in the vertebrate retina. Int. Rev. Cytol. Suppl. 7: 287–328. [PubMed] [Google Scholar]
- 3.Marmor M. F., Wolfensberger T. J., 1998. The Retinal Pigment Epithelium: Function and Disease. Oxford University Press, Oxford. [Google Scholar]
- 4.Masland R. H. 2001. Neuronal diversity in the retina. Curr. Opin. Neurobiol. 11: 431–436. [DOI] [PubMed] [Google Scholar]
- 5.Young R. W. 1976. Visual cells and the concept of renewal. Invest. Ophthalmol. Vis. Sci. 15: 700–725. [PubMed] [Google Scholar]
- 6.Besharse J. C. 1982. The daily light-dark cycle and rhythmic metabolism in the photoreceptor pigment epithelial complex. Prog. Retinal Res. 1: 81–124. [Google Scholar]
- 7.Björkhem I. 2006. Crossing the barrier: oxysterols as cholesterol transporters and metabolic modulators in the brain. J. Intern. Med. 260: 493–508. [DOI] [PubMed] [Google Scholar]
- 8.Dietschy J. M., Turley S. D. 2004. Thematic review series: Brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J. Lipid Res. 45: 1375–1397. [DOI] [PubMed] [Google Scholar]
- 9.Bretillon L., Diczfalusy U., Bjorkhem I., Maire M. A., Martine L., Joffre C., Acar N., Bron A., Creuzot-Garcher C. 2007. Cholesterol-24S-hydroxylase (CYP46A1) is specifically expressed in neurons of the neural retina. Curr. Eye Res. 32: 361–366. [DOI] [PubMed] [Google Scholar]
- 10.Fliesler S. J., Florman R., Rapp L. M., Pittler S. J., Keller R. K. 1993. In vivo biosynthesis of cholesterol in the rat retina. FEBS Lett. 335: 234–238. [DOI] [PubMed] [Google Scholar]
- 11.Keller R. K., Small M., Fliesler S. J. 2004. Enzyme blockade: a nonradioactive method to determine the absolute rate of cholesterol synthesis in the brain. J. Lipid Res. 45: 1952–1957. [DOI] [PubMed] [Google Scholar]
- 12.Tserentsoodol N., Sztein J., Campos M., Gordiyenko N. V., Fariss R. N., Lee J. W., Fliesler S. J., Rodriguez I. R. 2006. Uptake of cholesterol by the retina occurs primarily via a low density lipoprotein receptor-mediated process. Mol. Vis. 12: 1306–1318. [PubMed] [Google Scholar]
- 13.Fliesler S. J., Vaughan D. K., Jenewein E. C., Richards M. J., Peachey N. S. 2007. Partial rescue of retinal function and sterol steady-state in a rat model of Smith-Lemli-Opitz syndrome. Pediatr. Res. 61: 273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cunha-Vaz J. 2004. The blood-retinal barriers system: basic concepts and clinical evaluation. Exp. Eye Res. 78: 715–721. [DOI] [PubMed] [Google Scholar]
- 15.Törnquist P., Alm A., Bill A. 1990. Permeability of ocular vessels and transport across the blood-retinal barrier. Eye (Lond.). 4: 303–309. [DOI] [PubMed] [Google Scholar]
- 16.Andersson M., Elmberger P. G., Edlund C., Kristensson K., Dallner G. 1990. Rates of cholesterol, ubiquinone, dolichol and dolichyl-P biosynthesis in rat brain slices. FEBS Lett. 269: 15–18. [DOI] [PubMed] [Google Scholar]
- 17.Serougne-Gautheron C., Chevallier F. 1973. Time course of biosynthetic cholesterol in the adult rat brain. Biochim. Biophys. Acta. 316: 244–250. [DOI] [PubMed] [Google Scholar]
- 18.Wetzel M. G., Li J., Alvarez R. A., Anderson R. E., O'Brien P. J. 1991. Metabolism of linolenic acid and docosahexaenoic acid in rat retinas and rod outer segments. Exp. Eye Res. 53: 437–446. [DOI] [PubMed] [Google Scholar]
- 19.Fliesler S. J., Anderson R. E. 1983. Chemistry and metabolism of lipids in the vertebrate retina. Prog. Lipid Res. 22: 79–131. [DOI] [PubMed] [Google Scholar]
- 20.Bretillon L., Thuret G., Grégoire S., Acar N., Joffre C., Bron A., Gain P., Creuzot-Garcher C. 2008. Lipid and fatty acid profile of the retina, retinal pigment epithelium/choroid, and lacrimal gland, and associations with adipose tissue fatty acids in human subjects. Exp. Eye Res. 87: 521–528. [DOI] [PubMed] [Google Scholar]
- 21.Fliesler S. J., Schroepfer G. J., Jr 1982. Sterol composition of bovine retinal rod outer segment membranes and whole retinas. Biochim. Biophys. Acta. 711: 138–148. [DOI] [PubMed] [Google Scholar]
- 22.Curcio C. A., Johnson M., Huang J. D., Rudolf M. 2010. Apolipoprotein B-containing lipoproteins in retinal aging and age-related macular degeneration. J. Lipid Res. 51: 451–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodriguez I. R., Larrayoz I. M. 2010. Cholesterol oxidation in the retina: implications of 7-ketochoelsterol formation in chronic inflammation and age-related macular degeneration. J. Lipid Res. 51: 2847–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bretillon L., Acar N., Seeliger M. W., Santos M., Maire M. A., Juaneda P., Martine L., Gregoire S., Joffre C., Bron A. M., et al. 2008. ApoB100,LDLR−/− mice exhibit reduced electroretinographic response and cholesteryl esters deposits in the retina. Invest. Ophthalmol. Vis. Sci. 49: 1307–1314. [DOI] [PubMed] [Google Scholar]
- 25.Francis C. M. 1955. Lipids in the retina. J. Comp. Neurol. 103: 355–383. [DOI] [PubMed] [Google Scholar]
- 26.Caldwell R. B., McLaughlin B. J. 1985. Freeze-fracture study of filipin binding in photoreceptor outer segments and pigment epithelium of dystrophic and normal retinas. J. Comp. Neurol. 236: 523–537. [DOI] [PubMed] [Google Scholar]
- 27.Boesze-Battaglia K., Fliesler S. J., Albert A. D. 1990. Relationship of cholesterol content to spatial distribution and age of disc membranes in retinal rod outer segments. J. Biol. Chem. 265: 18867–18870. [PMC free article] [PubMed] [Google Scholar]
- 28.Curcio C. A., Presley J. B., Malek G., Medeiros N. E., Avery D. V., Kruth H. S. 2005. Esterified and unesterified cholesterol in drusen and basal deposits of eyes with age-related maculopathy. Exp. Eye Res. 81: 731–741. [DOI] [PubMed] [Google Scholar]
- 29.Boesze-Battaglia K., Hennessey T., Albert A. D. 1989. Cholesterol heterogeneity in bovine rod outer segment disk membranes. J. Biol. Chem. 264: 8151–8155. [PMC free article] [PubMed] [Google Scholar]
- 30.Albert A. D., Boesze-Battaglia K. 2005. The role of cholesterol in rod outer segment membranes. Prog. Lipid Res. 44: 99–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fliesler S. J., Keller R. K. 1997. Isoprenoid metabolism in the vertebrate retina. Int. J. Biochem. Cell Biol. 29: 877–894. [DOI] [PubMed] [Google Scholar]
- 32.Fliesler S. J., Schroepfer G. J., Jr 1986. In vitro metabolism of mevalonic acid in the bovine retina. J. Neurochem. 46: 448–460. [DOI] [PubMed] [Google Scholar]
- 33.Fliesler S. J., Schroepfer G. J., Jr 1983. Metabolism of mevalonic acid in cell-free homogenates of bovine retinas: formation of novel isoprenoid acids. J. Biol. Chem. 258: 15062–15070. [PubMed] [Google Scholar]
- 34.Keller R. K., Fliesler S. J., Nellis S. W. 1988. Isoprenoid biosynthesis in the retina: quantitation of the sterol and dolichol biosynthetic pathways. J. Biol. Chem. 263: 2250–2254. [PubMed] [Google Scholar]
- 35.Fliesler S. J., Florman R., Keller R. K. 1995. Isoprenoid metabolism in the retina: dynamics of squalene and cholesterol incorporation and turnover in frog rod outer segment membranes. Exp. Eye Res. 60: 57–69. [DOI] [PubMed] [Google Scholar]
- 36.Fliesler S. J., Keller R. K. 1995. Metabolism of [3H]farnesol to cholesterol and cholesterogenic intermediates in the living rat eye. Biochem. Biophys. Res. Commun. 210: 695–702. [DOI] [PubMed] [Google Scholar]
- 37.Rodwell V. W., Nordstrom J. L., Mitschelen J. J. 1976. Regulation of HMG-CoA reductase. Adv. Lipid Res. 14: 1–74. [DOI] [PubMed] [Google Scholar]
- 38.Beg Z. H., Brewer H. B., Jr 1981. Regulation of liver 3-hydroxy-3-methylglutaryl-CoA reductase. Curr. Top. Cell. Regul. 20: 139–184. [DOI] [PubMed] [Google Scholar]
- 39.Fliesler S. J. 2002. Effects of cholesterol biosynthesis inhibitors on retinal development, structure, and function. Sterols and Oxysterols: Chemistry, Biology and Pathobiology. Fliesler S. J., editor Research Signpost, Kerala, India: 77–109. [Google Scholar]
- 40.Grundy S. M., Metzger A. L. 1972. A physiological method for estimation of hepatic secretion of biliary lipids in man. Gastroenterology. 62: 1200–1217. [PubMed] [Google Scholar]
- 41.Wilson M. D., Rudel L. L. 1994. Review of cholesterol absorption with emphasis on dietary and biliary cholesterol. J. Lipid Res. 35: 943–955. [PubMed] [Google Scholar]
- 42.Bays H. 2002. Ezetimibe. Expert Opin. Investig. Drugs. 11: 1587–1604. [DOI] [PubMed] [Google Scholar]
- 43.Clearfield M. B. 2003. A novel therapeutic approach to dyslipidemia. J. Am. Osteopath. Assoc. 103: S16–S20. [PubMed] [Google Scholar]
- 44.Iqbal J., Hussain M. M. 2009. Intestinal lipid absorption. Am. J. Physiol. Endocrinol. Metab. 296: E1183–E1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mahley R. W., Innerarity T. L., Rall S. C., Jr., Weisgraber K. H. 1984. Plasma lipoproteins: apolipoprotein structure and function. J. Lipid Res. 25: 1277–1294. [PubMed] [Google Scholar]
- 46.Camps L., Reina M., Llobera M., Vilaro S., Olivecrona T. 1990. Lipoprotein lipase: cellular origin and functional distribution. Am. J. Physiol. 258: C673–C681. [DOI] [PubMed] [Google Scholar]
- 47.Hu W., Jiang A., Liang J., Meng H., Chang B., Gao H., Qiao X. 2008. Expression of VLDLR in the retina and evolution of subretinal neovascularization in the knockout mouse model's retinal angiomatous proliferation. Invest. Ophthalmol. Vis. Sci. 49: 407–415. [DOI] [PubMed] [Google Scholar]
- 48.Gordiyenko N., Campos M., Lee J. W., Fariss R. N., Sztein J., Rodriguez I. R. 2004. RPE cells internalize low-density lipoprotein (LDL) and oxidized LDL (oxLDL) in large quantities in vitro and in vivo. Invest. Ophthalmol. Vis. Sci. 45: 2822–2829. [DOI] [PubMed] [Google Scholar]
- 49.Yamada Y., Tian J., Yang Y., Cutler R. G., Wu T., Telljohann R. S., Mattson M. P., Handa J. T. 2008. Oxidized low density lipoproteins induce a pathologic response by retinal pigmented epithelial cells. J. Neurochem. 105: 1187–1197. [DOI] [PubMed] [Google Scholar]
- 50.Tserentsoodol N., Gordiyenko N. V., Pascual I., Lee J. W., Fliesler S. J., Rodriguez I. R. 2006. Intraretinal lipid transport is dependent on high density lipoprotein-like particles and class B scavenger receptors. Mol. Vis. 12: 1319–1333. [PubMed] [Google Scholar]
- 51.Houssier M., Raoul W., Lavalette S., Keller N., Guillonneau X., Baragatti B., Jonet L., Jeanny J. C., Behar-Cohen F., Coceani F., et al. 2008. CD36 deficiency leads to choroidal involution via COX2 down-regulation in rodents. PLoS Med. 5: e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duncan K. G., Bailey K. R., Kane J. P., Schwartz D. M. 2002. Human retinal pigment epithelial cells express scavenger receptors BI and BII. Biochem. Biophys. Res. Commun. 292: 1017–1022. [DOI] [PubMed] [Google Scholar]
- 53.Kociok N., Joussen A. 2007. Varied expression of functionally important genes of RPE and choroid in the macula and in the periphery of normal human eyes. Graefes Arch. Clin. Exp. Ophthalmol. 245: 101–113. [DOI] [PubMed] [Google Scholar]
- 54.Ryeom S. W., Sparrow J. R., Silverstein R. L. 1996. CD36 participates in the phagocytosis of rod outer segments by retinal pigment epithelium. J. Cell Sci. 109: 387–395. [DOI] [PubMed] [Google Scholar]
- 55.Provost A. C., Pequignot M. O., Sainton K. M., Gadin S., Salle S., Marchant D., Hales D. B., Abitbol M. 2003. Expression of SR-BI receptor and StAR protein in rat ocular tissues. C. R. Biol. 326: 841–851. [DOI] [PubMed] [Google Scholar]
- 56.Duncan K. G., Hosseini K., Bailey K. R., Yang H., Lowe R. J., Matthes M. T., Kane J. P., LaVail M. M., Schwartz D. M., Duncan J. L. 2009. Expression of reverse cholesterol transport proteins ATP-binding cassette A1 (ABCA1) and scavenger receptor BI (SR-BI) in the retina and retinal pigment epithelium. Br. J. Ophthalmol. 93: 1116–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Herz J. 2010. ApoE receptors in the nervous system. Curr. Opin. Lipidol. 20: 190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Herz J., Strickland D. K. 2001. LRP: a multifunctional scavenger and signaling receptor. J. Clin. Invest. 108: 779–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shi Z., Rudzinski M., Meerovitch K., Lebrun-Julien F. D. R., Birman E., Di Polo A., Saragovi H. U. 2008. alpha2-Macroglobulin is a mediator of retinal ganglion cell death in glaucoma. J. Biol. Chem. 283: 29156–29165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sánchez M. C., Barcelona P. F., Luna J. D., Ortiz S. G., Juarez P. C., Riera C. M., Chiabrando G. A. 2006. Low-density lipoprotein receptor-related protein-1 (LRP-1) expression in a rat model of oxygen-induced retinal neovascularization. Exp. Eye Res. 83: 1378–1385. [DOI] [PubMed] [Google Scholar]
- 61.Lillis A. P., Van Duyn L. B., Murphy-Ullrich J. E., Strickland D. K. 2008. LDL Receptor-Related Protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol. Rev. 88: 887–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang L., Li C. M., Rudolf M., Belyaeva O., Chung B. H., Messinger J. D., Kedishvili N. Y., Curcio C. A. 2009. Lipoprotein particles of intraocular origin in human Bruch membrane: an unusual lipid profile. Invest. Ophthalmol. Vis. Sci. 50: 870–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li C. M., Presley J. B., Zhang X., Dashti N., Chung B. H., Medeiros N. E., Guidry C., Curcio C. A. 2005. Retina expresses microsomal triglyceride transfer protein: implications for age-related maculopathy. J. Lipid Res. 46: 628–640. [DOI] [PubMed] [Google Scholar]
- 64.Li C. M., Chung B., Presley J. B., Malek G., Zhang X., Dashti N., Li L., Chen J., Bradley K., Kruth H. S., et al. 2005. Lipoprotein-like particles and cholesteryl esters in human Bruch's membrane: initial characterization. Invest. Ophthalmol. Vis. Sci. 46: 2576–2586. [DOI] [PubMed] [Google Scholar]
- 65.Curcio C. A., Millican C. L., Bailey T., Kruth H. S. 2001. Accumulation of cholesterol with age in human Bruch's membrane. Invest. Ophthalmol. Vis. Sci. 42: 265–274. [PubMed] [Google Scholar]
- 66.Malek G., Li C. M., Guidry C., Medeiros N. E., Curcio C. A. 2003. Apolipoprotein B in cholesterol-containing drusen and basal deposits of human eyes with age-related maculopathy. Am. J. Pathol. 162: 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pauleikhoff D., Harper C. A., Marshall J., Bird A. C. 1990. Aging changes in Bruch's membrane. A histochemical and morphologic study. Ophthalmology. 97: 171–178. [PubMed] [Google Scholar]
- 68.Haimovici R., Gantz D. L., Rumelt S., Freddo T. F., Small D. M. 2001. The lipid composition of drusen, Bruch's membrane, and sclera by hot stage polarizing light microscopy. Invest. Ophthalmol. Vis. Sci. 42: 1592–1599. [PubMed] [Google Scholar]
- 69.Huang J. D., Presley J. B., Chimento M. F., Curcio C. A., Johnson M. 2007. Age-related changes in human macular Bruch's membrane as seen by quick-freeze/deep-etch. Exp. Eye Res. 85: 202–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li C. M., Clark M. E., Rudolf M., Curcio C. A. 2007. Distribution and composition of esterified and unesterified cholesterol in extra-macular drusen. Exp. Eye Res. 85: 192–201. [DOI] [PubMed] [Google Scholar]
- 71.Moore D. J., Hussain A. A., Marshall J. 1995. Age-related variation in the hydraulic conductivity of Bruch's membrane. Invest. Ophthalmol. Vis. Sci. 36: 1290–1297. [PubMed] [Google Scholar]
- 72.Starita C., Hussain A. A., Pagliarini S., Marshall J. 1996. Hydrodynamics of ageing Bruch's membrane: implications for macular disease. Exp. Eye Res. 62: 565–572. [DOI] [PubMed] [Google Scholar]
- 73.Starita C., Hussain A. A., Patmore A., Marshall J. 1997. Localization of the site of major resistance to fluid transport in Bruch's membrane. Invest. Ophthalmol. Vis. Sci. 38: 762–767. [PubMed] [Google Scholar]
- 74.Schnebelen C., Viau S., Grégoire S., Joffre C., Creuzot-Garcher C. P., Bron A. M., Bretillon L., Acar N. 2009. Nutrition for the eye: different susceptibility of the retina and the lacrimal gland to dietary omega-6 and omega-3 polyunsaturated fatty acid incorporation. Ophthalmic Res. 41: 216–224. [DOI] [PubMed] [Google Scholar]
- 75.Bush R. A., Malnoe A., Reme C. E., Williams T. P. 1994. Dietary deficiency of N-3 fatty acids alters rhodopsin content and function in the rat retina. Invest. Ophthalmol. Vis. Sci. 35: 91–100. [PubMed] [Google Scholar]
- 76.Grossfield A., Feller S. E., Pitman M. C. 2006. A role for direct interactions in the modulation of rhodopsin by {omega}-3 polyunsaturated lipids. Proc. Natl. Acad. Sci. U.S.A. 103: 4888–4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pitman M. C., Grossfield A., Suits F., Feller S. E. 2005. Role of cholesterol and polyunsaturated chains in lipid-protein interactions: molecular dynamics simulation of rhodopsin in a realistic membrane environment. J. Am. Chem. Soc. 127: 4576–4577. [DOI] [PubMed] [Google Scholar]
- 78.Niu S-L., Mitchell D. C., Lim S-Y., Wen Z-M., Kim H-Y., Salem N., Jr., Litman B. J. 2004. Reduced G protein-coupled signaling efficiency in retinal rod outer segments in response to n-3 fatty acid deficiency. J. Biol. Chem. 279: 31098–31104. [DOI] [PubMed] [Google Scholar]
- 79.Bazan N. G. 1989. The metabolism of omega-3 polyunsaturated fatty acids in the eye: the possible role of docosahexaenoic acid and docosanoids in retinal physiology and ocular pathology. Prog. Clin. Biol. Res. 312: 95–112. [PubMed] [Google Scholar]
- 80.Bretillon L., Chardigny J. M., Noël J. P., Sébédio J. L. 1998. Desaturation and chain elongation of 1-14C mono-trans isomers of linoleic and α-linolenic acids in perfused rat liver. J. Lipid Res. 39: 2228–2239. [PubMed] [Google Scholar]
- 81.Burdge G. C. 2006. Metabolism of alpha-linolenic acid in humans. Prostaglandins Leukot. Essent. Fatty Acids. 75: 161–168. [DOI] [PubMed] [Google Scholar]
- 82.Burdge G. C., Calder P. C. 2005. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod. Nutr. Dev. 45: 581–597. [DOI] [PubMed] [Google Scholar]
- 83.Geerling B. J., Badart-Smook A., van Deursen C., van Houwelingen A. C., Russel M. G., Stockbrugger R. W., Brummer R. J. 2000. Nutritional supplementation with N-3 fatty acids and antioxidants in patients with Crohn's disease in remission: effects on antioxidant status and fatty acid profile. Inflamm. Bowel Dis. 6: 77–84. [DOI] [PubMed] [Google Scholar]
- 84.Katan M. B., Deslypere J. P., van Birgelen A. P. J. M., Penders M., Zegwaard M. 1997. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. J. Lipid Res. 38: 2012–2022. [PubMed] [Google Scholar]
- 85.Leaf D. A., Connor W. E., Barstad L., Sexton G. 1995. Incorporation of dietary n-3 fatty acids into the fatty acids of human adipose tissue and plasma lipid classes. Am. J. Clin. Nutr. 62: 68–73. [DOI] [PubMed] [Google Scholar]
- 86.Nelson G. J., Schmidt P. C., Bartolini G. L., Kelley D. S., Kyle D. 1997. The effect of dietary docosahexaenoic acid on plasma lipoproteins and tissue fatty acid composition in humans. Lipids. 32: 1137–1146. [DOI] [PubMed] [Google Scholar]
- 87.Meaney S., Lütjohann D., Diczfalusy U., Björkhem I. 2000. Formation of oxysterols from different pools of cholesterol as studied by stable isotope technique: cerebral origin of most circulating 24S-hydroxycholesterol in rats, but not in mice. Biochim. Biophys. Acta. 1486: 293–298. [DOI] [PubMed] [Google Scholar]
- 88.Lütjohann D., Stroick M., Bertsch T., Kuhl S., Lindenthal B., Thelen K., Andersson U., Björkhem I., Bergmann K., K. V., Fassbender K. 2004. High doses of simvastatin, pravastatin, and cholesterol reduce brain cholesterol synthesis in guinea pigs. Steroids. 69: 431–438. [DOI] [PubMed] [Google Scholar]
- 89.Haimovici R., Kramer M., Miller J. W., Hasan T., Flotte T. J., Schomacker K. T., Gragoudas E. S. 1997. Localization of lipoprotein-delivered benzoporphyrin derivative in the rabbit eye. Curr. Eye Res. 16: 83–90. [DOI] [PubMed] [Google Scholar]
- 90.Miller J. W., Walsh A. W., Kramer M., Hasan T., Michaud N., Flotte T. J., Haimovici R., Gragoudas E. S. 1995. Photodynamic therapy of experimental choroidal neovascularization using lipoprotein-delivered benzoporphyrin. Arch. Ophthalmol. 113: 810–818. [DOI] [PubMed] [Google Scholar]
- 91.Rudolf M., Winkler B., Aherrahou Z., Doehring L. C., Kaczmarek P., Schmidt-Erfurth U. 2005. Increased expression of vascular endothelial growth factor associated with accumulation of lipids in Bruch's membrane of LDL receptor knockout mice. Br. J. Ophthalmol. 89: 1627–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schmidt-Erfurth U., Rudolf M., Funk M., Hofmann-Rummelt C., Franz-Haas N. S., Aherrahrou Z., Schlotzer-Schrehardt U. 2008. Ultrastructural changes in a murine model of graded Bruch membrane lipoidal degeneration and corresponding VEGF164 detection. Invest. Ophthalmol. Vis. Sci. 49: 390–398. [DOI] [PubMed] [Google Scholar]
- 93.Goldberg I. J., Eckel R. H., Abumrad N. A. 2009. Regulation of fatty acid uptake into tissues: lipoprotein lipase- and CD36-mediated pathways. J. Lipid Res. 50 (Suppl.): S86–S90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pfrieger F. W. 2003. Outsourcing in the brain: do neurons depend on cholesterol delivery by astrocytes? Bioessays. 25: 72–78. [DOI] [PubMed] [Google Scholar]
- 95.Pfrieger F. W. 2010. Role of glial cells in the formation and maintenance of synapses. Brain Res. Rev. 63: 39–46. [DOI] [PubMed] [Google Scholar]
- 96.Pfrieger F. W., Barres B. A. 1997. Synaptic efficacy enhanced by glial cells in vitro. Science. 277: 1684–1687. [DOI] [PubMed] [Google Scholar]
- 97.Nieweg K., Schaller H., Pfrieger F. W. 2009. Marked differences in cholesterol synthesis between neurons and glial cells from postnatal rats. J. Neurochem. 109: 125–134. [DOI] [PubMed] [Google Scholar]
- 98.Pittler S. J., Fliesler S. J., Rapp L. M. 1992. Novel morphological changes in rat retina induced by intravitreal injection of lovastatin. Exp. Eye Res. 54: 149–152. [DOI] [PubMed] [Google Scholar]
- 99.Pittler S. J., Fliesler S. J., Fisher P. L., Keller R. K., Rapp L. M. 1995. In vivo requirement of protein prenylation for maintenance of retinal cytoarchitecture and photoreceptor structure. J. Cell Biol. 130: 431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Waterham H. R. 2006. Defects in cholesterol metabolism. FEBS Lett. 580: 5442–5449. [DOI] [PubMed] [Google Scholar]
- 101.Hennekam R. C. 2005. Congenital brain anomalies in distal cholesterol biosynthesis defects. J. Inherit. Metab. Dis. 28: 385–392. [DOI] [PubMed] [Google Scholar]
- 102.Porter F. D. 2003. Human malformation syndromes due to inborn errors of cholesterol synthesis. Curr. Opin. Pediatr. 15: 607–613. [DOI] [PubMed] [Google Scholar]
- 103.Smith D. W., Lemli L., Opitz J. M. 1964. A newly recognized syndrome of multiple congenital anomalies. J. Pediatr. 64: 210–217. [DOI] [PubMed] [Google Scholar]
- 104.Irons M., Elias E. R., Salen G., Tint G. S., Batta A. K. 1993. Defective cholesterol biosynthesis in Smith-Lemli-Opitz syndrome. Lancet. 341: 1414. [DOI] [PubMed] [Google Scholar]
- 105.Tint G. S., Irons M., Elias E. R., Batta A. K., Frieden R., Chen T. S., Salen G. 1994. Defective cholesterol biosynthesis associated with the Smith-Lemli-Opitz syndrome. N. Engl. J. Med. 330: 107–113. [DOI] [PubMed] [Google Scholar]
- 106.Correa-Cerro L. S., Porter F. D. 2005. 3beta-Hydroxysterol Delta7-reductase and the Smith-Lemli-Opitz syndrome. Mol. Genet. Metab. 84: 112–126. [DOI] [PubMed] [Google Scholar]
- 107.Yu H., Patel S. B. 2005. Recent insights into the Smith-Lemli-Opitz syndrome. Clin. Genet. 68: 383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jira P. E., Waterham H. R., Wanders R. J., Smeitink J. A., Sengers R. C., Wevers R. A. 2003. Smith-Lemli-Opitz syndrome and the DHCR7 gene. Ann. Hum. Genet. 67: 269–280. [DOI] [PubMed] [Google Scholar]
- 109.Kolf-Clauw M., Chevy F., Wolf C., Siliart B., Citadelle D., Roux C. 1996. Inhibition of 7-dehydrocholesterol reductase by the teratogen AY9944: a rat model for Smith-Lemli-Opitz syndrome. Teratology. 54: 115–125. [DOI] [PubMed] [Google Scholar]
- 110.Dvornik D., Kraml M., Dubuc J., Givner M., Gaudry R. 1963. A novel mode of inhibition of cholesterol biosynthesis. J. Am. Chem. Soc. 85: 3309. [Google Scholar]
- 111.Givner M. L., Dvornik D. 1965. Agents affecting lipid metabolism-XV. Biochemical studies with the cholesterol biosynthesis inhibitor AY-9944 in young and mature rats. Biochem. Pharmacol. 14: 611–619. [DOI] [PubMed] [Google Scholar]
- 112.Fliesler S. J., Richards M. J., Miller C-Y., Peachey N. S. 1999. Marked alteration of sterol metabolism and composition without compromising retinal development or function. Invest. Ophthalmol. Vis. Sci. 40: 1792–1801. [PubMed] [Google Scholar]
- 113.Fliesler S. J., Peachey N. S., Richards M. J., Nagel B. A., Vaughan D. K. 2004. Retinal degeneration in a rodent model of Smith-Lemli-Opitz syndrome: electrophysiologic, biochemical, and morphologic features. Arch. Ophthalmol. 122: 1190–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Merkens L. S., Wassif C., Healy K., Pappu A. S., DeBarber A. E., Penfield J. A., Lindsay R. A., Roullet J. B., Porter F. D., Steiner R. D. 2009. Smith-Lemli-Opitz syndrome and inborn errors of cholesterol synthesis: summary of the 2007 SLO/RSH Foundation scientific conference sponsored by the National Institutes of Health. Genet. Med. 11: 359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fliesler S. J. 2010. Retinal degeneration in a rat model of Smith-Lemli-Opitz syndrome: thinking beyond cholesterol. Adv. Exp. Med. Biol. 664: 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kretzer F. L., Hitner H. M., Mehta R. S. 1981. Ocular manifestations of the Smith-Lemli-Opitz syndrome. Arch. Ophthalmol. 99: 2000–2006. [DOI] [PubMed] [Google Scholar]
- 117.Atchaneeyasakul L. O., Linck L. M., Connor W. E., Weleber R. G., Steiner R. D. 1998. Eye findings in 8 children and a spontaneously aborted fetus with RSH/Smith-Lemli-Opitz syndrome. Am. J. Med. Genet. 80: 501–505. [DOI] [PubMed] [Google Scholar]
- 118.Elias E. R., Hansen R. M., Irons M., Quinn N. B., Fulton A. B. 2003. Rod photoreceptor responses in children with Smith-Lemli-Opitz syndrome. Arch. Ophthalmol. 121: 1738–1743. [DOI] [PubMed] [Google Scholar]
- 119.Garry D., Hansen R. M., Moskowitz A., Elias E. R., Irons M., Fulton A. B. 2010. Cone ERG responses in patients with Smith-Lemli-Opitz syndrome (SLOS). Doc Ophthalmol. Epub ahead of print May 4, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Phillips W. A., Avigan J. 1963. Inhibition of cholesterol biosynthesis in the rat by 3β-(diethylaminoethoxy) androst-5en-17-one hydrochloride. Proc. Soc. Exp. Biol. Med. 112: 233–236. [DOI] [PubMed] [Google Scholar]
- 121.Cenedella R.J., Bierkamper G. G. 1979. Mechanism of cataract production by 3-β(2-diethylaminoethoxy)androst-5-en-17-one hydrochloride, U18666A: an inhibitor of cholesterol biosynthesis. Exp. Eye Res. 28: 673–688. [DOI] [PubMed] [Google Scholar]
- 122.Clayton P., Mills K., Keeling J., FitzPatrick D. R. 1996. Desmosterolosis: a new inborn error of cholesterol metabolism. Lancet. 348: 404. [DOI] [PubMed] [Google Scholar]
- 123.FitzPatrick D. R., Keeling J. W., Evans M. J., Kan A. E., Bell J. E., Porteous M. E. M., Mills K., Winter R. M., Clayton P. T. 1998. Clinical phenotype of Desmosterolosis. Am. J. Med. Genet. 75: 145–152. [PubMed] [Google Scholar]
- 124.Fliesler S. J., Richards M. J., Miller C-Y., Peachey N. S., Cenedella R. J. 2000. Retinal structure and function in an animal model that replicates the biochemical hallmarks of desmosterolosis. Neurochem. Res. 25: 685–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cenedella R. J. 2009. Cholesterol synthesis inhibitor U18666A and the role of sterol metabolism and trafficking in numerous pathophysiological processes. Lipids. 44: 477–487. [DOI] [PubMed] [Google Scholar]
- 126.Paul C. A., Boegle A. K., Maue R. A. 2004. Before the loss: neuronal dysfunction in Niemann-Pick Type C disease. Biochim. Biophys. Acta. 1685: 63–76. [DOI] [PubMed] [Google Scholar]
- 127.Phillips S. E., Woodruff E. A., III, Liang P., Patten M., Broadie K. 2008. Neuronal loss of Drosophila NPC1a causes cholesterol aggregation and age-progressive neurodegeneration. J. Neurosci. 28: 6569–6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Claudepierre T., Paques M., Simonutti M., Buard I., Sahel J., Maue R. A., Picaud S., Pfrieger F. W. 2010. Lack of Niemann-Pick type C1 induces age-related degeneration in the mouse retina. Mol. Cell. Neurosci. 43: 164–176. [DOI] [PubMed] [Google Scholar]
- 129.Pitas R. E., Boyles J. K., Lee S. H., Foss D., Mahley R. W. 1987. Astrocytes synthesize apolipoprotein E and metabolize apolipoprotein E-containing lipoproteins. Biochim. Biophys. Acta. 917: 148–161. [DOI] [PubMed] [Google Scholar]
- 130.Björkhem I., Lütjohann D., Diczfalusy U., Stahle L., Ahlborg G., Wahren J. 1998. Cholesterol homeostasis in human brain: turnover of 24S-hydroxycholesterol and evidence for a cerebral origin of most of this oxysterol in the circulation. J. Lipid Res. 39: 1594–1600. [PubMed] [Google Scholar]
- 131.Lütjohann D., Breuer O., Ahlborg G., Nennesmo I., Siden A., Diczfalusy U., Björkhem I. 1996. Cholesterol homeostasis in human brain: evidence for an age-dependent flux of 24S-hydroxycholesterol from the brain into the circulation. Proc. Natl. Acad. Sci. USA. 93: 9799–9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lund E. G., Guileyardo J. M., Russell D. W. 1999. cDNA cloning of cholesterol 24-hydroxylase, a mediator of cholesterol homeostasis in the brain. Proc. Natl. Acad. Sci. USA. 96: 7238–7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ramirez D. M., Andersson S., Russell D. W. 2008. Neuronal expression and subcellular localization of cholesterol 24-hydroxylase in the mouse brain. J. Comp. Neurol. 507: 1676–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mast N., Reem R., Bederman I., Huang S., DiPatre P. L., Bjorkhem I., Pikuleva I. A. 2010. Cholestenoic acid is an important elimination product of cholesterol in the retina: comparison of retinal cholesterol metabolism to that in the brain. Invest. Ophthalmol. Vis. Sci. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Björkhem I., Lütjohann D., Breuer O., Sakinis A., Wennmalm A. 1997. Importance of a novel oxidative mechanism for elimination of brain cholesterol. Turnover of cholesterol and 24(S)-hydroxycholesterol in rat brain as measured with 18O2 techniques in vivo and in vitro. J. Biol. Chem. 272: 30178–30184. [DOI] [PubMed] [Google Scholar]
- 136.Lütjohann D., Papassotiropoulos A., Björkhem I., Locatelli S., Bagli M., Oehring R. D., Schlegel U., Jessen F., Rao M. L., von Bergmann K., et al. 2000. Plasma 24S-hydroxycholesterol (cerebrosterol) is increased in Alzheimer and vascular demented patients. J. Lipid Res. 41: 195–198. [PubMed] [Google Scholar]
- 137.Bretillon L., Sidén A., Wahlund L. O., Lütjohann D., Minthon L., Crisby M., Hillert J., Groth C. G., Diczfalusy U., Björkhem I. 2000. Plasma levels of 24S-hydroxycholesterol in patients with neurological diseases. Neurosci. Lett. 293: 87–90. [DOI] [PubMed] [Google Scholar]
- 138.Heverin M., Bogdanovic N., Lütjohann D., Bayer T., Pikuleva I., Bretillon L., Diczfalusy U., Winblad B., Björkhem I. 2004. Changes in the levels of cerebral and extracerebral sterols in the brain of patients with Alzheimer's disease. J. Lipid Res. 45: 186–193. [DOI] [PubMed] [Google Scholar]
- 139.Bogdanovic N., Bretillon L., Lund E. G., Diczfalusy U., Lannfelt L., Winblad B., Russell D. W., Björkhem I. 2001. On the turnover of brain cholesterol in patients with Alzheimer's disease. Abnormal induction of the cholesterol-catabolic enzyme CYP46 in glial cells. Neurosci. Lett. 314: 45–48. [DOI] [PubMed] [Google Scholar]
- 140.Brown J., III, Theisler C., Silberman S., Magnuson D., Gottardi-Littell N., Lee J. M., Yager D., Crowley J., Sambamurti K., Rahman M. M., et al. 2004. Differential expression of cholesterol hydroxylases in Alzheimer's disease. J. Biol. Chem. 279: 34674–34681. [DOI] [PubMed] [Google Scholar]
- 141.Bringmann A., Pannicke T., Grosche J., Francke M., Wiedemann P., Skatchkov S. N., Osborne N. N., Reichenbach A. 2006. Muller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 25: 397. [DOI] [PubMed] [Google Scholar]
- 142.Kolsch H., Lutjohann D., Tulke A., Bjorkhem I., Rao M. L. 1999. The neurotoxic effect of 24-hydroxycholesterol on SH-SY5Y human neuroblastoma cells. Brain Res. 818: 171–175. [DOI] [PubMed] [Google Scholar]
- 143.Joffre C., Leclere L., Buteau B., Martine L., Cabaret S., Malvitte L., Acar N., Lizard G., Bron A., Creuzot-Garcher C., et al. 2007. Oxysterols induced inflammation and oxidation in primary porcine retinal pigment epithelial cells. Curr. Eye Res. 32: 271–280. [DOI] [PubMed] [Google Scholar]
- 144.Ning A., Cui J., To E., Ashe K. H., Matsubara J. 2008. Amyloid-beta deposits lead to retinal degeneration in a mouse model of Alzheimer disease. Invest. Ophthalmol. Vis. Sci. 49: 5136–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pikuleva I., Javitt N. B. 2003. Novel sterols synthesized via the CYP27A1 metabolic pathway. Arch. Biochem. Biophys. 420: 35–39. [DOI] [PubMed] [Google Scholar]
- 146.Lee J. W., Fuda H., Javitt N. B., Strott C. A., Rodriguez I. R. 2005. Expression and localization of sterol 27-hydroxlase (CYP27A1) in monkey retina. Exp. Eye Res. 83: 465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xu L., Korade Z., Porter N. A. 2010. Oxysterols from free radical chain oxidation of 7-dehydrocholestero: product and mechanistic studies. J. Am. Chem. Soc. 132: 2222–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Korade Z., Xu L., Shelton R., Porter N. 2010. Biological activities of 7-dehydrocholesterol-derived oxysterols: implications for Smith-Lemli-Opitz syndrome. J. Lipid Res. Epub ahead of print. Aug 11, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fielding P., Fielding C. 1991. Dynamics of lipoprotein transport in the circulatory system. Biochemistry of Lipids, Lipoproteins and Membranes. Vance D., Vance J., Elsevier Science, Amsterdam: 427–459. [Google Scholar]