Abstract
Fructose intake is being discussed as a key dietary factor in the development of nonalcoholic fatty liver disease (NAFLD). Bile acids have been shown to modulate energy metabolism. We tested the effects of bile acids on fructose-induced hepatic steatosis. In C57BL/6J mice treated with a combination of chenodeoxycholic acid and cholic acid (100 mg/kg body weight each) while drinking water or a 30% fructose solution for eight weeks and appropriate controls, markers of hepatic steatosis, portal endotoxin levels, and markers of hepatic lipogenesis were determined. In mice concomitantly treated with bile acids, the onset of fructose-induced hepatic steatosis was markedly attenuated compared to mice only fed fructose. The protective effects of the bile acid treatment were associated with a downregulation of tumor necrosis factor (TNF)α, sterol regulatory element-binding protein (SREBP)1, FAS mRNA expression, and lipid peroxidation in the liver, whereas hepatic farnesoid X receptor (FXR) or short heterodimer partner (SHP) protein concentration did not differ between groups fed fructose. Rather, bile acid treatment normalized occludin protein concentration in the duodenum, portal endotoxin levels, and markers of Kupffer cell activation to the level of water controls. Taken together, these data suggest that bile acids prevent fructose-induced hepatic steatosis in mice through mechanisms involving protection against the fructose-induced translocation of intestinal bacterial endotoxin.
Keywords: nonalcoholic fatty liver disease, tumor necrosis factor α, occludin, endotoxin
Throughout the last three decades nonalcoholic fatty liver disease (NAFLD) has emerged to be among the leading liver diseases in the world (1). Fat accumulation in the liver, originally thought to be a benign, nonprogressive, histological state, is one of the first characteristics of the early phase of NAFLD (2). Furthermore, results of recent studies suggest that steatosis may play a critical role not only in the onset of NAFLD but also in its progression to later stages of the disease (e.g., fibrosis and cirrhosis (3)). Therefore, therapies protecting against the onset of NAFLD may also be beneficial for the later stages of the disease.
Results of several epidemiologic and clinical studies indicate that besides a general over-nutrition dietary intake of carbohydrates and fructose consumption in particular may play a critical role in the development of NAFLD in humans (4). The hypothesis that a diet rich in mono- and disaccharides, such as fructose and sucrose, might play a critical role in the pathogenesis of NAFLD is also supported by a number of studies performed in animals. In these studies, it was shown that an increased consumption of fructose (e.g., up to 60% of daily calories derived from fructose) resulted in an increased lipid accumulation in the liver, which was accompanied by insulin resistance, elevated plasma triglyceride levels, and oxidative stress (5–9). Recently, our group was able to show that hepatic steatosis resulting from chronic intake of fructose is associated with a loss of the tight junction protein occludin in the duodenum, an increased translocation of bacterial endotoxins from the intestine, and an induction of tumor necrosis factor (TNF)α in the liver of mice (7, 10, 11). However, while plasma levels of TNFα and retinol binding protein 4 were both increased under this feeding regimen, suggesting that fructose-fed mice were insulin-resistant, glucose levels in blood of food-deprived, fructose-fed mice did not differ from those of water-fed controls (7, 10). In these studies, the concomitant treatment with antibiotics or the loss of the endotoxin receptor toll-like receptor (TLR) 4 markedly attenuated the effect of fructose on mouse liver (10). Furthermore, we were able to show that the marked protection of tumor necrosis factor receptor (TNFR)1−/− mice against the onset of fructose-induced liver steatosis was associated with a protection against the fructose-induced upregulation of the expression of sterol regulator element-binding protein (SREBP)1 in the liver (12).
Primary bile acids (e.g., chenodeoxycholic acid and cholic acid) have long been known to be essential for the absorption of dietary lipids and cholesterol. In recent years, bile acids have been identified to play an important role as ligands for nuclear orphan receptors, such as the farnesoid X receptor (FXR) (13–15). Mainly through mechanisms involving the induction of the short heterodimer partner (SHP), FXR has been shown to downregulate hepatic fatty acid and triglyceride biosynthesis, as well as the production of very low density lipoproteins (VLDL) mediated by SREBP1c (16, 17). Recently it has been shown that, through binding to the G-protein-coupled receptor TGR5, bile acids may increase cyclic-AMP-dependent thyroid hormone activating enzyme type 2 iodothyronine deiodinase (D2) activity, thereby increasing energy expenditure in brown adipose tissue, preventing obesity and insulin resistance (18, 19). However, whether bile acids also protect the liver from the onset of fructose-induced NAFLD has not yet been clarified. The aim of the present study was to test if primary bile acids (e.g., cholic acid and chenodeoxycholic acid) protect against fructose-induced hepatic steatosis in a mouse model and if so, to identify underlying mechanisms.
MATERIAL AND METHODS
Animals and treatments
Mice were housed in a standard pathogen-free barrier facility accredited by the AAALAC with no positive testing for Helicobacter spp. All procedures were approved by the local IACUC. Six-week-old C57BL/6J mice (Jackson, Bar Harbor, ME) were fed either plain water or water containing 30% fructose for a period of 8 weeks. Some of the animals were concomitantly treated with a combination of bile acids (cholic acid, 100 mg/kg body weight; and chenodeoxycholic acid, 100 mg/ kg body weight) in drinking solutions throughout the entire feeding time. Similar doses of these bile acids have been used in rats by others (20). Animals were anesthetized with 80 mg ketamin/kg and 6 mg xylazin/kg body weight by intraperitoneal injection. Blood was collected just prior to euthanization. Portions of liver were either frozen immediately in liquid nitrogen, fixed in neutral-buffered formalin, or frozen-fixed in OCT mounting media (Medite, Burgdorf, Germany).
RNA isolation and realtime RT-PCR
Total RNA was extracted from liver samples using peqGOLD TriFast™ (PEQLAB, Erlangen, Germany). RNA concentrations were determined spectrophotometrically, and 1 µg total RNA was reverse-transcribed using a MuLV reverse transcriptase and oligo dT primers after a DNase digestion step (Fermentas, St. Leon-Rot, Germany). Polymerase chain reaction (PCR) primers for fatty acid synthase (FAS), FXR, myeloid differentiation factor (MyD)88, interferon regulatory factor (IRF)3 and 7, inducible nitric oxide synthase (iNOS), SHP, SREBP1, TNFα, X-box binding protein 1 (XBP1), spliced XBP1 (XBP1s), occludin, and β-actin (for primer sequences, see Table 1) were designed using Primer 3 software (Whitehead Institute for Biomedical Research). Sybr®Green Universal PCR Master Mix (Applied Biosystems, Darmstadt, Germany) was used to prepare the PCR mix. The amplification reactions were carried out in an iCycler (Bio-Rad Laboratories, Munich, Germany) with an initial hold step (95°C for 3 min) and 50 cycles of a three-step PCR (95°C for 15 s, 60°C for 15 s, 72°C for 30 s). The fluorescence intensity of each sample was measured at each temperature change to monitor amplification of the target gene. The comparative CT method was used to determine the amount of target gene, normalized to an endogenous reference (β-actin) and relative to a calibrator (2-ΔΔCt). The purity of PCR products was verified by melting curves and gel electrophoresis.
TABLE 1.
Primers used for real-time RT-PCR
| Mediator | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| FAS | TCT GGG CCA ACC TCA TTG GT | GAA GCT GGG GGT CCA TTG TG |
| iNOS | CAG CTG GGC TGT ACA AAC CTT | CAT TGG AAG TGA AGC GTT TCG |
| IRF3 | AAC CGG AAA GAA GTC TTG CG | GCA CCC AGA TGT ACG AAG TCC |
| IRF7 | ACA GGG CGT TTT ATC TTG CG | TCC AAG CTC CCG GCT AAG |
| MyD88 | CAA AAG TGG GGT GCC TTT GC | AAA TCC ACA GTG CCC CCA GA |
| SREBP1 | ACC GGC TAC TGC TGG ACT GC | AGA GCA AGA GGG TGC CAT CG |
| TNFα | CCA GGC GGT GCC TAT GTC TC | CAG CCA CTC CAG CTG CTC CT |
| Occludin | CAT CAG CCA TGT CCG TGA GG | GGG GCG ACG TCC ATT TGT AG |
| XBP1 | CCT GAG CCC GGA GGA GAA | CTC GAG CAG TCT GCG CTG |
| XBP1s | GAG TCC GCA GCA GGT G | GTG TCA GAG TCC ATG GGA |
| β-actin | GTA ACC CGT TGA ACC CCA TT | CCA TCC AAT CGG TAG TAG CG |
IRF3/7, interferon regulatory factor 3 or 7; MyD88, myeloid differentiation factor; iNOS, inducible nitric oxide synthase; SREBP, sterol regulator element-binding protein; TNF, tumor necrosis factor; XBP1, X-box binding protein 1; XBP1s, spliced X-box binding protein 1.
Fluorescent in situ hybridization
All chemicals used for fluorescent in situ hybridization (FISH) were obtained from Roth, Karlsruhe, Germany. Frozen sections (5 μm) of the first 5 cm of the small intestine were fixed with 4% PBS buffered formalin for 3 min. After incubation with lysozyme buffer (100 mM Tris-HCl, pH 8.0; 50 mM EDTA; 130.000 U/ml lysozyme) for 5 min, sections were washed in a buffer (20 mM Tris-HCl, pH 7.2; 225 mM NaCl; 0.01% SDS) and incubated with the Cy3-labeled RNA probe EUB338 (21) (5′- Cy3-GCTGCCTCCCGTAGGAGT-3′; 0.02 pmol/µl probe; 45% formamide; 900 mM NaCl; 0.01% SDS; 20 mM Tris-HCl, pH 7.2) for 2 h (46°C). Sections were washed and covered with Roti®-Mount FluorCare DAPI. In each sample, the number of bacteria was counted in 10 randomly selected fields per section at a 400× magnification using a fluorescence microscope (Axio Vert 200M, Zeiss, Jena, Germany). Data were pooled to determine means.
Triglyceride determination in liver
Liver samples were homogenized in ice-cold 2× PBS. Tissue lipids were extracted with methanol/chloroform (1:2), dried, and resuspended in 5% fat free BSA. Triglyceride levels were determined using a commercially available kit (Randox, Krefeld, Germany). Values were normalized to protein concentration determined by Bradford assay in liver homogenate (Bio-Rad Laboratories).
Oil Red O staining
To determine hepatic lipid accumulation, frozen sections of liver (10 µm) were stained with Oil Red O (Sigma, Steinheim, Germany) for 10 min, washed, and counterstained with hematoxylin for 45 s (Sigma, Steinheim, Germany) (7). Representative photomicrographs were captured at a 400× magnification using a system incorporated in a microscope (Axio Vert 200M, Zeiss, Jena, Germany). The extent of labeling in the liver lobule was defined as the percentage of the field area within the default color range determined by the software. Data from each tissue section (eight fields per section) were pooled to determine means.
Clinical chemistry and pathologic evaluation
Plasma alanine-aminotransferase (ALT) activity was determined using a commercially available kit (Randox, Krefeld, Germany). Paraffin sections of liver (5 µm) were stained for hematoxylin and eosin to assess liver histology (200×).
Endotoxin assay
Endotoxin was determined as described previously in detail (22). In brief, plasma samples were heated up to 70°C for 20 min. Plasma endotoxin levels were determined using a commercially available endpoint Limulus Amebocyte Lysate assay (Charles River, L‘Arbaesle, France) following the instructions of the manufacturer.
Immunohistochemical staining for 4-hydroxynonenal adducts
Paraffin-embedded liver sections (5 µm) were cut and stained for 4-hydroxynonenal (4-HNE) adducts using a polyclonal antibody (AG Scientific, San Diego, CA) as described previously (7). To detect specific binding of primary antibody, tissue sections were incubated with a peroxidase-linked secondary antibody and diaminobenzidine (Peroxidase Envision Kit; DAKO, Hamburg, Germany). Using an image acquisition and analysis system incorporated in the microscope, the extent of staining in liver sections was defined as percentage of the field area within the default color range determined by the software. To determine means, data from eight fields (200×) of each tissue section were used.
Immunoblots
To prepare nuclear protein lysates, liver tissue was homogenized first in Dignum A buffer (10 mM HEPES, 1.5 mM MgCl2, 5 mM KCl, 0.5 mM DTT) and then extracted with Dignum C (10 mM HEPES, 50% glycerol, 84 mM NaCl, 1.5 mM Mg2Cl, 0.2 mM EDTA, 0.5 mM DTT). All buffers contained a protease and phosphatase inhibitor mix (Sigma). To obtain samples of duodenal protein, snap-frozen samples of tissue obtained from the duodenum were homogenized in peqGOLD TriFastTM (PEQLAB, Erlangen, Germany), and protein was isolated according to the manufactures instructions. Protein lysates (10–30 µg protein/well) were separated in 10% SDS-PAGE and transferred to Hybond™-P polyvinylidene difluoride membranes. The resulting blots were then probed with antibodies against occludin (Zymed, San Francisco, CA), FXR, or SHP (Santa Cruz Biotechnology), respectively, and bands were visualized using a Super Signal Western Dura kit (Pierce, Perbio Science, Rockford, IL). To ensure equal loading, all blots were stained with Ponceau red. Signals of occludin were normalized to β-actin, which was detected using a commercially available antibody (New England Biolabs, Frankfurt, Germany) whereas bands detected for FXR and SHP, respectively, were normalized to histone H3 (3H1) Rabbit mAb (Cell Signaling Technology, Danvers, MA). Protein bands were analyzed densitometrically using Flurochem Software (Alpha Innotech, San Leandro, CA).
Statistical analyses
All results are reported as means ± SEM. Data were tested for equality of variances. If equality of variances was not given, data were log-transformed. Two-way ANOVA with Bonferroni post-hoc test was used to determine statistically significant differences between treatment groups. A P value < 0.05 was selected as the level of significance before the study.
RESULTS
Plasma and hepatic indices of liver damage
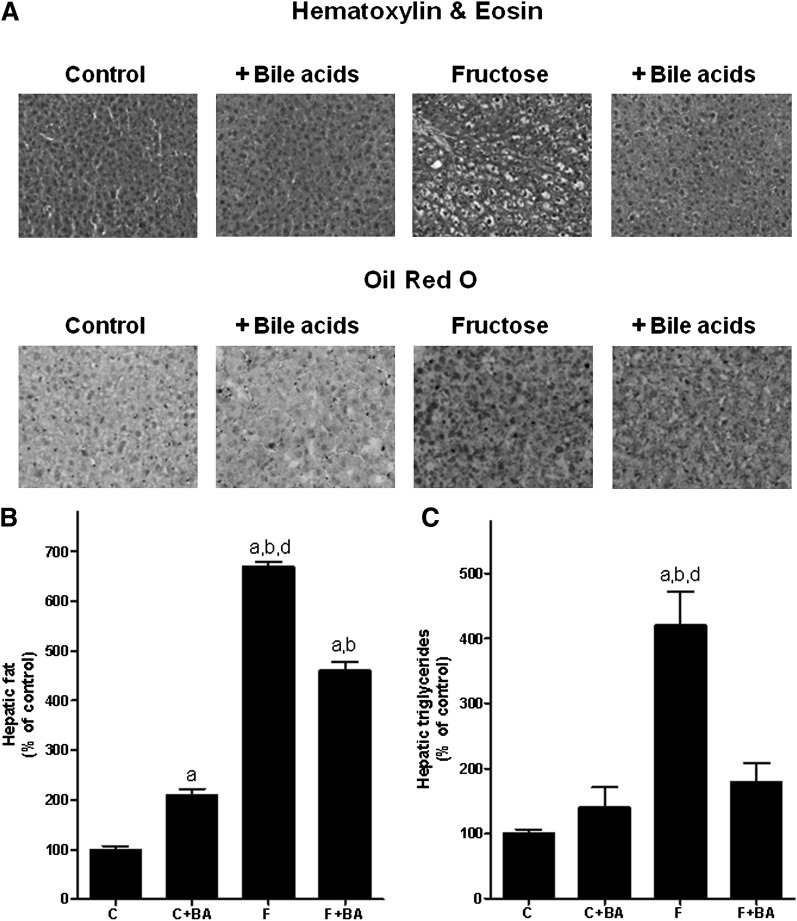
Fig. 1A shows representative pictures of Oil Red O and hematoxylin and eosin staining. Fig 1B and 1C show quantitation of hepatic lipid and triglyceride levels after chronic intake of water or water sweetened with 30% fructose. Chronic treatment of mice with bile acids resulted in a significant ∼2-fold increase in hepatic lipid content compared to water controls as determined by Oil Red O staining; however, staining was detected primarily around hepatocytes, indicating that lipid content of cells other than hepatocytes was affected by the bile acid treatment. Furthermore, a similar effect of bile acid treatment was not detected when determining total triglyceride content of liver. In line with our earlier findings using this mouse model, chronic intake of 30% fructose solution resulted in a significant ∼4.2-fold increase in triglyceride and ∼6.7-fold increase in lipid levels in the liver compared with controls. In contrast, in livers of fructose-fed mice concomitantly treated with bile acids, hepatic lipid and triglyceride levels were only increased by ∼2.2-fold and ∼1.3-fold, respectively, compared to controls treated with bile acids. In accordance with these findings, ALT levels were significantly higher in plasma of fructose-fed mice; however, plasma ALT levels did not differ between controls and fructose-fed mice treated with bile acids (Table 2). A similar protective effect of the bile acid treatment was not found when comparing absolute liver weights or liver-to-body weight ratios. Absolute weight gain of fructose-fed mice concomitantly treated with bile acids and that of mice fed only with fructose did not differ.
Fig. 1.
Effect of chronic consumption of 30% fructose solution and bile acids on the lipid accumulation in the liver. A: Representative photomicrographs of the hematoxylin and eosin (200×) as well as Oil Red O staining (400×) of liver sections. B and C: Quantitative analysis of Oil Red O staining and hepatic triglyceride content. Data are shown as means ± SEM (n = 5–6) and are normalized to percent of control. aP < 0.05 compared with mice fed water; bP < 0.05 compared with mice fed water + bile acids; dP < 0.05 compared with mice fed fructose solution + bile acids. C, water; BA, bile acid; F, 30% fructose solution.
TABLE 2.
Effect of chronic intake of sweetened water and bile acids on caloric intake and indices of hepatic lipogenesis
| Indices | Water | Water + Bile Acids | Fructose | Fructose + Bile Acids |
|---|---|---|---|---|
| Weight gain (g) | 3.29 ± 0.3 | 3.7 ± 0.5 | 5.0 ± 0.4 | 4.5 ± 0.5 |
| Liver weight (g) | 1.06 ± 0.03 | 1.11 ± 0.06 | 1.41 ± 0.07ab | 1.46 ± 0.06ab |
| Liver-to-body weight ratio | 4.88 ± 0.1 | 4.9 ± 0.2 | 6.01 ± 0.2ab | 6.4 ± 0.1ab |
| ALT(U/l)d | 1.6 ± 0.5 | 7.0 ± 3.1 | 35.3 ± 3.7abc | 8.0 ± 1.2 |
| FAS mRNA | 100 ± 21 | 199 ± 55 | 633 ± 84ab | 423 ± 53a |
| SREBP1 mRNA | 100 ± 12 | 113 ± 30 | 244 ± 47ab | 164 ± 33 |
Feeding of fructose-sweetened water and bile acids is described in Material and Methods. Data are means ± SEM (n = 6). ALT, alanine-aminotransferase; SREBP, sterol regulator element-binding protein.
P < 0.05 compared with mice fed water.
P < 0.05 compared with mice fed water enriched with bile acids
P < 0.05 compared with mice fed with 30% fructose solution.
ALT activity in plasma was determined in n = 3–6 mice per group.
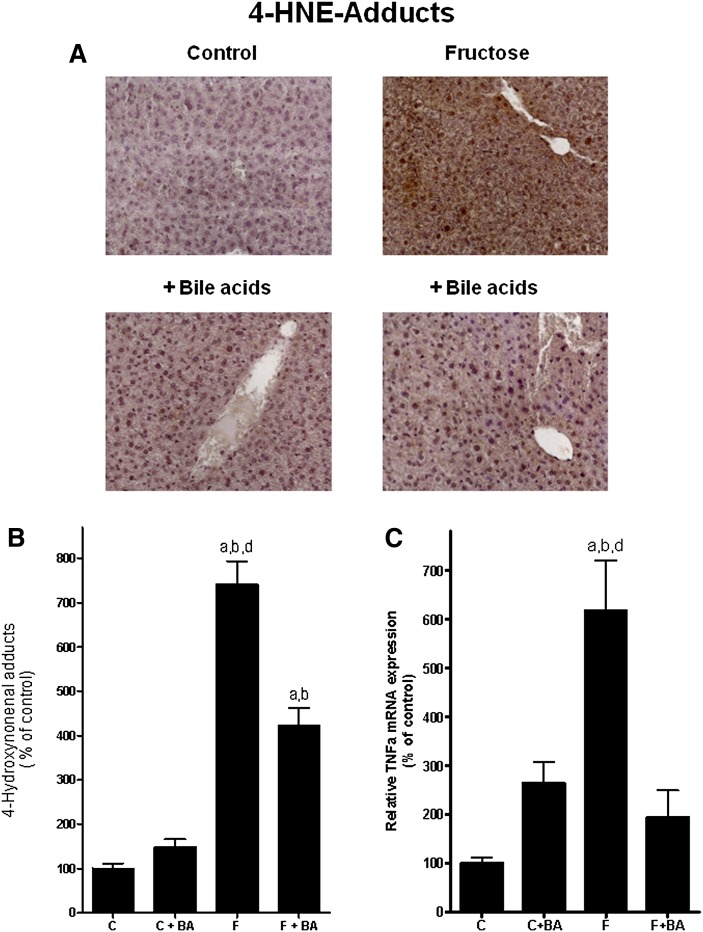
Concentration of 4-HNE adducts, mRNA expression of TNFα, and lipogenesis in the liver
Our results and those of other groups suggest that the pivotal effects of chronic fructose intake on the liver may at least partly depend on an increased formation of reactive oxygen species (ROS), induction of TNFα mRNA expression, and activation of hepatic lipogenesis (7, 10). Therefore, we determined levels of 4-HNE adducts and mRNA expression of TNFα (Fig. 2), as well as markers of lipogenesis (e.g., SREBP1 and FAS) (Table 2) in the liver. Representative pictures of 4-HNE adduct staining (brown) are depicted in Fig. 2A, and quantitative analysis of staining is summarized in Fig. 2B. Concentrations of 4-HNE adducts did not differ between control groups. In fructose-fed mice, levels of 4-HNE adducts in the liver were significantly increased by ∼7.4-fold compared to plain water controls. In fructose-fed mice concomitantly treated with bile acids, levels of 4-HNE adducts in the liver were only increased by ∼4-fold compared to controls; however, differences were still significant. In line with these findings, expression of TNFα in livers of fructose-fed mice was significantly increased by ∼6.2-fold compared to plain water controls (Fig. 2C). In contrast, TNFα mRNA expression levels in livers of fructose-fed mice treated with bile acids did not differ from those of controls. Expression of FAS was induced by ∼6.3-fold and ∼3.2-fold in livers of fructose-fed mice compared to water controls and water controls treated with bile acids (Table 2). In contrast, in livers of fructose-fed mice concomitantly treated with bile acids, FAS expression was only induced by ∼4.2 and ∼2.1-fold, respectively, compared with the two control groups. Expression of SREBP1 mRNA was also significantly induced in livers of fructose-fed mice compared to the two water control groups (Table 2). A similar effect was not found in livers of fructose-fed mice concomitantly treated with bile acids.
Fig. 2.
Effect of chronic consumption of 30% fructose solution and bile acids on the hepatic lipid peroxidation and TNFα mRNA levels in liver. A: Representative photomicrographs of immunostaining of 4-HNE adducts (200×) in liver sections. 4-HNE adducts are brown. B: Densitometric analysis of 4-HNE adduct staining. C: Relative hepatic TNFα mRNA expression as determined by realtime RT-PCR. Data for TNFα are normalized to β-actin. Data are shown as means ± SEM (n = 5–6) and are normalized to percent of control. aP < 0.05 compared with mice fed water; bP < 0.05 compared with mice fed water + bile acids; dP < 0.05 compared with mice fed fructose solution + bile acids. C, water; BA, bile acid; F, 30% fructose solution; 4-HNE, 4-Hydroxynonenal; TNF, tumor necrosis factor.
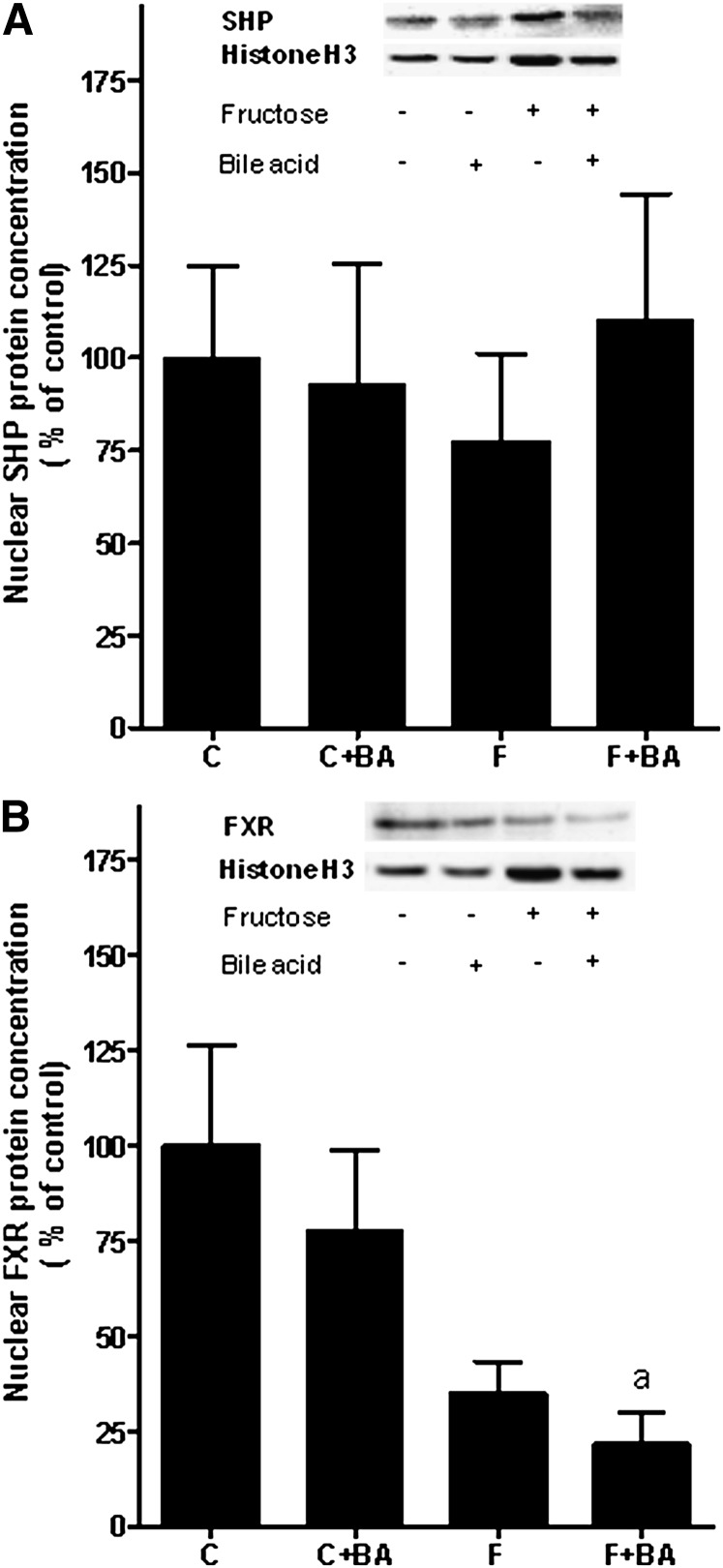
Levels of FXR and SHP in the liver
To determine whether the protective effects of the bile acid treatment found in fructose-fed mice were associated with an alternation of FXR and SHP in the liver protein, levels of these two nuclear receptors were determined in nuclear extracts. Results are summarized in Fig. 3. Protein levels of SHP (Fig. 3A) did not differ between groups. When determining protein levels of FXR in the liver, FXR protein concentration (Fig. 3B) was found to be markedly lower in livers of both fructose-fed groups compared to water-fed mice; however, as protein levels varied considerably between mice, differences only reached the level of significance for the comparison of FXR protein levels between water controls and fructose-fed mice concomitantly treated with bile acids (water controls versus fructose plus bile acids, P < 0.05; water controls versus fructose, P = 0.052).
Fig. 3.
Effect of chronic consumption of 30% fructose solution and bile acids on nuclear protein concentration of SHP and FXR in the liver. A: Representative Western blot of SHP and histone H3 (3H1) as well as quantitative analysis of blots. B: Representative Western blot of FXR and histone H3 (3H1) as well as quantitative analysis of blots. Data are shown as means ± SEM (n = 5–6) and are normalized to percent of control. aP < 0.05 compared with mice fed water. C, water; BA, bile acid; F, 30% fructose solution; FXR, farnesoid X receptor; SHP, short heterodimer partner.
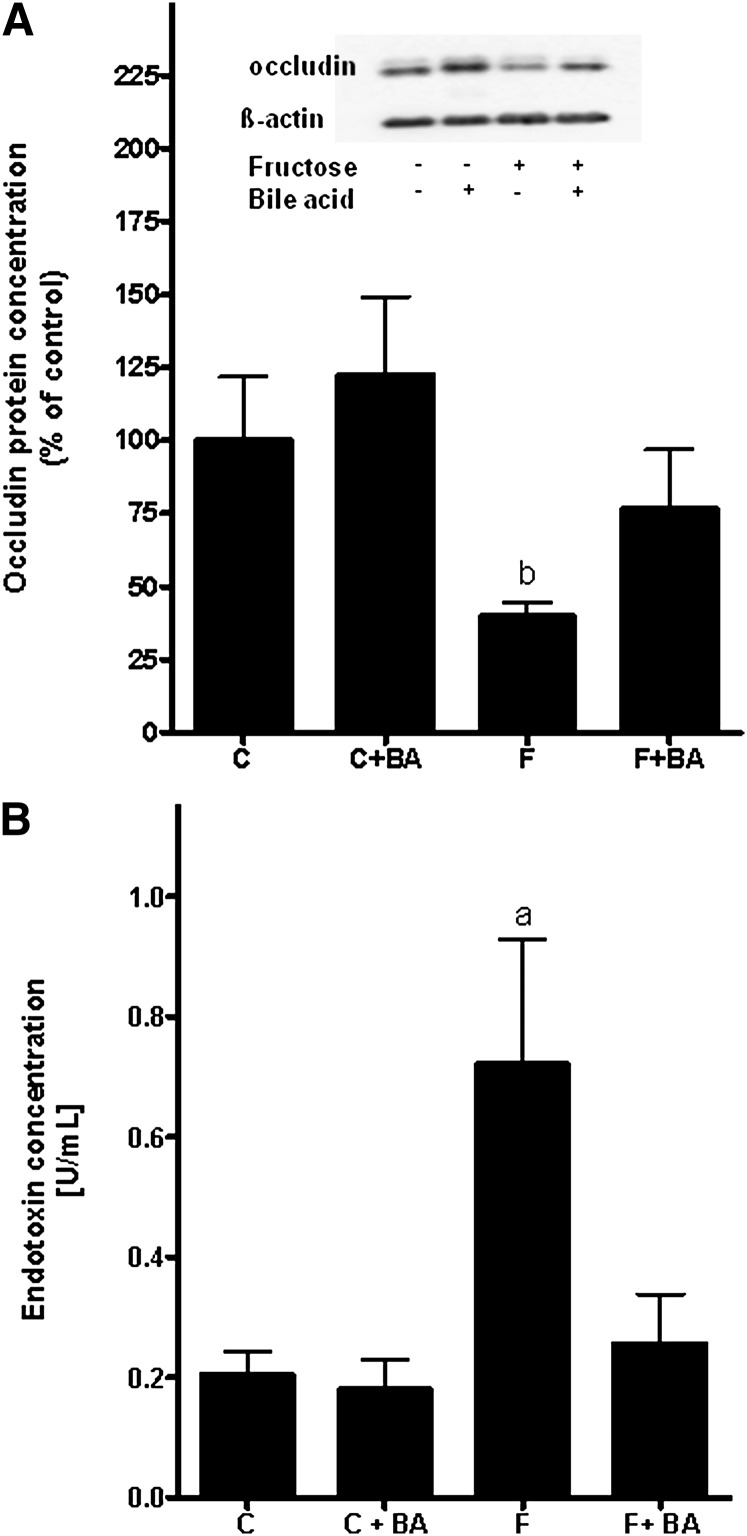
Protein concentration of occludin in the duodenum, portal endotoxin concentration, and expression of MyD88, IRF3/7, and iNOS in the liver
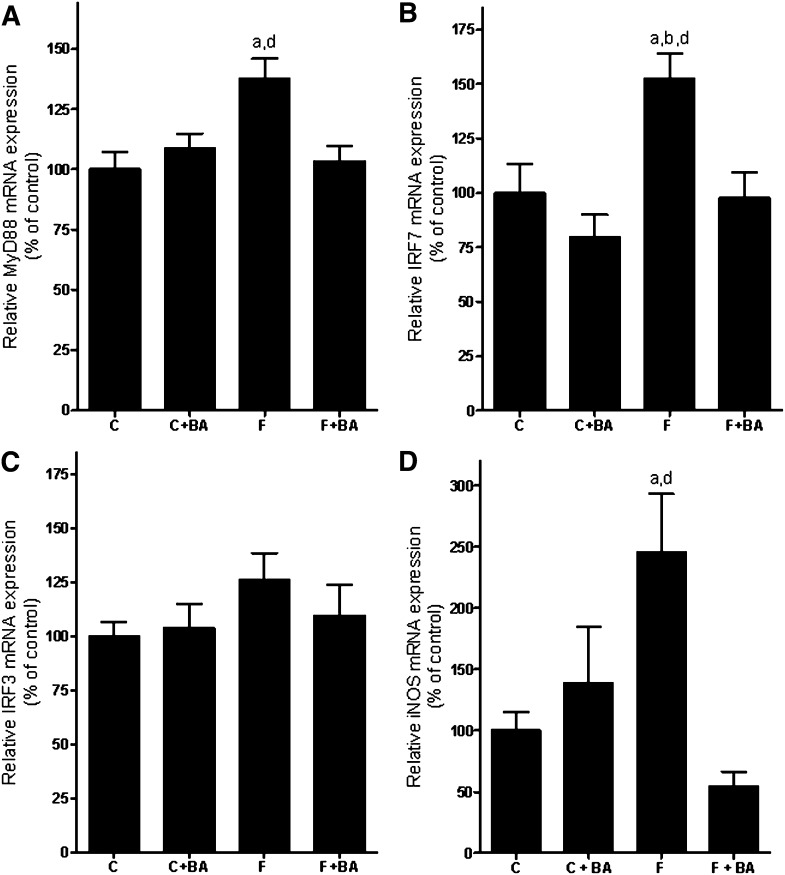
In line with our earlier findings (11), chronic intake of 30% fructose solution resulted in a marked loss of the tight junction protein occludin in the duodenum compared to controls (∼60% decrease compared to water controls and ∼83% decrease compared to water controls treated with bile acids, P < 0.05; data are summarized in Fig. 4A). Interestingly, in fructose-fed mice concomitantly treated with bile acids, the loss of occludin protein was prevented. Plasma endotoxin levels (Fig. 4B) and mRNA expression of the TLR4-adaptor protein MyD88 (Fig. 5A) were only found to be significantly elevated in mice exposed to 30% fructose solution, whereas both plasma endotoxin levels and mRNA expression of MyD88 were at the levels of controls in fructose-fed mice concomitantly treated with bile acids. Similar results were found for IRF7 mRNA expression (Fig. 5B), whereas no differences between groups were found for IRF3 mRNA expression (Fig. 5C). Furthermore, when determining iNOS expression in whole-liver RNA extracts (Fig. 5D), we only found the expression of iNOS to be significantly induced in livers of fructose-fed mice compared to water controls, whereas iNOS mRNA expression was found to be at the levels of controls in livers of fructose-fed mice concomitantly treated with bile acids.
Fig. 4.
Effect of chronic consumption of 30% fructose solution and bile acids on occludin concentration in the duodenum and portal endotoxin levels. A: Representative Western blots of occludin and quantitative analysis of blots. Data for occludin were normalized to β-actin. B: Portal endotoxin concentration. Data are shown as means ± SEM (occludin, n = 5–6; endotoxin, n = 3–6) and are normalized to percent of control. aP < 0.05 compared with mice fed water; bP < 0.05 compared with mice fed water + bile acids. C, water; BA, bile acid; F, 30% fructose solution.
Fig. 5.
Effect of chronic consumption of 30% fructose solution and bile acids on hepatic MyD88, IRF3, and IRF7 as well as iNOS levels. Expression of (A) MyD88, (B) IRF7, (C) IRF3, and (D) iNOS determined by realtime RT-PCR. Expression levels were normalized to β-actin expression. Data are expressed as means ± SEM (n = 4–6).aP < 0.05 compared with mice fed water; bP < 0.05 compared with mice fed water + bile acids; dP < 0.05 compared with mice fed fructose solution + bile acids. C, water; BA, bile acid; F, 30% fructose solution; IRF3/7, interferon regulatory factor 3 or 7; MyD88, myeloid differentiation factor; iNOS, inducible nitric oxide synthase.
Occludin mRNA expression, markers of ER stress, and number of bacteria in the duodenum
To further delineate how the treatment with bile acids may have protected mice from the onset of fructose-induced hepatic steatosis, we determined mRNA expression levels of occludin in the duodenum of mice. Interestingly, despite no difference in protein levels, mRNA expression of occludin in the duodenum of mice exposed to the bile acids were significantly lower than in those not treated with bile acids (Table 3). However, no differences in occludin mRNA expression were found when fructose-fed mice and fructose-fed mice concomitantly treated with bile acids were compared with their respective controls.
TABLE 3.
Effect of chronic intake of sweetened water and bile acids on mRNA expression
| Water | Water + Bile Acids | Fructose | Fructose + Bile Acids | |
|---|---|---|---|---|
| Occludin | 3.1 ± 0.5 | 1.7 ± 0.3a | 2.4 ± 0.2 | 1.4 ± 0.3a |
| Xbp1 | 7.7 ± 1.0 | 2.3 ± 0.5a | 10.5 ± 2.0bd | 4.1 ± 1.2 |
| Xbp1s | 7.1 ± 0.7 | 2.3 ± 0.7ac | 9.7 ± 1.5 | 5.8 ± 1.6 |
Feeding of fructose-sweetened water and bile acids is described in Material and Methods. Data are means ± SEM (n = 6). XBP1, X-box binding protein 1; XBP1s, spliced X-box binding protein 1.
P < 0.05 compared with mice fed water.
P < 0.05 compared with mice fed water enriched with bile acids.
P < 0.05 compared with mice fed with 30% fructose solution.
P < 0.05 compared with mice fed fructose solution enriched with bile acids.
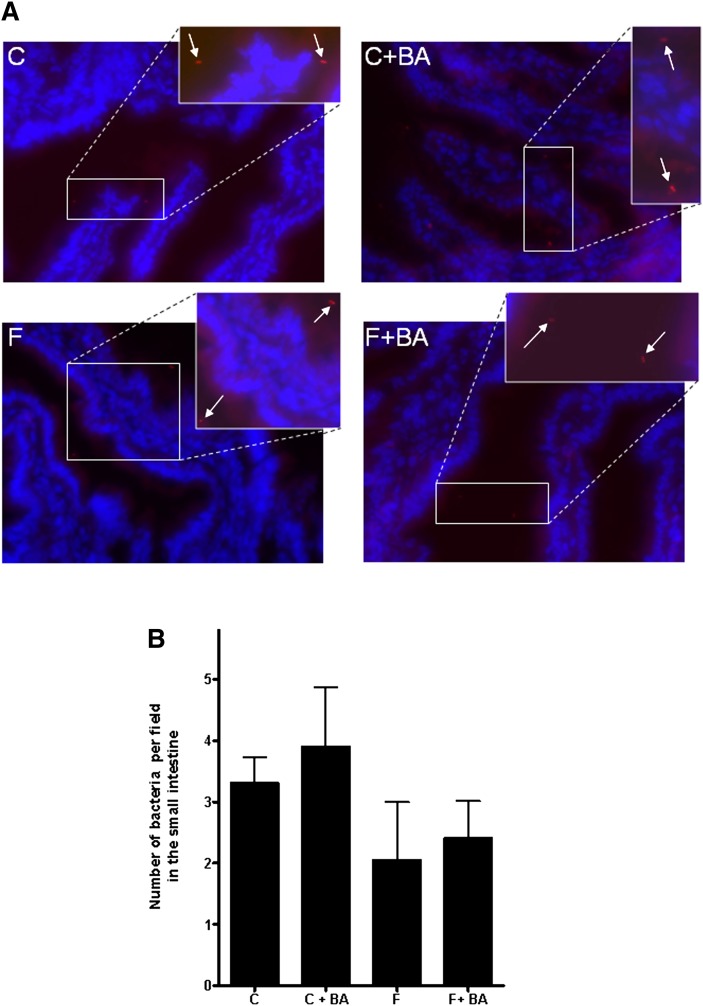
To determine whether the downregulation of occludin protein found in fructose-fed mice and the protective effect of the bile acid treatment thereon was associated with a protection against endoplasmic reticulum (ER) stress, we determined expression levels of XBP1 and XBP1s (Table 3). Both XBP1 and XBP1s have been used by others as markers of ER stress (23–25). Interestingly, the chronic treatment of mice with the two bile acids led to a marked downregulation of the expression of XBP1 and XBP1s in the duodenum, regardless of additional feeding regiments (XBP1: water plus bile acids, ∼70% decrease, P < 0.05; bile acids plus fructose, ∼46% decrease, P = 0.052, compared to water controls; XBP1s: water plus bile acids,∼67% decrease, P < 0.05; fructose plus bile acids, ∼19% decrease, P = 0.43, compared to water controls). However, no differences were found between groups when fructose-fed mice and fructose-fed mice concomitantly treated with bile acids were compared to their respective controls. As bile acids have been shown by others (26) to inhibit growth of intestinal bacteria, the number of bacteria in the duodenum was determined by FISH. Representative pictures and quantitative analysis of FISH are shown in Fig. 6. Regardless of additional treatments, number of bacteria in the duodenum did not differ among groups.
Fig. 6.
Effect of chronic consumption of 30% fructose solution and bile acids on number of bacteria in the small intestine. A: Representative pictures of FISH. B: Quantitative analysis of FISH. Data are expressed as means ± SEM (n = 4–6). C, water; BA, bile acid; F, 30% fructose solution; FISH, fluorescent in situ hybridization.
DISCUSSION
Results of several human and animal studies propose a role of dietary fructose intake in the development of NAFLD (for reviews, see Refs. 27 and 28). Furthermore, results of a small human intervention trial suggest that a reduction of fructose intake may indeed have a beneficial effect on the progression of NAFLD (29). The results of animal studies of our own group and those of others indicate that the onset of fructose-induced NAFLD in mice may not only be a result of a general over nutrition and the insulin-independent metabolism of fructose but also may partly result from molecular alterations caused either directly or indirectly by fructose (for an overview, see Ref. 30). Furthermore, results of several in vivo and in vitro studies suggest that bile acids, through an activation of FXR- and/or TGR5-dependent pathways, can alter hepatic lipid and energy metabolism (e.g., through a negative regulation of SREBP1). Recently, it has been shown by Leikin-Frenkel et al. (31) in rats with fat-induced NAFLD that oral treatment with fatty acid-bile acid conjugates may lower fat content in the liver. Results obtained from animals with bile duct ligation further suggest that treatment of animals with bile acids (e.g., taurocholic acid) may protect livers from TNFα-induced apoptosis (32). However, whether treatment with bile acids (e.g., cholic acid and chenodeoxycholic acid) protects mice from the onset of fructose-induced NAFLD has not yet been investigated. In the present study, we tested the hypothesis that a combined treatment of mice with two bile acids (cholic acid and chenodeoxycholic acid) at rather low doses (100 mg/kg body weight each) compared to other studies (18, 33–35) protects mice from the onset of fructose-induced NAFLD. Indeed, despite no effect on absolute body weight gain, the increase in hepatic lipid content and plasma ALT levels found in mice only fed fructose was markedly attenuated in fructose-fed animals concomitantly treated with the two bile acids. Interestingly, in livers of controls treated with bile acids, Oil Red O staining revealed a marked increase in lipid content compared to water controls; however, differences seemed to stem primarily from a more intense staining of cells surrounding hepatocytes (e.g., stellate cells) rather than from lipids stored within hepatocytes. An effect of bile acids on other hepatic cell populations, such as stellate cells, has been suggested by the results of several in vivo and in vitro studies (36, 37). However, exact origin and underlying mechanisms of the more intense Oil Red O staining in control mice treated with bile acids remains to be determined. Furthermore, treatment with bile acids also protected livers of fructose-fed mice from increased formation of ROS, as determined by measuring 4-HNE adducts and the induction of TNFa, SREBP1, and FAS mRNA expression found in mice fed only fructose. Taken together, our data suggest that bile acid treatment may protect against the onset of fructose-induced NAFLD without affecting body weight gain at rather low doses. These data further suggest that bile acids under the present conditions may have protected the liver from the pivotal effects of fructose, at least in part through mechanisms involving protection against the fructose-induced formation of ROS and induction of TNFα as well as lipogenesis in the liver. These results by no means preclude that treatment with bile acids may add to the formation of ROS, as shown by others in in vitro studies (38–40); rather, these results suggest that the effect of bile acids may depend on dosage, duration, and disease to be treated. The differences found regarding the effect of the bile acid treatment on weight gain between our study and those of others (19), who found a marked protection against high fat diet-induced obesity, might have resulted from differences in diet (high-fat chow versus 30% fructose solution as the only source of liquid in the present study) and a markedly lower dosage of bile acids in the present study (e.g., 100 mg/kg body weight versus 0.5% w/w in Ref. 19).
One mechanism by which bile acids may alter lipid metabolism in the liver is through the nuclear receptor FXR. Excess triglyceride deposition in the liver can be the consequence of impaired hepatic fatty acid oxidation or enhanced fatty acid extraction and triglyceride synthesis (41). FXR activation induced by bile acids can improve hypertriglyceridemia in mice and in in vitro models (18). Furthermore it has been shown that FXR controls triglyceride homeostasis via the negative regulation of SREBP1, which controls the expression of various genes involved in lipogenesis [e.g., FAS and acetyl-CoA carboxylase ACC] (17). In mice it has been shown that the FXR-induced suppression of SREBP1 by endogenous bile acids is ineffective in SHP−/− mice (18). These results further suggest that the FXR-SHP-SREBP1 cascade may play a critical role in the regulation of hepatic lipogenesis. However, in the present study, despite a markedly lower SREBP1 and FAS mRNA expression in livers of fructose-fed mice concomitantly treated with bile acids, nuclear concentration of SHP and FXR protein were similar in livers of mice fed with fructose and mice fed with fructose concomitantly treated with bile acids. It may be that dosages of bile acids used in the present study were not sufficient to alter the protein levels of FXR in the liver or to prevent the marked downregulation found in fructose-fed animals. Indeed, in the studies of Watanabe et al. (18), who fed mice ∼0.5 g cholic acid/kg body weight with chow, no changes in FXR mRNA in the liver were detected after one week of feeding. Contrary to the results of the present study, mRNA expression of SHP was markedly increased in the study of Watanabe et al. (18); however, differences between the two studies might have resulted from the different detection levels used (nuclear protein versus RNA). Furthermore, neither mRNA expression nor protein levels directly reflect activity levels of nuclear receptors. Taken together, these data suggest that the protective effects of bile acids under these conditions are perhaps independent of the FXR-SHP cascade and their dependent pathways. These results do not preclude a role of FXR-SHP signaling in NAFLD; rather, they suggest that additional pathways may contribute to lipid accumulation.
Results of in vitro studies suggest that bile acids, in addition to exerting their effects through FXR, may also reduce the permeation of endotoxin through intestinal epithelial cell layers by binding it to micelles formed by conjugated primary bile salts or through modulating transepithelial permeability (34, 42). Indeed, it has been shown that unconjugated cholic acid may decrease transepithelial permeability in differentiated Caco-2 cells through mechanisms involving an increased intracellular ROS generation (42). In line with our earlier findings (7, 10, 11), portal endotoxin levels were significantly higher in fructose-fed mice, whereas portal endotoxin levels of fructose-fed mice treated with bile acids were at the level of controls. Expression of the TLR-adaptor proteins MyD88 and IRF7 were only found to be induced in fructose-fed mice. Furthermore, mRNA expression of iNOS, which has been shown to be induced through endotoxin/TLR4-dependent pathways (10, 43), was at the level of controls in livers of fructose-fed mice concomitantly treated with bile acids, whereas in livers of mice fed only fructose, it was found to be significantly induced. The protection against the increased translocation of intestinal bacterial endotoxin was associated with a protection of fructose-fed mice concomitantly treated with bile acids against the loss of the tight junction protein occludin in the duodenum. Interestingly, a similar effect was not found at the level of occludin mRNA expression, which did not differ between fructose-treated mice and their respective controls. However, it has been suggested by the results of others that occludin is not solely regulated at the level of transcription (44, 45). For instance, it was recently shown by Coeffier et al. (44) that a proteasome-mediated modulation of protein degradation may contribute to the regulation of occludin protein levels in patients with inflammatory bowel disease. Interestingly, in these studies, mRNA expression levels of occludin did not differ between groups, whereas protein levels were markedly lower in patients with inflammatory bowel disease than in controls. Another factor that may have contributed to the protective effects of the two bile acids on the loss of intestinal integrity found in the present study may have been the protection against ER stress. Elevated ER stress seems to be associated with an impaired barrier function (46), and some bile acids haven been shown to inhibit ER stress (47). In the present study, expression of markers of ER stress were generally lower in mice treated with bile acids; however, fructose feeding seemed not to have been associated with an enhancement of ER stress in the duodenum of mice compared to water controls. It also has been suggested by the results of other groups that bile acids may inhibit bacterial growth in the intestine (26, 48, 49). However, in the present study, the protective effects found in fructose-fed mice concomitantly treated with bile acids were not associated with changes in number of bacteria in the duodenum. These results do not preclude that bacterial flora may have been altered in mice treated with bile acids and/or fructose. Indeed, it has been suggested by the results of Pumbwe et al. (50) that bile salts, for instance, can enhance intestinal colonization with B. fragilis. Taken together, these findings suggest that bile acids under the present conditions may protect the liver from the onset of fructose-induced NAFLD through normalizing intestinal transepithelial permeability, thereby decreasing the translocation of bacterial endotoxin from the gut into the portal plasma and subsequently decreasing the activation of hepatic Kupffer cells. However, the mechanisms underlying the protective effect(s) of bile acids on the fructose-induced downregulation of tight junctions in the small intestine remain to be determined.
The data of the present study add further weight to the hypothesis that besides the insulin-independent metabolism of fructose, an increased translocation of bacterial endotoxin is a key factor in the onset of fructose-induced NAFLD. Furthermore, the results of the present study suggest a novel mechanism by which bile acids may protect the liver from the onset of NAFLD. Protection of the intestinal epithelium against the loss of tight junction proteins (e.g., occludin), which results from either direct or indirect effects of the chronic intake of fructose on the intestinal mucosa, seems to be a key factor. Additional studies are necessary to further explore this concept in humans and to determine mechanisms underlying the protective effects of bile acids on tight junctions in the intestine.
Footnotes
Abbreviations:
- ALT
- alanine-aminotransferase
- BA
- bile acid
- ER
- endoplasmic reticulum
- FISH
- fluorescent in situ hybridization
- FXR
- farnesoid X receptor
- 4-HNE
- 4-hydroxynonenal
- IRF3/7
- interferon regulatory factor 3 or 7
- MyD88
- myeloid differentiation factor
- NAFLD
- non-alcoholic fatty liver disease
- iNOS
- inducible nitric oxide synthase
- ROS
- reactive oxygen species
- SHP
- short heterodimer partner
- SREBP
- sterol regulator element-binding protein
- TNF
- tumor necrosis factor
- TNFR
- tumor necrosis factor receptor
- XBP1
- X-box binding protein 1
- XBP1s
- spliced X-box binding protein 1
This work was supported by a grant from the German Ministry of Education and Science (BMBF) (03105084 to I.B.).
REFERENCES
- 1.Clark J. M. 2006. The epidemiology of nonalcoholic fatty liver disease in adults. J. Clin. Gastroenterol. 40(Suppl. 1): S5–S10. [DOI] [PubMed] [Google Scholar]
- 2.Yang S. Q., Lin H. Z., Mandal A. K., Huang J., Diehl A. M. 2001. Disrupted signaling and inhibited regeneration in obese mice with fatty livers: implications for nonalcoholic fatty liver disease pathophysiology. Hepatology. 34: 694–706. [DOI] [PubMed] [Google Scholar]
- 3.Adams L. A., Lymp J. F., St S. J., Sanderson S. O., Lindor K. D., Feldstein A., Angulo P. 2005. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 129: 113–121. [DOI] [PubMed] [Google Scholar]
- 4.Ouyang X., Cirillo P., Sautin Y., McCall S., Bruchette J. L., Diehl A. M., Johnson R. J., Abdelmalek M. F. 2008. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J. Hepatol. 48: 993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ackerman Z., Oron-Herman M., Grozovski M., Rosenthal T., Pappo O., Link G., Sela B. A. 2005. Fructose-induced fatty liver disease: hepatic effects of blood pressure and plasma triglyceride reduction. Hypertension. 45: 1012–1018. [DOI] [PubMed] [Google Scholar]
- 6.Armutcu F., Coskun O., Gurel A., Kanter M., Can M., Ucar F., Unalacak M. 2005. Thymosin alpha 1 attenuates lipid peroxidation and improves fructose-induced steatohepatitis in rats. Clin. Biochem. 38: 540–547. [DOI] [PubMed] [Google Scholar]
- 7.Bergheim I., Weber S., Vos M., Kramer S., Volynets V., Kaserouni S., McClain C. J., Bischoff S. C. 2008. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. J. Hepatol. 48: 983–992. [DOI] [PubMed] [Google Scholar]
- 8.Jurgens H., Haass W., Castaneda T. R., Schurmann A., Koebnick C., Dombrowski F., Otto B., Nawrocki A. R., Scherer P. E., Spranger J., et al. 2005. Consuming fructose-sweetened beverages increases body adiposity in mice. Obes. Res. 13: 1146–1156. [DOI] [PubMed] [Google Scholar]
- 9.Elliott S. S., Keim N. L., Stern J. S., Teff K., Havel P. J. 2002. Fructose, weight gain, and the insulin resistance syndrome. 176. Am. J. Clin. Nutr. 76: 911–922. [DOI] [PubMed] [Google Scholar]
- 10.Spruss A., Kanuri G., Wagnerberger S., Haub S., Bischoff S. C., Bergheim I. 2009. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology. 50: 1094–1104. [DOI] [PubMed] [Google Scholar]
- 11.Haub S., Kanuri G., Volynets V., Brune T., Bischoff S. C., Bergheim I. 2010. Serotonin reuptake transporter (SERT) plays a critical role in the onset of fructose-induced hepatic steatosis in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 298: G335–G344. [DOI] [PubMed] [Google Scholar]
- 12.Kanuri G., Spruss A., Haub S., Wagneberger S., Bischoff S. C., Bergheim I. Role of TNF alpha in the onset of fructose-induced non-alcoholic fatty liver disease. J. Nutr. Biochem. Epub ahead of print. August 27, 2010. [Google Scholar]
- 13.Makishima M., Okamoto A. Y., Repa J. J., Tu H., Learned R. M., Luk A., Hull M. V., Lustig K. D., Mangelsdorf D. J., Shan B. 1999. Identification of a nuclear receptor for bile acids. Science. 284: 1362–1365. [DOI] [PubMed] [Google Scholar]
- 14.Parks D. J., Blanchard S. G., Bledsoe R. K., Chandra G., Consler T. G., Kliewer S. A., Stimmel J. B., Willson T. M., Zavacki A. M., Moore D. D., et al. 1999. Bile acids: natural ligands for an orphan nuclear receptor. Science. 284: 1365–1368. [DOI] [PubMed] [Google Scholar]
- 15.Wang L., Huang J., Saha P., Kulkarni R. N., Hu M., Kim Y., Park K., Chan L., Rajan A. S., Lee I., et al. 2006. Orphan receptor small heterodimer partner is an important mediator of glucose homeostasis. Mol. Endocrinol. 20: 2671–2681. [DOI] [PubMed] [Google Scholar]
- 16.Brown M. S., Goldstein J. L. 1997. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 89: 331–340. [DOI] [PubMed] [Google Scholar]
- 17.Horton J. D., Goldstein J. L., Brown M. S. 2002. SREBPs: transcriptional mediators of lipid homeostasis. Cold Spring Harb. Symp. Quant. Biol. 67: 491–498. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe M., Houten S. M., Wang L., Moschetta A., Mangelsdorf D. J., Heyman R. A., Moore D. D., Auwerx J. 2004. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J. Clin. Invest. 113: 1408–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watanabe M., Houten S. M., Mataki C., Christoffolete M. A., Kim B. W., Sato H., Messaddeq N., Harney J. W., Ezaki O., Kodama T., et al. 2006. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 439: 484–489. [DOI] [PubMed] [Google Scholar]
- 20.Montet A. M., Oliva L., Beauge F., Montet J. C. 2002. Bile salts modulate chronic ethanol-induced hepatotoxicity. 3. Alcohol Alcohol. 37: 25–29. [DOI] [PubMed] [Google Scholar]
- 21.Amann R. I., Binder B. J., Olson R. J., Chisholm S. W., Devereux R., Stahl D. A. 1990. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl. Environ. Microbiol. 56: 1919–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thuy S., Ladurner R., Volynets V., Wagner S., Strahl S., Konigsrainer A., Maier K. P., Bischoff S. C., Bergheim I. 2008. Nonalcoholic fatty liver disease in humans is associated with increased plasma endotoxin and plasminogen activator inhibitor 1 concentrations and with fructose intake. J. Nutr. 138: 1452–1455. [DOI] [PubMed] [Google Scholar]
- 23.Sha H., He Y., Chen H., Wang C., Zenno A., Shi H., Yang X., Zhang X., Qi L. 2009. The IRE1alpha-XBP1 pathway of the unfolded protein response is required for adipogenesis. Cell Metab. 9: 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lisbona F., Rojas-Rivera D., Thielen P., Zamorano S., Todd D., Martinon F., Glavic A., Kress C., Lin J. H., Walter P., et al. 2009. BAX inhibitor-1 is a negative regulator of the ER stress sensor IRE1alpha. 3. Mol. Cell. 33: 679–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaser A., Lee A. H., Franke A., Glickman J. N., Zeissig S., Tilg H., Nieuwenhuis E. E., Higgins D. E., Schreiber S., Glimcher L. H., et al. 2008. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. 17. Cell. 134: 743–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lorenzo-Zuniga V., Bartoli R., Planas R., Hofmann A. F., Vinado B., Hagey L. R., Hernandez J. M., Mane J., Alvarez M. A., Ausina V., et al. 2003. Oral bile acids reduce bacterial overgrowth, bacterial translocation, and endotoxemia in cirrhotic rats. Hepatology. 37: 551–557. [DOI] [PubMed] [Google Scholar]
- 27.Cave M., Deaciuc I., Mendez C., Song Z., Joshi-Barve S., Barve S., McClain C. 2007. Nonalcoholic fatty liver disease: predisposing factors and the role of nutrition. 163. J. Nutr. Biochem. 18: 184–195. [DOI] [PubMed] [Google Scholar]
- 28.Le K. A., Bortolotti M. 2008. Role of dietary carbohydrates and macronutrients in the pathogenesis of nonalcoholic fatty liver disease. 164. Curr. Opin. Clin. Nutr. Metab. Care. 11: 477–482. [DOI] [PubMed] [Google Scholar]
- 29.Assy N., Nasser G., Kamayse I., Nseir W., Beniashvili Z., Djibre A., Grosovski M. 2008. Soft drink consumption linked with fatty liver in the absence of traditional risk factors. 177. Can. J. Gastroenterol. 22: 811–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spruss A., Bergheim I. 2009. Dietary fructose and intestinal barrier: potential risk factor in the pathogenesis of nonalcoholic fatty liver disease. J. Nutr. Biochem. 20: 657–662. [DOI] [PubMed] [Google Scholar]
- 31.Leikin-Frenkel A., Goldiner I., Leikin-Gobbi D., Rosenberg R., Bonen H., Litvak A., Bernheim J., Konikoff F. M., Gilat T. 2008. Treatment of preestablished diet-induced fatty liver by oral fatty acid-bile acid conjugates in rodents. 3. Eur. J. Gastroenterol. Hepatol. 20: 1205–1213. [DOI] [PubMed] [Google Scholar]
- 32.Ueno Y., Francis H., Glaser S., DeMorrow S., Venter J., Benedetti A., Fava G., Marzioni M., Alpini G. 2007. Taurocholic acid feeding prevents tumor necrosis factor-alpha-induced damage of cholangiocytes by a PI3K-mediated pathway. 22. Exp. Biol. Med. (Maywood). 232: 942–949. [PubMed] [Google Scholar]
- 33.Lam I. P., Lee L. T., Choi H. S., Alpini G., Chow B. K. 2009. Bile acids inhibit duodenal secretin expression via orphan nuclear receptor small heterodimer partner (SHP). 2. Am. J. Physiol. Gastrointest. Liver Physiol. 297: G90–G97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parlesak A., Schaeckeler S., Moser L., Bode C. 2007. Conjugated primary bile salts reduce permeability of endotoxin through intestinal epithelial cells and synergize with phosphatidylcholine in suppression of inflammatory cytokine production. Crit. Care Med. 35: 2367–2374. [DOI] [PubMed] [Google Scholar]
- 35.Zollner G., Wagner M., Moustafa T., Fickert P., Silbert D., Gumhold J., Fuchsbichler A., Halilbasic E., Denk H., Marschall H. U., et al. 2006. Coordinated induction of bile acid detoxification and alternative elimination in mice: role of FXR-regulated organic solute transporter-alpha/beta in the adaptive response to bile acids. 29. Am. J. Physiol. Gastrointest. Liver Physiol. 290: G923–G932. [DOI] [PubMed] [Google Scholar]
- 36.Sommerfeld A., Reinehr R., Haussinger D. 2009. Bile acid-induced epidermal growth factor receptor activation in quiescent rat hepatic stellate cells can trigger both proliferation and apoptosis. 3. J. Biol. Chem. 284: 22173–22183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fiorucci S., Antonelli E., Rizzo G., Renga B., Mencarelli A., Riccardi L., Orlandi S., Pellicciari R., Morelli A. 2004. The nuclear receptor SHP mediates inhibition of hepatic stellate cells by FXR and protects against liver fibrosis. Gastroenterology. 127: 1497–1512. [DOI] [PubMed] [Google Scholar]
- 38.Fang Y., Han S. I., Mitchell C., Gupta S., Studer E., Grant S., Hylemon P. B., Dent P. 2004. Bile acids induce mitochondrial ROS, which promote activation of receptor tyrosine kinases and signaling pathways in rat hepatocytes. Hepatology. 40: 961–971. [DOI] [PubMed] [Google Scholar]
- 39.Kobak G. E., Dahl R., Devereaux M. W., Gumpricht E., Traber M., Doctor R. B., Sokol R. J. 2005. Increased susceptibility of fatladen Zucker-rat hepatocytes to bile acid-induced oncotic necrosis: an in vitro model of steatocholestasis. 52. J. Lab. Clin. Med. 145: 247–262. [DOI] [PubMed] [Google Scholar]
- 40.Yerushalmi B., Dahl R., Devereaux M. W., Gumpricht E., Sokol R. J. 2001. Bile acid-induced rat hepatocyte apoptosis is inhibited by antioxidants and blockers of the mitochondrial permeability transition. Hepatology. 33: 616–626. [DOI] [PubMed] [Google Scholar]
- 41.Yki-Jarvinen H. 2005. Fat in the liver and insulin resistance. Ann. Med. 37: 347–356. [DOI] [PubMed] [Google Scholar]
- 42.Araki Y., Andoh A., Sasaki A., Shimada M., Bamba S., Fujino S., Fujiyama Y. 2002. Dietary bile acids inhibit potentially elemental diet-induced small intestinal atrophy in rats. Int. J. Mol. Med. 10: 623–626. [PubMed] [Google Scholar]
- 43.McKim S. E., Gabele E., Isayama F., Lambert J. C., Tucker L. M., Wheeler M. D., Connor H. D., Mason R. P., Doll M. A., Hein D. W., et al. 2003. Inducible nitric oxide synthase is required in alcohol-induced liver injury: studies with knockout mice. 443. Gastroenterology. 125: 1834–1844. [DOI] [PubMed] [Google Scholar]
- 44.Coeffier M., Gloro R., Boukhettala N., Aziz M., Lecleire S., Vandaele N., Antonietti M., Savoye G., Bole-Feysot C., Dechelotte P., et al. 2010. Increased proteasome-mediated degradation of occludin in irritable bowel syndrome. 2. Am. J. Gastroenterol. 105: 1181–1188. [DOI] [PubMed] [Google Scholar]
- 45.Dalton J. E., Cruickshank S. M., Egan C. E., Mears R., Newton D. J., Andrew E. M., Lawrence B., Howell G., Else K. J., Gubbels M. J., et al. 2006. Intraepithelial gammadelta+ lymphocytes maintain the integrity of intestinal epithelial tight junctions in response to infection. Gastroenterology. 131: 818–829. [DOI] [PubMed] [Google Scholar]
- 46.Lauer M. E., Erzurum S. C., Mukhopadhyay D., Vasanji A., Drazba J., Wang A., Fulop C., Hascall V. C. 2008. Differentiated murine airway epithelial cells synthesize a leukocyte-adhesive hyaluronan matrix in response to endoplasmic reticulum stress. 3. J. Biol. Chem. 283: 26283–26296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xie Q., Khaoustov V. I., Chung C. C., Sohn J., Krishnan B., Lewis D. E., Yoffe B. 2002. Effect of tauroursodeoxycholic acid on endoplasmic reticulum stress-induced caspase-12 activation. Hepatology. 36: 592–601. [DOI] [PubMed] [Google Scholar]
- 48.Cahill C. J. 1983. Prevention of postoperative renal failure in patients with obstructive jaundice–the role of bile salts. 29. Br. J. Surg. 70: 590–595. [DOI] [PubMed] [Google Scholar]
- 49.Sheen-Chen S. M., Chau P., Harris H. W. 1998. Obstructive jaundice alters Kupffer cell function independent of bacterial translocation. J. Surg. Res. 80: 205–209. [DOI] [PubMed] [Google Scholar]
- 50.Pumbwe L., Skilbeck C. A., Nakano V., Avila-Campos M. J., Piazza R. M., Wexler H. M.2007. Bile salts enhance bacterial co-aggregation, bacterial-intestinal epithelial cell adhesion, biofilm formation and antimicrobial resistance of Bacteroides fragilis. 7. Microb. Pathog. 43: 78–87. [DOI] [PubMed] [Google Scholar]