Abstract
The previous studies in our laboratory revealed that seven cysteine mutants of apolipoprotein A-I (apoA-I) have different structural features and biological activities in vitro and in vivo. To investigate the potential cardioprotective effects of apolipoprotein A-I(N74C) [apoA-I(N74C)], we examined the anti-inflammatory, antioxidant, and antiatherosclerotic effects of this cysteine mutant in a rapid atherosclerosis model induced by perivascular carotid collar placement in apoE−/− mice. Lipid-free apoA-I(N74C) showed a significant increased antioxidant potency in low density lipoprotein (LDL) oxidation in vitro and reduced intracellular lipid accumulation in THP-1-derived macrophages, relative to wild-type apoA-I (apoA-Iwt). Mice injected with recombinant HDL (rHDL) reconstituted with apoA-I(N74C) (named rHDL74) through tail veins (40 mg/kg of body weight, three injections) had a significant lower level of serum interleukin-6 (IL-6) and enhanced serum antioxidation compared with mice receiving rHDL reconstituted with apoA-Iwt (named rHDLwt). Moreover, compared with rHDLwt, the rHDL74 in vivo injection resulted in a significant decrease in plaque size, ratio of aorta intima to media, arterial remodeling, and macrophage content in lesions. In summary, intravenous injection with rHDL74 reconstituted with apoA-I cysteine mutant apoA-I (N74C) dramatically delays the development of atherosclerosis induced by perivascular carotid collar placement and reduces vascular remodeling in the carotid artery in apoE−/− mice.
Keywords: apolipoprotein, antioxidant, cytokines
It has been well documented that the plasma level of high density lipoprotein (HDL) is inversely correlated with the incidence of atherosclerosis and coronary artery disease (1–3). Apolipoprotein A-I (apoA-I), the major protein component of HDL, is known to play an important role in reverse cholesterol transport (RCT), which delivers cholesterol from peripheral tissues, such as macrophages and foam cells in atherosclerotic lesions, to liver (4). In addition to promoting RCT, apoA-I also exerts cardioprotection against atherosclerosis via anti-inflammation and anti-oxidation (5–8).
Mature apoA-I contains a series of 10 repeating units that compose approximately 80% of the protein separated by helix-breaking amino acids such as proline. Eight of these repeats contain 22 amino acids, while two have 11 amino acids. Apolipoprotein A-IMilano (apoA-IMilano) and apoA-IParis, two natural cysteine mutants of apoA-I with dimers as their effective forms, have been shown to have enhanced cardiovascular protective activities (9–11). More comprehensive studies have also been carried out to identify the cardioprotective activities of apoA-IMilano in animals (12–14) and humans (15, 16). Repeated doses (40 mg/kg) and a single high dose of recombinant apoA-IMilano/PC (400 mg/kg) prevented the progression of aortic atherosclerosis and reduced the lipid and macrophage content in plaques of apoE-deficient (apoE−/−) mice compared with the control group (12, 17). Furthermore, infusion of recombinant human apoA-IMilano resulted in significant regression of coronary atherosclerosis accompanied by reverse remodeling of the external elastic membrane (EEM) in human patients as measured by intravascular ultrasound (IVUS) (15, 16). These studies highlight the significant role of apoA-I and its mutants in atherosclerotic protection.
According to the Edmundson wheel (18), the construction features of apoA-I, and the unique cysteine mutation positioning of apoA-IMilano and apoA-IParis at the polar-nonpolar interface on the helix, seven cysteine mutants of apoA-I were designed and constructed in our laboratory with mutant positions similar to apoA-IMilano on the α-helices (19). We have shown that monomeric cysteine mutant proteins in a lipid-free state exhibited different structural features as well as biological functions of lipid binding, anti-oxidation, and promotion of cholesterol efflux from THP-1-derived macrophages (19). Furthermore, our in vivo study documented that apoA-I(N74C) had an increased anti-inflammatory capability in an LPS-induced endotoxemia mouse model compared with wild-type apoA-I (apoA-Iwt) and apoA-IMilano (20), suggesting a potential role of apoA-I(N74C) in anti-inflammation in innate immunity as well as in chronic inflammatory diseases such as atherosclerosis.
ApoE−/− mice, which are prone to develop diet-induced hypercholesterolemia and atherosclerosis, are largely used in atherosclerosis study as an atherogenic mice model (12, 17). Perivascular carotid collar placement in apoE−/− mice is able to induce rapid, site-controlled lesion formation and has been shown to have similar plaque morphology, endothelial expression pattern, and plaque pathogenesis to human lesion (21, 22). To test our hypothesis that the administration of recombinant phospholipid apoA-I (N74C) complex will improve the progression of atherosclerosis and reduce injury-induced arterial remodeling, we used apoE−/− mice with perivascular carotid collar placement in this study.
METHODS
Animals
Male apoE−/− mice (C57BL/6J strain, SPF grade, 10 weeks old, 26–28 g), obtained from Department of Laboratory Animal Science of Peking University Health Science Center, were fed a high-fat, high-cholesterol (HF/HC) Western-type diet (21% (wt/wt) fat and 0.15% cholesterol) for two weeks before being used in the study (12) and maintained throughout the duration of the experiment. All animal experimental work was approved by the animal care committee of the Chinese Academy of Medical Science.
Preparation of recombinant HDL
The expression, purification, and endotoxin removal of recombinant proteins, including apoA-Iwt, apoA-I(N74C), and apoA-IMilano, were performed as described previously (19, 20). Subsequently, recombinant HDLs were prepared via sodium cholate dialysis (23) with soybean lecithin (SPC, at least 90% purification) with a mass ratio of SPC:apoA-I of 3.35:1 (24). rHDL was sterilized by filtering through a 0.22 μm membrane (Pall).
In vitro antioxidation against low density lipoprotein
LDL (1.019<d<1.063) was isolated by density gradient ultracentrifugation from healthy human plasma, and then dialyzed against 0.01 M PBS (PH 7.4). To evaluate the antioxidation ability of lipid-free cysteine mutant proteins, we used copper-mediated LDL oxidation system and thiobarbituric acid-reacting substance (TBARS) assay to measure the oxidation extent of LDL in vitro (25). Briefly, fresh human LDL (100 mg/l) was incubated with 5 μM of copper sulfate (CuSO4) in the presence of recombinant proteins (10, 50, 100, and 300 mg/l) for 4 h at 37°C. After incubation, an aliquot of LDL (150 μl) was first mixed with 1.5 ml of 0.8% thiobarbituric acid (TBA, Merck), 1.5 ml of 20% glacial acetic acid, and 150 μl of 0.2% ethylenediamine tetraacetic acid. It was then heated at 95°C for 50 min and then cooled down on ice. After centrifugation at 14, 000 g for 10 min, the supernatant was monitored at 532 nm to assess the oxidation extent, with 1,1,3,3-Tetraethoxypropane (Merck) as a standard. At least three independent assays were performed for each sample. The half-maximal inhibitory concentration (IC50) values were calculated for comparison of the antioxidant capacities of the tested recombinant apolipoproteins. To determine whether the antioxidant effect might be the result of chelating Cu2+ of the free thiols induced by cysteine mutation in the recombinant apoA-I cysteine mutants, we included L-glutathione (GSH, Roche) in the apoA-Iwt reaction at the same molar concentration of thiols as the mutants have to counteract the potential antioxidant role of thiols.
Cellular uptake of oxidized LDL and intracellular lipid accumulation in vitro
Oxidized LDL (oxLDL) was generated by incubating LDL with CuSO4 at 37°C and then filtering prior to use (26). Macrophage uptake of oxLDL and intracellular lipid accumulation assay were performed as described previously (26). THP-1 cells (Cell Culture Center, Chinese Academy of Medical Science) were incubated in PRMI-1640 medium containing 10% fetal bovine serum (FBS) and 160 nM phorbol 12-myristate 13-acetate (PMA) in 12-well plates for 48 h at 37°C to induce differentiation into macrophages. Subsequently, the differentiated macrophages were washed with PBS and incubated with oxLDL (100 mg/l) in 1 ml medium containing PBS or each recombinant apolipoprotein (150 mg/l) for another 48 h at 37°C. After incubation, the macrophages were washed with PBS three times and fixed in 4% paraformaldehyde for 10 min. The fixed cells were stained with 0.3% Oil Red O solution for 30 min and then washed with distilled water. The stained cells were photographed and the mean area of Oil Red O-stained region per cell was quantified with 20 representative cells using the Image-Pro Plus software 6.0. To confirm the Oil Red O staining data, the cells were lysed with 1% TritonX-100, and the protein concentrations were determined by the BCA method. The intracellular lipids were extracted from the cells with a chloroform/methanol (2:1) mixture and dried under nitrogen gas stream (27). Subsequently, the lipid extracts were solubilized in deionized water with 3% Triton X-100, and the intracellular total cholesterol (TC) levels were determined using a commercially available enzymatic assay kit. The data was expressed as total cholesterol (mg) per cellular protein (g).
Carotid collar placement
Silicone tube for carotid collar placement (length, 5 mm; internal diameter, 0.3 mm; external diameter, 0.6 mm) was obtained from Beijing Rubber Products Institute and stored at 95% ethanol. The surgery was based on a previously described method (21). Briefly, mice were anesthetized by pentobarbital (50 mg/kg) via intraperitoneal injection, and both of the common carotid arteries were dissected from the surrounding connective tissue. Collars were placed carefully around the right carotid arteries and then tied with three circumferential silk ties at their axial edges. Subsequently, the entry wound was closed, and the animals continued to feed on a Western-type diet throughout the duration of the experiment.
In vivo injection of rHDL
rHDL was prepared with apoA-Iwt, apoA-I (N74C), and apoA-IMilano (named rHDLwt, rHDL74, and rHDLmilano, respectively) as described above and adjusted to appropriate concentration for tail vein injection. Carotid collars were first placed in the right carotid arteries of the mice, with the left carotid arteries as sham operation as described above. Five days after surgery, mice were injected through the tail vein with indicated apolipoproteins every 10 days for a total of three injections per mouse before mice were euthanized. Briefly, 28 male apoE−/− mice fed a HF/HC diet for two weeks were divided randomly into five groups and received via tail vein injections with saline (n = 5, 0.3 ml), soybean lecithin (n = 5, 134 mg/kg body weight in 0.3 ml), rHDLwt (n = 6, 40 mg/kg body weight in 0.3ml), rHDL74 (n = 6, 40 mg/kg body weight in 0.3ml), or rHDLmilano (n = 6, 40 mg/kg body weight in 0.3ml).
Serum lipid and cytokine analysis
Twenty-four h after the third tail vein injection of rHDL, blood samples were obtained from the retro-orbital plexus. Serum was isolated and stored at −80°C until analysis. Serum total cholesterol (TC) and triglyceride (TG) concentrations were determined by standard enzymatic methods. Serum HDL levels were measured using a direct HDL-cholesterol reagent kit (BioSino Bio-technology and Science). NonHDL cholesterol (nonHDL-C) was determined as the difference between serum total cholesterol and HDL cholesterol. Serum interleukin-6 (IL-6), an inflammatory molecular marker most strongly associated with atherosclerosis and its progression, was measured using a commercially available ELISA kit (Boster Biological Technology).
Ferric-reducing ability of serum assay
The ferric-reducing ability of serum (FRAS) assay, a known, rapid, reproducible method for determining the antioxidant values of serum described by Benzie and Strain (28), was used to determine the antioxidant activity of recombinant proteins in vivo. FRAS regents were freshly prepared by mixing 25 ml 0.3M acetate buffer, 2.5 ml 10 mM 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ, Sigma) solution and 2.5 ml 20 mM FeCl3·6H2O solution. Freshly prepared FRAS regents (300 μl, prewarmed to 37°C) were mixed with 10 μl serum isolated from mice receiving rHDL (described above) and 30 μl distilled water and then monitored at 593 nm every 20 s over a 10 min period at 37°C.
Arylesterase activity measurements
Paraoxonase (PON) activity was measured as arylesterase activity with phenylacetate as substrate in the presence of 0.9 mM CaCl2 in 20 mM Tris-HCl, as described by Aviram et al. (29). Activity was determined by monitoring the increase in absorption at 270 nm for 60 s. One unit of arylesterase activity is equal to 1 mmol of phenylacetate hydrolyzed/min.
Tissue harvesting and histological analysis
Mice were euthanized 24 h after the last injection. The thoracic cavity was exposed immediately, and in situ perfusion fixation through the left cardiac ventricle was performed by thorough perfusion with PBS for 10 min, followed by 10% neutral buffered formalin for 30 min (21). Subsequently, both carotid arteries were removed and fixed in 10% formaldehyde solution and embedded in paraffin. Carotid artery sections were stained with hematoxylin and eosin (HE) to observe the neointima formation and vascular remodeling. Images of HE-stained sections were analyzed using Image-Pro Plus 6.0 software to measure the areas of the intima and media divided by internal elastic membrane (IEM) and EEM, and the intima/media ratios (I/M) were calculated. Sections were digitally processed into monochrome with Image-Pro Plus software, showing tissues as black and delipidated areas as white. Percentage of total white area in the total plaque area was calculated as the fractional lipid content (30). At least two to three sections of each animal were used for quantitative measurement of plaque size and lipid content.
To detect macrophage content in atherosclerotic plaques, sections were stained immunohistochemically with rat monoclonal anti-mouse F4/80 antibody (Abcam, dilution 1:10) (31). In brief, enzymatic antigen retrieval (Proteinase K in 0.05 M Tris-HCl, 15 mM sodium azide, pH 7.5) at room temperature for 10 min was carried out on the sections. Endogenous peroxidase was blocked using 3% hydrogen peroxide for 10 min. Sections were incubated at 4°C overnight with monoclonal rat anti-mouse F4/80 antibody, followed by incubation with nonbiotin labeled polymer conjugated to goat anti-rat IgG and then stained with 3,3’-diaminobenzidine (DAB). The stained sections were visualized with an inverted microscope (Leica DMI 4000 B, Germany) and photographed. Results are expressed as the percentage of the total plaque area stained with DAB as measured with the Image-Pro Plus software.
Statistical analysis
Data were expressed as mean ± SD. Differences among the experimental groups were examined for significance using one-way ANOVA (ANOVA) followed by multiple comparisons test. P < 0.05 was considered statistically significant.
RESULTS
Antioxidant activity against LDL in vitro
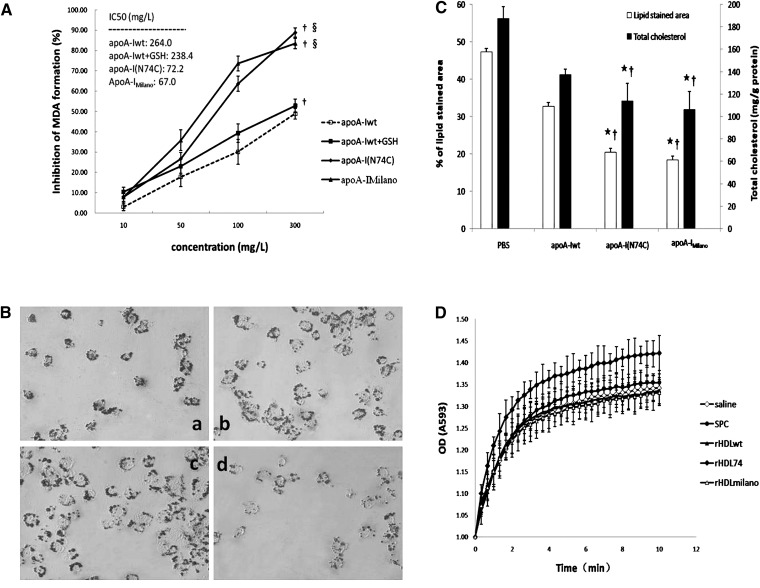
HDL and rHDL have been shown to have atheroprotective effects by inhibiting LDL oxidation in vitro. As shown in Fig. 1A, apoA-Iwt, as well as apoA-I(N74C) and apoA-IMilano, inhibited the malondialdehyde (MDA) formation in a dose-dependent pattern in the presence of 5 μM CuSO4 when apolipoproteins were used at a final concentration varying from 10 to 300 mg/l. Under Cu2+-mediated oxidation, apoA-Iwt, apoA-I(N74C), and apoA-IMilano showed antioxidant activities with IC50 values of 264, 72, and 67 mg/L, respectively (supplementary Table I). Adding the same molar concentration of GSH to apoA-Iwt to counteract the role of thiols induced by the cysteine mutant of apoA-I resulted in a decrease in IC50 value from 264 to 238 mg/l (supplementary Table I). Our findings suggested that the antioxidation of cysteine mutants resulted not only from free thiols induced by cysteine mutation but also from structural change and interaction of cysteine mutants with other substrates.
Fig. 1.
Antioxidant activity of apoA-I(N74C) in lipid-free state in vitro and in lipid-bound state in vivo. A: Potent inhibitory effect of recombinant apolipoproteins against LDL oxidation in the presence of 5 μM CuSO4 in vitro. Freshly prepared LDL (100 mg/l) was incubated with each apolipoprotein (10–300 mg/l) for 4 h at 37°C. After incubation, samples were assayed for MDA formation and expressed as percentage of inhibition relative to the Cu2+-only controls. The IC50 values were calculated from the linear slopes of the graphs. To preclude the effects of thiols induced by cysteine mutation, the same molar concentrations of GSH as the apolipoproteins tested were added to apoA-Iwt reaction system and analyzed the antioxidant effects. B: The Oil Red O staining in THP-1-derived macrophages treated with PBS at 40× magnification: (a) apoA-Iwt (control); (b) apoA-Iwt + GSH; (c) apoA-I(N74C); and (d) apoA-IMilano. C: Quantitative analysis of the intracellular lipid accumulation in THP-1-derived macrophages. The areas of Oil Red O staining were calculated using the Image-Pro Plus software. To verify the data of lipid staining area, the intracellular total cholesterol level expressed as the ratio between total cholesterol and total protein was analyzed. D: Antioxidant ability of mice serum measured by FRAS. Absorption of rHDL74-injected serum at 593 nm for up to 10 min exhibited of up to 42% more than the initial values. Data are expressed as mean ± SD. ★P < 0.05 versus PBS; †P < 0.05 versus apoA-Iwt; §P < 0.01 versus apoA-Iwt + GSH. Apo, apolipoprotein; apoA-I(N74C), apolipoprotein A-I(N74C); apoA-Iwt, wild-type apoA-I; FRAS, ferric reducing ability of serum; GSH, L-glutathione; rHDL, recombinant HDL; IC50, half-maximal inhibitory concentration; MDA, malondialdehyde.
Intracellular lipid accumulation in THP-1-derived macrophages
Macrophage uptake of oxLDL is thought to play a central role in foam cell formation and the pathogenesis of atherosclerosis. To investigate whether recombinant apolipoproteins can inhibit lipid accumulation in macrophages, we incubated THP-1-derived macrophages with our reconstituted apoA-Iwt, apoA-I(N74C), and apoA-IMilano. As shown in Fig. 1B and 1C, compared with the PBS control group [Fig. 1B(a)], the Oil Red O staining area was significantly less in THP-1-derived macrophages treated with the recombinant apolipoproteins. ApoA-I(N74C) [Fig. 1B(c)] and apoA-IMilano [Fig. 1B(d)] significantly inhibited intracellular lipid accumulation, with a 56.78% and 61.08% decrease, respectively, in the Oil Red O staining area, whereas apoA-Iwt decreased only 30.65% [Fig. 1B(b)]. Consistent with the Oil Red O staining results, apoA-I(N74C)- and apoA-IMilano-treated macrophages also showed a significant lower level of intracellular cholesterol accumulation relative to the PBS and apoA-Iwt control groups (Fig. 1C, P < 0.05).
Serum lipid profile of rHDL-treated apoE−/− mice
The protective effect of HDL against atherosclerosis can largely be attributed to its ability to promote macrophage cholesterol efflux and RCT. As shown in Table 1, HF/HC diet feeding for six weeks dramatically increased serum TC concentration in saline- and SPC-treated apoE−/− mice. Interestingly, in vivo injection of rHDLmilano significantly reduced the serum TC concentration by 48.4% relative to the saline group, whereas rHDL74 and rHDLwt showed a similar TC-lowering ability with a 31.9% and 27.6% reduction of serum TC, respectively. No significant difference in the serum TG concentration was detected between the saline- and rHDL-treated groups; however, SPC injection led to an acute increase in serum TG. In addition, rHDLmilano-treated animals had a slightly, but significantly, lower serum HDL level compared with other groups, but there was no statistic difference in HDL level among groups. Consistent with the TC results, rHDLmilano-treated mice showed a significantly lower level of nonHDL-C relative to the saline and rHDLwt groups, whereas rHDL74 and rHDLwt showed a similar nonHDL-C-lowering ability, indicating that rHDL74 is as effective as rHDLwt in lowering serum TC and nonHDL-C levels, which were lower than that of rHDLmilano.
TABLE 1.
Serum lipid profile of the hypercholesterolaemic mice 24 h after the last injection
| TC |
TG |
HDL-C |
NonHDL-C |
|
|---|---|---|---|---|
| Group (n) | mM | mM | mM | mM |
| Saline (5) | 15.93 ± 2.07c | 1.12 ± 0.24b | 3.36 ± 0.36 | 12.57 ± 1.97bc |
| SPC (5) | 14.55 ± 1.39 | 1.74 ± 0.34 | 3.58 ± 0.35 | 10.97 ± 1.20ac |
| rHDLwt (6) | 11.52 ± 0.55ab | 1.05 ± 0.07b | 3.16 ± 0.23 | 8.36 ± 0.39ab |
| rHDL74 (6) | 10.84 ± 0.48ab | 0.95 ± 0.17b | 3.20 ± 0.24 | 7.64 ± 0.39ab |
| rHDLmilano (6) | 8.23 ± 0.56abc | 0.94 ± 0.21b | 2.75 ± 0.13ab | 5.48 ± 0.90abc |
Results expressed as mean ± SD. HDL-C, HDL-cholesterol; rHDL, recombinant HDL; SPC, soybean lecithin; TC, total cholesterol; TG, triglyceride.
P < 0.05 compared with saline group.
P < 0.05 compared with SPC group.
P < 0.05 compared with rHDLwt group.
Serum antioxidant ability in vivo
To assess the antioxidant activity of rHDL in vivo, we used the FRAS method to determine the serum antioxidant ability by monitoring absorption at 593 nm every 20 s for up to 10 min as described in “Methods.” As shown in Fig. 1D, the rHDL74 injection group showed a dramatic increase in absorption at 593 nm (by 42% compared with the initial values), which was significantly higher compared with other groups. No difference was detected among other groups regarding serum antioxidation ability.
Serum anti-inflammatory and PON arylesterase activity in vivo
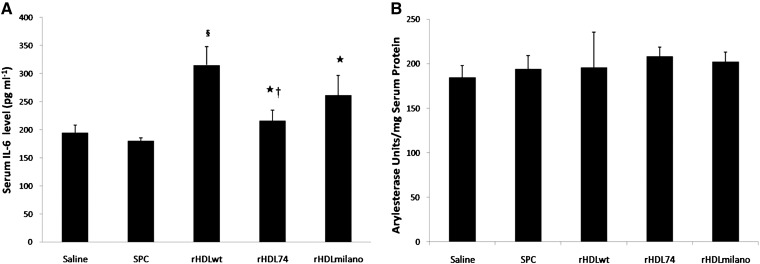
To evaluate the impact of rHDL on inflammation induced by a HF/HC diet and carotid collar replacement in apoE−/− mice, we measured by ELISA the concentration of serum IL-6 from different rHDL-treated mice. As shown in Fig. 2, 24 h after the last injection, rHDLwt-treated mice exhibited an acute increase in serum IL-6 (315.0 ± 32.5 pg/ml) compared with the saline- and SPC-treated mice. Administration of rHDLmilano resulted in a 17% decrease (261.5 ± 35.2 pg/ml), whereas rHDL74 resulted in a 32% decrease (215.6 ± 19.4 pg/ml) in mouse serum IL-6 compared with mice that received with rHDLwt. The saline and SPC groups exhibited the lowest IL-6 serum concentration of all treatment groups, which is consistent with the previous findings of Cho et al. (32–34). Since all recombinant apolipoproteins were prepared using the same E. coli expression, purification system, and endotoxin removal procedure, we do not think that there was more contamination in apoA-Iwt than in apoA-I(N74C) or apoA-IMilano. It has been reported that residual endotoxin in rHDL does not damage cultured cell and animal tissue because of the ability of rHDL to neutralize the toxicity of endotoxin or lipopolysaccharide (7, 35, 36). The endotoxin level of recombinant apolipoproteins used in our study was checked with tachypleus amebocyte lysate before injection, and residual endotoxin in rHDL should not lead to a significant difference in the level of serum IL-6. These results suggested that rHDL74 or rHDLmilano may have an anti-inflammatory function compared with rHDLwt. However, we still do not know the mechanism by which rHDLwt injection induces acute inflammation.
Fig. 2.
Serum IL-6 level and PON arylesterase activity in apoE−/− mice. A: The serum IL-6 levels at 24 h after the last injection. Mouse IL-6 concentration was determined with mouse IL-6 ELISA kit. The serum IL-6 concentrations increased 24 h after injection with rHDLwt and rHDLmilano complexes, whereas rHDL74 treatment only exhibited a level similar to the saline treatment group. Data are expressed as mean ± SD. ★P < 0.01 versus rHDLwt; †P < 0.05 and §P < 0.01versus rHDLmilano. B: Arylesterase activity. Serum was added to 1 ml of the 1 mM phenylacetate-containing solution (20 mM Tris-HCl/0.9 mM CaCl2, pH 8.0). A PON-1 activity of 1 U/l is equal to 1 mmol of phenylacetate hydrolyzed/min; arylesterase activity was expressed as units per mg serum proteins. rHDL, recombinant HDL; IL-6, interleukin-6; PON, paraoxonase.
As shown in Fig. 2B, all rHDL groups showed a slightly higher serum PON activity than the saline group; however, there is no statistically significant relationship among rHDL groups.
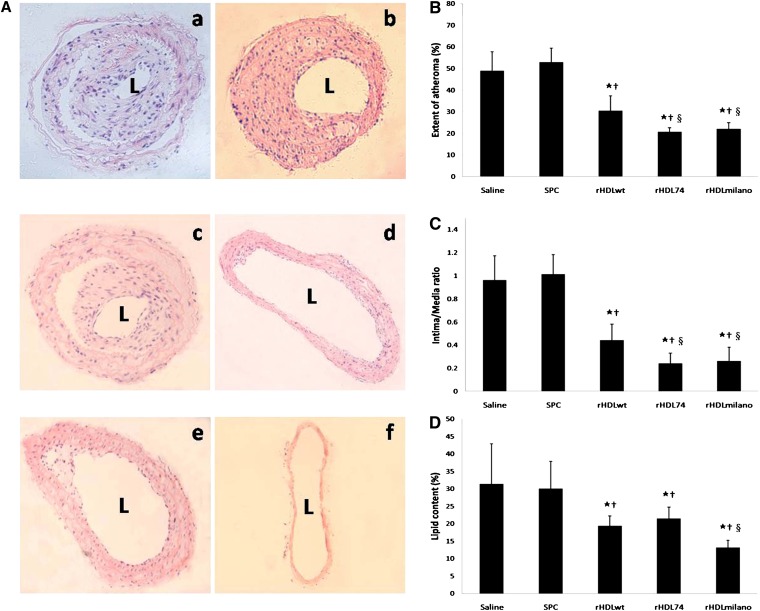
Extent of carotid atherosclerosis and vascular remodeling in atherosclerotic plaques
Arterial remodeling in response to atherosclerotic plaque formation is currently appreciated as a causal factor for arterial occlusive disease (37). It has recently become clear that the geometrical change in arterial size and plaque area may equally contribute to the luminal narrowing in atherosclerotic disease (37). In the saline- and SPC-treated mice, collared carotid arteries showed the formation of large intimal lesions [Fig. 3A(a, b)]. Compared with the saline group, the rHDLwt injection [Fig. 3A(c)] reduced the extent of atheroma by 38%, whereas treatment with rHDL74 [Fig. 3A(d)] and rHDLmilano [Fig. 3A(e)] resulted in a further 58% and 55% reduction, respectively, with no significance between these two groups (Fig. 3B). Not surprisingly, there was no apparent neointimal thickening in sham-operated left carotid arteries [Fig. 3A(f)]. As shown in Fig. 3C, the mean intima-to-media ratio increased dramatically in the saline-treated mice, similar to the SPC-treated group (0.96 versus 1.01). Compared with saline group, rHDLwt injection reduced I/M ratio by 54% to 0.44, whereas rHDL74 resulted in a further 75% reduction to 0.24. Treatment with rHDLmilano resulted in a reduction similar to rHDL74 (0.26 versus 0.24). Our results indicated that rHDL74 and rHDLmilano injections significantly slow the progression of atherosclerosis and reduce injury-induced arterial remodeling to a similar extent compared with rHDLwt.
Fig. 3.
Effects of rHDL on vascular remodeling and carotid atherosclerosis in apoE−/− mice. A: Representative hematoxylin and eosin staining of collared right carotid arteries at 20× magnification after injection with (a) saline (n = 5); (b) SPC (n = 5); (c) rHDLwt (n = 6); (d) rHDL74 (n = 6); and (e) rHDLmilano (n = 6). Sham-operated left carotid arteries from saline group did not show neointima formation (f) at 10× magnification. B-D: Quantitative analysis of the extent of atheroma, intima/media ratio, and lipid content in neointima. Results are expressed as mean ± SD. ★P < 0.05 compared with saline group; †P < 0.05 compared with SPC group; §P < 0.05 compared with rHDLwt group. rHDL, recombinant HDL; L, lumen; SPC, soybean lecithin.
Lipid content in atherosclerotic plaques
As shown in Fig. 3D, injection of all rHDL significantly reduced the lipid content in the carotid neointima compared with the saline and SPC control groups. The rHDLwt and rHDL74 injections resulted in a similar reduction in lipid content (19% versus 21%), whereas the rHDLmilano injection decreased the lipid content to 13% compared with the control groups. Our finding is quite consistent with the results of the serum lipid profile (Table 1), in which rHDL74 was evidenced to have a lipid-lowering ability similar to that of rHDLwt.
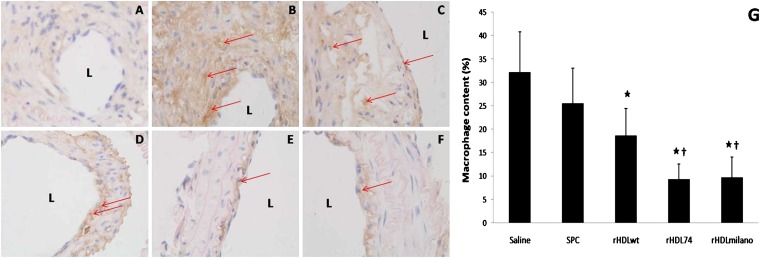
Macrophage content in atherosclerotic plaques
The infiltration of monocyte/macrophages is an early event in the development of atherosclerosis and accelerates the progression of atherosclerosis by promoting the formation of foam cells and secretion of cytokines and chemotactic factors (38). In the saline-treated mice, the macrophages stained with the macrophage-specific marker F4/80 were detected throughout the neointima (Fig. 4B). Fig. 4C–F shows representative neointima macrophage staining in mice injected with SPC, rHDLwt, rHDL74, or rHDLmilano, respectively. As summarized in Fig. 4F, relative to the saline control, rHDLwt injection resulted in a 42% reduction of macrophage accumulation in plaques, whereas injection with rHDL74 and rHDLmilano resulted in more dramatic reduction in macrophage content in the neointima, with a 71% and 70% decrease, respectively (P < 0.05), suggesting that rHDL74 and rHDLmilano may protect animals from atherosclerosis by decreasing macrophage accumulation in plaque lesions.
Fig. 4.
Effects of rHDL on macrophage content in neointima staining with macrophage antibody F4/80 (40× magnification). The carotid plaques were immunostained with F4/80 antibody to identify macrophages as brown (red arrows). The macrophage content is expressed as the ratio between the staining area in the neointima and the neointima area. A: Negative control. B-F: Groups treated with saline, SPC, rHDLwt, rHDL74, and rHDLmilano, respectively. G: Quantitative analysis of the macrophage content in neointima measured with Image-Pro Plus software. ★P < 0.05 compared with saline group. †P < 0.05 compared with rHDLwt group. rHDL, recombinant HDL; L, lumen; SPC, soybean lecithin.
DISCUSSION
The cardioprotective effects of HDL and apoA-I have been attributed to their functions on RCT, inhibition of inflammation and LDL oxidation, reduction of thrombus formation, etc. (4–8). However, the role of HDL with apoA-I or its cysteine mutants in the pathogenesis of atherosclerosis is still not fully understood. In the present study, we reconstituted three recombinant HDL (named rHDLwt, rHDL74, and rHDLmilano) by mixing SPC with apoA-Iwt or its cysteine mutants [apoA-I(N74C) and apoA-IMilano] to investigate their antioxidant, anti-inflammatory, and anti-atherosclerotic activities in vitro and in vivo. Our results showed that apoA-I(N74C) dramatically inhibited the formation of MDA in LDL and decreased the intracellular lipid accumulation in vitro compared with apoA-Iwt. In addition, our in vivo study showed that rHDL74 injection resulted in increased serum antioxidation in HF/HC diet fed apoE−/− mice compared with the rHDLwt or rHDLmilano groups. rHDL74 treatment also induced lower serum IL-6 production in HF/HC diet fed apoE−/− mice compared with the rHDLwt or rHDLmilano groups. Injecting mice with rHDL74 after carotid artery collar placement resulted in significant reduction of the development of carotid atherosclerosis, improvement of atheroma extent and I/M ratio, and reduction of lipid and macrophages content that was as effective as rHDLmilano. All together, our findings suggest that rHDL74 may function as a potential atheroprotective inhibitor to attenuate the development of atherosclerosis by inhibiting inflammation, oxidant factors, and accumulation of lipid and macrophages in artery lesions.
The oxidation of LDL is known to be associated with the development of atherosclerotic lesions, especially in the early stages of progression, and plasma level of oxLDL is a risk marker for cardiovascular diseases (39). In the present study, we showed that apoA-I(N74C) dramatically inhibited LDL-oxidation, decreased intracellular lipid accumulation in macrophages, and decreased serum total antioxidant level in animals, suggesting a broad anti-oxidation capability of apoA-I(N74C) both in vitro and in vivo. Note that we did not see the difference in anti-oxidation between apoA-I cysteine mutants in our previous study (19), perhaps due to the following reasons: (1) the antioxidant system used previously was the lipoxygenase-mediated oxidation of PLPC, while the current study used Cu2+-mediated LDL oxidation, which plays a critical role at the beginning of atherosclerosis; (2) the apolipoproteins used in our previous study mainly existed in monomeric form, while the proteins used here were in monomeric and dimeric forms; and (3) only lipid-free proteins were used in our previous in vitro study, whereas the present study evaluated the antioxidant activity of cysteine mutants both in lipid-free status in vitro and lipid-bound status in vivo. It is thought that the antioxidant activity of HDL in vivo is partly attributed to the paraoxonase-1 (PON-1) and platelet-activating factor acetylhydrolase (PAF-AH), two antioxidant-like enzymes associated with natural HDL. It has been reported that maximal PON activity is lipid-dependent and requires co-assembly of PON and apoA-I on nascent HDL (40). In addition, it is reported that the cysteine mutations in the N-terminal region of apoA-I significantly increased PON activity relative to wild-type (40). However, we could not see the same phenomenon in the cysteine mutant of apoA-I(N74C) in our experiment (Fig. 2B). Therefore, the higher antioxidant activity of apoA-I(N74C) than that of apoA-IMilano in vivo may be attributed to its lipid-dependent mechanism.
Atherosclerosis is considered as a chronic inflammatory disease with recruitment of circulating inflammatory monocytes into the arterial wall, migration and proliferation of smooth muscle cells, and formation of fibrous tissue (41). IL-6, a key pro-inflammatory cytokine mediating expression of acute-phase proteins, is strongly associated with atherosclerosis and its progression (42). Recently, it is reported that IL-6 is required to modulate lipid homeostasis, vascular remodeling, and plaque inflammation in atherosclerosis (43) and that it exacerbates atherosclerotic lesion development (44). Our data showed that, compared with rHDLwt and rHDLmilano, administration of rHDL74 significantly decreased serum IL-6 production in HF/HC diet fed apoE−/− mice, consistent with our previous findings using endotoxemia mice model (20). HDL or rHDL are well-known to neutralize endotoxin and lipopolysaccharides, decrease inflammatory cytokine production, and inhibit the expression of cell adhesion molecules in vitro and in vivo (7, 35, 36). Our findings indicate that rHDL74 might have an enhanced neutralizing effect on endotoxin and the subsequent inflammatory cascade. Likewise, injection of rHDL74 decreased the macrophage content in neointima, which implied an enhanced protective role from inflammatory cytokine and its effect on the artery wall.
Evidence suggests that rHDL reconstituted with apoA-IMilano exhibited better anti-inflammatory and anti-atherosclerotic activities compared with apoA-Iwt (12–14). More interestingly, intravenous injection with rHDLmilano not only dramatically stopped the progression of atherosclerosis but it also reversed preformed coronary atherosclerosis in animal models and in patients with acute coronary syndromes (15, 16). Consistently, our current results showed that intravenous injection with rHDLmilano significantly inhibited the development of atherosclerosis in a carotid artery collar placement mouse model compared with rHDLwt. Furthermore, rHDL74 injection exerted similar cardioprotective effects as rHDLmilano, based on assessment of formation of plaques, vascular remodeling, and macrophage content in neointima. Our previous study also indicated that the cysteine substitution in apoA-I(N74C) had no significant effect on its cholesterol efflux ability (19). Therefore, we assume that reduction of the intracellular lipid accumulation in the apoA-I(N74C)-treatment group might result from inhibition of LDL oxidation and cholesterol uptake instead of promotion of cholesterol efflux. Thus, the inhibition of rHDL74 in vascular remodeling and progression of atherosclerosis may be due to the enhanced anti-inflammatory and antioxidant activities of apoA-I(N74C) instead of its ability in reverse cholesterol transport.
In conclusion, the present study shows that treatment with rHDL74 reconstituted with apoA-I cysteine mutant apoA-I(N74C) and SPC dramatically delays the development of atherosclerosis induced by perivascular carotid collar placement and reduces vascular remodeling in the carotid artery in apoE−/− mice. Our findings suggest that rHDL74 might be a potential therapeutic target for the prevention of chronic inflammatory-related diseases, such as atherosclerosis.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the technical assistance and helpful suggestions of Hong Xue.
Footnotes
Abbreviations:
- Apo
- apolipoprotein
- apoA-I(N74C)
- apolipoprotein A-I(N74C)
- apoA-Iwt
- wild-type apoA-I
- FRAS
- ferric-reducing ability of serum
- GSH
- L-glutathione
- rHDL
- recombinant HDL
- HDL-C
- HDL-cholesterol
- IC50
- half-maximal inhibitory concentration
- IL-6
- interleukin-6
- oxLDL
- oxidized LDL
- MDA
- malondialdehyde
- IVUS
- intravascular ultrasound
- PON
- paraoxonase
- RCT
- reverse cholesterol transport
- SPC
- soybean lecithin
- TC
- total cholesterol
- TG
- triglyceride
This work was supported by National Basic Research Grant 973 of China (2006CB503801).
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two tables.
REFERENCES
- 1.Gordon T., Castelli W. P., Hjortland M. C., Kannel W. B., Dawber T. R. 1977. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am. J. Med. 62: 707–714. [DOI] [PubMed] [Google Scholar]
- 2.Miller N. E., Thelle D. S., Forde O. H., Mjos O. D. 1977. The Tromso heart-study. High-density lipoprotein and coronary heart-disease: a prospective case-control study. Lancet. 1: 965–968. [DOI] [PubMed] [Google Scholar]
- 3.Tall A. R. 1990. Plasma high density lipoproteins. Metabolism and relationship to atherogenesis. J. Clin. Invest. 86: 379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boisvert W. A., Black A. S., Curtiss L. K. 1999. ApoA1 reduces free cholesterol accumulation in atherosclerotic lesions of ApoE-deficient mice transplanted with ApoE-expressing macrophages. Arterioscler. Thromb. Vasc. Biol. 19: 525–530. [DOI] [PubMed] [Google Scholar]
- 5.Pajkrt D., Doran J. E., Koster F., Lerch P. G., Arnet B., van der Poll T., ten Cate J. W., van Deventer S. J. 1996. Antiinflammatory effects of reconstituted high-density lipoprotein during human endotoxemia. J. Exp. Med. 184: 1601–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barter P. J., Nicholls S., Rye K. A., Anantharamaiah G. M., Navab M., Fogelman A. M. 2004. Antiinflammatory properties of HDL. Circ. Res. 95: 764–772. [DOI] [PubMed] [Google Scholar]
- 7.Parker T. S., Levine D. M., Chang J. C., Laxer J., Coffin C. C., Rubin A. L. 1995. Reconstituted high-density lipoprotein neutralizes gram-negative bacterial lipopolysaccharides in human whole blood. Infect. Immun. 63: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicholls S. J., Dusting G. J., Cutri B., Bao S., Drummond G. R., Rye K. A., Barter P. J. 2005. Reconstituted high-density lipoproteins inhibit the acute pro-oxidant and proinflammatory vascular changes induced by a periarterial collar in normocholesterolemic rabbits. Circulation. 111: 1543–1550. [DOI] [PubMed] [Google Scholar]
- 9.Weisgraber K. H., Rall S. C., Jr., Bersot T. P., Mahley R. W., Franceschini G., Sirtori C. R. 1983. Apolipoprotein A-IMilano. Detection of normal A-I in affected subjects and evidence for a cysteine for arginine substitution in the variant A-I. J. Biol. Chem. 258: 2508–2513. [PubMed] [Google Scholar]
- 10.Sirtori C. R., Calabresi L., Franceschini G., Baldassarre D., Amato M., Johansson J., Salvetti M., Monteduro C., Zulli R., Muiesan M. L., et al. 2001. Cardiovascular status of carriers of the apolipoprotein A-I(Milano) mutant: the Limone sul Garda study. Circulation. 103: 1949–1954. [DOI] [PubMed] [Google Scholar]
- 11.Bruckert E., von Eckardstein A., Funke H., Beucler I., Wiebusch H., Turpin G., Assmann G. 1997. The replacement of arginine by cysteine at residue 151 in apolipoprotein A-I produces a phenotype similar to that of apolipoprotein A-IMilano. Atherosclerosis. 128: 121–128. [DOI] [PubMed] [Google Scholar]
- 12.Shah P. K., Nilsson J., Kaul S., Fishbein M. C., Ageland H., Hamsten A., Johansson J., Karpe F., Cercek B. 1998. Effects of recombinant apolipoprotein A-I(Milano) on aortic atherosclerosis in apolipoprotein E-deficient mice. Circulation. 97: 780–785. [DOI] [PubMed] [Google Scholar]
- 13.Li D., Weng S., Yang B., Zander D. S., Saldeen T., Nichols W. W., Khan S., Mehta J. L. 1999. Inhibition of arterial thrombus formation by ApoA1 Milano. Arterioscler. Thromb. Vasc. Biol. 19: 378–383. [DOI] [PubMed] [Google Scholar]
- 14.Kaul S., Rukshin V., Santos R., Azarbal B., Bisgaier C. L., Johansson J., Tsang V. T., Chyu K. Y., Cercek B., Mirocha J., et al. 2003. Intramural delivery of recombinant apolipoprotein A-IMilano/phospholipid complex (ETC-216) inhibits in-stent stenosis in porcine coronary arteries. Circulation. 107: 2551–2554. [DOI] [PubMed] [Google Scholar]
- 15.Nissen S. E., Tsunoda T., Tuzcu E. M., Schoenhagen P., Cooper C. J., Yasin M., Eaton G. M., Lauer M. A., Sheldon W. S., Grines C. L., et al. 2003. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 290: 2292–2300. [DOI] [PubMed] [Google Scholar]
- 16.Nicholls S. J., Tuzcu E. M., Sipahi I., Schoenhagen P., Crowe T., Kapadia S., Nissen S. E. 2006. Relationship between atheroma regression and change in lumen size after infusion of apolipoprotein A-I Milano. J. Am. Coll. Cardiol. 47: 992–997. [DOI] [PubMed] [Google Scholar]
- 17.Shah P. K., Yano J., Reyes O., Chyu K. Y., Kaul S., Bisgaier C. L., Drake S., Cercek B. 2001. High-dose recombinant apolipoprotein A-I(milano) mobilizes tissue cholesterol and rapidly reduces plaque lipid and macrophage content in apolipoprotein e-deficient mice. Potential implications for acute plaque stabilization. Circulation. 103: 3047–3050. [DOI] [PubMed] [Google Scholar]
- 18.Segrest J. P., Garber D. W., Brouillette C. G., Harvey S. C., Anantharamaiah G. M. 1994. The amphipathic alpha helix: a multifunctional structural motif in plasma apolipoproteins. Adv. Protein Chem. 45: 303–369. [DOI] [PubMed] [Google Scholar]
- 19.Zhu X., Wu G., Zeng W., Xue H., Chen B. 2005. Cysteine mutants of human apolipoprotein A-I: a study of secondary structural and functional properties. J. Lipid Res. 46: 1303–1311. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y., Zhu X., Wu G., Shen L., Chen B. 2008. Effect of lipid-bound apoA-I cysteine mutants on lipopolysaccharide-induced endotoxemia in mice. J. Lipid Res. 49: 1640–1645. [DOI] [PubMed] [Google Scholar]
- 21.von der Thusen J. H., van Berkel T. J., Biessen E. A. 2001. Induction of rapid atherogenesis by perivascular carotid collar placement in apolipoprotein E-deficient and low-density lipoprotein receptor-deficient mice. Circulation. 103: 1164–1170. [DOI] [PubMed] [Google Scholar]
- 22.Baetta R., Silva F., Comparato C., Uzzo M., Eberini I., Bellosta S., Donetti E., Corsini A. 2007. Perivascular carotid collar placement induces neointima formation and outward arterial remodeling in mice independent of apolipoprotein E deficiency or Western-type diet feeding. Atherosclerosis. 195: e112–e124. [DOI] [PubMed] [Google Scholar]
- 23.Chen C. H., Albers J. J. 1982. Characterization of proteoliposomes containing apoprotein A-I: a new substrate for the measurement of lecithin:cholesterol acyltransferase activity. J. Lipid Res. 23: 680–691. [PubMed] [Google Scholar]
- 24.Koizumi J., Kano M., Okabayashi K., Jadhav A., Thompson G. R. 1988. Behavior of human apolipoprotein A-I: phospholipid and apoHDL:phospholipid complexes in vitro and after injection into rabbits. J. Lipid Res. 29: 1405–1415. [PubMed] [Google Scholar]
- 25.Draper H. H., Hadley M. 1990. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 186: 421–431. [DOI] [PubMed] [Google Scholar]
- 26.Cho K. H. 2009. Synthesis of reconstituted high density lipoprotein (rHDL) containing apoA-I and apoC-III: the functional role of apoC-III in rHDL. Mol. Cells. 27: 291–297. [DOI] [PubMed] [Google Scholar]
- 27.Guo G. L., Santamarina-Fojo S., Akiyama T. E., Amar M. J., Paigen B. J., Brewer B., Jr., Gonzalez F. J. 2006. Effects of FXR in foam-cell formation and atherosclerosis development. Biochim. Biophys. Acta. 1761: 1401–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benzie I. F., Strain J. J. 1996. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 239: 70–76. [DOI] [PubMed] [Google Scholar]
- 29.Aviram M., Billecke S., Sorenson R., Bisgaier C., Newton R., Rosenblat M., Erogul J., Hsu C., Dunlop C., La Du B. 1998. Paraoxonase active site required for protection against LDL oxidation involves its free sulfhydryl group and is different from that required for its arylesterase/paraoxonase activities: selective action of human paraoxonase allozymes Q and R. Arterioscler. Thromb. Vasc. Biol. 18: 1617–1624. [DOI] [PubMed] [Google Scholar]
- 30.Johnson J., Carson K., Williams H., Karanam S., Newby A., Angelini G., George S., Jackson C. 2005. Plaque rupture after short periods of fat feeding in the apolipoprotein E-knockout mouse: model characterization and effects of pravastatin treatment. Circulation. 111: 1422–1430. [DOI] [PubMed] [Google Scholar]
- 31.Naghavi M., Wyde P., Litoversusky S., Madjid M., Akhtar A., Naguib S., Siadaty M. S., Sanati S., Casscells W. 2003. Influenza infection exerts prominent inflammatory and thrombotic effects on the atherosclerotic plaques of apolipoprotein E-deficient mice. Circulation. 107: 762–768. [DOI] [PubMed] [Google Scholar]
- 32.Cho K. H., Park S. H., Han J. M., Kim H. C., Choi Y. K., Choi I. 2006. ApoA-I mutants V156K and R173C promote anti-inflammatory function and antioxidant activities. Eur. J. Clin. Invest. 36: 875–882. [DOI] [PubMed] [Google Scholar]
- 33.Cho K. H. 2009. A reconstituted high density lipoprotein containing the V156E mutant of apolipoprotein A-I exhibits anti-atherosclerotic activity in Apo-E deficient mice. J. Atheroscler. Thromb. 16: 217–229. [DOI] [PubMed] [Google Scholar]
- 34.Cho K. H., Kim J. R. 2009. A reconstituted HDL containing V156K or R173C apoA-I exhibited anti-inflammatory activity in apo-E deficient mice and showed resistance to myeloperoxidase-mediated oxidation. Exp. Mol. Med. 41: 417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baker P. W., Rye K. A., Gamble J. R., Vadas M. A., Barter P. J. 1999. Ability of reconstituted high density lipoproteins to inhibit cytokine-induced expression of vascular cell adhesion molecule-1 in human umbilical vein endothelial cells. J. Lipid Res. 40: 345–353. [PubMed] [Google Scholar]
- 36.Cockerill G. W., Huehns T. Y., Weerasinghe A., Stocker C., Lerch P. G., Miller N. E., Haskard D. O. 2001. Elevation of plasma high-density lipoprotein concentration reduces interleukin-1-induced expression of E-selectin in an in vivo model of acute inflammation. Circulation. 103: 108–112. [DOI] [PubMed] [Google Scholar]
- 37.Pasterkamp G., Galis Z. S., de Kleijn D. P. 2004. Expansive arterial remodeling: location, location, location. Arterioscler. Thromb. Vasc. Biol. 24: 650–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woollard K. J., Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat. Rev. Cardiol. 7: 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toshima S., Hasegawa A., Kurabayashi M., Itabe H., Takano T., Sugano J., Shimamura K., Kimura J., Michishita I., Suzuki T., et al. 2000. Circulating oxidized low density lipoprotein levels. A biochemical risk marker for coronary heart disease. Arterioscler. Thromb. Vasc. Biol. 20: 2243–2247. [DOI] [PubMed] [Google Scholar]
- 40.Oda M. N., Bielicki J. K., Berger T., Forte T. M. 2001. Cysteine substitutions in apolipoprotein A-I primary structure modulate paraoxonase activity. Biochemistry. 40: 1710–1718. [DOI] [PubMed] [Google Scholar]
- 41.Ross R. 1999. Atherosclerosis–an inflammatory disease. N. Engl. J. Med. 340: 115–126. [DOI] [PubMed] [Google Scholar]
- 42.Tzoulaki I., Murray G. D., Lee A. J., Rumley A., Lowe G. D., Fowkes F. G. 2005. C-reactive protein, interleukin-6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population: Edinburgh Artery Study. Circulation. 112: 976–983. [DOI] [PubMed] [Google Scholar]
- 43.Schieffer B., Selle T., Hilfiker A., Hilfiker-Kleiner D., Grote K., Tietge U. J., Trautwein C., Luchtefeld M., Schmittkamp C., Heeneman S., et al. 2004. Impact of interleukin-6 on plaque development and morphology in experimental atherosclerosis. Circulation. 110: 3493–3500. [DOI] [PubMed] [Google Scholar]
- 44.Huber S. A., Sakkinen P., Conze D., Hardin N., Tracy R. 1999. Interleukin-6 exacerbates early atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 19: 2364–2367. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.