Abstract
The mechanism by which cholesteryl ester transfer protein (CETP) activity affects HDL metabolism was investigated using agents that selectively target CETP (dalcetrapib, torcetrapib, anacetrapib). In contrast with torcetrapib and anacetrapib, dalcetrapib requires cysteine 13 to decrease CETP activity, measured as transfer of cholesteryl ester (CE) from HDL to LDL, and does not affect transfer of CE from HDL3 to HDL2. Only dalcetrapib induced a conformational change in CETP, when added to human plasma in vitro, also observed in vivo and correlated with CETP activity. CETP-induced pre-β-HDL formation in vitro in human plasma was unchanged by dalcetrapib ≤3 µM and increased at 10 µM. A dose-dependent inhibition of pre-β-HDL formation by torcetrapib and anacetrapib (0.1 to 10 µM) suggested that dalcetrapib modulates CETP activity. In hamsters injected with [3H]cholesterol-labeled autologous macrophages, and given dalcetrapib (100 mg twice daily), torcetrapib [30 mg once daily (QD)], or anacetrapib (30 mg QD), only dalcetrapib significantly increased fecal elimination of both [3H]neutral sterols and [3H]bile acids, whereas all compounds increased plasma HDL-[3H]cholesterol. These data suggest that modulation of CETP activity by dalcetrapib does not inhibit CETP-induced pre-β-HDL formation, which may be required to increase reverse cholesterol transport.
Keywords: dalcetrapib, torcetrapib, anacetrapib, high density lipoprotein-functionality, apolipoproteins, bile acids and salts/metabolism, lipoproteins/metabolism, CETP
The inverse relationship between HDL-cholesterol (HDL-C) and cardiovascular (CV) disease risk (1) suggests that increasing HDL-C could potentially reduce CV risk (2). This has been postulated to be related to the multiple CV benefits of HDL and, perhaps most importantly, the efflux of cholesterol from peripheral tissue. A variety of agents have been developed that increase HDL-C, including niacin and fibrates, and more recently, agents that decrease cholesteryl ester transfer protein (CETP) activity and are generically referred to as CETP inhibitors.
CETP inhibitors from two different chemical classes have been developed and have reached late clinical development. The first class are potent CETP inhibitors, which are 3,5-bis-trifluoromethyl-benzene derivatives such as torcetrapib (3) and anacetrapib (4) that have been shown to raise plasma HDL-C by up to 130% in humans (4). The Phase III mortality/morbidity study of torcetrapib was terminated prematurely due to increased CV and non-CV deaths and events, most likely due to off-target effects (5) unrelated to CETP inhibition (6–8); anacetrapib is currently undergoing Phase II/III investigations. The second chemical class represented by dalcetrapib (JTT-705/R1658/RO4607381), are benzenethiol derivatives (9–11). Dalcetrapib, which raises HDL-C by up to 36% in humans at a dose of 600 mg (12), is currently being evaluated in a Phase III outcomes study (13).
In addition to differences in potency, the mechanism of CETP inhibition by compounds derived from these two chemical classes is also likely to be different. The binding of torcetrapib to CETP increases its affinity for lipoproteins and induces the formation of an inactive high-affinity complex between CETP and lipoproteins such as HDL (14). The CETP-lipoprotein complex, similar to the one induced by the CETP-inhibitory antibody TP2 (15), cannot efficiently exchange neutral lipids between different lipoprotein particles. Although the exact mechanism by which dalcetrapib decreases CETP activity has not been elucidated, the mode of action has been suggested to involve the direct specific interaction between cysteine 13 (Cys13) of CETP and the benzenethiol moiety of dalcetrapib (9). The thioester bond of dalcetrapib is cleaved by nonspecific esterases in the gastrointestinal tract and in plasma, generating reactive dalcetrapib-thiol and allowing formation of a disulfide bond with Cys13 (9).
Qiu et al. (16) suggested that CETP adopts a new conformation to enable binding to lipoproteins of larger size than HDL, such as VLDL. We therefore investigated potential differences in changes in CETP conformation induced by dalcetrapib or torcetrapib using monoclonal antibodies against CETP.
The significance of the different modes of interaction of various CETP inhibitors and the relative impact of decreased CETP activity on lipoprotein metabolism have not been compared previously. Synthetic CETP inhibitors were optimized for the inhibition of neutral lipid transfer between HDL and apolipoprotein B (apoB)-containing lipoproteins (heterotypic transfer), but their effect on transfer of cholesteryl ester (CE) among HDL subparticles (homotypic transfer) and CETP-dependent HDL remodeling have not been studied. CETP facilitates the extensive remodeling of plasma HDL particles by promoting the interconversion of apoA-I-containing α-HDL to small, lipid-poor, pre-β-HDL (17, 18) and transfering CE among HDL subparticles (19, 20). This dynamic remodeling of HDL is a key aspect of its function in reverse cholesterol transport (RCT), leading to the removal of excess cholesterol from tissue and its delivery to the liver (21). CETP has been shown to impact these two processes by generating small lipid-poor pre-β-HDL particles, which are preferred acceptors of ABCA1-dependent cholesterol efflux (22), and to facilitate HDL-CE uptake via the scavenger receptor class B type I (SR-BI) pathway (23). In addition, CE transferred by CETP from HDL to LDL and subsequent uptake via the LDL receptor (LDLR) might be an important aspect of RCT (24). Thus, the observation that CETP mediates HDL remodeling and cholesterol clearance via the LDLR raised concern that inhibition of CETP activity may adversely impact cholesterol efflux capacity and RCT (21).
Indeed, torcetrapib treatment was not associated with an increase in fecal sterol excretion in clinical studies (25), supporting the need for a relevant animal model to investigate the effects of compounds affecting CETP activity on macrophage RCT. A macrophage RCT model has been extensively characterized (albeit in mice naturally deficient in CETP) and confirmed as a valid model for assessing promotion of RCT (26). In a recent study where torcetrapib was tested in a new hamster macrophage model, although treatment was associated with increased excretion of labeled cholesterol in feces, there was no correlation between fecal sterol excretion and torcetrapib dose. This raised the query as to whether marked inhibition of CETP activity was associated with an increase in HDL of normal composition and function (27).
CETP inhibitors represent a valuable tool for investigating the mechanism by which changes in CETP activity affects HDL metabolism in vitro. We therefore compared the potent CETP inhibitors torcetrapib and anacetrapib with the structurally dissimilar benzenethiol derivative dalcetrapib (10) in terms of respective modes of interaction with CETP, effect on transfer of CE from HDL to LDL versus from HDL3 to HDL2, binding sites, change in CETP conformation, and effect on CETP-induced formation of pre-β-HDL. In addition, the potential relevance of these differences was evaluated in vivo by assessing changes in macrophage RCT in CETP inhibitor-treated hamsters.
MATERIALS AND METHODS
All animal studies complied with the guidelines for animal experimentation of our laboratories and were approved by institutional review boards. Human plasma samples were provided by volunteers or were from clinical trials conducted in accordance with the Declaration of Helsinki and approved by independent ethics committees, where participants provided written informed consent.
All compounds studied (dalcetrapib, dalcetrapib-thiol, dalcetrapib-disulfide, torcetrapib, and anacetrapib) were synthesized using standard procedures. Chemical structures of test compounds are provided (see supplementary Table I). All additional materials were sourced from recognized suppliers, and commercially available reagents and kits were used according to manufacturers’ instructions unless otherwise indicated.
Mode of action
Selective binding of dalcetrapib to Cys13.
CETP containing a serine residue instead of Cys13 (C13S CETP) was constructed by site-directed mutagenesis. The protein was expressed in HEK293EBNA cells from large-scale transient transfection, and purified as described below for recombinant human (rh)CETP.
Inhibition of rhCETP and C13S CETP-mediated transfer of CE from HDL to LDL.
The inhibitory potency (IC50) of dalcetrapib, torcetrapib, and anacetrapib to decrease CE transfer from HDL to LDL by rhCETP and C13S CETP was measured using a scintillation proximity assay kit, #TRKQ7015 (GE Healthcare; Waukesha, WI) (28). Briefly, [3H]CE-labeled HDL donor particles were incubated in the presence of purified CETP proteins (final concentration 0.5 µg/ml) and biotinylated LDL acceptor particles for 3 h at 37°C. Subsequently, streptavidin-coupled polyvinyltoluene beads containing liquid scintillation cocktail binding selectively to biotinylated LDL were added, and the amount of [3H]CE molecules transferred to LDL was measured by β counting.
Inhibition of transfer of CE from HDL3 to HDL2.
Assessment of lipid movement among HDL subfractions was performed using radiolabeled lipid transfer assays as previously described (20). Lipoprotein subfraction (d > 1.063 g/ml) was labeled with [3H]CE. [3H]CE-labeled HDL3 (1.125 < d < 1.210 g/ml) was prepared by sequential ultracentrifugation (29). [3H]CE-labeled HDL3 and nonradiolabeled HDL2 (1.063 < d < 1.125 g/ml) were added on an equal phospholipid basis (2.3 μg/tube). The lipoprotein mixture was incubated in the presence of 1% BSA, 21 mM tris-HCl (pH 7.4), 0.5% NaCl, and 0.006% EDTA with or without rhCETP (0.5 μg/tube). Dalcetrapib, torcetrapib, and anacetrapib were tested at concentrations of 0.001, 0.01, 0.1, 1, and 10 µM in a total volume of 0.715 ml and incubated for 4 h at 37°C. After incubation, HDL2 and HDL3 fractions were separated by ultracentrifugation (d = 1.125 g/ml) for 19 h at 4°C. Total radioactivity in the HDL2 (upper layer) and HDL3 (lower layer) subfractions was measured by scintillation counting. CETP activity was expressed as the percentage of total radioactivity recovered in the HDL2 fraction.
Binding sites of compounds on CETP: competition for binding site on sepharose-immobilized rhCETP.
Binding studies were performed according to Connolly et al. (30) using rhCETP expressed by a cell line described by Weinberg et al. (31), kindly provided by Professor Alan Tall (Columbia University) and purified by hydrophobic interaction chromatography and size exclusion chromatography (SEC) [modified from Ohnishi, Yokoyama, and Yamamoto (32)]. BSA and rhCETP were immobilized on CNBr-activated sepharoseTM 4 Fast Flow (GE Healthcare).
Both competition (co-incubation experiments) and displacement after preincubation [the latter with or without the reducing agent tris(2-carboxyethyl)phosphine (TCEP)] with radioactive compound were determined for 300 pmol immobilized rhCETP (3 μM) or the same mass of BSA mixed with 0.25 μM [14C]torcetrapib or 2.5 μM [14C]dalcetrapib, respectively, and unlabeled CETP inhibitors in a total volume of 100 μl. [14C]dalcetrapib was treated with pancreatic lipase to produce [14C]dalcetrapib-thiol prior to incubation with CETP. Radioactivity bound to sepharose was measured by scintillation counting.
Changes in conformation of CETP assessed by ELISA.
Anti-CETP antibody production.
Antibodies were raised in NMRI mice injected intraperitoneally with 20 µg rhCETP emulsified in aluminum hydroxide gel (Alhydrogel-2%; Brenntag Biosector, Frederikssund, Denmark) containing CpG oligodeoxynucleotides according to Davis et al. (33). The animals received four booster injections each at 3-week intervals with the same antigen preparation.
As soon as the animals showed a specific immune response to the rhCETP, the best responders were boosted, and after 3 days, the spleens were removed and the isolated cells were fused to PAI myeloma cells, a variant of the P3-x63-AG8 myeloma (34). The monoclonal antibodies 6/2, 6/6, and 6/17 were selected for further characterization, together with clone JHC-1 (35).
CETP ELISA using different capture antibodies.
Two types of ELISA were performed, one using clone JHC-1 as capture antibody and clone 6/6 conjugated to HRP as detection antibody, and the other with clone 6/2 as capture antibody and clone 6/17 conjugated to HRP as detection antibody.
In vitro addition of CETP inhibitors: plasma samples from seven healthy donors were prediluted 1:10 with blocking buffer (50 mM Tris, 140 mM NaCl, 5 mM EDTA, 0.05% Nonidet P40, 0.25% gelatin, 1% BSA, pH 7.4). A dilution series of the test compounds was prepared from this prediluted plasma and incubated for 3 h at 37°C, then 50 µl of each dilution was analyzed by both types of ELISA.
These ELISAs were applied to plasma samples from 22 healthy subjects receiving a single oral dose of 600 mg dalcetrapib (batch F49, PK study WP22305, Roche data on file). Plasma samples were collected before, and 2, 4, 6, 8, 12, and 24 h after dalcetrapib intake. Dalcetrapib level was measured as described elsewhere (36) and CETP immunoreactivity was determined using the ELISA described above with JHC-1 or 6/2 as capture antibody and 6/6 or 6/17 for detection. CETP activity was determined by an ex vivo CETP activity assay kit (Roar Biomedical, Inc., New York, NY).
Quantification of pre-beta-HDL by ELISA.
Samples with or without added rhCETP were incubated for 21 h in the presence of torcetrapib, anacetrapib, and dalcetrapib (0.10 µM to 10 µM). Pre-β-HDL concentration was measured by ELISA (Daiichi Pure Chemicals Co. Ltd, Tokyo, Japan) as previously described (37).
Additional methodology relating to characterization of pre-β-HDL by SEC/reverse-phase protein array and the assessment of pre-β-HDL generation by Western blot are provided as supplementary materials.
Statistical analysis was performed by an ANCOVA model in which the slope of pre-β-HDL formation as a function of CETP concentration was compared between the tested doses of each compound.
In vivo RCT study.
To investigate the effect of dalcetrapib, torcetrapib, and anacetrapib on macrophage-to-feces RCT, radiolabeled macrophages from the peritoneal cavity of donor Golden Syrian hamsters preinjected with [3H]cholesterol were prepared as previously described (38). Male recipient Golden Syrian hamsters, 8 weeks old, on a standard chow diet were preadministered dalcetrapib [100 mg/kg twice daily (BID)], torcetrapib [30 mg/kg once daily (QD)], anacetrapib (30 mg/kg QD), or vehicle (0.5% methylcellulose BID) for 7 days by oral gavage before intraperitoneal injection of [3H]cholesterol-labeled macrophages (3.8 × 106 cells/90.6 kBq/0.5 ml per animal) at day 0. The percentage of esterified cholesterol in injected macrophages was 21% (mass) and 16% (labeled). Animals continued to receive vehicle or test compounds daily for 10 days. Samples for plasma lipid analysis were obtained on days −7, 0, 3, 7, and 10 and for radioactivity levels on days 3, 7, and 10. Total cholesterol and HDL-C were measured by enzymatic methods (Roche Diagnostics, Burgess Hill, West Sussex, UK). HDL-C was measured as the cholesterol concentration in the HDL fraction separated by polyethylene glycol 6000 solution. The area under the plasma HDL-C concentration-time curve (HDL-C·AUC) during the RCT study period (day 0 to day 10) was calculated from plasma HDL-C levels (at day 0, 3, 7, and 10) by the trapezoidal method.
Feces were collected continuously from day 0 to day 10. Fecal neutral sterols were extracted by a 2:1 mixture of chloroform-methanol (39) and measured by enzymatic methods (Roche Diagnostics), and fecal [3H]neutral sterols and [3H]bile acids were determined by previously described methods (40). Fecal bile acids extracted by ethanol were measured by an enzymatic method (Wako Pure Chemical Industries Ltd, Osaka, Japan).
Additional methodology relating to the measurement of fecal sterols is provided in Supplementary Materials.
Statistical analysis
The relationship between dose of CETP inhibitor and pre-β-HDL formation was assessed by ANCOVA with nested factor (PROC MIXED from SASv8) and on CE transfer from HDL3 to HDL2 by Student's t-test or Dunnett test.
For RCT studies, differences between control and test compound groups were assessed using the Bartlett test followed by the Steel test. The Dunnett test was applied when no differences were found with the Bartlett test. Differences were considered significant when P < 0.05 (two-sided).
RESULTS
Mode of action
Role of CETP Cys13 in the inhibitory activity of dalcetrapib versus torcetrapib and anacetrapib toward CE transfer from HDL to LDL.
It was shown previously that mutation of Cys13 to Ser (C13S), but not mutation of Cys1 or Cys131, abolishes the CETP inhibitory activity of dalcetrapib (9). Using a synthetic assay system, we determined the IC50 of torcetrapib, anacetrapib, and dalcetrapib for transfer of CE from HDL to LDL by rhCETP and C13S CETP mutant. In this assay system, the mean ± SEM IC50 of dalcetrapib, dalcetrapib-thiol, and dalcetrapib-disulfide was found to be 204.6 ± 96.3 nM, 23.7 ± 1.9 nM, and 35.2 ± 2.8 nM, respectively, for rhCETP, whereas this was >100,000 nM for the same compounds for the C13S CETP mutant. Torcetrapib and anacetrapib were potent inhibitors of rhCETP with an IC50 of 7.4 ± 2.6 nM and 7.9 ± 2.5 nM, respectively, while their inhibitory activity was virtually unchanged for the C13S CETP mutant (11.8 ± 5.2 nM and 11.8 ± 1.9 nM, respectively).
Differential inhibitory activity of dalcetrapib, torcetrapib, and anacetrapib toward CE transfer from HDL3 to HDL2.
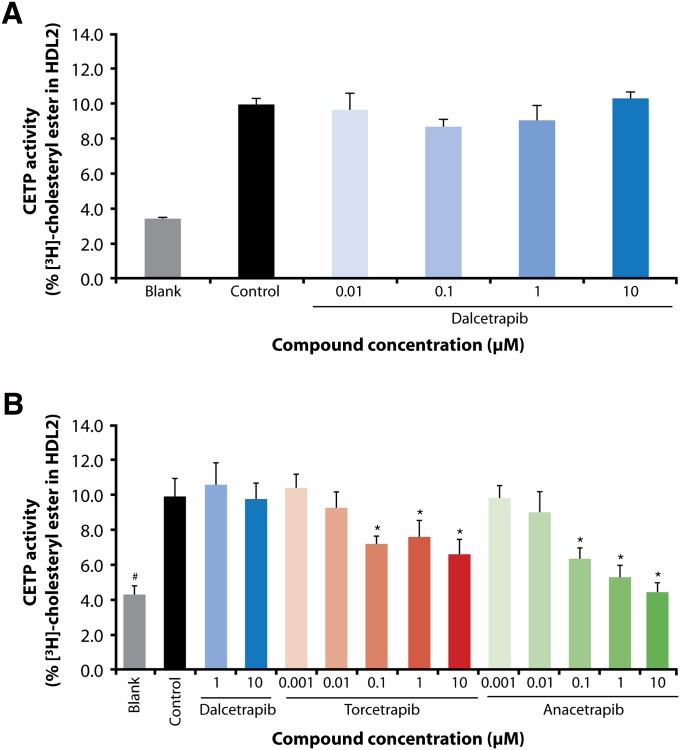
Dalcetrapib was first tested at concentrations of 0.01, 0.1, 1, and 10 μM for its effect on CE transfer from HDL3 to HDL2. As displayed in Fig. 1A, no effect of dalcetrapib was observed up to 10 μM. In a second series of experiments, torcetrapib and anacetrapib were tested at 0.001, 0.01, 0.1, 1, and 10 μM, and dalcetrapib at 1 and 10 μM. As shown in Fig. 1B, torcetrapib and anacetrapib dose-dependently and significantly decreased the transfer of CE from HDL3 to HDL2 (P < 0.001 for concentrations equal to and higher than 0.1 µM), which remained unchanged with dalcetrapib at concentrations of 1 and 10 µM.
Fig. 1.
[3H]cholesteryl ester-labeled HDL3 was incubated with unlabeled HDL2 and recombinant human cholesteryl ester transfer protein [(rh)CETP] in the presence of: (A) dalcetrapib, 0.01 µM to 10 µM (n = 3); (B) dalcetrapib, 1 µM and 10 µM, torcetrapib and anacetrapib, 0.001 µM to 10 µM (n = 4–6). Data are expressed as radioactivity recovered in the HDL2 fraction as a percentage of total radioactivity [mean (SD)]. Blank value: without added rhCETP; control value: with added rhCETP. (*P < 0.01 vs. control, Student's t-test; #P < 0.01 vs. control, Dunnett test).
Competition for binding to rhCETP of 14C-labeled and unlabeled CETP inhibitors.
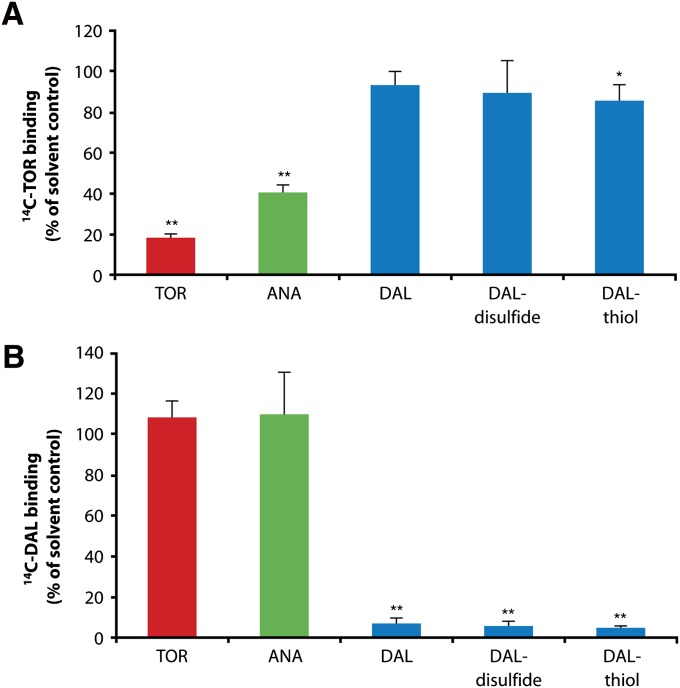
To determine whether the compounds interact with the same binding site, we conducted competition experiments. Excess torcetrapib and anacetrapib (25 µM) decreased the amount of [14C]torcetrapib (0.25 µM) bound to immobilized rhCETP by 82% and 60%, respectively. In contrast, binding of [14C]torcetrapib was unaffected by 25 µM of unlabeled dalcetrapib and dalcetrapib-disulfide, and was only marginally decreased by dalcetrapib-thiol (Fig. 2A). Displacement of [14C]dalcetrapib-thiol (2.5 μM) by excess unlabeled dalcetrapib-thiol (25 μM) was not observed in the absence of the reducing agent TCEP (data not shown), but did occur when TCEP was present. Under similar conditions, neither torcetrapib nor anacetrapib affected the amount of [14C]dalcetrapib-thiol bound to rhCETP (Fig. 2B).
Fig. 2.
A: Competition of [14C]torcetrapib (0.25 μM) and unlabeled CETP inhibitors for binding to rhCETP. Radioactivity bound to CETP sepharose after coincubation with 0.25 µM [14C]torcetrapib and 25 μM (100-fold excess) unlabeled CETP inhibitors (TOR, torcetrapib; ANA, anacetrapib; DAL, dalcetrapib). Data shown are the mean of three independent experiments with duplicates of rhCETP-sepharose (n = 6). *P < 0.01; **P < 0.001 (unpaired t-test). B: Displacement of [14C]dalcetrapib in the presence of reducing agent tris(2-carboxyethyl)phosphine (TCEP) by CETP inhibitors after preincubation. Radioactivity bound to rhCETP sepharose after preincubation of sepharose with 2.5 μM [14C]dalcetrapib followed by incubation with 25 μM of unlabeled CETP inhibitors (TOR, torcetrapib; ANA, anacetrapib; DAL, dalcetrapib) with addition of 5 mM TCEP. Data shown are the mean of three independent experiments with singlets of rhCETP-sepharose (n = 3). Error bars represent the SD of all values (all corrected for the corresponding BSA control). **P < 0.001 (unpaired t-test).
Changes in CETP conformation assessed by ELISA using different anti-CETP monoclonal antibodies.
A series of anti-CETP monoclonal antibodies were produced in an attempt to select those directed toward epitopes potentially modified following the binding of different CETP inhibitors. Binding characteristics of capture antibodies JHC-1 and 6/2 to rhCETP were established by surface plasmon resonance (SPR). The association rate constant (ka) of the antibody JHC-1 was 3.1 × 104 M−1 · s−1 and 1.8 × 104 M−1 · s−1 and the dissociation rate constant (kd) was 4.5 × 10−3 s−1 and 1.3 × 10−2 s−1 in the absence and presence of dalcetrapib-thiol, respectively (see supplementary Table IIA). The dissociation constant [Kd (kd/ka)] was then calculated to be 148 nM and 743 nM in the absence and presence of dalcetrapib-thiol, respectively, indicating a 5-fold increase in dissociation rate of JHC-1 in the presence of dalcetrapib (this parameter was not changed for torcetrapib and decreased for anacetrapib). For antibody 6/2, Kd was 5.5 nM and 14 nM in the absence and presence of dalcetrapib-thiol, respectively. A decrease in Kd values was measured upon addition of either torcetrapib or anacetrapib.
Dalcetrapib-thiol did not bind to JHC-1. JHC-1 bound with comparable affinity to wild-type and the C13S mutant of CETP (see supplementary Table IIB) and did not bind to any cysteine-containing peptide of CETP (see supplementary Table IIC).
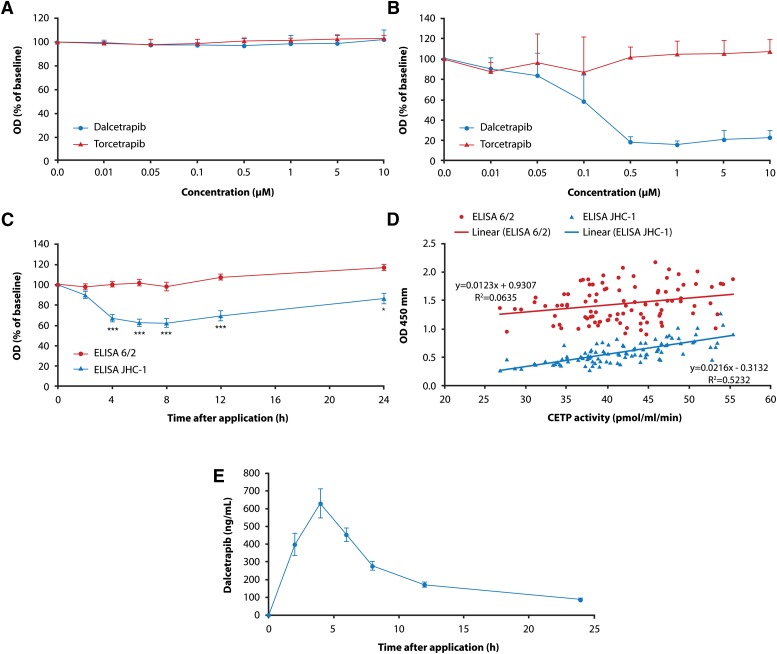
The antibody JHC-1 was selected as a capture antibody for development of an ELISA to detect an epitope selective for the dalcetrapib-CETP interaction, whereas antibody 6/2 was used as capture antibody for the reference ELISA. Plasma samples from seven healthy volunteers were incubated in vitro with dalcetrapib and torcetrapib (0.01 µM to 10 µM). Using antibody 6/2, no change in epitope detection was observed with concentrations of dalcetrapib and torcetrapib up to 10 µM (Fig. 3A). On the contrary, dalcetrapib decreased CETP detection by JHC-1 starting at a concentration of 0.1 µM (Fig. 3B). Torcetrapib did not change epitope detection by JHC-1 antibody over the concentration range 0.01 µM to 10 µM (Fig. 3B).
Fig. 3.
Comparison of effects of dalcetrapib and torcetrapib. A: Using clone 6/2 as capture antibody. B: Using clone JHC-1 as capture antibody. Data shown in A and B are the mean of independent measurements with duplicates using seven individual human plasma samples (n = 14). Error bars represent the SD of all values. C: ELISA using clone 6/2 (circles) and clone JHC-1 (triangles) applied to plasma of volunteers receiving a single oral dose (600 mg) of dalcetrapib. D: Correlation of CETP ELISA and CETP activity. E: Dalcetrapib concentration in plasma of healthy volunteers treated with 600 mg dalcetrapib. Data shown in C, D, and E are the mean ± SEM for n = 22; statistical analysis of ELISA data in Fig. 2C resulted in *P < 0.01 and ***P < 0.0001 (paired Student's t-test).
Change in conformation of CETP in plasma samples from volunteers receiving a single 600 mg oral dose of dalcetrapib assessed by ELISA.
A decrease in CETP detection by the JHC-1-based ELISA was also observed in plasma from 22 healthy volunteers who received a single oral dose of 600 mg dalcetrapib (30%, P < 0.001), confirming in the clinical setting the change in epitope detection by JHC-1 induced by dalcetrapib in vitro (Fig. 3C). In addition, the decrease in detection of CETP by JHC-1 antibody paralleled that of CETP activity and correlated with the reduction in CETP activity (Fig. 3D). As expected, no change was detected in the same plasma when 6/2 was used as capture antibody.
In the same subjects, the plasma dalcetrapib-thiol level reached a maximum of 628.7 ng/ml at 4 h (Fig. 3E), the mean ± SD t1/2 of 25.5 ± 3.9 h suggesting that the induced change in conformation was maintained at low plasma dalcetrapib concentrations, as evidenced at the 12 h time point.
In vitro generation of pre-beta-HDL by CETP in human plasma: effect of CETP inhibitors.
In vitro incubation of human plasma with added increasing concentrations of purified rhCETP led to a dose-dependent increase in lipid-poor apoA-I-containing particles (SEC fraction 29), characterized as pre-β-HDL by agarose gel electrophoresis (see supplementary ).
Quantification of pre-beta-HDL by ELISA.
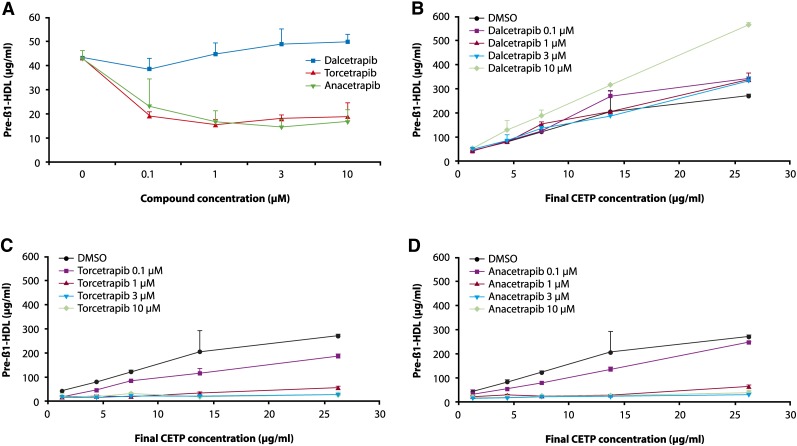
To better evaluate the role of CETP in the generation of pre-β-HDL in human plasma, and to quantify the amount of pre-β-HDL generated, we incubated human plasma with or without added rhCETP and quantified pre-β-HDL by a pre-β1-selective ELISA. Incubation of human plasma for 21 h at 37°C without added exogenous CETP, increased the level of pre-β-HDL by 43%. Dalcetrapib 1, 3, and 10 μM dose-dependently increased pre-β-HDL by 4, 13, and 16% (NS, P < 0.05, P < 0.01, respectively). Torcetrapib and anacetrapib decreased pre-β-HDL formation by more than 46% (P < 0.001) at all concentrations tested (Fig. 4A).
Fig. 4.
A: Human plasma with an endogenous CETP level of 1.25 μg/ml was incubated for 21 h with and without test compounds, dalcetrapib, torcetrapib, and anacetrapib (0.1, 1, 3, and 10 µM). Dalcetrapib 1, 3, and 10 µM dose-dependently increased pre-β-HDL, as quantified by ELISA, by 4, 13, and 16% (NS, P < 0.05, and 0.01, respectively). Human plasma with added recombinant human CETP (final concentration 4.38, 7.5, 13.75, and 26.25 μg/ml) was incubated for 21 h with and without test compounds (0.1, 1, 3, and 10 µM). B: Dalcetrapib significantly increased pre-β-HDL formation at concentrations of 0.1, 1, 3, and 10 µM (P < 0.001, 0.001, 0.05, and 0.001, respectively). C: Torcetrapib significantly and dose-dependently decreased pre-β-HDL formation at all concentrations tested (P < 0.01 at 0.1 µM and P < 0.001 at 1, 3, and 10 µM). D: Anacetrapib significantly decreased pre-β-HDL formation at concentrations of 1, 3, and 10 µM (each P < 0.001). Error bars represent the SD of four replicates.
The dose-related effect of dalcetrapib, torcetrapib, and anacetrapib on the amount of pre-β-HDL at different concentrations of added rhCETP was evaluated. Data were analyzed by an ANCOVA model in which slopes of pre-β-HDL as a function of CETP were compared between doses by compound. A statistically significant increase in pre-β-HDL formation was observed with dalcetrapib (P < 0.001, 0.001, 0.05, and 0.001 for concentrations of 0.1, 1, 3, and 10 μM, respectively) (Fig. 4B). In the case of torcetrapib, a dose-dependent decrease in pre-β-HDL formation was observed even at the lowest concentration of 0.1 μM (P < 0.004), and at 1, 3, and 10 μM (each P < 0.001) (Fig. 4C). Similar reductions in pre-β-HDL formation were observed for anacetrapib at 1, 3, and 10 μM (each P < 0.001) (Fig. 4D).
In vivo RCT study.
Effect of dalcetrapib, torcetrapib, and anacetrapib on RCT in hamsters.
Pilot dose-response studies with dalcetrapib, torcetrapib, and anacetrapib showed that dalcetrapib 100 mg/kg BID, torcetrapib 30 mg/kg QD, and anacetrapib 30 mg/kg QD provided similar reductions in the area under the concentration time curve AUC0–24 (% transfer·h) for CETP inhibitory activity although HDL-C levels were not comparable (data not shown). These doses were therefore selected for in vivo RCT studies in hamsters to assess if differences among the three compounds observed in vitro would manifest as differences in macrophage RCT. Hamsters were given dalcetrapib, torcetrapib, or anacetrapib for 7 days before injection of [3H]cholesterol-labeled macrophages (day 0). Treatment with dalcetrapib, torcetrapib, or anacetrapib led to significant increases in HDL-C levels at day 0 (Table 1). The efficacy of dalcetrapib appeared to be less than that of torcetrapib and anacetrapib. At day 3, [3H]cholesterol radioactivity in the HDL fraction was significantly increased from control values for dalcetrapib, torcetrapib, and anacetrapib (Table 1).
TABLE 1.
Plasma total cholesterol, HDL-C, [3H]plasma and [3H]HDL in hamster RCT study
| Control (n = 6) | Dalcetrapib (n = 6) | Torcetrapib (n = 5) | Anacetrapib (n = 6) | |
|---|---|---|---|---|
| 100 mg/kg, BID | 30 mg/kg, QD | 30 mg/kg, QD | ||
| Day 0 | ||||
| Total cholesterol (mg/dl) | 167.1 ± 8.0 | 186.3 ± 10.1a | 183.4 ± 11.4a | 220.7 ± 12.2b |
| HDL-C (mg/dl) | 82.7 ± 6.0 | 102.1 ± 11.3a | 112.7 ± 7.4b | 137.1 ± 15.4b |
| Day 3 | ||||
| [3H]plasma (kBq/ml) | 0.70 ± 0.07 | 0.74 ± 0.05 | 0.77 ± 0.11 | 0.79 ± 0.11 |
| [3H]HDL (kBq/ml) | 0.19 ± 0.01 | 0.28 ± 0.04c | 0.32 ± 0.07c | 0.34 ± 0.07c |
Syrian hamsters were treated with CETP inhibitors (dalcetrapib: 100 mg/kg BID; torcetrapib: 30 mg/kg QD; anacetrapib: 30 mg/kg QD. Day: after labeled macrophage injection. Data presented as mean ± SD. BID, twice daily; QD, once daily.
P < 0.05.
P < 0.01 vs. control (Dunnett test).
P < 0.05 vs. control (Steel test).
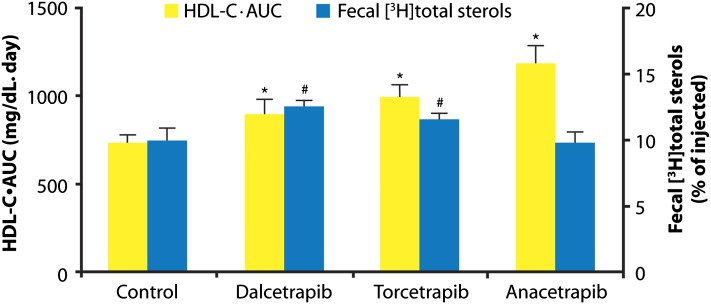
At the end of the study (day 10), liver radioactivity was significantly reduced by dalcetrapib and torcetrapib compared with vehicle (−42.2% and −28.1%, respectively; both P < 0.01), whereas the reduction with anacetrapib (−9.2%) was not statistically significant (data not shown). Total fecal radioactivity over the period from day 0 to day 10 was significantly increased versus control in dalcetrapib- and torcetrapib-treated animals (Table 2). Although torcetrapib significantly increased only the radioactivity of fecal bile acids and not fecal neutral sterols, dalcetrapib significantly increased the radioactivity of both components. Moreover, the mass of total fecal bile acids (nonradiolabeled) was significantly increased only in the dalcetrapib group. For anacetrapib, there were no significant changes in any of the fecal parameters described in Table 2, compared with control. We evaluated the relationship between HDL-C raised by dalcetrapib, torcetrapib, or anacetrapib for the RCT observation period (day 0 to day 10), RCT promotion by calculating the HDL-C·AUC over 0 to 10 days, and also fecal total radioactive sterols for each compound, as shown in Fig. 5. It can be seen that dalcetrapib-treated hamsters had the highest fecal radioactivity despite a lower HDL-C·AUC than torcetrapib and anacetrapib.
TABLE 2.
Fecal neutral sterols and bile acids in hamster RCT study
| Control | Dalcetrapib | Torcetrapib | Anacetrapib | |
|---|---|---|---|---|
| 100 mg/kg BID | 30 mg/kg QD | 30 mg/kg QD | ||
| Neutral sterols (mg/total feces) | 40.4 ± 1.1 | 38.7 ± 3.0 | 41.2 ± 2.3 | 40.9 ± 2.8 |
| Bile acids (µmol/total feces) | 67.0 ± 6.6 | 86.0 ± 6.0b | 74.6 ± 5.6 | 69.8 ± 7.2 |
| Total radioactivity (% of injected) | 13.3 ± 1.3 | 17.5 ± 0.5c | 16.5 ± 0.5c | 13.2 ± 1.5 |
| [3H]neutral sterols (% of injected) | 3.0 ± 0.3 | 3.6 ± 0.3b | 3.3 ± 0.3 | 3.0 ± 0.3 |
| [3H]bile acids (% of injected) | 6.9 ± 0.8 | 8.8 ± 0.7b | 8.2 ± 0.3a | 6.7 ± 0.7 |
| [3H]total sterols (% of injected) | 9.9 ± 0.9 | 12.5 ± 0.5b | 11.5 ± 0.5b | 9.7 ± 0.9 |
Feces were collected for 10 days after injection of [3H]cholesterol-labeled macrophages. Total radioactivity is the count of whole fecal homogenates. [3H]total sterols is the sum of [3H]neutral sterols and [3H]bile acids. The data are presented as mean ± SD, n = 5–6. RCT, reverse cholesterol transport.
P < 0.05.
P < 0.01 vs. control (Dunnett test).
P < 0.05 vs. control (Steel test).
Fig. 5.
Comparison of the effect of dalcetrapib, torcetrapib, and anacetrapib on HDL-C·AUC and radioactivity of fecal total sterols as a percentage of injected radioactivity in the hamster macrophage reverse cholesterol transport (RCT) study. HDL-C·AUC during the RCT study period was calculated from plasma HDL-C levels (day 0, 3, 7, and 10) by the trapezoidal method. Error bars represent the SD for n = 5 or 6 animals. *P < 0.01 (HDL·AUC); #P < 0.01 (radioactivity of fecal total sterols) vs. control (Dunnett test).
In the absence of significant and consistent changes in the expression of genes involved in hepatic and adipose tissue lipid metabolism (hepatic: CETP, apoA-I, apoB, apoE, CYP7A1, CYP8B1, HMG-CoA reductase, LDLR; adipose tissue: CETP, apoA-I, apoE, SR-BI) for all treatment groups, we consider the differences observed with the three CETP inhibitors to be more related to their effect on plasma lipoproteins than on hepatic lipid metabolism (see supplementary Table IIIB).
DISCUSSION
Although CETP inhibition is an accepted means to reproducibly increase plasma HDL-C, the mechanism and extent of inhibition required to optimally increase HDL and maintain HDL functionality has proven controversial and, to date, inadequately addressed. We attempted to resolve this by taking advantage of the availability of agents derived from different chemical classes that specifically target CETP activity. We compared compounds representative of the main chemical classes, the 3,5-bis-trifluoromethyl-benzene derivatives torcetrapib and anacetrapib, and the benzenethiol derivative dalcetrapib, for biochemical characteristics underlying their interaction with CETP, and for their effect on CETP-induced pre-β-HDL formation and RCT in vivo in a macrophage-to-feces RCT hamster model.
Mechanisms leading to a decrease in CETP activity
Several thiol-containing compounds have previously been shown to affect CETP activity (11) via an interaction with free cysteine residues of CETP that play an important role in its transfer activity (41). In agreement with previous studies (9), the benzenethiol derivative dalcetrapib decreased CETP activity, measured as transfer of CE from HDL to LDL of wild-type rhCETP, but did not inhibit CETP activity of the C13S mutant (IC50 of 204.6 ± 96.3 and >100,000 nM, respectively). On the contrary, both torcetrapib and anacetrapib were active and equipotent for recombinant wild-type and the C13S CETP mutant (IC50 of 7.4 ± 2.6 and 11.8 ± 5.2 nM, respectively, for torcetrapib and 7.9 ± 2.5 and 11.8 ± 1.9 nM, respectively, for anacetrapib).
Surprisingly, dalcetrapib at concentrations up to 10 μM did not affect homotypic CE transfer from HDL3 to HDL2. Such transfer was potently inhibited by torcetrapib and anacetrapib at concentrations equal to and higher than 0.1 μM, suggesting that dalcetrapib, by not interfering with transfer among HDL subparticles, is more likely a modulator than an inhibitor of CETP activity and that dalcetrapib may interact with and decrease CETP activity by a unique mechanism.
Competitive binding studies showed that torcetrapib and anacetrapib decreased binding of radiolabeled [14C]torcetrapib to CETP, whereas dalcetrapib, dalcetrapib-thiol, or dalcetrapib-disulfide had no, or only a marginal, effect. Conversely [14C]dalcetrapib-thiol bound to CETP was displaced in the presence of reducing agent by dalcetrapib and dalcetrapib-thiol but not by torcetrapib and anacetrapib.
SPR investigations confirmed that the binding of dalcetrapib-thiol to the CETP C13S mutant was negligible when compared with rhCETP (see supplementary ). As reported, a single peptide with dalcetrapib-thiol bound to Cys13 was identified by mass spectrometry (data not shown). The binding of dalcetrapib-thiol to Cys13 could prevent its potential critical interaction with Cys1 and/or Cys333 as observed by Hope et al. (42).
As expected, torcetrapib was shown to interact similarly with rhCETP and the C13S CETP mutant, excluding a potential interaction with Cys13 as a major mechanism associated with CETP inhibition by torcetrapib and torcetrapib-related compounds. The monitoring of binding interactions, kinetic rate constants, and thermodynamic parameters in real time by SPR further confirmed this finding, inasmuch as following saturation of the dalcetrapib binding site (see supplementary ) the binding of torcetrapib remained unchanged.
We observed a decreased binding affinity of the anti-CETP monoclonal antibody JHC-1 to CETP incubated with dalcetrapib-thiol, as judged by SPR. Because JHC-1 does not bind to dalcetrapib-thiol, and does not bind to any cysteine-containing polypeptide of CETP, as judged by SPR (see supplementary Table IIC), these results support the hypothesis that the binding of dalcetrapib changes exposure of the epitope recognized by the JHC-1 antibody. An ELISA using JHC-1 as a capture antibody confirmed a marked decrease in the detection of this epitope in human plasma incubated with increasing concentrations of dalcetrapib (0.01 µM to 10 µM), with a maximum effect reached at 0.5 µM. The relevance of this finding to the clinical situation was examined using plasma samples from 22 healthy volunteers who each received a single oral dose of 600 mg dalcetrapib. A highly significant decrease in the detection of the JHC-1-specific epitope was observed at 4, 6, 8, and 12 h postdose at plasma levels of dalcetrapib-thiol between 200 and 700 ng/ml (0.63 µM to 2.2 µM). These observations support the hypothesis of a change in conformation of CETP upon binding of dalcetrapib-thiol to Cys13 in vitro and in vivo at therapeutic concentrations. It should be noted that in spite of the covalent nature of the binding of dalcetrapib-thiol to CETP and potentially to other plasma proteins (43), the compound is cleared with a relatively short half life (t1/2 25.5 h), and the resulting decrease in CETP activity and change in conformation are only transient. The results indicate that the covalent binding of dalcetrapib is weak and fully reversible. The observed correlation between the change in conformation and the decrease in CETP activity suggests that dalcetrapib-thiol binding to CETP could impair or limit the changes in conformation needed for the optimal activity of CETP, i.e., in accordance with the model of Bruce, Beamer, and Tall (44) or the specific increase in curvature suggested by Qiu et al. (16) to be required for binding to VLDL.
Although potent or nonselective CETP inhibitors may affect transfer among all lipoproteins and subparticle classes, the dalcetrapib-dependent conformational change of CETP may lead to a modulation of CETP activity, decreasing neutral lipid transfer between HDL and LDL (heterotypic transfer) while preserving transfer between other lipoprotein classes, such as between HDL3 and HDL2 (homotypic transfer) (45). Change in CETP conformation induced by dalcetrapib could also explain the subsequent decrease in the binding of the torcetrapib analog by hindrance of its binding site under the experimental conditions used by Cunningham et al. (46).
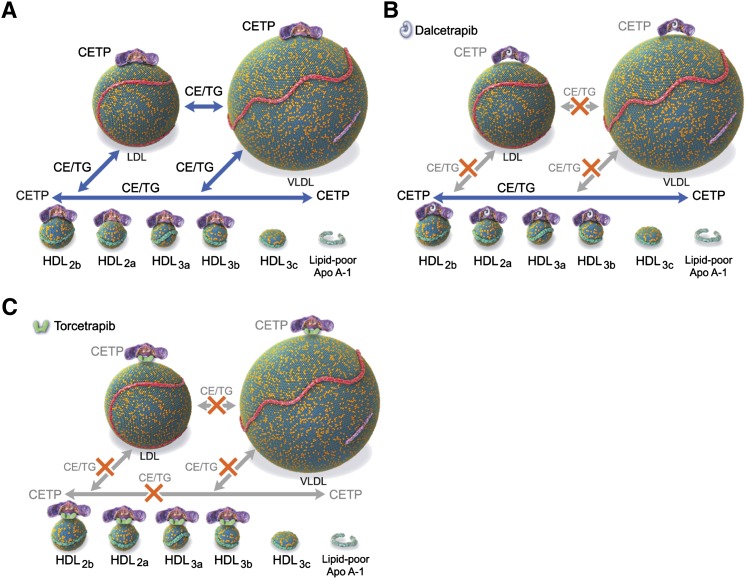
Of the seven cysteines of human CETP, only two are involved in stable S-S bond formation (Cys143 and Cys184). The presence of the five additional cysteines suggests that some might be involved in the allosteric regulation of CETP activity. Indeed, it has been demonstrated that modification of the N-terminal Cys1 of CETP is involved in the selective inhibition of triglyceride versus CE transfer activity (47); on the contrary, selective inhibition of CE transfer can be achieved by compounds binding to Cys333 (41). These data suggest that both Cys1 and Cys333 are involved in the selective transfer of neutral lipids. We may hypothesize, therefore, that Cys13 plays a role different from that of either Cys1 or Cys333 in modulating CETP activity by facilitating or preventing the transfer of neutral lipids between lipoproteins of selective size and/or composition, as illustrated in Fig. 6.
Fig. 6.
Schematic depicting action of CETP and proposed effects of dalcetrapib and torcetrapib. A: CETP transfers neutral lipids [cholesteryl ester (CE) and triglycerides (TGs)] among HDL and HDL subparticles and changes conformation to accommodate LDL and VLDL. B: Dalcetrapib prevents the change in conformation required for transfer activity to LDL and VLDL, leaving unaffected CETP-mediated exchange among HDL subparticles. C: Torcetrapib increases binding affinity of CETP for lipoproteins, decreasing CE and TG exchange between lipoproteins, including among HDL subparticles.
CETP-induced pre-beta-HDL formation
In addition to its well-established role in the transfer of neutral lipids from HDL to apoB-containing lipoproteins, CETP has been shown in experiments using a purified HDL fraction (i.e., in the absence of LDL and VLDL) to be involved in lipid transfer among HDL particles, and also to play an important role in HDL remodeling and the generation of pre-β-HDL (17, 18, 48, 49). The increase in pre-β-HDL, measured by a specific and sensitive ELISA detecting a specific epitope exposed only in pre-β1-HDL (37) following incubation of human plasma, is most likely generated by endogenous CETP, inasmuch as it is abolished by the two CETP inhibitors torcetrapib and anacetrapib. A similar inhibition of pre-β-HDL formation using the monoclonal antibody TP1 to inhibit CETP activity was first described by Lagrost et al. (49), albeit using a purified HDL fraction.
Surprisingly, in contrast to torcetrapib and anacetrapib, dalcetrapib up to 10 µM did not prevent pre-β-HDL formation produced by endogeneous CETP but significantly increased pre-β-HDL generation. Pre-β-HDL formation induced by rhCETP (4 to 25 times normal CETP level) added to human plasma was also dose-dependently and markedly decreased by torcetrapib and anacetrapib, whereas such an effect was not observed with dalcetrapib. These differences could be linked to the strong inhibitory effect of torcetrapib and anacetrapib on CE transfer from HDL3 to HDL2, which is absent with dalcetrapib.
Phospholipid transfer protein (PLTP) has also been shown to play an important role in HDL remodeling (50). Because dalcetrapib (data not shown) and torcetrapib (14) neither inhibit nor increase PLTP activity at concentrations up to 30 μM, we conclude that in human plasma CETP is a major component of HDL remodeling and that this process is highly sensitive to nonselective or potent CETP inhibitors.
Our findings suggest that, in contrast to both torcetrapib and anacetrapib, dalcetrapib may behave like a direct CETP modulator (20, 51) in not affecting CETP activity among HDL particles and preserving pre-β-HDL formation, thus, potentially maintaining or enhancing the first step of RCT.
To determine the potential biological relevance of the biochemical differences described above, the impact of the three agents affecting CETP activity on macrophage RCT was assessed in vivo using a hamster model (27) derived from the well-characterized mouse model (26, 52). In contrast to mice, hamsters express CETP endogenously and have been used previously to study the effect of CETP inhibition on plasma lipoproteins (53). We further developed this model by using in vivo [3H]cholesterol-labeled autologous hamster macrophages.
Although dalcetrapib provided a relatively smaller increase in HDL-C than did torcetrapib and anacetrapib, it was the most effective in promoting fecal excretion of macrophage [3H]cholesterol, mainly as bile acids. In addition, only dalcetrapib was shown to increase total fecal bile acid mass, which may be due to a higher turnover of HDL-C. Indeed, Schwartz et al. (54) showed in humans that HDL-C is preferentially used for bile acid synthesis, whereas Nanjee and coworkers showed that infusion of apoA-I in man generates pre-β-HDL and increases bile acid elimination (55). This is the first report, to our knowledge, in which an agent targeting CETP activity is shown to affect macrophage RCT. In accordance with previous studies of torcetrapib (27), a weak increase in RCT was observed, whereas we found that anacetrapib treatment had no effect, despite being associated with the greatest increase in HDL-C levels. In addition, a dose-response study with dalcetrapib confirmed the absence of any correlation between [3H]HDL-C levels and fecal elimination of labeled sterols (data not shown).
These findings are consistent with the hypothesis that direct modulation of CETP activity by dalcetrapib increased RCT in vivo by maintaining pre-β-HDL formation and CE transfer between HDL subparticles, thus preserving the first step of RCT: efflux from peripheral tissues via the ABCA1 transporter. Interestingly, it has been proposed by Morton and Greene (51) that apoF acts as a physiologic CETP modulator by preferentially diminishing transfer events involving LDL, especially those between VLDL and LDL, and indirectly enhancing the ability of CETP to remove CE from HDL subparticles and to remodel HDL. For these reasons, the authors suggested that modulation of CETP activity by apoF could be a very efficient way to increase RCT (20, 51).
In summary, we have demonstrated that compounds that decrease CETP activity, derived from two different chemical classes, have different binding sites to CETP and differentially affect homotypic and heterotypic CE transfer. In addition, we have shown that dalcetrapib induces a specific conformational change of CETP not observed with torcetrapib. These different modes of action have opposing effects on the in vitro process of HDL remodeling and pre-β-HDL generation, which is important in HDL-induced RCT and may explain the stronger effect of dalcetrapib on in vivo RCT in hamsters. These results suggest that compounds originally described as CETP inhibitors can be differentiated into CETP modulators such as dalcetrapib, and CETP inhibitors exemplified by torcetrapib. The clinical relevance of these in vitro properties, and the differences observed in macrophage RCT in hamsters, remains to be determined. The ongoing Phase III placebo-controlled dal-OUTCOMES study (13) evaluating the effect of 600 mg dalcetrapib in clinically stable patients (n = 15,600) following a recent acute coronary syndrome will provide more information as to the clinical significance of modulation of CETP activity by dalcetrapib.
Supplementary Material
Acknowledgments
The authors thank the following colleagues from F. Hoffmann-La Roche Ltd for their exceptional scientific expertise, assistance, and support: Marie Brousse, Arno Friedlein, Christophe Gardes, Cyrill Mangold, Stefan Obermüller, Anne Perez, Gabriele Raphael, Bernard Rutten, Heidi Stoll, Andrea Wiget, Doris Zulauf, Andrea Stauffer, Gonzalo Duran Pacheco, and the Roche Protein Sciences Group. The authors also thank Dr. D. Bentley and Dr. O. Kuhlman for providing the Phase I plasma samples. Editorial assistance was provided by Teresa Haigh and Carl Felton (Prime Healthcare).
Footnotes
Abbreviations:
- apo
- apolipoprotein
- BID
- twice daily
- CE
- cholesteryl ester
- CETP
- cholesteryl ester transfer protein
- CV
- cardiovascular
- Cys13
- cysteine 13
- HDL-C
- HDL-cholesterol
- LDLR
- LDL receptor
- PLTP
- phospholipid transfer protein
- QD
- once daily
- RCT
- reverse cholesterol transport
- rh
- recombinant human
- SEC
- size exclusion chromatography
- SPR
- surface plasmon resonance
- SR-BI
- scavenger receptor class B type I
- TCEP
- tris(2-carboxyethyl)phosphine
These studies were supported by F. Hoffmann-La Roche Ltd and Japan Tobacco, Inc.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of three figures and three tables.
REFERENCES
- 1.Gordon T., Castelli W. P., Hjortland M. C., Kannel W. B., Dawber T. R. 1977. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am. J. Med. 62: 707–714. [DOI] [PubMed] [Google Scholar]
- 2.Neeli H., Rader D. J. 2008. Cholesteryl ester transfer protein (CETP) inhibitors: is there life after torcetrapib? Cardiol. Clin. 26: 537–546. [DOI] [PubMed] [Google Scholar]
- 3.Clark R. W., Sutfin T. A., Ruggeri R. B., Willauer A. T., Sugarman E. D., Magnus-Aryitey G., Cosgrove P. G., Sand T. M., Wester R. T., Williams J. A., et al. 2004. Raising high-density lipoprotein in humans through inhibition of cholesteryl ester transfer protein: an initial multidose study of torcetrapib. Arterioscler. Thromb. Vasc. Biol. 24: 490–497. [DOI] [PubMed] [Google Scholar]
- 4.Krishna R., Bergman A. J., Jin B., Fallon M., Cote J., Van Hoydonck P., Laethem T., Gendrano I. N., III, Van Dyck K., Hilliard D., et al. 2008. Multiple-dose pharmacodynamics and pharmacokinetics of anacetrapib, a potent cholesteryl ester transfer protein (CETP) inhibitor, in healthy subjects. Clin. Pharmacol. Ther. 84: 679–683. [DOI] [PubMed] [Google Scholar]
- 5.Barter P. J., Caulfield M., Eriksson M., Grundy S. M., Kastelein J. J., Komajda M., Lopez-Sendon J., Mosca L., Tardif J. C., Waters D. D., et al. 2007. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 357: 2109–2122. [DOI] [PubMed] [Google Scholar]
- 6.Hu X., Dietz J. D., Xia C., Knight D. R., Loging W. T., Smith A. H., Yuan H., Perry D. A., Keiser J. 2009. Torcetrapib induces aldosterone and cortisol production by an intracellular calcium-mediated mechanism independently of cholesteryl ester transfer protein inhibition. Endocrinology. 150: 2211–2219. [DOI] [PubMed] [Google Scholar]
- 7.Stroes E. S. G., Kastelein J. J. P., Bernardeau A., Kuhlmann O., Blum D., Campos L. A., Clerc R. G., Niesor E. J. 2009. Dalcetrapib: no off-target toxicity on blood pressure or renin-angiotensin aldosterone system-related genes in rats. Br. J. Pharmacol. 158: 1763–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forrest M. J., Bloomfield D., Briscoe R. J., Brown P. N., Cumiskey A. M., Ehrhart J., Hershey J. C., Keller W. J., Ma X., McPherson H. E., et al. 2008. Torcetrapib-induced blood pressure elevation is independent of CETP inhibition and is accompanied by increased circulating levels of aldosterone. Br. J. Pharmacol. 154: 1465–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okamoto H., Yonemori F., Wakitani K., Minowa T., Maeda K., Shinkai H. 2000. A cholesteryl ester transfer protein inhibitor attenuates atherosclerosis in rabbits. Nature. 406: 203–207. [DOI] [PubMed] [Google Scholar]
- 10.Shinkai H. 2009. Cholesteryl ester transfer protein inhibitors as high-density lipoprotein raising agents. Expert Opin. Ther. Pat. 19: 1229–1237. [DOI] [PubMed] [Google Scholar]
- 11.Connolly D. T., Heuvelman D., Glenn K. 1996. Inactivation of cholesteryl ester transfer protein by cysteine modification. Biochem. Biophys. Res. Commun. 223: 42–47. [DOI] [PubMed] [Google Scholar]
- 12.Stein E. A., Stroes E. S., Steiner G., Buckley B. M., Capponi A. M., Burgess T., Niesor E. J., Kallend D., Kastelein J. J. 2009. Safety and tolerability of dalcetrapib. Am. J. Cardiol. 104: 82–91. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz G.G., Olsson A.G., Ballantyne C.M., Barter P.J., Holme I.M., Kallend D., Leiter L.A., Leitersdorf E., McMurray J.J.V., Shah P.K., et al. 2009. Rationale and design of the dal-OUTCOMES trial: efficacy and safety of dalcetrapib in patients with recent acute coronary syndrome. Am. Heart J. 158: 896–901. [DOI] [PubMed] [Google Scholar]
- 14.Clark R. W., Ruggeri R. B., Cunningham D., Bamberger M. J. 2006. Description of the torcetrapib series of cholesteryl ester transfer protein inhibitors, including mechanism of action. J. Lipid Res. 47: 537–552. [DOI] [PubMed] [Google Scholar]
- 15.Swenson T. L., Hesler C. B., Brown M. L., Quinet E., Trotta P. P., Haslanger M. F., Gaeta F. C., Marcel Y. L., Milne R. W., Tall A. R. 1989. Mechanism of cholesteryl ester transfer protein inhibition by a neutralizing monoclonal antibody and mapping of the monoclonal antibody epitope. J. Biol. Chem. 264: 14318–14326. [PubMed] [Google Scholar]
- 16.Qiu X., Mistry A., Ammirati M. J., Chrunyk B. A., Clark R. W., Cong Y., Culp J. S., Danley D. E., Freeman T. B., Geoghegan K. F., et al. 2007. Crystal structure of cholesteryl ester transfer protein reveals a long tunnel and four bound lipid molecules. Nat. Struct. Mol. Biol. 14: 106–113. [DOI] [PubMed] [Google Scholar]
- 17.Kunitake S. T., Mendel C. M., Hennessy L. K. 1992. Interconversion between apolipoprotein A-I-containing lipoproteins of pre-beta and alpha electrophoretic mobilities. J. Lipid Res. 33: 1807–1816. [PubMed] [Google Scholar]
- 18.Rye K. A., Barter P. J. 2004. Formation and metabolism of prebeta-migrating, lipid-poor apolipoprotein A-I. Arterioscler. Thromb. Vasc. Biol. 24: 421–428. [DOI] [PubMed] [Google Scholar]
- 19.Ha Y. C., Gorjatschko L., Barter P. J. 1983. Changes in the density distribution of pig high density lipoproteins during incubation in vitro. Influence of esterified cholesterol transfer activity. Atherosclerosis. 48: 253–263. [DOI] [PubMed] [Google Scholar]
- 20.Paromov V. M., Morton R. E. 2003. Lipid transfer inhibitor protein defines the participation of high-density lipoprotein subfractions in lipid transfer reactions mediated by cholesterol ester transfer protein (CETP). J. Biol. Chem. 278: 40859–40866. [DOI] [PubMed] [Google Scholar]
- 21.Sacks F. M., Rudel L. L., Conner A., Akeefe H., Kostner G., Baki T., Rothblat G., de la Llera-Moya M., Asztalos B., Perlman T., et al. 2009. Selective delipidation of plasma HDL enhances reverse cholesterol transport in vivo. J. Lipid Res. 50: 894–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tall A. R., Yvan-Charvet L., Terasaka N., Pagler T., Wang N. 2008. HDL, ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metab. 7: 365–375. [DOI] [PubMed] [Google Scholar]
- 23.Collet X., Tall A. R., Serajuddin H., Guendouzi K., Royer L., Oliveira H., Barbaras R., Jiang X. C., Francone O. L. 1999. Remodeling of HDL by CETP in vivo and by CETP and hepatic lipase in vitro results in enhanced uptake of HDL CE by cells expressing scavenger receptor B-I. J. Lipid Res. 40: 1185–1193. [PubMed] [Google Scholar]
- 24.Tanigawa H., Billheimer J. T., Tohyama J., Zhang Y., Rothblat G., Rader D. J. 2007. Expression of cholesteryl ester transfer protein in mice promotes macrophage reverse cholesterol transport. Circulation. 116: 1267–1273. [DOI] [PubMed] [Google Scholar]
- 25.Brousseau M. E., Diffenderfer M. R., Millar J. S., Nartsupha C., Asztalos B. F., Welty F. K., Wolfe M. L., Rudling M., Björkhem I., Angelin B., et al. 2005. Effects of cholesteryl ester transfer protein inhibition on high-density lipoprotein subspecies, apolipoprotein A-I metabolism, and fecal sterol excretion. Arterioscler. Thromb. Vasc. Biol. 25: 1057–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rader D. J., Alexander E. T., Weibel G. L., Billheimer J., Rothblat G. H. 2009. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J. Lipid Res. 50(Suppl.): 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tchoua U., D'Souza W., Mukhamedova N., Blum D., Niesor E., Mizrahi J., Maugeais C., Sviridov D. 2008. The effect of cholesteryl ester transfer protein overexpression and inhibition on reverse cholesterol transport. Cardiovasc. Res. 77: 732–739. [DOI] [PubMed] [Google Scholar]
- 28.Roy P., MacKenzie R., Hirama T., Jiang X-C., Kussie P., Tall A., Rassart E., Milne R. 1996. Structure-function relationships of human cholesteryl ester transfer protein: analysis using monoclonal antibodies. J. Lipid Res. 37: 22–34. [PubMed] [Google Scholar]
- 29.Tollefson J. H., Albers J. J. 1986. Isolation, characterization, and assay of plasma lipid transfer proteins. Methods Enzymol. 129: 797–816. [DOI] [PubMed] [Google Scholar]
- 30.Connolly D. T., Witherbee B. J., Melton M. A., Durley R. C., Grapperhaus M. L., McKinnis B. R., Vernier W. F., Babler M. A., Shieh J. J., Smith M. E., et al. 2000. Stereospecific inhibition of CETP by chiral N,N-disubstituted trifluoro-3-amino-2-propanols. Biochemistry. 39: 13870–13879. [DOI] [PubMed] [Google Scholar]
- 31.Weinberg R. B., Cook V. R., Jones J. B., Kussie P., Tall A. R. 1994. Interfacial properties of recombinant human cholesterol ester transfer protein. J. Biol. Chem. 269: 29588–29591. [PubMed] [Google Scholar]
- 32.Ohnishi T., Yokoyama S., Yamamoto A. 1990. Rapid purification of human plasma lipid transfer proteins. J. Lipid Res. 31: 397–406. [PubMed] [Google Scholar]
- 33.Davis H. L., Weeratna R., Waldschmidt T. J., Tygrett L., Schorr J., Krieg A. M. 1998. CpG DNA is a potent enhancer of specific immunity in mice immunized with recombinant hepatitis B surface antigen. J. Immunol. 160: 870–876. [PubMed] [Google Scholar]
- 34.Köhler G., Milstein C. 1975. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 256: 495–497. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi H., Takahashi A., Maki M., Sasai H., Kamada M. 2001. Effect of CETP on the plasma lipoprotein profile in four strains of transgenic mouse. Biochem. Biophys. Res. Commun. 283: 118–123. [DOI] [PubMed] [Google Scholar]
- 36.Derks M., Fowler S., Kuhlmann O. 2009. A single-center, open-label, one-sequence study of dalcetrapib coadministered with ketoconazole, and an in vitro study of the S-methyl metabolite of dalcetrapib. Clin. Ther. 31: 586–599. [DOI] [PubMed] [Google Scholar]
- 37.Miida T., Miyazaki O., Nakamura Y., Hirayama S., Hanyu O., Fukamachi I., Okada M. 2003. Analytical performance of a sandwich enzyme immunoassay for pre beta1-HDL in stabilized plasma. J. Lipid Res. 44: 645–650. [DOI] [PubMed] [Google Scholar]
- 38.Kleinman Y., Halperin G., Stein O., Stein Y. 1982. Long-lived labeling of phagocytic cells with analogs of atheroma lipids. Atherosclerosis. 43: 1–6. [DOI] [PubMed] [Google Scholar]
- 39.Folch J., Lees M., Sloane Stanley G. H. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226: 497–509. [PubMed] [Google Scholar]
- 40.Naik S. U., Wang X., Da Silva J. S., Jaye M., Macphee C. H., Reilly M. P., Billheimer J. T., Rothblat G. H., Rader D. J. 2006. Pharmacological activation of liver X receptors promotes reverse cholesterol transport in vivo. Circulation. 113: 90–97. [DOI] [PubMed] [Google Scholar]
- 41.Epps D. E., Vosters A. F. 2002. The essential role of a free sulfhydryl group in blocking the cholesteryl site of cholesteryl ester transfer protein (CETP). Chem. Phys. Lipids. 114: 113–122. [DOI] [PubMed] [Google Scholar]
- 42.Hope H. R., Heuvelman D., Duffin K., Smith C., Zablocki J., Schilling R., Hegde S., Lee L., Witherbee B., Baganoff M., et al. 2000. Inhibition of cholesteryl ester transfer protein by substituted dithiobisnicotinic acid dimethyl ester: involvement of a critical cysteine. J. Lipid Res. 41: 1604–1614. [PubMed] [Google Scholar]
- 43.Ranalletta M., Bierilo K. K., Chen Y., Milot D., Chen Q., Tung E., Houde C., Elowe N. H., Garcia-Calvo M., Porter G., et al. 2010. Biochemical characterization of cholesteryl ester transfer protein inhibitors. J. Lipid Res. 51: 2739–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bruce C., Beamer L. J., Tall A. R. 1998. The implications of the structure of the bactericidal/permeability-increasing protein on the lipid-transfer function of the cholesteryl ester transfer protein. Curr. Opin. Struct. Biol. 8: 426–434. [DOI] [PubMed] [Google Scholar]
- 45.Connolly D. T., McIntyre J., Heuvelman D., Remsen E. E., McKinnie R. E., Vu L., Melton M., Monsell R., Krul E. S., Glenn K. 1996. Physical and kinetic characterization of recombinant human cholesteryl ester transfer protein. Biochem. J. 390: 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cunningham D., Lin W., Hoth L. R., Danley D. E., Ruggeri R. B., Geoghegan K. F., Chrunyk B. A., Boyd J. G. 2008. Biophysical and biochemical approach to locating an inhibitor binding site on cholesteryl ester transfer protein. Bioconjug. Chem. 19: 1604–1613. [DOI] [PubMed] [Google Scholar]
- 47.Kotake H., Agellon L. B., Yokoyama S. 1997. Modification of the N-terminal cysteine of plasma cholesteryl ester transfer protein selectively inhibits triglyceride transfer activity. Biochim. Biophys. Acta. 1347: 69–74. [DOI] [PubMed] [Google Scholar]
- 48.Barter P. J. 2002. Hugh Sinclair lecture: the regulation and remodelling of HDL by plasma factors. Atheroscler. Suppl. 3: 39–47. [DOI] [PubMed] [Google Scholar]
- 49.Lagrost L., Gambert P., Dangremont V., Athias A., Lallemant C. 1990. Role of cholesteryl ester transfer protein (CETP) in the HDL conversion process as evidenced by using anti-CETP monoclonal antibodies. J. Lipid Res. 31: 1569–1575. [PubMed] [Google Scholar]
- 50.Rye K. A., Jauhiainen M., Barter P. J., Ehnholm C. 1998. Triglyceride-enrichment of high density lipoproteins enhances their remodelling by phospholipid transfer protein. J. Lipid Res. 39: 613–622. [PubMed] [Google Scholar]
- 51.Morton R. E., Greene D. J. 1994. Regulation of lipid transfer between lipoproteins by an endogenous plasma protein: selective inhibition among lipoprotein classes. J. Lipid Res. 35: 836–847. [PubMed] [Google Scholar]
- 52.Zhang Y., Zanotti I., Reilly M. P., Glick J. M., Rothblat G. H., Rader D. J. 2003. Overexpression of apolipoprotein A-I promotes reverse transport of cholesterol from macrophages to feces in vivo. Circulation. 108: 661–663. [DOI] [PubMed] [Google Scholar]
- 53.Zuckerman S. H., Evans G. F. 1995. Cholesteryl ester transfer protein inhibition in hypercholesterolemic hamsters: kinetics of apoprotein changes. Lipids. 30: 307–311. [DOI] [PubMed] [Google Scholar]
- 54.Schwartz C. C., Halloran L. G., Vlahcevic Z. R., Gregory D. H., Swell L. 1978. Preferential utilization of free cholesterol from high-density lipoproteins for biliary cholesterol secretion in man. Science. 200: 62–64. [DOI] [PubMed] [Google Scholar]
- 55.Nanjee M. N., Cooke C. J., Garvin R., Semeria F., Lewis G., Olszewski W. L., Miller N. E. 2001. Intravenous apoA-I/lecithin discs increase pre-β-HDL concentration in tissue fluid and stimulate reverse cholesterol transport in humans. J. Lipid Res. 42: 1586–1593. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.