Abstract
Cidea, the cell death-inducing DNA fragmentation factor-α-like effector (CIDE) domain-containing protein, is targeted to lipid droplets in mouse adipocytes, where it inhibits triglyceride hydrolysis and promotes lipid storage. In mice, Cidea may prevent lipolysis by binding and shielding lipid droplets from lipase association. Here we demonstrate that human Cidea localizes with lipid droplets in both adipocyte and nonadipocyte cell lines, and we ascribe specific functions to its protein domains. Expression of full-length Cidea in undifferentiated 3T3-L1 cells or COS-1 cells increases total cellular triglyceride and strikingly alters the morphology of lipid droplets by enhancing their size and reducing their number. Remarkably, both lipid droplet binding and increased triglyceride accumulation are also elicited by expression of only the carboxy-terminal 104 amino acids, indicating this small domain directs lipid droplet targeting and triglyceride shielding. However, unlike the full-length protein, expression of the carboxy-terminus causes clustering of small lipid droplets but not the formation of large droplets, identifying a novel function of the N terminus. Furthermore, human Cidea promotes lipid storage via lipolysis inhibition, as the expression of human Cidea in fully differentiated 3T3-L1 adipocytes causes a significant decrease in basal glycerol release. Taken together, these data indicate that the carboxy-terminal domain of Cidea directs lipid droplet targeting, lipid droplet clustering, and triglyceride accumulation, whereas the amino terminal domain is required for Cidea-mediated development of enlarged lipid droplets.
Keywords: adipocytes, triglycerides, lipase/lipoprotein
An important mechanism to survive cycles of nutrient scarcity and abundance is the ability to sequester energy stores and readily access them. One efficient method of storing excess energy is the conversion of nutrients into triglyceride, which provides 9,300 kcalories of energy per kilogram of fatty acid (1). The major triglyceride storage site in mammals is adipose tissue, which expresses many lipid-metabolizing enzymes and is able to accumulate large amounts of lipid without causing cellular toxicity. However, under conditions where adipose tissue becomes deficient in the ability to store triglyceride, fatty acids may accumulate in peripheral tissues, such as muscle and liver, causing lipotoxicity and metabolic perturbations (2–4). This phenomenon is commonly associated with obesity, in which enlarged adipocytes exhibit low triglyceride synthetic capability. Obesity is now a global health epidemic as are its associated medical disorders, including type 2 diabetes, hypertension, dyslipidemia, and atherosclerosis (5, 6). Understanding how adipocytes regulate fatty acid storage and release is important in identifying how obesity causes these serious metabolic abnormalities.
The adipose tissue stores triglyceride and other neutral lipids in lipid droplets that initially emanate from the endoplasmic reticulum within adipocytes and enlarge as fat is deposited. Such lipid droplets are composed of a neutral lipid core surrounded by a phospholipid monolayer and associated proteins (7, 8). The best characterized lipid droplet-associated protein is perilipin, which shares sequence similarity with two other lipid droplet-associated proteins, adipophilin/adipocyte differentiation-related protein (Adrp) and tail-interacting protein 47 (Tip47) (9, 10). Together, these proteins compose the PAT (perilipin-adipophilin-Tip47) family of proteins, which are involved in promoting triglyceride storage. Perilipin, however, is unique among the PAT proteins, because it is also required for triglyceride hydrolysis in response to β-adrenergic stimuli (11–13). The PAT family now includes other lipid droplet-associated proteins, such as S3-12 and Oxpat (14, 15). In addition to the PAT family of proteins, there have been many proteins found associated with lipid droplets, including the CIDE family [Cidea, Cideb, and Cidec (human); Fsp27 (mouse)]; caveolin 1; SNARE proteins; various lipid-synthesizing enzymes, lipases (Hsl and Atgl); the RAB family of GTPases; and the small GTPase Arf1 (10, 16–28). This growing list of lipid droplet-associated proteins includes members that regulate lipid droplet formation and movement and control lipid storage and turnover. Importantly, through lipolysis, lipid droplets provide fatty acids for energy production, phospholipid and membrane biosynthesis, and cholesterol for membrane rigidity. They may also act as a depot for proteins involved in transcription and apoptosis (7, 29).
Not only do lipid droplet proteins regulate many cellular processes, but they also dramatically regulate whole body processes, such as energy homeostasis. For example, Perilipin−/−, Cidea−/−, and Fsp27−/− mice are lean and insulin-sensitive on a high-fat diet. These mice have lower intracellular triglyceride levels and white adipose tissue weight, but they have increased fatty acid oxidation rates (11, 30, 31). Thus, these proteins may promote obesity and insulin resistance by increasing fat storage and decreasing fat oxidation in mice. Nordstrom et al. found that small interfering RNA (siRNA)-mediated depletion of Cidea in human preadipocytes resulted in increased lipolysis, supporting the idea that, similar to mouse Cidea, human Cidea enhances adipocyte lipid storage (32). In obese humans, however, lipid droplet protein levels in adipose tissue appear to correlate with increased insulin sensitivity. For example, Puri et al. found that in body mass index (BMI)-matched obese patients, Cidea, Cidec, and perilipin expression was elevated in adipose tissue of subjects with low homeostatic model assessment of insulin resistance (HOMA-IR) scores (increased insulin sensitivity) (18). Therefore, these proteins may protect obese humans from developing insulin resistance by promoting the sequestration of fat into adipocyte lipid droplets, thereby preventing lipotoxicity in peripheral tissues.
Due to the impact that Cidea has on whole body metabolism in mice and possibly humans, it is important to understand how Cidea regulates adipocyte lipid storage. In the present study, we demonstrate that human Cidea reduces lipolysis, increases triglyceride accumulation, and associates with lipid droplets in adipocytes as well as other cell types by a mechanism that is directed by its carboxy-terminal domain. Additionally, Cidea expression in COS cells or in preadipocytes simultaneously induces the appearance of large lipid droplets and reduces the number of lipid droplets. This change in lipid droplet morphology is dependent on both the amino and carboxy-terminal domains. Thus, the various functions of Cidea to target to lipid droplets, increase triglyceride deposition, and promote the development of very large lipid droplets can be ascribed to specific domains of the protein.
MATERIALS AND METHODS
Materials
Rabbit anti-human calreticulin IgG was purchased from Calbiochem. Mouse anti-hemagglutinin (HA)-epitope IgG (monoclonal HA.11) was purchased from Covance. Alexa 568 donkey anti-rabbit IgG, Alexa 488 chicken anti-mouse IgG, and Prolong Gold anti-fade reagent were purchased from Invitrogen. The glycerol and triglyceride determination kit and Oil Red O were purchased from Sigma.
Plasmids
The Cidea cDNA was purchased from Open Biosystems. PCR was performed to generate DNA encoding a full-length or truncated product by using a 5′-linker containing a Bgl2 restriction site and a 3′-linker containing a Sal1 site. After cutting with the restriction enzymes, the purified PCR fragments were cloned into pEGFPC1 vector (Clontech). For the generation of HA-tagged proteins, PCR was performed using a 5′-linker containing an Mlu1 restriction site and a 3′-linker containing a Sal1 site. After cleavage with the restriction enzymes, the purified PCR fragments were cloned into the 3XHA-pCMV5 (F3) vector. This vector was generated from the pCMV5 vector (gift from Dr. David Russel, Southwestern Medical Center, Dallas, TX) by inserting a linker containing the DNA sequences for a 3 HA tag at the EcoR1 and Mlu1 sites.
Cell culture and electroporation
Cells were cultured in DMEM supplemented with 10% FBS, 50 μg/ml streptomycin, and 50 units/ml penicillin (33). For experiments performed in preadipocytes, 2.5 × 106 cells were electroporated with 8 μg of plasmid DNA. The electroporation was performed using a Bio-Rad gene pulser II at the setting of 0.18 kV and 960 microfarads. Immediately after electroporation, the cells were reseeded into 1 well of a 6-well plate. For experiments in mature adipocytes, fibroblasts were cultured for eight days, differentiated into mature adipocytes by adding differentiation media (2.5 μg/ml insulin, 0.25 μM dexamethasone, and 0.5 mM isobutylmethylxanthine in the culture media described above) for 72 h, then cultured for an additional 24–48 h. Adipocytes were then electroporated as described above using 5 × 106 cells and 15 μg of plasmid DNA. After electroporation, cells were reseeded into 1 well of a 6-well plate. For experiments in COS-7 cells, transfection was performed using TransIT-LT1 transfection reagent (Mirus Bio) according to the manufacturer's instructions.
Oil Red O staining for intracellular triglycerides
To stain neutral lipids, cells were first washed with PBS and then fixed in 4% formaldehyde solution in PBS (1 h). After three PBS washes, the cells were stained with Oil Red O solution (5 mg/ml Oil Red O solid dissolved in isopropanol, then diluted to a 60% working solution with ddH20 and filtered through a 0.45 μm filter) for 30 min at room temperature, followed by six washes with water.
Immunofluorescence
Cells were washed with PBS and fixed in 4% paraformaldehyde in PBS (1 h), then washed three times with PBS, permeabilized with 0.05% Triton X-100 in PBS containing 1% FBS (15 min), and incubated overnight at 4°C with primary antibody against HA or calreticulin in permeabilization buffer. Cells were then washed, labeled with secondary antibody, and washed again with permeabilization buffer before mounting on slides using Prolong Gold (Invitrogen).
Confocal microscopy
Images were taken on a Zeiss Axiophot microscope equipped with a Hamamatsu digital camera and processed using Metamorph imaging software, version 6.1 (Universal Imaging, Downingtown, PA).
Fluorescence-activated cell sorting (FACS) analysis
Green-fluorescent protein (GFP) positive cells were sorted on a BD Biosciences (San Jose, CA) FACS Vantage DiVa Cell Sorter. The acquisition software used was FACS DiVa 6.0.
Triglyceride determination
Cells were transfected as described above, and GFP positive cells were sorted by FACS. The cells were then lysed with water, sonicated, and quantitated for triglyceride using the Triglyceride Determination Kit (Sigma) according to the manufacturer's instructions.
Measurement of glycerol release
3T3-L1 cells were grown and differentiated in 12-well plates. At day four after differentiation, HA-control or Cidea adenovirus (1013 viral particles/well) was added. After 44–48 h, the cells were washed three times in PBS, and then incubated in either phenol red-free DMEM or Krebs-Ringer Hepes buffer supplemented with 4% BSA. Glycerol content in the medium was determined after 2.5 h using the free glycerol determination kit (Sigma Chemical) according to the manufacturer's protocol.
Statistical analysis
Quantitative data is represented as mean ± SEM. For statistical analysis, the differences between groups were examined with Student's paired t-test. P < 0.05 was considered statistically significant.
RESULTS
The carboxy-terminus of human Cidea is necessary and sufficient for lipid droplet targeting
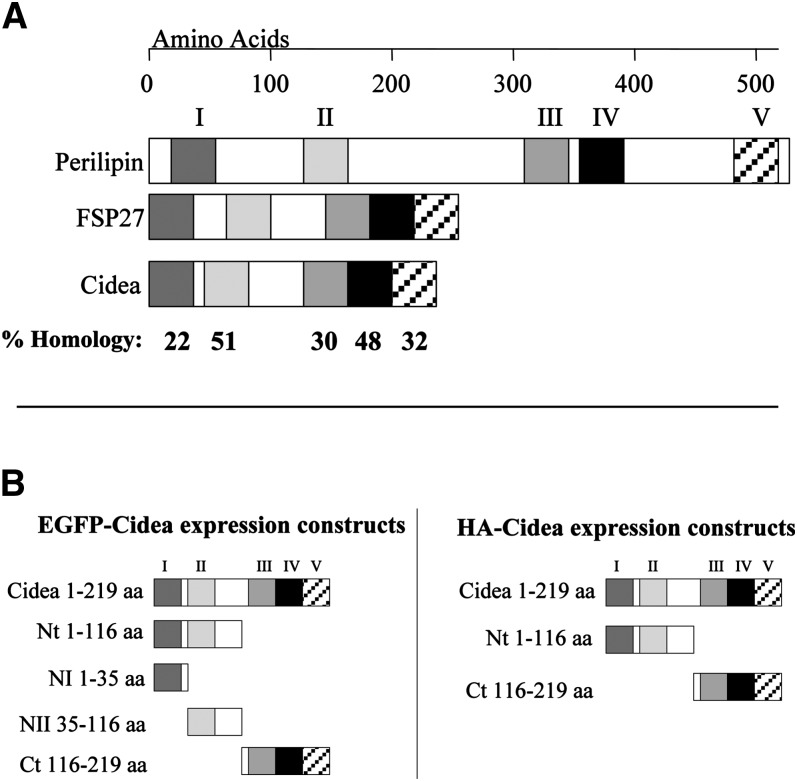
Although the functional domains of many lipid droplet-associated proteins have been identified (34–37), analysis of the functional domains of Cidea has not yet been performed. To address this, various domains of human Cidea were fused to GFP or HA-epitope to identify which regions are required for lipid droplet targeting and triglyceride shielding (Fig. 1). As human and mouse Cidea are approximately 90% homologous, the domains were chosen based on the sequence similarity previously described for mouse Cidea to the PAT family protein perilipin (18). These regions also share homology with Fsp27, another CIDE family protein that binds lipid droplets (18). The first region of Cidea (domain I) shares an approximately 22% homology with perilipin in a short amino terminal sequence that also shares homology with adipophilin and Tip47, the other PAT family proteins. No function has yet been identified for this region, but the shared homology among the lipid droplet proteins may indicate a conserved function for this motif. The second region of Cidea (domain II) shares 51% homology with the amino terminal triglyceride-shielding domain of perilipin. This segment of perilipin is required for blocking lipase association with the lipid droplet and preventing lipolysis (35). Because Cidea also inhibits lipolysis (30), this region is potentially important for Cidea function. The third and fourth regions of Cidea (domains III and IV) share approximately 30% and 48% homology, respectively, with the two regions of perilipin responsible for lipid droplet targeting and anchoring and, therefore, may be required to target Cidea to the lipid droplet (18). Finally, the fifth region of Cidea (domain V) shares 32% homology with the second triglyceride-shielding domain found in the carboxy-terminal portion of perilipin (30). The constructs generated include the full-length protein (Cidea), the amino terminal 116 amino acids (Nt), which encompass regions I and II, and the carboxy-terminal 104 amino acids (Ct), which encompass regions III and IV fused to either enhanced green fluorescent protein (EGFP) or HA. To further analyze the amino terminal region, EGFP constructs were generated to express amino acids 1–35 (NI) or amino acids 35–116 (NII) (Fig. 1).
Fig. 1.
Diagram showing the predicted amino acid (aa) similarity of Cidea and Fsp27 to perilipin and the Cidea constructs generated based on these motifs. A: The adipophilin-like sequence of perilipin (aa 11–38; region I) shows a sequence similarity of 32% with Fsp27 (aa 2–29) and 22% with Cidea (aa 2–28). Region II of perilipin (aa 120–152) is responsible for shielding triglyceride from cytosolic lipases and has a sequence similarity of 40% with Fsp27 (aa 46–77) and 51% with Cidea (aa 38–69). Regions III (aa 313–352) and IV (aa 365–391) of perilipin are responsible for lipid droplet targeting and anchoring and have 40% and 30% similarity with the respective sequences of Fsp27 (aa 137–173 and aa 174–200) and 38% and 48% similarity with Cidea (aa 122–158 and 159–185). The homology between Cidea and perilipin within each domain is shown. B: Cidea constructs of EGFP or hemagglutinin (HA) fused to the full length or various fragments of Cidea were generated based on the homology to the known perilipin domains.
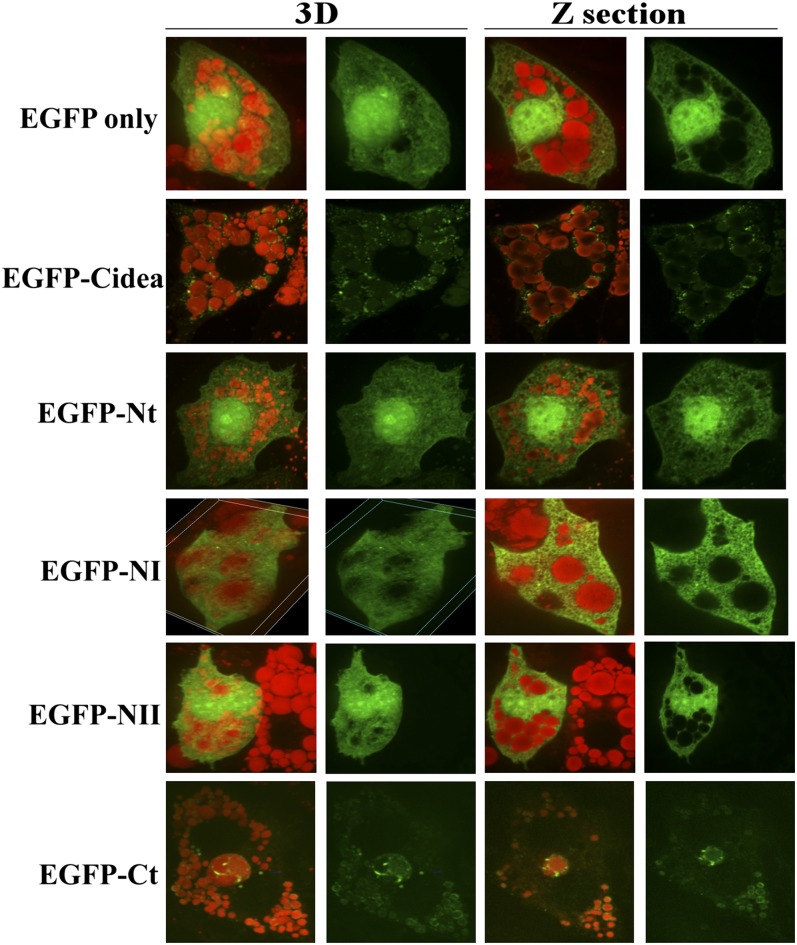
Since previous findings by Puri et al. demonstrated that the endogenous Cidea protein targets to the periphery of lipid droplets in primary human adipocytes, it was expected that the ectopic expression of the full-length human Cidea fused to EGFP would also colocalize to lipid droplets in 3T3-L1 adipocytes (18). As expected, Cidea concentrated around the periphery of lipid droplets stained with Oil Red O, a hydrophobic dye that binds to neutral lipids, such as triglyceride and cholesterol esters (Fig. 2 and supplementary Fig. I). Conversely, the EGFP control, EGFP-Nt, EGFP-NI, and EGFP-NII showed diffuse cytoplasmic and nuclear staining, indicating no distinct intracellular localization. In contrast, EGFP-Ct has a localization pattern similar to EGFP-Cidea, with ring-like labeling surrounding lipid droplets (Fig. 2 and supplementary Fig. I).
Fig. 2.
The carboxy-terminus of Cidea (aa 116–219) is necessary and sufficient for lipid droplet localization in 3T3-L1 adipocytes. EGFP only or the EGFP-Cidea constructs were expressed in day 5 adipocytes for 24 h. Then the cells were fixed, and the neutral lipid was labeled with Oil Red O. In the left two panels, 3D images with both green and red visualize the Cidea localization to lipid droplets; images with green only visualize the cellular distribution of Cidea. The right two panels show confocal Z sections with both green and red or green only. All images are 100× magnification.
To ensure that these findings were not an artifact of the EGFP fused to the Cidea segments, the experiments were repeated with HA-Cidea, HA-Nt, and HA-Ct. These experiments showed a similar intracellular localization pattern for the EGFP- and HA-tagged proteins, indicating that Cidea localization around lipid droplets is not influenced by the presence of these modifications (supplementary Fig. II). Furthermore, these experiments reveal that Cidea targeting to lipid droplets in adipocytes is solely dependent on the 104 amino acid carboxy-terminus.
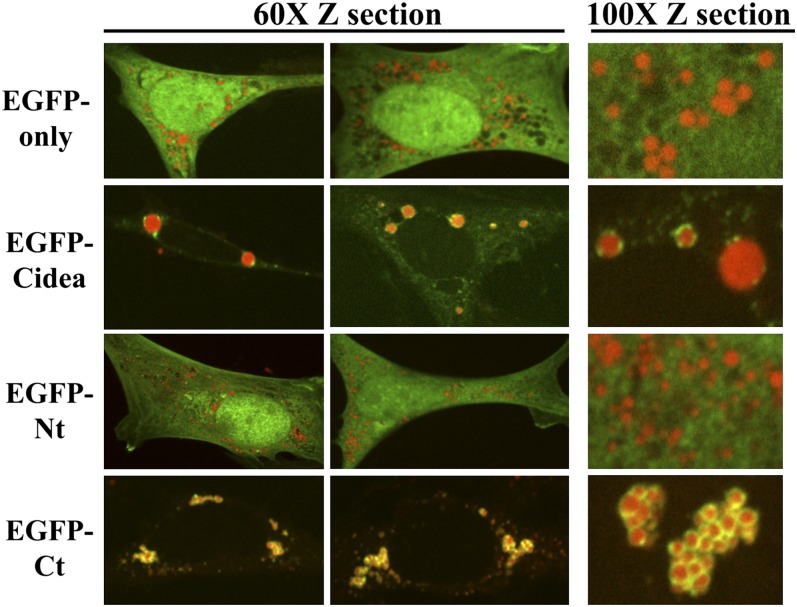
To determine if the lipid droplet targeting of human Cidea is an adipocyte-specific phenomenon, EGFP-tagged constructs were expressed in 3T3-L1 fibroblasts and COS-7 monkey kidney cells. Similar to 3T3-L1 adipocytes, in 3T3-L1 fibroblasts, both the EGFP-Cidea and EGFP-Ct were targeted to lipid droplets, while the EGFP protein alone and EGFP-Nt showed diffuse cytoplasmic and nuclear staining (Fig. 3). These findings suggest that Cidea targeting to lipid droplets is not fully dependent on a mechanism specific to differentiated adipocytes. To eliminate the possibility that 3T3-L1 fibroblasts may express low levels of an adipocyte-specific protein that facilitates targeting of Cidea to lipid droplets, COS-7 kidney cells were also analyzed. These experiments again showed that the EGFP control and EGFP-Nt do not have distinct localization patterns but that EGFP-Cidea and EGFP-Ct localize to the lipid droplets. Although EGFP-Cidea and EGFP-Ct partially colocalize with lipid droplets in a punctate staining pattern, they do not show as tight an association with droplets in COS cells as they do in adipocytes or preadipocytes. In particular, they do not form the same ring-like structures surrounding the lipid droplets, suggesting there may be components or mechanisms in adipocytes and preadipocytes that enhances Cidea association or prevents Cidea dissociation from lipid droplets (Fig. 4 and supplementary Fig. III).
Fig. 3.
The carboxy-terminus of Cidea (aa 116–219) is necessary and sufficient for lipid droplet localization in 3T3-L1 preadipocytes. EGFP only or the EGFP-Cidea constructs were expressed in preadipocytes for 24 h. Then the cells were fixed, and the neutral lipid was labeled with Oil Red O. The left two panels show a 60× confocal Z section of two different cells. The right panel shows a 100× confocal Z section for closer inspection of Cidea localization to the lipid droplet. The images for each construct are from three different cells.
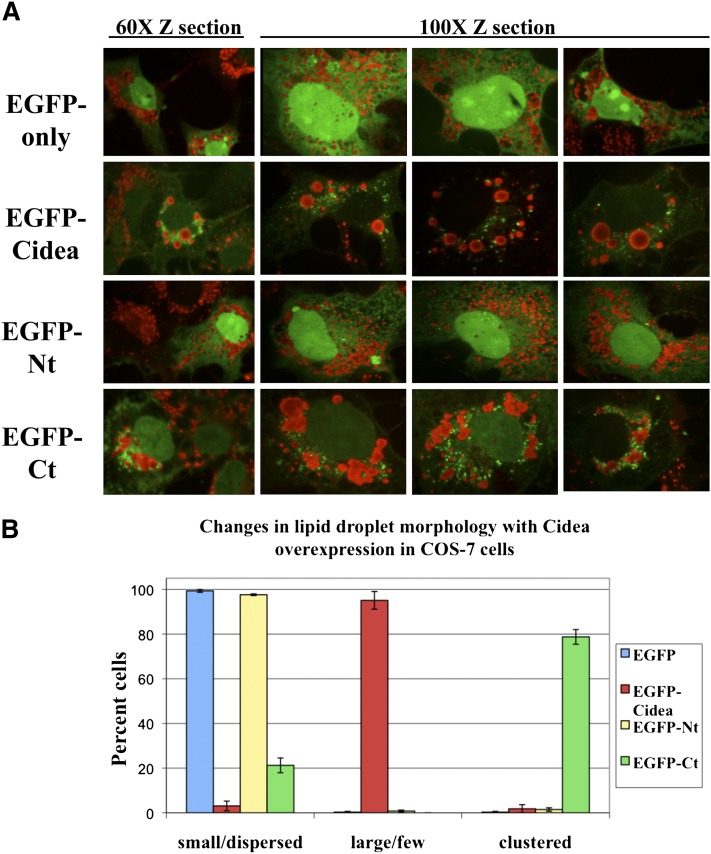
Fig. 4.
Cidea changes lipid droplet morphology in COS-7 cells but does not tightly associate with lipid droplets. A: EGFP only or the EGFP-Cidea constructs were expressed in COS-7 for 24 h in the presence of 400 μM oleate. Then the cells were fixed, and the neutral lipid was labeled with Oil Red O. The left panel shows a 60× confocal Z section of a cell expressing EGFP. The right panel shows a 100× confocal Z section for closer inspection of Cidea localization to the lipid droplet. The images for each construct are from four different cells. B: Lipid droplet morphology was determined in 200 cells from three independent experiments as being small and dispersed, large and few, or clustered.
Cidea and the carboxy-terminus of Cidea induce distinct changes in lipid droplet morphology
Expression of full-length Cidea or its carboxy-terminus in 3T3-L1 preadipocytes and COS-7 cells also revealed a striking change in lipid droplet morphology (Fig. 4A). In cells expressing the EGFP control or EGFP-Nt, lipid droplets remained small and dispersed throughout the cytoplasm. However, in cells expressing EGFP-Cidea, the lipid droplets tended to be fewer in number and much larger in size (Fig. 4B). This increase in lipid droplet size is also seen when mouse Cidea is expressed in 3T3-L1 adipocytes and COS-7 cells (18). In cells expressing EGFP-Ct, the lipid droplets form distinct clusters, but they do not form large droplets during the time course analyzed (Fig. 4B). This clustered morphology has been reported for other lipid droplet-shielding proteins, such as perilipin and Mldp, suggesting that the carboxy-terminus may contain the shielding domain required to inhibit lipase association with the droplet (38, 39). These results were confirmed by expressing HA-Cidea, HA-Nt, and HA-Ct (supplementary Fig. IV). Even after 72 h of expression, the carboxy-terminus did not induce the appearance of large lipid droplets observed with the full-length protein. To determine if the carboxy-terminus could induce large lipid droplet formation in the presence of the N-terminus, these constructs were coexpressed in COS-7 cells. In cells expressing both constructs, lipid droplets were clustered but remained small, similar to what was observed upon expressing the carboxy-terminus alone (data not shown). Therefore, dual expression of the amino and carboxy-termini together did not result in the full-length phenotype, suggesting the carboxy-terminus is required for the proper targeting of the N terminus for large lipid droplet formation.
The C terminus of Cidea is necessary and sufficient to stimulate triglyceride storage
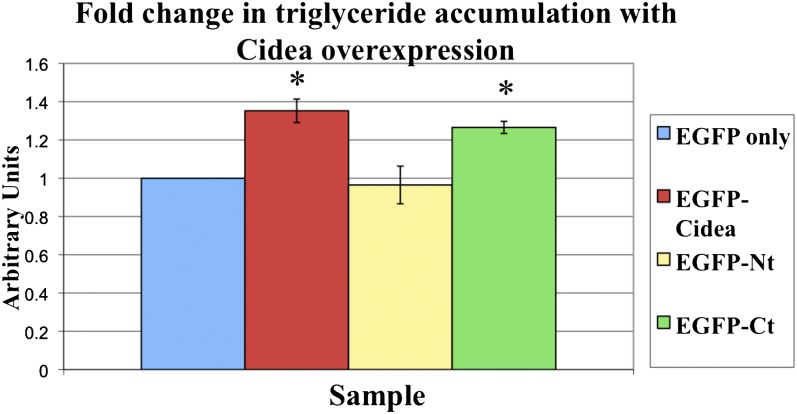
Multiple lines of evidence indicate that Cidea promotes lipid storage by inhibiting lipolysis. To date, support for this Cidea function has been obtained through loss of function studies (30, 32). To determine if Cidea can also promote lipid storage, the various EGFP-Cidea constructs were expressed in COS-7 cells. The EGFP-positive cells were then isolated through FACS and the triglyceride levels were analyzed in cells expressing each of the constructs. Not surprisingly, expression of EGFP-Nt causes no significant change in triglyceride level compared with the EGFP control. However, expression of either EGFP-Cidea or EGFP-Ct causes a significant increase in triglyceride accumulation above the EGFP control (an increase of 35% and 27%, respectively). Although expression of the carboxy-terminus causes slightly less triglyceride accumulation, this is not statistically different from the full-length protein, suggesting that the carboxy-terminus of Cidea is necessary and sufficient to inhibit lipolysis and promote triglyceride accumulation (Fig. 5). These data also suggest that while the N terminus is required to promote large lipid droplet formation seen in response to the full-length protein, this change in morphology is not required to promote triglyceride accumulation.
Fig. 5.
Cidea overexpression causes increased triglyceride accumulation. EGFP only or the EGFP-Cidea constructs were expressed in COS-7 for 24 h in the presence of 400 μM oleate, and then triglyceride levels were determined. The triglyceride levels with each condition were normalized to cell number and then expressed as a fold change compared with the EGFP-only control. The values represent the average of three experiments. There is no significant difference in triglyceride in cells expressing the full length or carboxy-terminal domain. The asterisks denote a P value < 0.01.
Human Cidea promotes lipid storage by inhibiting lipolysis
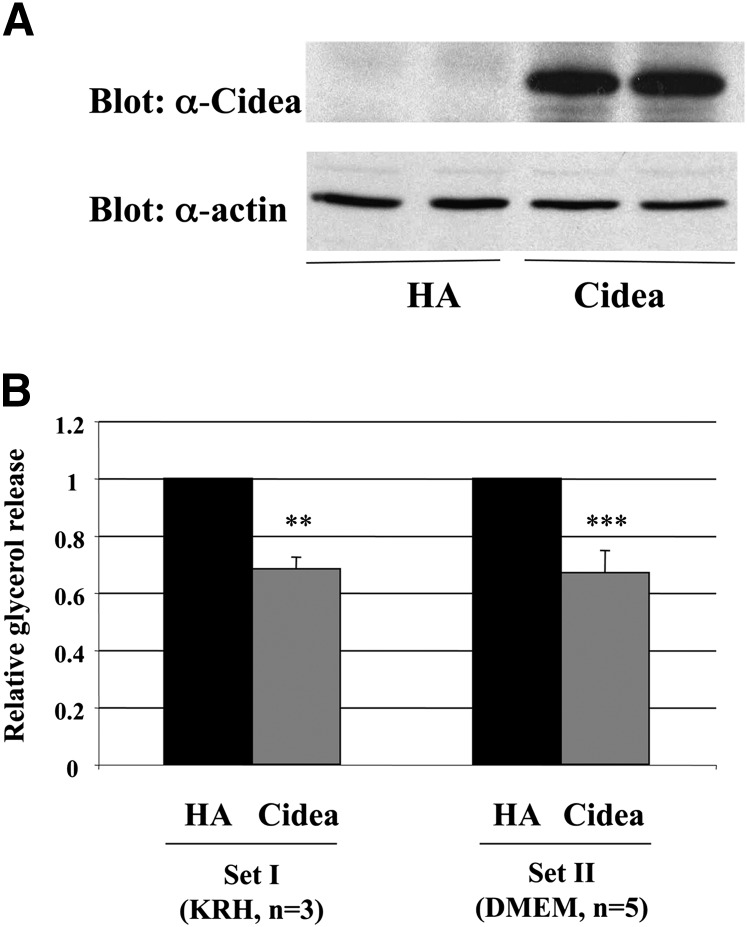
While previous studies indicate that a loss in Cidea function results in increased lipolysis and decreased triglyceride storage, it is necessary to verify that the converse is also true; i.e., that the expression of Cidea inhibits lipolysis and promotes lipid storage. During basal conditions, adipocytes undergo a low rate of lipolysis to release free fatty acids for both peripheral and cellular energy production. During the hydrolysis of triglyceride, glycerol is released along with the free fatty acids, and the glycerol can be measured to determine the lipolytic state of the cells. To determine if human Cidea reduces lipolysis, adenoviral constructs expressing HA control or HA-Cidea were expressed in fully differentiated 3T3-L1 adipocytes, and the glycerol release was assayed. As seen in Fig. 6, the expression of Cidea results in an approximately 32% reduction in basal glycerol release compared with the HA control, indicating that Cidea does inhibit lipolysis. In addition, this effect was not dependent on a particular media formulation, as a similar effect was found in cells cultured in KRH or DMEM (Fig. 6).
Fig. 6.
Expression of full-length Cidea attenuates glycerol release from 3T3-L1 adipocytes. Differentiated adipocytes were infected with hemagglutinin (HA)-control or Cidea adenovirus for 48 h. Then cells were incubated in Krebs-Ringer Hepes (KRH) (Set I) or DMEM (Set II), and glycerol concentration in the media after 2.5 h was assayed. Cells were then harvested for total protein measurement and immunoblotting. A: Representative immunoblot of protein lysates from cells infected with HA-control or Cidea adenovirus (experimental duplicates of each). Upper panel, anti-Cidea antibody. Lower panel, actin (loading control) antibody. B: Glycerol release normalized to total protein from cells infected as in (A) is shown relative to HA-adenovirus control. Averages ± SEM of three or five experiments per set. The P value was calculated using Student's t-test. **P < 0.01; ***P < 0.001.
DISCUSSION
Currently, published reports suggest that Cidea promotes lipid storage by inhibiting lipolysis, but the mechanism driving this function is still unknown (30, 32). In this study, we show that the expression of either human Cidea or a domain comprising its carboxy-terminal 104 amino acids directs both lipid droplet targeting (Figs. 2 and 3) and triglyceride accumulation (Fig. 5), which identifies the carboxy-terminus as both necessary and sufficient for these two functions. As expected, the triglyceride accumulation due to Cidea expression occurs, at least partially, through lipolysis inhibition (Fig. 6). Interestingly, the 32% reduction in lipolysis with Cidea expression in adipocytes is very similar to the 35% percent increase in triglyceride accumulation found with Cidea expression in COS cells, which suggests that Cidea promotes triglyceride accumulation largely through lipolysis inhibition rather than triglyceride synthesis (Figs. 5 and 6). Nevertheless, this conclusion cannot be definitively drawn because different cell lines were used to assay triglyceride accumulation and lipolysis.
However, many studies have shown that inhibiting lipolysis by various means stimulates triglyceride accumulation in isolated adipocytes. For example, the depletion of Atgl expression with siRNA inhibits lipolysis, resulting in increased triglyceride levels (40, 41). Conversely, expression of lipid droplet-shielding proteins, such as perilipin, Adrp, or Mldp, increases triglyceride storage in a variety of nonadipocyte cell lines (35, 39, 42, 43). It is not surprising that the carboxy-terminus of Cidea is responsible for both lipid droplet localization and triglyceride accumulation, as this region of Cidea shares sequence similarity to the carboxy-terminal triglyceride-shielding domain and the targeting and anchoring domains of perilipin (Fig. 1) (35, 36).
Fsp27, another protein of the CIDE family, also binds lipid droplets and negatively regulates lipolysis (17, 31). This protein exhibits 65% sequence similarity to Cidea, and the two proteins show a similar phenotype when depleted or expressed. Interestingly, expression of Fsp27 in 3T3-L1 preadipocytes increases triglyceride storage to an extent similar to Cidea expression, suggesting these two proteins may have redundant functions (19).
Despite the presence of a motif in the N-terminus of Cidea that has some similarity to the amino-terminal triglyceride-shielding domain of perilipin (35), this Cidea region is not required for lipid droplet targeting or triglyceride accumulation ( ). Nevertheless, the N-terminus is required within the full-length Cidea protein to induce large lipid droplet formation and reduce lipid droplet number. These effects were not observed upon expression of the carboxy-terminus or N-terminus alone, indicating that both termini are required to catalyze these events (Fig. 4B).
The lipid droplets that are regulated by Cidea might be used for energy storage, phospholipid and membrane synthesis, cholesterol supply for membrane rigidity, or perhaps the generation of lipid-signaling molecules. Because Cidea expression does not cause a striking increase in lipid storage, as reported for perilipin (35), it may be that Cidea promotes lipid storage for usages other than energy production. Therefore, a loss in Cidea function may lead to dramatic effects on cellular function, despite only a modest effect on lipid storage.
Interestingly, the ability of Cidea to increase lipid droplet size has not yet been reported for any other canonical lipid droplet-associated protein, and this finding identifies a novel function for this class of proteins. As lipid droplets can occupy much of the intracellular volume, especially in adipocytes, storing the lipids in few rather than several droplets would allow more cytoplasmic space for other cellular processes. It is possible that the formation of large and few lipid droplets by Cidea expression may be due to lipid droplet fusion. However, if lipid droplet fusion explains the change in lipid droplet morphology, cells expressing full-length Cidea would be expected to accumulate more triglyceride than cells expressing the carboxy-terminus, but this is not the case (Fig. 5). Because a large droplet has less surface area to undergo lipolytic activity than a small droplet, forming larger droplets could further reduce lipolysis and increase lipid storage. However, experimental limitations may explain the lack in differences seen in triglyceride accumulation between the full-length versus the carboxy-terminus of Cidea. For example, the experiments in this study were performed for 24 h as this time point yielded the optimal parameters for analysis: the cells had adequate EGFP-Cidea expression, showed phenotypic changes, and were still fully viable; longer timepoints result in increased cell toxicity. However, 24 h may not be sufficient time for the large lipid droplets to form and lipids to accumulate to a significant degree above the anti-lipolytic carboxy-terminus. This issue will be interesting to address in future experiments.
These studies outline the mechanisms by which human Cidea regulates lipid storage and may be an important aspect in the maintenance of human adipocyte function and glucose homeostasis. In fact, Cidea expression is increased in lean versus obese humans, with expression 2-fold less in obese, insulin-resistant humans (32). However, when Cidea expression is maintained during the obese state, insulin sensitivity is also maintained (18). Therefore, these studies strongly support the idea that Cidea is an important regulator of insulin sensitivity and glucose homeostasis in humans.
Perhaps Cidea regulates whole body insulin sensitivity by controlling the rate of lipolysis in the adipose tissue and promoting proper sequestration of fatty acids as triglyceride within lipid droplets. This function would be particularly important during the obese state to prevent the elevation of free fatty acid levels in serum, which could then accumulate in the liver and muscle, causing lipotoxicity and insulin resistance (4). Additionally, increasing the free fatty acid concentration within adipocyte lipid droplets may lead to changes in membrane phospholipid composition and alter cell signaling events, negatively affecting adipocyte function. Furthermore, because increasing evidence suggests that different populations of droplets are involved in different cellular functions, Cidea may function on lipid droplets that mediate a specific cell function. Interestingly, the carboxy-terminus of Cidea induces apoptosis when ectopically expressed at high levels (44). This effect could be due to the mislocalization of Cidea to lipid droplets due to the absence of the amino terminal localization signal, altering cell signaling events and leading to apoptosis. Further mechanistic studies of lipid droplet-associated proteins, such as Cidea, will enhance our understanding of these newly recognized organelles that may influence many disease states.
Supplementary Material
Acknowledgments
The authors thank Drs. Silvia Corvera, Heidi Tissenbaum, Stephen Doxsey, Yong-Xu Wang, Srijana Ranjit, and Joseph Virbasius for significant input into the development of this project.
Footnotes
Abbreviations:
- ADRP
- adipocyte differentiation-related protein
- ATGL
- adipose triglyceride lipase
- CIDE
- cell death-inducing DFF effector
- DFF40/45
- DNA fragmentation factor 40/45
- EGFP
- enhanced green fluorescent protein
- FACS
- fluorescence-activated cell sorting
- GFP
- green fluorescent protein
- HA
- hemagglutinin
- HOMA-IR
- homeostatic model assessment of insulin resistance
- HSL
- hormone sensitive lipase
- OXPAT
- PAT family member associated with oxidative metabolism
- PAT
- perilipin/ADRP/TIP47 family
- SNARE
- soluble N-ethylmaleimide-sensitive factor-attachment protein receptor
- TIP47
- Mannose-6-phosphate receptor binding protein 1
This work was supported by National Institutes of Health Grants DK-30898; DK-32520 (FACS, Genomics and Bioinformatics Core Facilities of the University of Massachusetts Diabetes and Endocrinology Center); and DK-60837 (Diabetes Genome Anatomy Project). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of four figures.
REFERENCES
- 1.Wang S., Soni K. G., Semache M., Casavant S., Fortier M., Pan L., Mitchell G. A. 2008. Lipolysis and the integrated physiology of lipid energy metabolism. Mol. Genet. Metab. 95: 117–126. [DOI] [PubMed] [Google Scholar]
- 2.Griffin M. E., Marcucci M. J., Cline G. W., Bell K., Barucci N., Lee D., Goodyear L. J., Kraegen E. W., White M. F., Shulman G. I. 1999. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 48: 1270–1274. [DOI] [PubMed] [Google Scholar]
- 3.Listenberger L. L., Han X., Lewis S. E., Cases S., Farese R. V., Jr., Ory D. S., Schaffer J. E. 2003. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl. Acad. Sci. USA. 100: 3077–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rasouli N., Kern P. A. 2008. Adipocytokines and the metabolic complications of obesity. J. Clin. Endocrinol. Metab. 93: S64–S73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma A. M., Staels B. 2007. Review: peroxisome proliferator-activated receptor gamma and adipose tissue–understanding obesity-related changes in regulation of lipid and glucose metabolism. J. Clin. Endocrinol. Metab. 92: 386–395. [DOI] [PubMed] [Google Scholar]
- 6.Wisse B. E., Kim F., Schwartz M. W. 2007. Physiology. An integrative view of obesity. Science. 318: 928–929. [DOI] [PubMed] [Google Scholar]
- 7.Martin S., Parton R. G. 2006. Lipid droplets: a unified view of a dynamic organelle. Nat. Rev. Mol. Cell Biol. 7: 373–378. [DOI] [PubMed] [Google Scholar]
- 8.Wolins N. E., Brasaemle D. L., Bickel P. E. 2006. A proposed model of fat packaging by exchangeable lipid droplet proteins. FEBS Lett. 580: 5484–5491. [DOI] [PubMed] [Google Scholar]
- 9.Miura S., Gan J. W., Brzostowski J., Parisi M. J., Schultz C. J., Londos C., Oliver B., Kimmel A. R. 2002. Functional conservation for lipid storage droplet association among Perilipin, ADRP, and TIP47 (PAT)-related proteins in mammals, Drosophila, and Dictyostelium. J. Biol. Chem. 277: 32253–32257. [DOI] [PubMed] [Google Scholar]
- 10.Londos C., Brasaemle D. L., Schultz C. J., Segrest J. P., Kimmel A. R. 1999. Perilipins, ADRP, and other proteins that associate with intracellular neutral lipid droplets in animal cells. Semin. Cell Dev. Biol. 10: 51–58. [DOI] [PubMed] [Google Scholar]
- 11.Tansey J. T., Sztalryd C., Gruia-Gray J., Roush D. L., Zee J. V., Gavrilova O., Reitman M. L., Deng C. X., Li C., Kimmel A. R., et al. 2001. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc. Natl. Acad. Sci. USA. 98: 6494–6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sztalryd C., Xu G., Dorward H., Tansey J. T., Contreras J. A., Kimmel A. R., Londos C. 2003. Perilipin A is essential for the translocation of hormone-sensitive lipase during lipolytic activation. J. Cell Biol. 161: 1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyoshi H., Souza S. C., Zhang H. H., Strissel K. J., Christoffolete M. A., Kovsan J., Rudich A., Kraemer F. B., Bianco A. C., Obin M. S., et al. 2006. Perilipin promotes hormone-sensitive lipase-mediated adipocyte lipolysis via phosphorylation-dependent and -independent mechanisms. J. Biol. Chem. 281: 15837–15844. [DOI] [PubMed] [Google Scholar]
- 14.Dalen K. T., Dahl T., Holter E., Arntsen B., Londos C., Sztalryd C., Nebb H. I. 2007. LSDP5 is a PAT protein specifically expressed in fatty acid oxidizing tissues. Biochim. Biophys. Acta. 1771: 210–227. [DOI] [PubMed] [Google Scholar]
- 15.Wolins N. E., Quaynor B. K., Skinner J. R., Schoenfish M. J., Tzekov A., Bickel P. E. 2005. S3 -12, Adipophilin, and TIP47 package lipid in adipocytes. J. Biol. Chem. 280: 19146–19155. [DOI] [PubMed] [Google Scholar]
- 16.Martin S., Driessen K., Nixon S. J., Zerial M., Parton R. G. 2005. Regulated localization of Rab18 to lipid droplets: effects of lipolytic stimulation and inhibition of lipid droplet catabolism. J. Biol. Chem. 280: 42325–42335. [DOI] [PubMed] [Google Scholar]
- 17.Puri V., Czech M. P. 2008. Lipid droplets: FSP27 knockout enhances their sizzle. J. Clin. Invest. 118: 2693–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puri V., Ranjit S., Konda S., Nicoloro S. M., Straubhaar J., Chawla A., Chouinard M., Lin C., Burkart A., Corvera S., et al. 2008. Cidea is associated with lipid droplets and insulin sensitivity in humans. Proc. Natl. Acad. Sci. USA. 105: 7833–7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keller P., Petrie J. T., De Rose P., Gerin I., Wright W. S., Chiang S. H., Nielsen A. R., Fischer C. P., Pedersen B. K., MacDougald O. A. 2008. Fat-specific protein 27 regulates storage of triacylglycerol. J. Biol. Chem. 283: 14355–14365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolins N. E., Quaynor B. K., Skinner J. R., Tzekov A., Croce M. A., Gropler M. C., Varma V., Yao-Borengasser A., Rasouli N., Kern P. A., et al. 2006. OXPAT/PAT-1 is a PPAR-induced lipid droplet protein that promotes fatty acid utilization. Diabetes. 55: 3418–3428. [DOI] [PubMed] [Google Scholar]
- 21.Martin S., Parton R. G. 2005. Caveolin, cholesterol, and lipid bodies. Semin. Cell Dev. Biol. 16: 163–174. [DOI] [PubMed] [Google Scholar]
- 22.Liu P., Bartz R., Zehmer J. K., Ying Y. S., Zhu M., Serrero G., Anderson R. G. 2007. Rab-regulated interaction of early endosomes with lipid droplets. Biochim. Biophys. Acta. 1773: 784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen A. W., Razani B., Schubert W., Williams T. M., Wang X. B., Iyengar P., Brasaemle D. L., Scherer P. E., Lisanti M. P. 2004. Role of caveolin-1 in the modulation of lipolysis and lipid droplet formation. Diabetes. 53: 1261–1270. [DOI] [PubMed] [Google Scholar]
- 24.Bostrom P., Andersson L., Rutberg M., Perman J., Lidberg U., Johansson B. R., Fernandez-Rodriguez J., Ericson J., Nilsson T., Boren J., et al. 2007. SNARE proteins mediate fusion between cytosolic lipid droplets and are implicated in insulin sensitivity. Nat. Cell Biol. 9: 1286–1293. [DOI] [PubMed] [Google Scholar]
- 25.Brasaemle D. L., Dolios G., Shapiro L., Wang R. 2004. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J. Biol. Chem. 279: 46835–46842. [DOI] [PubMed] [Google Scholar]
- 26.Ducharme N. A., Bickel P. E. 2008. Lipid droplets in lipogenesis and lipolysis. Endocrinology. 149: 942–949. [DOI] [PubMed] [Google Scholar]
- 27.Murphy S., Martin S., Parton R. G. 2009. Lipid droplet-organelle interactions; sharing the fats. Biochim. Biophys. Acta. 1791: 441–447. [DOI] [PubMed] [Google Scholar]
- 28.Brasaemle D. L. 2007. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J. Lipid Res. 48: 2547–2559. [DOI] [PubMed] [Google Scholar]
- 29.Cermelli S., Guo Y., Gross S. P., Welte M. A. 2006. The lipid-droplet proteome reveals that droplets are a protein-storage depot. Curr. Biol. 16: 1783–1795. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Z., Yon Toh S., Chen Z., Guo K., Ng C. P., Ponniah S., Lin S. C., Hong W., Li P. 2003. Cidea-deficient mice have lean phenotype and are resistant to obesity. Nat. Genet. 35: 49–56. [DOI] [PubMed] [Google Scholar]
- 31.Nishino N., Tamori Y., Tateya S., Kawaguchi T., Shibakusa T., Mizunoya W., Inoue K., Kitazawa R., Kitazawa S., Matsuki Y., et al. 2008. FSP27 contributes to efficient energy storage in murine white adipocytes by promoting the formation of unilocular lipid droplets. J. Clin. Invest. 118: 2808–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nordstrom E. A., Ryden M., Backlund E. C., Dahlman I., Kaaman M., Blomqvist L., Cannon B., Nedergaard J., Arner P. 2005. A human-specific role of cell death-inducing DFFA (DNA fragmentation factor-alpha)-like effector A (CIDEA) in adipocyte lipolysis and obesity. Diabetes. 54: 1726–1734. [DOI] [PubMed] [Google Scholar]
- 33.Jiang Z. Y., Zhou Q. L., Coleman K. A., Chouinard M., Boese Q., Czech M. P. 2003. Insulin signaling through Akt/protein kinase B analyzed by small interfering RNA-mediated gene silencing. Proc. Natl. Acad. Sci. USA. 100: 7569–7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye J., Li J. Z., Liu Y., Li X., Yang T., Ma X., Li Q., Yao Z., Li P. 2009. Cideb, an ER- and lipid droplet-associated protein, mediates VLDL lipidation and maturation by interacting with apolipoprotein B. Cell Metab. 9: 177–190. [DOI] [PubMed] [Google Scholar]
- 35.Garcia A., Subramanian V., Sekowski A., Bhattacharyya S., Love M. W., Brasaemle D. L. 2004. The amino and carboxyl termini of perilipin a facilitate the storage of triacylglycerols. J. Biol. Chem. 279: 8409–8416. [DOI] [PubMed] [Google Scholar]
- 36.Garcia A., Sekowski A., Subramanian V., Brasaemle D. L. 2003. The central domain is required to target and anchor perilipin A to lipid droplets. J. Biol. Chem. 278: 625–635. [DOI] [PubMed] [Google Scholar]
- 37.McManaman J. L., Zabaronick W., Schaack J., Orlicky D. J. 2003. Lipid droplet targeting domains of adipophilin. J. Lipid Res. 44: 668–673. [DOI] [PubMed] [Google Scholar]
- 38.Brasaemle D. L., Rubin B., Harten I. A., Gruia-Gray J., Kimmel A. R., Londos C. 2000. Perilipin A increases triacylglycerol storage by decreasing the rate of triacylglycerol hydrolysis. J. Biol. Chem. 275: 38486–38493. [DOI] [PubMed] [Google Scholar]
- 39.Granneman J. G., Moore H. P., Mottillo E. P., Zhu Z. 2009. Functional interactions between Mldp (LSDP5) and Abhd5 in the control of intracellular lipid accumulation. J. Biol. Chem. 284: 3049–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gandhi P. 2008. Regulation of Lipid Metabolism by Fat Specific Protein-FSP27. MSc Thesis. University of Massachusetts Medical School, Worcester, MA. [Google Scholar]
- 41.Haemmerle G., Lass A., Zimmermann R., Gorkiewicz G., Meyer C., Rozman J., Heldmaier G., Maier R., Theussl C., Eder S., et al. 2006. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 312: 734–737. [DOI] [PubMed] [Google Scholar]
- 42.Larigauderie G., Cuaz-Perolin C., Younes A. B., Furman C., Lasselin C., Copin C., Jaye M., Fruchart J. C., Rouis M. 2006. Adipophilin increases triglyceride storage in human macrophages by stimulation of biosynthesis and inhibition of beta-oxidation. FEBS J. 273: 3498–3510. [DOI] [PubMed] [Google Scholar]
- 43.Larigauderie G., Bouhlel M. A., Furman C., Jaye M., Fruchart J. C., Rouis M. 2006. Perilipin, a potential substitute for adipophilin in triglyceride storage in human macrophages. Atherosclerosis. 189: 142–148. [DOI] [PubMed] [Google Scholar]
- 44.Inohara N., Koseki T., Chen S., Wu X., Nunez G. 1998. CIDE, a novel family of cell death activators with homology to the 45 kDa subunit of the DNA fragmentation factor. EMBO J. 17: 2526–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.