Abstract
The overproduction of intestinal lipoproteins may contribute to the dyslipidemia found in diabetes. We studied the influence of diabetes on the fasting jejunal lipid content and its association with plasma lipids and the expression of genes involved in the synthesis and secretion of these lipoproteins. The study was undertaken in 27 morbidly obese persons, 12 of whom had type 2 diabetes mellitus (T2DM). The morbidly obese persons with diabetes had higher levels of chylomicron (CM) triglycerides (P < 0.001) and apolipoprotein (apo)B48 (P = 0.012). The jejunum samples obtained from the subjects with diabetes had a lower jejunal triglyceride content (P = 0.012) and angiopoietin-like protein 4 (ANGPTL4) mRNA expression (P = 0.043). However, the apoA-IV mRNA expression was significantly greater (P = 0.036). The jejunal triglyceride content correlated negatively with apoA-IV mRNA expression (r = −0.587, P = 0.027). The variables that explained the jejunal triglyceride content in a multiple linear regression model were the insulin resistance state and the apoA-IV mRNA expression. Our results show that the morbidly obese subjects with diabetes had lower jejunal lipid content and that this correlated negatively with apoA-IV mRNA expression. These findings show that the jejunum appears to play an active role in lipid homeostasis in the fasting state.
Keywords: morbid obesity, insulin resistance, intestine, chylomicron, apolipoprotein B48, apolipoprotein A-IV, angiopoietin-like protein 4
The insulin-resistance state and type 2 diabetes mellitus (T2DM) are closely related with an increase in cardiovascular disease. Factors conditioning this increase in cardiovascular disease include metabolic dyslipidemia associated with insulin resistance. It has recently been suggested that the overproduction of intestinally derived apolipoprotein (apo)B48-containing lipoproteins may contribute greatly to the dyslipidemia found in both the fasting and postprandial states in insulin resistance (1–3). However, the underlying mechanisms are still not fully understood.
Most studies undertaken in this area have been carried out in the postprandial state (3, 4). The intestine maintains a basal production of apoB48, even in fasting states, synthesizing small triglyceride-rich lipoprotein (TRL) particles (4, 5). Animal studies have shown that the contribution of intestinal lipoproteins to the total fasting plasma triglycerides is from 10% to 20% (4, 5), increasing in the case of diabetes (4, 6, 7). Fructose-fed hamsters experience an increase in the assembly and secretion of apoB48-containing lipoproteins in insulin-resistant states (2). Duez et al. showed that the production of chylomicrons (CM) was upregulated in humans with insulin resistance (8). Studies undertaken in fructose-fed, insulin-resistant Syrian golden hamsters in both the fasted and fed states found that, in these insulin-resistant animals, the increased production of small TRL was greater in the fasted state than the fed state (1). Nevertheless, caution should be exercised when extrapolating results from experimental animal models to humans, due to the differences between species.
Advances in the understanding of TRL assembly have shown the roles of apoA-IV and the microsomal triglyceride transfer protein (MTP) in the regulation of the synthesis and secretion of TRL. MTP is responsible for the lipidation of the chylomicron particle and apoA-IV has an important role in enhancing the secretion of chylomicrons and associated lipids in newborn enterocytes (9–11). Recently, it has been shown that angiopoietin-like protein 4 (ANGPTL4), an inhibitor of lipoprotein lipase activity, is a regulator of glucose homeostasis, lipid metabolism, and insulin sensitivity and is also involved in the metabolism of TRL (12).
To date, most studies have addressed the clearing processes of these apoB48-containing intestinal lipoproteins and their degree of production (7). However, as far as we are aware, no study has yet attempted to determine whether there is an alteration in the lipid content in the human gut associated with the dyslipidemia found in persons with T2DM. The aim of this study was to determine whether there is an alteration in the lipid content in the jejunum of persons with morbid obesity, with or without T2DM, and its association with plasma lipids and the expression of genes involved in the synthesis and secretion of TRL.
METHODS
Subjects
The study was undertaken in 27 morbidly obese persons who underwent a Roux-en-Y gastric bypass, 12 of whom had T2DM. Eight morbidly obese patients with T2DM were receiving metformin. None of the morbidly obese persons with T2DM was receiving insulin therapy, nor were any of the morbidly obese persons receiving estrogens, statins, or other cholesterol-lowering agents. All the morbidly obese persons followed a similar low-calorie diet for one week prior to surgery, independently of their states of obesity and insulin resistance (630 Kcal/day, Optisource, Novartis Consumer Health S.A.). Blood samples were drawn on the day before surgery after a 10–12 h fast. The plasma was separated and immediately frozen at –80°C. An unfrozen aliquot of plasma was used to isolate the lipoproteins. Jejunum samples were obtained from the morbidly obese persons during gastric bypass, 40 cm from the ligament of Treitz. Samples were washed in physiological saline, fractionated, and then prepared for histochemical and immunohistochemical analysis, quantification of the jejunal triglycerides, and mRNA extraction. All the participants gave their informed consent, and the study was reviewed and approved by the Ethics and Research Committee of Carlos Haya Regional University Hospital (Malaga, Spain).
Histochemical and immunohistochemical analysis
For histochemical and immunohistochemical analysis, jejunum samples were frozen in liquid nitrogen. From each tissue, serial 4 μm transverse sections were cut using a cryostat at –20°C, placed in an Oil Red-O staining solution, and briefly washed. A counterstain in Harris hematoxylin was performed. For immunohistochemical analysis, all steps were performed at room temperature in a humid chamber. Sections were first fixed for 30 min with formol, and then washed in 0.1M phosphate buffered saline (PBS). The sections were then incubated for 30 min with 3% H2O2/methanol with PBS to inactivate endogenous peroxidase. After washing, antigen retrieval procedures were performed. After several washes with PBS, the sections were exposed to the primary antibodies overnight (ab7616, goat anti-human apoB) (Santa Cruz Biotechnology, Santa Cruz, CA). Washed sections were then incubated with the appropriate biotinylated secondary antibodies for 1 h [biotin-conjugated rabbit anti-goat immunoglobulin (Ig) (DakoCytomation, Glostrup, Denmark)]. The samples were washed with PBS, and ExtrAvidin-peroxidase (Sigma) was applied. The visualization of the signal was done by using 3,3′-diaminobenzidine substrate kit (Sigma). All the antibodies were used according to the manufacturer's instructions. Omission of the primary antibody resulted in no detectable staining. These sections were counterstained with hematoxylin. The slides were viewed using an Olympus BX41 microscope (Olympus, UK) with an Olympus DP70 digital camera (Olympus, UK). ImageJ version 1.32j software was used to analyze the relative quantity of apoB in the lamina propria of the samples.
Quantification of the jejunal triglycerides
A sample of full-thickness jejunum was taken to analyze the triglyceride content. Briefly, lipids were extracted with chloroform-methanol 2:1 (v/v) after sample homogenization. The organic phase was collected and evaporated under a current of nitrogen. The extracts were resuspended in isopropanol, and the concentration of triglycerides was determined by using commercial kits (Randox Laboratories, Antrium, UK) (13). The results were expressed as mmol of triglycerides/g of jejunal tissue (jejunal triglycerides).
mRNA extraction and real-time RT-PCR analysis
The biopsy samples were washed in physiological saline, the mucosa was scraped, and samples were immediately frozen in liquid nitrogen. Biopsy samples were maintained at –80°C until analysis. Total RNA isolation was obtained using RNeasy Lipid Tissue Mini Kit (Qiagen, Germany), as described (14). First strand cDNA was synthesized by retrotranscription using the M-MLV retrotranscriptase (Sigma). Gene expression levels were analyzed by quantitative real-time reverse transcriptase-polymerase chain reaction (RT-PCR) using a DNA Engine Opticon® System (MJ Research, Waltham, MA). Primers for the PCR reaction were designed based on NCBI database sequences and obtained from Proligo (Sigma) (18s: NM_014473.2; apoA-IV: NM_000482.3; ANGPTL4: NM_139314; and MTP: NM_000253). Calculation of relative expression levels of the different transcripts was performed based on the cycle threshold (CT) method. Thus, the CT value for each sample was calculated using the DNA Engine Opticon® System software with an automatic fluorescence threshold setting. Reactions were done in triplicate. Standard curves were constructed for the studied genes and 18S (internal control) by plotting values of CT (the cycle in which the fluorescence signal exceeds background) versus logcDNAinput (in nanograms). CT values from each experimental sample were then used to calculate the amount of apoA-IV, ANGPTL4, and MTP. The gene expression of cells was expressed as the percentage of relative gene expression referred to the internal calibrator included in each experiment.
Separation of plasma lipoproteins
Lipoproteins were separated from plasma by ultracentrifugation. Briefly, the chylomicron fraction was separated from the plasma by flotation at 35,000 rpm in a 45° rotor (Beckman TLA 100.3) (15). Then, the very low-density lipoprotein (VLDL) fraction was separated from the plasma by flotation at 55,000 rpm in a 45° rotor (Beckman TLA 100.3). After separation of the VLDL, the density of the infranatant was adjusted to 1.30 g/ml, separating the rest of the lipoproteins, intermediate-density lipoproteins (IDL), low-density lipoproteins (LDL), and high-density lipoproteins (HDL) by density gradient centrifugation (16). The concentrations of cholesterol and triglycerides were measured in each fraction.
ApoB48 Western blot
ApoB48 in plasma chylomicron was analyzed on a 5% sodium dodecyl sulfate (SDS) polyacrylamide gel as described (17). Briefly, the chylomicron fractions were isolated from the same amount of plasma in each patient. The chylomicron fraction was delipidated with methanol/diethylether solvent system by gently dipping the sample into 4 ml methanol. A volume of 4 ml ice-cold diethylether was added. The delipidation cocktail was mixed and centrifuged for 48 min at 2,500 g at 4°C. The supernatant was removed, and 4 ml of ice-cold diethylether was added. The sample was mixed and again centrifuged for 32 min at 2,500 g at 4°C, and the supernatant was again removed. The sample was dried by lyophilization and dissolved in sample buffer for 1 h at room temperature and then heated at 80°C for 15 min. Samples and a protein standard were loaded onto the gel, followed by electrophoretic transfer to a polyvinylidene difluoride membrane at 15 V for 1 h. The membranes were blocked in Protein-Free Tween 20 Blocking Buffer (Pierce, Rockford, IL) overnight at 4°C. After washing with PBS plus 0.05% Tween 20, membranes were incubated with a polyclonal anti apoB (Santa Cruz Biotechnology) at a dilution of 1:200 for 1 h at room temperature. This antibody is an affinity-purified goat polyclonal antibody used in other studies to identify apoB48 (18). Membranes were washed and incubated with HRP-conjugated donkey anti-goat IgG antibody (Santa Cruz Biotechnology) at a dilution of 1:2000 for 1 h at room temperature. The proteins were visualized with SuperSignal® West Pico Chemiluminescent Substrate (Pierce, Rockford, IL) in an ImageQuant LAS 4000 with the ImageQuant TL software (GE Healthcare, Uppsala, Sweden).
Laboratory measurements
Plasma biochemical variables were measured in duplicate. Glucose, cholesterol, triglycerides (Randox Laboratories, Antrium, UK) and free fatty acids (Wako Bioproducts, Richmond, VA) were determined by standard enzymatic methods. The insulin was analyzed by an immunoradiometric assay (BioSource International, Camarillo, CA). ApoB48 was analyzed by enzyme immunoassay (ELISA) (Shibayagi, Japan). Cross-reaction with human apoB-100 was less than the lower detection limit. The intra- and inter-assay CV was 3.5% and 5.7%, respectively. The sensitivity of the technique was 2.5 ng/ml. ApoA-IV was analyzed by ELISA (Millipore Corporation, Billerica, MA). The intra- and inter-assay CV was 4.6% and 12.2%, respectively. The sensitivity of the technique was 0.078 μg/ml. The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated from fasting insulin and glucose with the following equation:
HOMA-IR = fasting insulin (μIU/ml) × fasting glucose (mmol/l) / 22.5
Statistical analysis
The statistical analysis was done with SPSS version 11.5 for Windows (SPSS, Chicago, IL). Comparison between the results of the different groups was made with the Mann-Whitney test. The Spearman correlation coefficient was calculated to estimate the linear correlations between variables. Multiple linear regressions were used to determine the association between variables. Values were considered statistically significant when P ≤ 0.05. The results are given as the mean ± SD.
RESULTS
Anthropometric and biochemical variables
No significant differences were found between the two groups of morbidly obese persons (with or without T2DM) in the anthropometric variables studied (age, weight, BMI, and waist and hip circumferences) (Table 1). Plasma glucose, insulin, triglycerides, apoB48 and apoA-IV levels, HOMA-IR, chylomicron cholesterol, and triglycerides were significantly higher in the morbidly obese persons with T2DM (Table 1). The results of serum apoB48 by ELISA were confirmed by a Western blot of the proteins isolated from plasma chylomicrons (Fig. 1).
TABLE 1.
Clinical and biochemical variables in the morbidly obese persons
| Variable | Morbidly Obese Persons without T2DM | Morbidly Obese Persons with T2DM |
|---|---|---|
| N (men/women) | 14 (4/10) | 13 (3/10) |
| Age (years) | 44.4 ± 7.7 | 48.5 ± 9.0 |
| Weight (kg) | 143.3 ± 22.2 | 139.4 ± 24.3 |
| Body mass index (kg/m ) | 55.0 ± 7.8 | 50.5 ± 6.2 |
| Waist (cm) | 140.1 ± 14.8 | 142.3 ± 12.2 |
| Hip (cm) | 154.1 ± 18.5 | 141.4 ± 16.2 |
| Glucose (mmol/l) | 5.66 ± 0.47 | 9.61 ± 2.60c |
| Insulin (pmol/l) | 172.3 ± 84.7 | 234.6 ± 114.1a |
| Free fatty acids (mmol/l) | 0.680 ± 0.237 | 0.751 ± 0.142 |
| Cholesterol (mmol/l) | 5.55 ± 1.55 | 5.64 ± 1.06 |
| HDL cholesterol (mmol/l) | 1.23 ± 0.31 | 1.01 ± 0.23 |
| LDL cholesterol (mmol/l) | 3.25 ± 1.32 | 3.22 ± 0.34 |
| IDL cholesterol (mmol/l) | 0.20 ± 0.06 | 0.16 ± 0.06 |
| VLDL cholesterol (mmol/l) | 0.53 ± 0.27 | 0.65 ± 0.31 |
| CM cholesterol (mmol/l) | 0.34 ± 0.24 | 0.60 ± 0.22a |
| Triglycerides (mmol/l) | 1.46 ± 0.43 | 3.35 ± 0.91c |
| HDL triglycerides (mmol/l) | 0.25 ± 0.08 | 0.29 ± 0.06 |
| LDL triglycerides (mmol/l) | 0.31 ± 0.07 | 0.32 ± 0.07 |
| IDL triglycerides (mmol/l) | 0.12 ± 0.03 | 0.10 ± 0.03 |
| VLDL triglycerides (mmol/l) | 0.45 ± 0.15 | 0.68 ± 0.35 |
| CM triglycerides (mmol/l) | 0.33 ± 0.13 | 1.96 ± 0.25b |
| ApoB48 (μg/ml) | 8.64 ± 4.96 | 43.6 ± 16.3b |
| ApoB-100 (mg/dl) | 127.0 ± 27.8 | 131.2 ± 20.1 |
| ApoA-IV (mg/dl) | 20.2 ± 7.3 | 27.4 ± 3.7c |
| HOMA-IR | 5.9 ± 2.7 | 14.2 ± 7.7b |
| Jejunal triglycerides (mmol/g) | 7.12 ± 3.50 | 2.84 ± 0.76c |
| Jejunal apoB signal intensity | 155.6 ± 8.1 | 140.8 ± 7.5b |
Data are expressed as mean ± SD. Results adjusted for age and body mass index. Apo, apolipoprotein; CM, chylomicron; HDL, high density lipoprotein; HOMA-IR, homeostatic model assessment of insulin resistance; IDL, intermediate density lipoprotein; LDL, low density lipoprotein; T2DM, type 2 diabetes mellitus; VLDL, very low density lipoprotein.
P < 0.05.
P < 0.01.
P < 0.001.
Fig. 1.
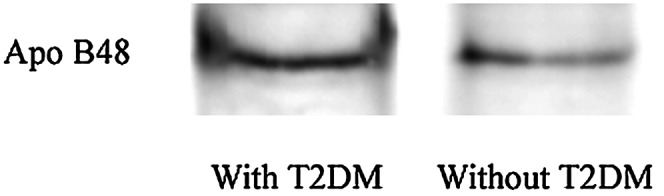
ApoB48 in the plasma chylomicron fraction from morbidly obese patients with or without T2DM. The plasma chylomicron fractions from both groups of patients (n = 6 per group) were delipidated, mixed, and subjected to 5% SDS-PAGE and Western blot. Apo, apolipoprotein; T2DM, type 2 diabetes mellitus.
Jejunal lipid content
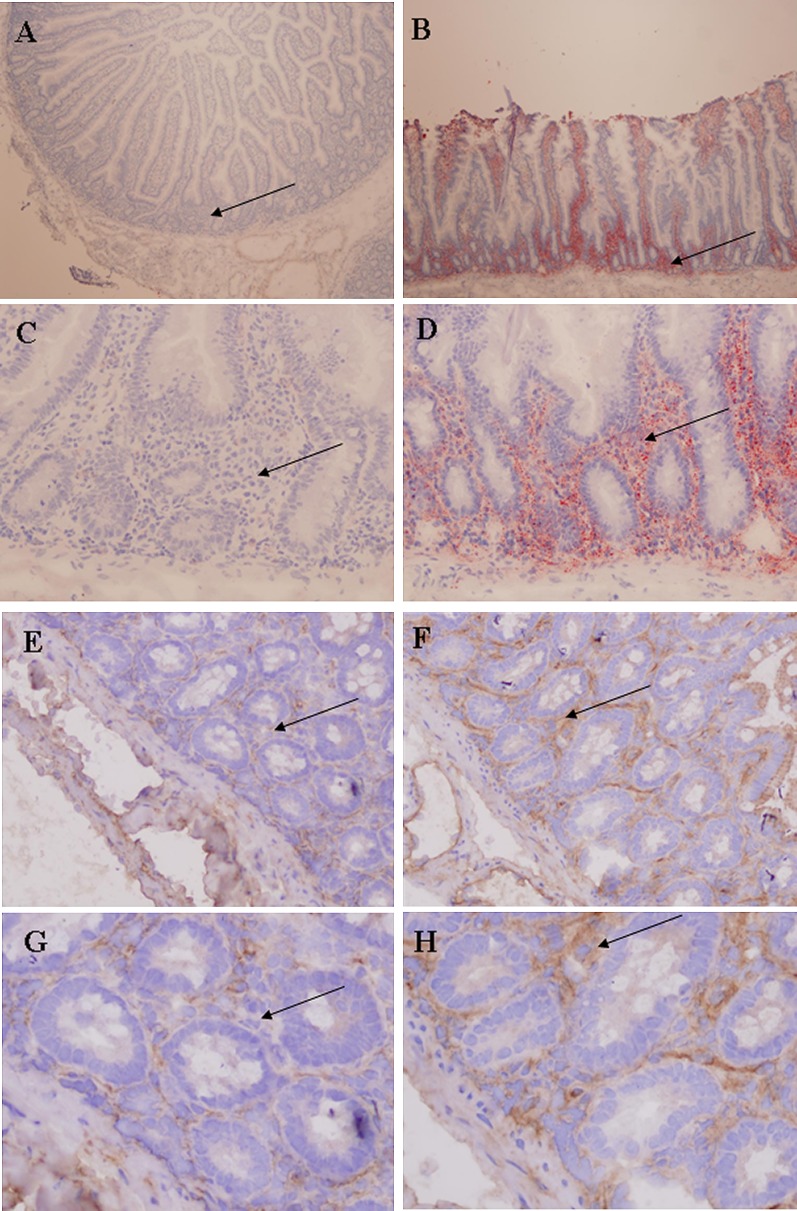
The jejunal triglyceride content differed significantly between the two groups of morbidly obese persons (P = 0.012) (Table 1). The lower content in the morbidly obese persons with T2DM was corroborated by the reduction in neutral lipids seen on Oil Red-O staining of the jejunum [T2DM versus non-T2DM (Fig. 2A, B); and T2DM versus non-T2DM (Fig. 2C, D)]. suggests that most of the triglycerides are not inside enterocytes. To determine whether these lipids were in the form of lipoproteins, we performed an immunohistochemical stain with an antibody against apoB. As can be seen from Table 1 and Fig. 2 [morbidly obese persons with T2DM (Fig. 2E, G) versus morbidly obese persons without T2DM (Fig. 2F, H)], the signal obtained in the morbidly obese persons with T2DM was lower than that in the persons without T2DM (P = 0.008). Both the apoB signal and the neutral lipids were mainly located in the lamina propria of the jejunal samples. Immunohistochemical analysis showed a slight, nonsignificant increase in the signal obtained in enterocyte apoB staining in the morbidly obese persons with T2DM (118.8 ± 8.7 versus 110.4 ± 4.5, P = 0.101). No significant differences were found between morbidly obese persons with T2DM according to whether or not they were on metformin (data not shown).
Fig. 2.
Lower jejunal fat accumulation in morbidly obese persons with T2DM. A and C: Oil Red-O staining of jejunum from morbidly obese persons with T2DM. B and D: Oil Red-O staining of jejunum from morbidly obese persons without T2DM. E and G: Jejunal apoB signal intensity of jejunum from morbidly obese persons with T2DM. F and H: Jejunal apoB signal intensity of jejunum from morbidly obese persons without T2DM. The lamina propria (indicated by →) of the morbidly obese persons with T2DM show markedly reduced Oil Red-O staining and jejunal apoB signal intensity, consistent with a decreased jejunal triglyceride concentration. A and B magnifications are 4×; C, D, E, and F magnifications, 20×; and G and H magnifications, 40×. Apo, apolipoprotein; T2DM, type 2 diabetes mellitus.
mRNA expression
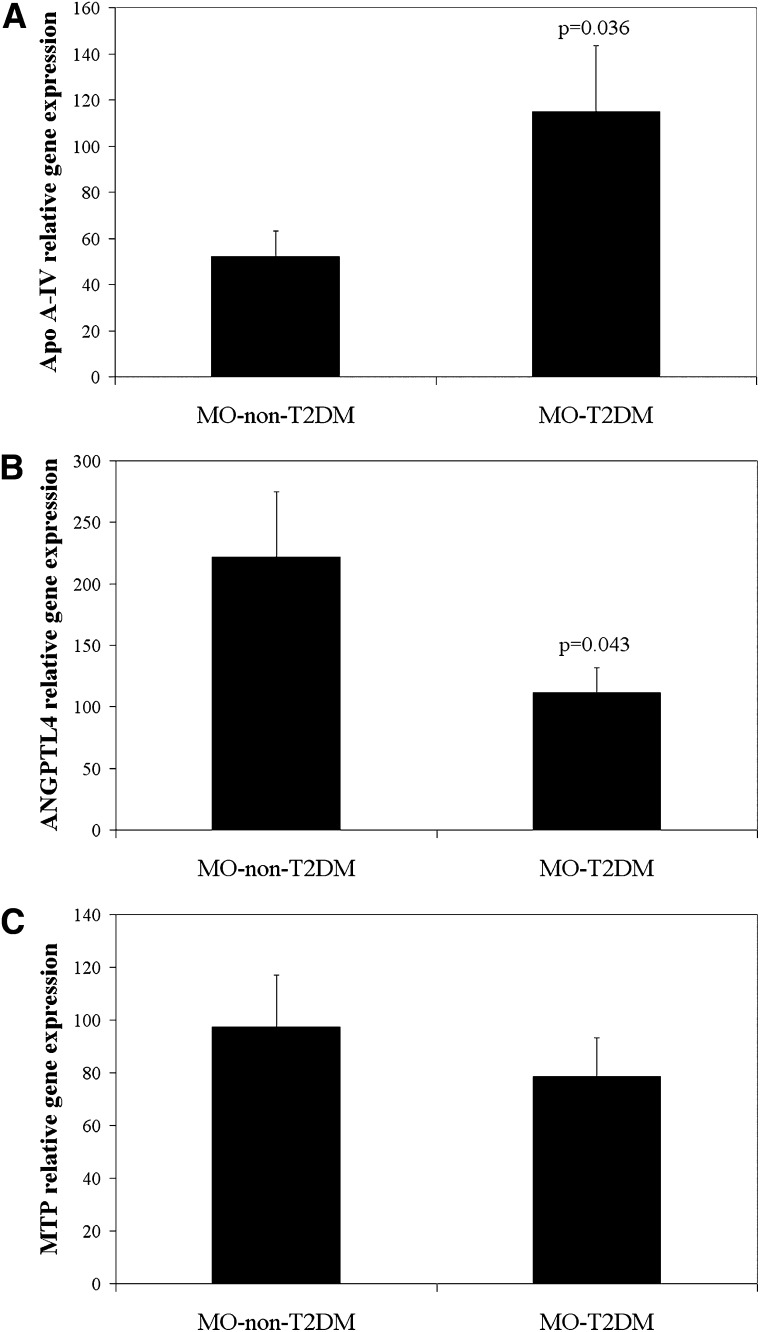
In the morbidly obese persons with T2DM, the apoA-IV mRNA expression was significantly greater (P = 0.036) (Fig. 3A) and ANGPTL4 mRNA expression significantly lower (P = 0.043) (Fig. 3B) than these expressions in the morbidly obese patients without T2DM. No significant differences were detected between the two groups of morbidly obese patients in MTP mRNA expression (P = 0.456) (Fig. 3C). No significant differences were found between the morbidly obese persons with T2DM according to whether or not they were on metformin (data not shown).
Fig. 3.
mRNA expression levels in jejunum of morbidly obese persons with or without T2DM. A: ApoA-IV relative gene expression. B: ANGPTL4 relative gene expression. C: MTP relative gene expression. Results are mean ± SD. ANGPTL4, angiopoietin-like protein 4; apo, apolipoprotein; MTP, microsomal triglyceride transfer protein; T2DM, type 2 diabetes mellitus.
Association between jejunal lipid content and other variables
Significant associations of plasma apoB48, apoA-IV, and chylomicron triglycerides with plasma variables and mRNA expression are shown in Table 2. There were no significant correlations with the remaining variables (data not shown).
TABLE 2.
Significant correlations (P) between plasma levels of apoA-IV, apoB48, CM triglycerides, jejunal triglyceride content, and jejunal apoB signal intensity and other anthropometric and biochemical variables
| Variable | ApoA-IV | ApoB48 | CM Triglycerides | Jejunal Triglycerides | Jejunal ApoBSignal Intensity |
|---|---|---|---|---|---|
| Glucose | 0.495 (0.001) | 0.596 (0.020) | 0.875 (<0.001) | −0.786 (<0.001) | −0.675 (0.006) |
| Insulin | 0.354 (0.009) | 0.561 (0.030) | 0.521 (0.046) | −0.632 (0.011) | −0.625 (0.013) |
| CM cholesterol | NS | 0.514 (0.040) | 0.670 (0.009) | −0.596 (0.025) | −0.771 (0.001) |
| Triglycerides | 0.513 (0.030) | 0.5974(0.020) | 0.836 (<0.001) | −0.692 (0.003) | −0.661 (0.005) |
| CM triglycerides | NS | 0.774 (<0.001) | – | −0.791 (<0.001) | −0.724 (0.002) |
| ApoB48 | 0.471 (0.023) | – | 0.774 (<0.001) | −0.768 (0.001) | −0.674 (0.004) |
| HOMA-IR | 0.560 (<0.001) | 0.688 (0.003) | 0.700 (0.003) | −0.774 (0.001) | −0.747 (0.001) |
| Jejunal triglycerides | NS | −0.768 (0.001) | −0.791 (<0.001) | – | 0.776 (<0.001) |
| ApoA-IV mRNA | NS | NS | NS | −0.587 (0.027) | NS |
| ANGPTL4 mRNA | NS | NS | NS | NS | NS |
| MTP mRNA | NS | −0.539 (0.038) | −0.564 (0.028) | NS | NS |
ANGPTL4, angiopoietin-like protein 4; apo, apolipoprotein; CM, chylomicron; HOMA-IR, homeostatic model assessment of insulin resistance; MTP, microsomal triglyceride transfer protein; NS, not significant.
ApoA-IV mRNA expression correlated with weight (r = −0.513, P = 0.013) and the jejunal triglyceride content (r = −0.587, P = 0.027). ANGPTL4 mRNA expression correlated with glucose (r = −0.676, P < 0.001), waist (r = 0.583, P = 0.011), and hip circumference (r = 0.573, P = 0.013). MTP mRNA expression also correlated with apoB (r = 0.558, P = 0.047), apoB48 (r = −0.539, P = 0.038), and chylomicron triglycerides (r = −0.564, P = 0.028).
Significant correlations were found between the jejunal triglyceride content and jejunal apoB signal intensity and the plasma levels of glucose, insulin, triglycerides, chylomicron triglycerides, apoB48, and HOMA-IR (Table 2). Jejunal triglycerides also correlated with apoA-IV mRNA expression (Table 2). The variables that best explained the jejunal triglyceride content in a multiple linear regression model were the HOMA-IR and the apoA-IV mRNA expression (Table 3).
TABLE 3.
Multiple linear regression analysis of jejunal triglyceride content
| Variable | B coefficient | SE B | β | 95% CI | P |
|---|---|---|---|---|---|
| Constant | 4.686 | 3.957 | (−4.670) – (14.042) | 0.275 | |
| Age | 0.034 | 0.065 | 0.115 | (−0.118) – (0.187) | 0.611 |
| Sex | 0.853 | 1.620 | 0.113 | (−2.977) – (4.683) | 0.615 |
| MTP mRNA | 0.020 | 0.013 | 0.335 | (−0.011) – (0.050) | 0.166 |
| ANGPTL4 mRNA | −0.001 | 0.006 | −0.024 | (−0.014) – (0.013) | 0.919 |
| ApoA-IV mRNA | −0.022 | 0.009 | −0.532 | (−0.043) – (−0.001) | 0.041 |
| HOMA-IR | −0.349 | 0.129 | −0.586 | (−0.654) – (−0.045) | 0.030 |
R2 for the model: 0.712. Sex: men = 1, women = 2. ANGPTL4, angiopoietin-like protein 4; apo, apolipoprotein; HOMA-IR, homeostatic model assessment of insulin resistance; MTP, microsomal triglyceride transfer protein; SE, standard error.
DISCUSSION
This study shows that the lipid content of the jejunum in morbidly obese persons varies significantly between patients with or without T2DM, and it is inversely related with the plasma levels of chylomicrons, HOMA-IR, and apoA-IV mRNA expression.
We found that morbidly obese persons with T2DM had higher plasma concentrations of triglycerides and chylomicrons, both of which correlated positively with the level of insulin resistance. Like others (19), we found no significant association between apoB48 concentrations and body mass index (BMI). However, we cannot conclude that this increase is the result of greater synthesis. Although different studies show an increase in the intestinal production of apoB48-containing lipoproteins in an animal model of insulin resistance (1), other studies show that this rise in plasma apoB48 in states of insulin resistance may be mainly due to lower plasma clearance of the remnant-like lipoprotein particles (7).
Recent research has focused on the physiological processes associated with how the intestine may exacerbate dyslipidemia in obesity and insulin-resistance (20). In our study we show that, when fasting, the morbidly obese persons without T2DM had a higher intestinal content of triglycerides. This finding agrees with the marked increase in the accumulation of neutral lipids in the lamina propria detected by Oil Red-O staining of the jejunum (Fig. 2B, D). Consistent with these results obtained with Oil Red-O staining, the jejunal samples from the morbidly obese persons without T2DM contained more signal corresponding to apoB and in the same place as the neutral lipids (Fig. 2F, H). These data support the suggestion that the lipid droplets detected with Oil Red-O staining correspond to intestinal lipoproteins. If the jejunal triglycerides were in the lamina propria blood vessels, they should reflect the plasma triglyceride levels in the fasted state. However, the levels of plasma triglycerides in the morbidly obese subjects with T2DM were higher, and the jejunal triglycerides were lower. This finding is also supported by the results showing a negative correlation between jejunal triglycerides and plasma apoB48 and plasma and chylomicron triglycerides, as well as between apoB signal intensity and plasma apoB48 and plasma and chylomicron triglycerides.
Once the chylomicrons are synthesized, they are secreted into the mesenteric lymph. During active lipid absorption in the rat, mucosal triglycerides can be divided into at least two pools (21). The first pool subserves the chylomicrons, with eventual delivery of the triglycerides into the lymph. The other pool appears not to be transported from the intestinal cell into the lymph but, rather, exits the mucosa via the portal vein (22). Little else is known about this. We hypothesize that intestinal lipoproteins may be more rapidly transferred out of the intestine in diabetic patients. The smaller size of these lipoproteins in the fasting state in these insulin-resistant persons may be involved in their reduced intestinal accumulation (1–3). The lipoproteins are more easily transported because they cross the endothelial barrier better and thus are not so readily retained in the lamina propria.
Few human studies so far have shown the production of intestinal lipoproteins in states of insulin resistance and/or diabetes (8, 23). As far as we are aware, this is the first study to show that the concentration of jejunal triglycerides is inversely associated with the levels of glycemia and insulin resistance. Nevertheless, little information is available about the intracellular mechanisms involved in the synthesis, assembly, and secretion of these TRL in the intestine in states of insulin resistance. This insulin resistance increases the secretion of TRL (23, 24); however, the mechanism responsible is not fully known. Evidence from fructose-fed hamster models suggests that the intestinal overproduction of TRL may result from an interaction among the induction of de novo lipogenesis, greater bioavailability of core lipids, greater intracellular apoB48 stability, and the abundance of MTP and apoA-IV, all of which produce greater lipoprotein assembly and secretion (1).
This possible increase in the synthesis and secretion of TRL may be supported by the results found in apoA-IV expression, which is increased in morbidly obese persons with T2DM (25). ApoA-IV synthesis and secretion by the gastrointestinal tract are upregulated by insulin (25–27). ApoA-IV enhances basolateral TG secretion in a dose-dependent manner by increasing the size of secreted lipoproteins (10). However, this experiment was undertaken in a newborn swine enterocyte cell line (IPEC-1) cultured with oleic acid. Our study conditions were different. In our study, samples were taken during fasting from human patients with morbid obesity, and in this state, the enterocytes mostly produce VLDL (28), a lipoprotein that is smaller and contains less mass than chylomicrons produced during feeding. Reports support the idea that intestinal chylomicron and VLDL can be produced and regulated by separate pathways (29). In addition, MTP is responsible for the lipidation of the immature chylomicron particle (30). Although previous studies have shown an increased intestinal MTP expression in an animal model of insulin resistance (31) and in humans (32), our results showed no significant differences between the two morbidly obese groups.
Some studies refer to the possibility that highly truncated forms of apoB can be assembled independently of MTP (i.e., that the initiation of an apoB proteolipid complex could be MTP-independent (33, 34)). Kulinski et al. (35) show that MTP does not appear to transfer the majority of triglycerides to apoB. They propose that when MTP is inhibited, the accumulation of apoB-independent triglycerides in the endoplasmic reticulum lumen is compromised, resulting in the availability of less triglycerides for assembly with apoB. However, it is widely accepted that MTP is essential for the transport of triglycerides into the endoplasmic reticulum for second-step assembly (35, 36). The greater apoA-IV expression in morbidly obese patients with T2DM, together with the same MTP expression, could contribute to a greater synthesis of these TRL but with fewer triglycerides.
ANGPTL4 may also play an important role in the size of the TRL. This protein is an inhibitor of lipoprotein lipase (LPL) activity (37, 38). In this study, we showed that the jejunum expressed ANGPTL4, significantly less so in the morbidly obese patients with T2DM, and that it was inversely associated with the glucose concentrations and the HOMA-IR. Previous studies have shown that serum levels of ANGPTL4 correlated inversely with plasma glucose concentrations and HOMA-IR (39) and that they were significantly lower in patients with T2DM. This lower expression of ANGPTL4 in the intestine of morbidly obese patients with T2DM would result in greater intestinal LPL activity than that which is found in the intestine of the patients without T2DM. Thus, a greater number of recently secreted TRL triglycerides could be being hydrolyzed, in this way indirectly contributing to a smaller size of these TRL. However, further studies are required to examine this hypothesis and the mechanisms involved. In other tissues, LPL and ANGPTL4 regulation can differ according to the tissue involved.
The lower fat concentration found in the intestine of the morbidly obese persons with T2DM was unexpected. In other tissues, such as the muscle or pancreatic islets, the accumulation of fat is accompanied by a state of insulin resistance and forms part of the mechanism explaining the pathophysiology of the metabolic syndrome. This intestinal fat pattern could be part of the mechanism of the metabolic syndrome, or insulin resistance, or both, or else a consequence. However, our study did not show whether the low jejunal triglyceride levels reported in the morbidly obese patients with T2DM were due to altered production and/or secretion. This is difficult to estimate from a single-time sample. Although most of the morbidly obese patients with T2DM were receiving treatment with metformin, we found no significant differences in the variables according to whether or not they were receiving it. However, studies with more patients are required to determine the effect of metformin treatment on jejunal triglyceride content. A limitation of this study concerns the lack of a control group to see the effect of obesity on the jejunal triglyceride content.
In conclusion, our results show an inverse correlation between the jejunal lipid content and the presence of T2DM. These findings show that the small intestine appears to play an active role in lipid homeostasis in the fasting state. However, further studies are required to determine the different intestinal regulatory mechanisms and factors involved. Caution should be exercised when extrapolating results from morbidly obese patients to less pathological forms of obesity/diabetes. Better awareness of these processes is fundamental to understanding how lipidemia due to greater production and/or secretion of these intestinal lipoproteins is related to the cardiovascular complications found in persons with metabolic syndrome or diabetes.
Acknowledgments
The authors thank Lourdes Sanchez and Ana Gomez for technical assistance and Ian Johnstone for help with the English-language version of the text.
Footnotes
Abbreviations:
- ANGPTL4
- angiopoietin-like protein 4
- apo
- apolipoprotein
- BMI
- body mass index
- CM
- chylomicron
- HOMA-IR
- homeostasis model assessment of insulin resistance
- LPL
- lipoprotein lipase
- MTP
- microsomal triglyceride transfer protein
- T2DM
- type 2 diabetes mellitus
- TRL
- triglyceride-rich lipoprotein
This work was supported by Instituto de Salud Carlos III (ISCIII) Grant CP04/00133. CIBER Fisiopatología de la Obesidad y Nutrición (CIBEROBN) and CIBER de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM) are ISCIII projects.
REFERENCES
- 1.Haidari M., Leung N., Mahbub F., Uffelman K. D., Kohen-Avramoglu R., Lewis G. F., Adeli K. 2002. Fasting and postprandial overproduction of intestinally derived lipoproteins in an animal model of insulin resistance. Evidence that chronic fructose feeding in the hamster is accompanied by enhanced intestinal de novo lipogenesis and ApoB48-containing lipoprotein overproduction. J. Biol. Chem. 277: 31646–31655. [DOI] [PubMed] [Google Scholar]
- 2.Guo Q., Avramoglu R. K., Adeli K. 2005. Intestinal assembly and secretion of highly dense/lipid-poor apolipoprotein B48-containing lipoprotein particles in the fasting state: evidence for induction by insulin resistance and exogenous fatty acids. Metabolism. 54: 689–697. [DOI] [PubMed] [Google Scholar]
- 3.Lewis G. F., Uffelman K., Naples M., Szeto L., Haidari M., Adeli K. 2005. Intestinal lipoprotein overproduction, a newly recognized component of insulin resistance, is ameliorated by the insulin sensitizer rosiglitazone: studies in the fructose-fed Syrian golden hamster. Endocrinology. 146: 247–255. [DOI] [PubMed] [Google Scholar]
- 4.Risser T. R., Reaven G. M., Reaven E. P. 1978. Intestinal contribution to secretion of very low density lipoproteins into plasma. Am. J. Physiol. 234: E277–E281. [DOI] [PubMed] [Google Scholar]
- 5.Ockner R. K., Hughes F. B., Isselbacher K. J. 1969. Very low density lipoproteins in intestinal lymph: origin, composition, and role in lipid transport in the fasting state. J. Clin. Invest. 48: 2079–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curtin A., Deegan P., Owens D., Collins P., Johnson A., Tomkin G. H. 1996. Elevated triglyceride-rich lipoproteins in diabetes. A study of apolipoprotein B-48. Acta Diabetol. 33: 205–210. [DOI] [PubMed] [Google Scholar]
- 7.Haffner S. M., Foster D. M., Kushwaha R. S., Hazzard W. R. 1984. Retarded chylomicron apolipoprotein-B catabolism in type 2 (non-insulin-dependent) diabetic subjects with lipaemia. Diabetologia. 26: 349–354. [DOI] [PubMed] [Google Scholar]
- 8.Duez H., Lamarche B., Valéro R., Pavlic M., Proctor S., Xiao C., Szeto L., Patterson B. W., Lewis G. F. 2008. Both intestinal and hepatic lipoprotein production are stimulated by an acute elevation of plasma free fatty acids in humans. Circulation. 117: 2369–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black D. D. 2007. Development and physiological regulation of intestinal lipid absorption. I. Development of intestinal lipid absorption: cellular events in chylomicron assembly and secretion. Am. J. Physiol. Gastrointest. Liver Physiol. 293: G519–G524. [DOI] [PubMed] [Google Scholar]
- 10.Lu S., Yao Y., Cheng X., Mitchell S., Leng S., Meng S., Gallagher J. W., Shelness G. S., Morris G. S., Mahan J., et al. 2006. Overexpression of apolipoprotein A-IV enhances lipid secretion in IPEC-1 cells by increasing chylomicron size. J. Biol. Chem. 281: 3473–3483. [DOI] [PubMed] [Google Scholar]
- 11.Lu S., Yao Y., Meng S., Cheng X., Black D. D. 2002. Overexpression of apolipoprotein A-IV enhances lipid transport in newborn swine intestinal epithelial cells. J. Biol. Chem. 277: 31929–31937. [DOI] [PubMed] [Google Scholar]
- 12.Ge H., Yang G., Yu X., Pourbahrami T., Li C. 2004. Oligomerization state-dependent hyperlipidemic effect of angiopoietin-like protein 4. J. Lipid Res. 45: 2071–2079. [DOI] [PubMed] [Google Scholar]
- 13.Gil-Villarino A., Garcia-Fuentes E., Zafra F., Garcia-Peregrin E. 1998. Production of a rapid hypercholesterolemia in young chick by feeding coconut oil from two different sources and fatty acid composition. Nutr. Res. 18: 1273–1285. [Google Scholar]
- 14.Macias-Gonzalez M., Moreno-Santos I., García-Almeida J. M., Tinahones F. J., Garcia-Fuentes E. 2009. PPARgamma2 protects against obesity by means of a mechanism that mediates insulin resistance. Eur. J. Clin. Invest. 39: 972–979. [DOI] [PubMed] [Google Scholar]
- 15.Cardona F., Tinahones F. J., Collantes E., Escudero A., García-Fuentes E., Soriguer F. J. 2003. The elevated prevalence of apolipoprotein E2 in patients with gout is associated with reduced renal excretion of urates. Rheumatology (Oxford). 42: 468–472. [PubMed] [Google Scholar]
- 16.García-Fuentes E., Gil-Villarino A., Zafra M. F., García-Peregrín E. 2000. Hypocholesterolemic activity of dipyridamole: effects on chick plasma and lipoprotein composition and arachidonic acid levels. Environ. Toxicol. Pharmacol. 8: 261–266. [DOI] [PubMed] [Google Scholar]
- 17.Verseyden C., Meijssen S., Castro Cabezas M. 2002. Postprandial changes of apoB-100 and apoB-48 in TG rich lipoproteins in familial combined hyperlipidemia. J. Lipid Res. 43: 274–280. [PubMed] [Google Scholar]
- 18.Vine D. F., Takechi R., Russell J. C., Proctor S. D. 2007. Impaired postprandial apolipoprotein-B48 metabolism in the obese, insulin-resistant JCR:LA-cp rat: increased atherogenicity for the metabolic syndrome. Atherosclerosis. 190: 282–290. [DOI] [PubMed] [Google Scholar]
- 19.Hogue J. C., Lamarche B., Tremblay A. J., Bergeron J., Gagne C., Couture P. 2007. Evidence of increased secretion of apolipoprotein B-48-containing lipoproteins in subjects with type 2 diabetes. J. Lipid Res. 48: 1336–1342. [DOI] [PubMed] [Google Scholar]
- 20.Vine D. F., Glimm D. R., Proctor S. D. 2008. Intestinal lipid transport and chylomicron production: possible links to exacerbated atherogenesis in a rodent model of the metabolic syndrome. Atheroscler. Suppl. 9: 69–76. [DOI] [PubMed] [Google Scholar]
- 21.Nevin P., Koelsch D., Mansbach C. M., 2nd 1995. Intestinal triacylglycerol storage pool size changes under differing physiological conditions. J. Lipid Res. 36: 2405–2412. [PubMed] [Google Scholar]
- 22.Mansbach C. M., II, Dowell R. F., Pritchett D. 1991. Portal transport of absorbed lipids in rats. Am. J. Physiol. 261: G530–G538. [DOI] [PubMed] [Google Scholar]
- 23.Duez H., Lamarche B., Uffelman K. D., Valero R., Cohn J. S., Lewis G. F. 2006. Hyperinsulinemia is associated with increased production rate of intestinal apolipoprotein B-48-containing lipoproteins in humans. Arterioscler. Thromb. Vasc. Biol. 26: 1357–1363. [DOI] [PubMed] [Google Scholar]
- 24.Federico L. M., Naples M., Taylor D., Adeli K. 2006. Intestinal insulin resistance and aberrant production of apolipoprotein B48 lipoproteins in an animal model of insulin resistance and metabolic dyslipidemia: evidence for activation of protein tyrosine phosphatase-1B, extracellular signal-related kinase, and sterol regulatory element-binding protein-1c in the fructose-fed hamster intestine. Diabetes. 55: 1316–1326. [DOI] [PubMed] [Google Scholar]
- 25.Hanniman E. A., Lambert G., Inoue Y., Gonzalez F. J., Sinal C. J. 2006. Apolipoprotein A-IV is regulated by nutritional and metabolic stress: involvement of glucocorticoids, HNF-4a, and PGC-1a. J. Lipid Res. 47: 2503–2514. [DOI] [PubMed] [Google Scholar]
- 26.Attia N., Touzani A., Lahrichi M., Balafrej A., Kabbaj O., Girard-Globa A. 1997. Response of apolipoprotein AIV and lipoproteins to glycaemic control in young people with insulin-dependent diabetes mellitus. Diabet. Med. 14: 242–247. [DOI] [PubMed] [Google Scholar]
- 27.Black D. D., Ellinas H. 1992. Apolipoprotein synthesis in newborn piglet intestinal explants. Pediatr. Res. 32: 553–558. [DOI] [PubMed] [Google Scholar]
- 28.Ockner R. K., Manning J. A. 1974. Fatty acid-binding protein in small intestine. Identification, isolation, and evidence for its role in cellular fatty acid transport. J. Clin. Invest. 54: 326–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kindel T., Lee D. M., Tso P. 2010. The mechanism of the formation and secretion of chylomicrons. Atheroscler. Suppl. 11: 11–16. [DOI] [PubMed] [Google Scholar]
- 30.Wetterau J. R., Aggerbeck L. P., Bouma M. E., Eisenberg C., Munck A., Hermier M., Schmitz J., Gay G., Rader D. J., Gregg R. E. 1992. Absence of microsomal triglyceride transfer protein in individuals with abetalipoproteinemia. Science. 258: 999–1001. [DOI] [PubMed] [Google Scholar]
- 31.Lewis G. F., Naples M., Uffelman K., Leung N., Szeto L., Adeli K. 2004. Intestinal lipoprotein production is stimulated by an acute elevation of plasma free fatty acids in the fasting state: studies in insulin-resistant and insulin-sensitized Syrian golden hamsters. Endocrinology. 145: 5006–5012. [DOI] [PubMed] [Google Scholar]
- 32.Phillips C., Mullan K., Owens D., Tomkin G. H. 2006. Intestinal microsomal triglyceride transfer protein in type 2 diabetic and non-diabetic subjects: the relationship to triglyceride-rich postprandial lipoprotein composition. Atherosclerosis. 187: 57–64. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y., Manchekar M., Sun Z., Richardson P. E., Dashti N. 2010. Apolipoprotein B-containing lipoprotein assembly in microsomal triglyceride transfer protein-deficient McA-RH7777 cells. J. Lipid Res. 51: 2253–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dashti N., Manchekar M., Liu Y., Sun Z., Segrest J. P. 2007. Microsomal triglyceride transfer protein activity is not required for the initiation of apolipoprotein B-containing lipoprotein assembly in McA-RH7777 cells. J. Biol. Chem. 282: 28597–28608. [DOI] [PubMed] [Google Scholar]
- 35.Kulinski A., Rustaeus S., Vance J. E. 2002. Microsomal triacylglycerol transfer protein is required for lumenal accretion of triacylglycerol not associated with ApoB, as well as for ApoB lipidation. J. Biol. Chem. 277: 31516–31525. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y., Tran K., Yao Z. 1999. The activity of microsomal triglyceride transfer protein is essential for accumulation of triglyceride within microsomes in McA-RH7777 cells. A unified model for the assembly of very low density lipoproteins. J. Biol. Chem. 274: 27793–27800. [DOI] [PubMed] [Google Scholar]
- 37.Mandard S., Zandbergen F., van Straten E., Wahli W., Kuipers F., Muller M., Kersten S. 2006. The fasting-induced adipose factor/angiopoietin-like protein 4 is physically associated with lipoproteins and governs plasma lipid levels and adiposity. J. Biol. Chem. 281: 934–944. [Erratum. 2006. J. Biol. Chem. 281: 21575.] [DOI] [PubMed] [Google Scholar]
- 38.Yoshida K., Shimizugawa T., Ono M., Furukawa H. 2002. Angiopoietin-like protein 4 is a potent hyperlipidemia inducing factor in mice and inhibitor of lipoprotein lipase. J. Lipid Res. 43: 1770–1772. [DOI] [PubMed] [Google Scholar]
- 39.Xu A., Lam M. C., Chan K. W., Wang Y., Zhang J., Hoo R. L., Xu J. Y., Chen B., Chow W. S., Tso A. W., et al. 2005. Angiopoietin-like protein 4 decreases blood glucose and improves glucose tolerance but induces hyperlipidemia and hepatic steatosis in mice. Proc. Natl. Acad. Sci. USA. 102: 6086–6091. [DOI] [PMC free article] [PubMed] [Google Scholar]