Abstract
A low level of HDL-C is the most common plasma lipid abnormality observed in men with established coronary heart disease (CHD). To identify allelic variants associated with susceptibility to low HDL-C and CHD, we examined 60 candidate genes with key roles in HDL metabolism, insulin resistance, and inflammation using samples from the Veterans Affairs HDL Intervention Trial (VA-HIT; cases, n = 699) and the Framingham Offspring Study (FOS; controls, n = 705). VA-HIT was designed to examine the benefits of HDL-raising with gemfibrozil in men with low HDL-C (≤40 mg/dl) and established CHD. After adjustment for multiple testing within each gene, single-nucleotide polymorphisms (SNP) significantly associated with case status were identified in the genes encoding LIPC (rs4775065, P < 0.0001); CETP (rs5882, P = 0.0002); RXRA (rs11185660, P = 0.0021); ABCA1 (rs2249891, P = 0.0126); ABCC6 (rs150468, P = 0.0206; rs212077, P = 0.0443); CUBN (rs7893395, P = 0.0246); APOA2 (rs3813627, P = 0.0324); SELP (rs732314, P = 0.0376); and APOC4 (rs10413089, P = 0.0425). Included among the novel findings of this study are the identification of susceptibility alleles for low HDL-C/CHD risk in the genes encoding CUBN and RXRA, and the observation that genetic variation in SELP may influence CHD risk through its effects on HDL.
Keywords: genetics, high density lipoprotein cholesterol, candidate genes
Coronary heart disease (CHD) is a major cause of death and disability in our society, resulting in approximately 450,000 deaths per year in the United States (2009 Heart and Stroke Statistical Update). Numerous population studies have shown that a strong inverse relationship exists between plasma high density lipoprotein cholesterol (HDL-C) concentrations and CHD risk (1–4). Approximately 50% of men with CHD have a low level of HDL-C, and not elevated low density lipoprotein cholesterol (LDL-C), as their primary lipid abnormality (5). In fact, a study in men has shown that a low level of HDL-C better distinguishes CHD status than a high level of LDL-C (6).
Environmental and metabolic factors that are commonly associated with low HDL-C concentrations include alcohol consumption, dietary saturated fat, decreased exercise, cigarette smoking, obesity, and diabetes (7–9). Low HDL-C is commonly seen in association with the metabolic syndrome, which is characterized by visceral obesity, elevated glucose and blood pressure, dyslipidemia, and frequently, insulin resistance and a pro-inflammatory state (10). In addition to environmental factors, strong evidence also exists for the role of genetics in the determination of HDL-C levels. HDL-C level is a heritable characteristic, with heritability estimates in the range of 40-60% (11). Most of the variation in HDL-C observed at the population level is of multifactorial origin and is the result of the complex interaction between genetic and environmental factors.
In the present study, we utilized a candidate gene approach to extend our earlier work (12–14) with cases recruited through the Veterans Affairs HDL Intervention Trial (VA-HIT) and controls selected from the Framingham Offspring Study (FOS). VA-HIT was designed to examine the benefits of HDL-raising with gemfibrozil in men with low HDL-C (≤40 mg/dl) and prevalent CHD (15). To identify susceptibility loci for the low HDL-C/CHD trait, we examined the allelic variation of 1,114 single-nucleotide polymorphisms (SNP) in 60 candidate genes using cases from VA-HIT before treatment and controls selected from the FOS. We hypothesized that susceptibility to the low HDL-C/CHD trait would be associated with common variants derived from genes involved in HDL metabolism, insulin resistance, and inflammation. We further compared the cases to controls having an HDL-C of < 40 mg/dl, as well as to controls having an HDL-C of ≥ 40 mg/dl. These comparisons allowed us to evaluate whether the genetic variants were influencing CHD through HDL-C levels or via alternative mechanisms.
METHODS
Study design
The rationale, design, and methods for VA-HIT have been described elsewhere in detail (15). Briefly, men were recruited at 20 Veterans Affairs (VA) medical centers throughout the United States. Eligibility for the trial required a documented history of CHD (defined as a history of myocardial infarction, angina corroborated by objective evidence of ischemia, coronary revascularization, or angiographic evidence of stenosis greater than 50% of the luminal diameter in one or more major epicardial coronary arteries); age < 74 years; HDL-C level ≤ 40 mg/dl (1.0 mmol/l); LDL-C level ≤ 140 mg/dl (3.6 mmol/l); plasma triglyceride concentration ≤ 300 mg/dl (3.4 mmol/l); and absence of coexisting conditions (cancer, excluding skin; other life-threatening diseases, such as chronic pulmonary or kidney disease; alcohol or substance abuse; cholelithiasis; or marked left ventricular dysfunction). Information on age, alcohol consumption, smoking status, blood pressure, body mass index (BMI), and diabetes was available for all subjects enrolled in VA-HIT. Based on the existence of informed consent for genotyping analysis, 870 subjects from the VA-HIT study were available for the present study as cases. Subjects were self-identified as Caucasian (n = 808) or nonCaucasian race (n = 58) (4 missing information). Data used in our statistical analyses were obtained at baseline, before treatment with gemfibrozil. The characteristics of the cases at baseline, as well as basic demographic information, are provided in Table 1.
TABLE 1.
Baseline characteristics of subjects
| Characteristic | Cases(n = 699) | Controls(n = 705) | Controls HDL < 40 (n = 270) | Controls HDL ≥ 40 (n = 435) | Pa |
|---|---|---|---|---|---|
| Age (years) | 64.29 ± 7.2 | 58.67 ± 8.82 | 58.8 ± 8.7 | 58.59 ± 8.9 | <0.0001 |
| Age category (years) | <0.0001 | ||||
| ≤50 | 41 (5.87) | 132 (18.72) | 45 (16.67) | 87 (20.00) | |
| 51–60 | 133 (19.03) | 294 (41.7) | 121 (44.81) | 173 (39.77) | |
| 61–70 | 375 (53.65) | 201 (28.51) | 73 (27.04) | 128 (29.43) | |
| Over 70 | 150 (21.46) | 78 (11.06) | 31 (11.48) | 47 (10.80) | |
| Body mass index (kg/m2) | 29.28 ± 4.56 | 28.47 ± 4.3 | 29.6 ± 4.4 | 27.77 ± 4.09 | 0.0006 |
| Alcohol (>1 drink/day) | 44 (6.29) | 49 (7.05) | 4 (1.5) | 45 (10.51) | 0.5718 |
| Current smoker | 123 (17.6) | 99 (14.06) | 42 (15.61) | 57 (13.1) | 0.0697 |
| Diabetic | 190 (27.18) | 54 (7.66) | 29 (10.74) | 25 (5.75) | <0.0001 |
| Hypertensive | 636 (90.99) | 290 (41.19) | 125 (46.47) | 165 (37.93) | <0.0001 |
| Cholesterol | |||||
| Total (mg/dl) | 178.75 ± 24.37 | 201.56 ± 34.2 | 196.4 ± 34.87 | 204.77 ± 33.42 | <0.0001 |
| LDL (mg/dl) | 114.45 ± 22.36 | 130.11 ± 30.7 | 128.29 ± 30.62 | 131.21 ± 30.73 | <0.0001 |
| HDL (mg/dl) | 31.64 ± 4.99 | 44.29 ± 11.96 | 33.03 ± 4.63 | 51.27 ± 9.54 | <0.0001 |
| HDL < 40 | 270 (38.3) | –b | |||
| HDL ≥ 40 | 435 (61.7) | –b | |||
| Triglycerides (mg/dl) | 163.94 ± 63.86 | 140.97 ± 101.46 | 186.06 ± 130.52 | 112.99 ± 63.95 | <0.0001 |
| Blood pressure | |||||
| Systolic (mmHg) | 131.95 ± 18.48 | 130.28 ± 16.88 | 130.78 ± 16.61 | 129.97 ± 17.06 | 0.0782 |
| Diastolic (mmHg) | 76.64 ± 9.82 | 77.97 ± 8.96 | 78.69 ± 8.82 | 77.52 ± 9.03 | 0.008 |
| Drug therapy | |||||
| Diabetes medication | 114 (16.31) | 28 (3.97) | 18 (6.67) | 10 (2.3) | <0.0001 |
| Antihypertensive | 570 (81.55) | 180 (25.53) | 81 (30) | 99 (22.76) | <0.0001 |
Values are either mean ± SD or n (%).
A two sample t-test was used to obtain the P value for continuous variables, and a chi-square test was used to obtain significance for categorical variables to compare cases (n = 699) to all controls (n = 705).
Cannot perform test due to missing information.
The Framingham Offspring Study is a longitudinal study designed to identify risk factors for cardiovascular disease in subjects from Framingham, MA (16). A total of 866 males from FOS were selected for use as controls in our case-control analysis. Controls were free of CHD, at least 39 years old at exam six (data collected between 1995 and 1998), and self-reported Caucasian. Plasma lipid and demographic information for the controls at exam six are provided in Table 1.
All participants in both cohorts provided written informed consent. The VA-HIT study was approved by the Human Rights Committee of the Cooperative Studies Program Coordinating Center and by the Institutional Review Board (IRB) of each VA medical center. Study protocols were approved by the IRB for human research at Tufts University, Boston University, and the Broad Institute.
Biochemical analyses
Plasma lipid concentrations were determined from blood samples collected from subjects, after a 12-14 h fast, into tubes containing 0.1% EDTA. Plasma was isolated and frozen for subsequent analysis of plasma lipid, lipoprotein, and apolipoprotein (apo) concentrations. Plasma total cholesterol and triglyceride concentrations were determined using enzymatic assays (17). Plasma HDL-C concentrations were measured after dextran sulfate-magnesium precipitation of apoB-containing lipoproteins (18). LDL-C levels were calculated with the equation of Friedewald et al. (19).
SNP selection and genotyping
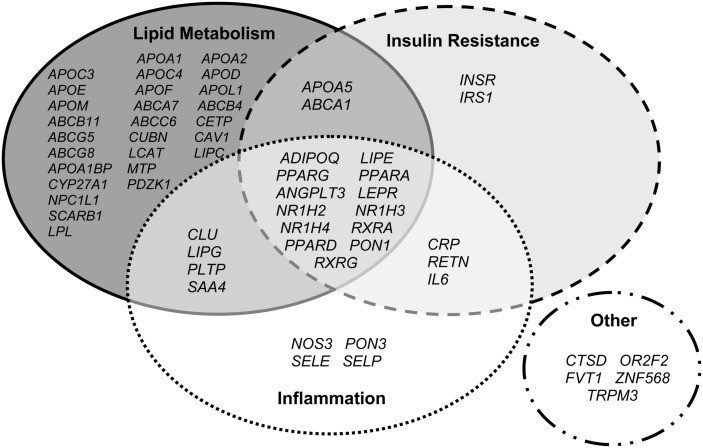
Because HDL-C is a complex trait, we considered it important to explore common variation not only in genes whose products directly influence HDL metabolism but also in genes whose products may indirectly influence HDL concentrations. Thus, candidate genes included those associated with diabetes and/or insulin resistance, as well as those involved in inflammatory pathways. As illustrated in Fig. 1, many of the candidate genes have been associated with more than one phenotype (i.e., LIPG with lipoprotein metabolism and inflammation). For each candidate gene, the entire coding length of the gene (from the first 5′ and 3′ UTR) plus 10 kb upstream and downstream of the gene were downloaded from HapMap for the CEU sample (Genome Build 36, and Version 126 of dbSNP), allowing us to search for variation in the promoter and other regulatory regions. SNPs were selected using the Tagger algorithm (www.broad.mit.edu/mpg/tagger) to identify SNPs (tagSNP) within each gene having a pair-wise r2 > 0.9 (20). Priority was given to SNPs that had higher design scores for Illumina genotyping. A list of all the candidate genes and number of tagSNPs selected in each gene is presented in supplementary Table I. To address potential population structure, 274 ancestry informative markers (AIM) were selected for genotyping (21–23). Inclusion criteria for AIMs included 1) not within 60 base pairs (bp) of another AIM SNP; 2) not in a candidate gene; 3) a minor allele frequency > 0.05; 4) an Illumina design score > 0.6; and 5) an appropriate Illumina validation class.
Fig. 1.
Candidate genes selected for analysis. Because HDL-C is a complex trait, we explored common variations in genes whose products directly influence HDL metabolism, as well as in genes whose products may indirectly influence HDL concentrations (diabetes, insulin resistance, and inflammation).
Genomic DNA was extracted from whole blood samples using either QIAamp mini kits (Qiagen) or Generation Capture Column kits (Gentra Systems). DNA isolated from the case samples was of low concentration and was whole-genome-amplified (WGA) to provide sufficient DNA for genotyping. For consistency, control DNA samples underwent WGA as well. Genotyping was performed using Illumina GoldenGate Bead Array platform with a 1536-SNP OPA (GS0010268-OPA). Sentrix Array Matrix (SAM) fiber-optic bundles manufactured at Illumina using two lots of oligo-beads (beadtype v4 or v5) were used to image the genotypes. The use of different beadtypes is not expected to affect the performance of SNP assays. No duplicated samples were processed on both beadtypes, but allele frequencies were similar between beadtypes. Individuals with a call rate of < 90%, missing phenotype information, or ≥ 50% genotype data inconsistent with previous genotyping were omitted from analysis (supplementary Table II). A total of 1,404 individuals (cases, n = 699; controls, n = 705) met quality control criteria and were included in the final analysis. SNPs were excluded based on 1) call rate of < 0.90; 2) minor allele frequency (MAF) < 0.05; or 3) Hardy Weinberg Equilibrium (HWE) P value in the control sample < 0.001. A total of 1,355 SNPs (1,114 candidate gene SNPs and 241 AIMs) met these quality control criteria and were used in our analysis.
Assessment of population stratification
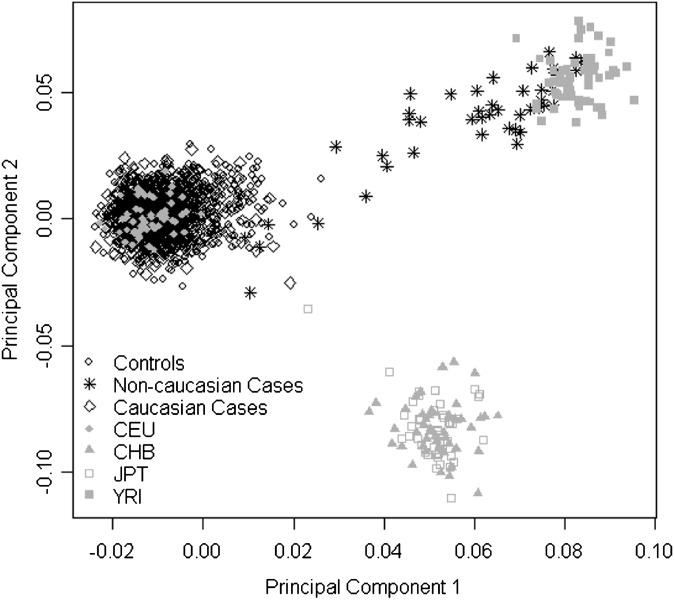
Population structure, or stratification, is the presence of a systematic difference in allele frequencies between subpopulations in a sample, possibly due to differences in ancestry. Population structure can cause bias in the results of an association study and can lead to false positive findings. The smartpca package in the software EIGENSTRAT (24, 25) was used on the AIMs to determine population structure within the sample. Smartpca performs efficient principal components analysis (PCA) on the genotype matrix and provides principal components (PC) that describe the genetic variability in the sample. A modified PCA (26), described below, was conducted on the study sample plus the four HapMap samples [Utah residents with Northern and Western European ancestry from the CEPH collection (CEU); Yoruba in Ibadan, Nigeria (West Africa) (YRI); Japanese in Tokyo, Japan (JPT); and Han Chinese in Beijing, China (CHB)] using the selected AIMs. Plotting PC1 versus PC2 shows that some subjects in our sample are near the YRI individuals (Fig. 2). Subjects clustering near the YRI sample (PC1 > 0.03), denoted with a nonCaucasian race, or removed based on outlier detection (see below), were excluded from analysis to obtain a homogeneous sample of Caucasian individuals for analysis (supplementary Table II). The rationale for excluding these subjects is based on the fact that, due to a lack of control subjects having a PC1 of > 0.03, we cannot adequately adjust for the nonCaucasian subjects in the cases. Including multiple population groups in a sample without appropriate adjustment leads to an increase in Type I error (27); therefore, only Caucasian individuals were included in the analyses.
Fig. 2.
Plot of PC1 and PC2 from PCA of study subjects and HapMap samples using ancestry informative markers to detect population structure. CEU, Utah residents with Northern and Western European ancestry from the CEPH collection; CHB, Han Chinese in Beijing, China; JPT, Japanese in Tokyo, Japan; YRI, PC, principal component; PCA, principal component analysis; Yoruba in Ibadan, Nigeria.
A modified PCA on the study subjects was performed (26). A few family members in a sample are known to bias a PCA, and the entire control sample may have extended family members. To take this into account, we performed PCA on the case sample and a subset of unrelated controls based on the full Framingham pedigree file, and then inferred the PCs for the remaining individuals based on the SNP weights. Thirty-three subjects were removed during EIGENSTRAT's default outlier removal process, which iteratively performs PCA, removing subjects that are more than six standard deviations from the mean for the top ten PCs over five iterations.
Statistical analyses
The primary outcome was the combined trait of low HDL-C/CHD. Low HDL-C was defined as HDL-C < 40 mg/dl. Subgroup analysis included comparing 1) low HDL-C/CHD and control subjects with HDL-C < 40 mg/dl; 2) low HDL-C/CHD and control subjects with HDL-C ≥ 40 mg/dl; and 3) control subjects with HDL-C < 40 mg/dl and control subjects with HDL-C ≥ 40 mg/dl. These comparisons allowed us to evaluate whether the genetic variants were influencing CHD through HDL-C levels or via alternative mechanisms. The first subgroup analysis provided a comparison of those with low HDL-C and CHD to those who have neither, whereas the second analysis provided a comparison of those who have CHD versus those without CHD among men with low HDL-C. The intra-cohort comparison within FOS allowed us to identify SNPs associated with HDL-C levels. Three covariate adjustments were made: 1) age and PC1-4 (labeled “MinAdj”); 2) age, PC1-4, body mass index (BMI), systolic blood pressure (SBP), diabetes status, smoking status, and alcohol use (“MultiAdj”); and 3) age, PC1-4, BMI, SBP, diabetes status, smoking status, alcohol use, HDL-C, triglycerides, and total cholesterol (“LipidAdj”). Adjusting for lipid levels allowed us to compare CHD cases to controls, after controlling for the differences in lipid levels. We note that differences in unmeasured exposures are always a concern and recognize this as a limitation of our work. All analyses used logistic regression in PLINK (28) with a general genetic model. A general model was used to avoid power loss due to mis-specifying the genetic model (29). We did not account for family structure in the association analyses, because only extended familial relationships (i.e., cousins) existed among some of the control subjects.
Statistical significance was determined using an empirical P value (AdjP) obtained by a permutation strategy (30) to adjust for testing multiple SNPs within each gene. A null distribution of minimum P values was generated based on permuting the outcome phenotype (keeping the corresponding covariate values) and performing the analysis as described above. We did not permute the genotypes to maintain the linkage disequilibrium within the genes intact. The minimum P value across all the SNPs in the same gene was documented for 10,000 permutations of the phenotype and compared with the observed P value. This approach provides correction for testing multiple SNPs within each gene. To take into account multiple outcomes, we only conducted subgroup analysis for SNPs that were significant in the primary analysis, as an approach to refine the association. False discovery rate (FDR) (31) was calculated in SAS based on the 60 genes (1,114 candidate gene SNPs) analyzed using the observed P values.
SNP interaction with age analyses were conducted with SNPs that were significantly different between cases and controls, after adjustment for multiple testing within each gene (PAdj < 0.05), and in silico function predictions were obtained (32).
RESULTS
Baseline characteristics of the cases (VA-HIT) and controls (FOS) are presented in Table 1. Significant differences in baseline characteristics were observed between the cases (n = 699) and controls (n = 705). Cases tended to be older and have higher BMI; there are a greater percentage of diabetic and hypertensive subjects relative to controls. Consistent with the selection criteria of VA-HIT, cases also had lower plasma concentrations of total, LDL-, and HDL-cholesterol and higher triglyceride levels compared with controls. Cases were also more likely to be on drug therapy (diabetes medication or antihypertensive medication) than their control counterparts. Note that statistical adjustments were made to account for the differences in these measures when testing for associations between each candidate gene and case status.
Table 2 shows the SNPs that were significantly different between cases and controls, after adjustment for multiple testing within each gene (PAdj < 0.05). SNPs significantly associated with case status were located in the genes encoding hepatic lipase (LIPC; rs4775065; P < 0.0001); cholesteryl ester transfer protein (CETP; rs5882; P = 0.0002); retinoid X receptor α (RXRA; rs11185660; P = 0.0021); ATP-binding cassette transporter A1 (ABCA1; rs2249891; P = 0.0126); ATP-binding cassette transporter C6 (ABCC6; rs150468 and rs212077; P = 0.0206 and P = 0.0443); cubilin (CUBN; rs7893395; P = 0.0246); apolipoprotein A-II (APOA2; rs3813627; P = 0.0324); P-selectin (SELP; rs732314; P = 0.0376); and apoC-IV (APOC4; rs10413089; P = 0.0425). All SNPs in Table 2 were in HWE, and similar results were seen with the minimal adjustment model (results not shown). None of the reported SNPs had a significant interaction with age (all interaction P values > 0.37). A model fit with the ten reported variants for the primary outcome of low HDL-C/CHD indicated a generalized-R2 of 0.14, indicating a 14% improvement over a null model for low HDL-C/CHD, with an area under the curve (AUC) of 0.687. Addition of risk factors (age, BMI, SBP, diabetic status, smoking status, alcohol consumption) to the ten reported variants provides a 37% improvement over the null model with an AUC of 0.810, while a model with just risk factors has a 24% improvement over the null model and an AUC of 0.751. Note, in this context a generalized-R2 should not be confused with the percent of variability explained in ordinary least square regression.
TABLE 2.
SNPs significantly associated with low HDL-C/CHD
| Chr | Location | Gene | SNP | Major/Minor Allele | Minor Allele Frequency in VA-HIT | Minor Allele Frequency in FOS | ORheta | ORhomb | P2dfc | PAdjd | PFDRe | PlipidAdjf | PHWEg |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15 | 56589235 | LIPC | rs4775065 | G/A | 0.33 | 0.23 | 2.36 (1.83, 3.05) | 1.54 (0.82, 2.89) | 3.82E-10 | < 0.0001 | 4.26E-07 | 1.15E-06 | 0.075 |
| 16 | 55573593 | CETP | rs5882 | A/G | 0.32 | 0.40 | 0.63 (0.48, 0.82) | 0.39 (0.26, 0.60) | 1.06E-05 | 0.0002 | 5.90E-03 | 0.0101 | 0.673 |
| 9 | 136399813 | RXRA | rs11185660 | T/C | 0.16 | 0.24 | 0.72 (0.55, 0.94) | 0.22 (0.10, 0.50) | 0.0002 | 0.0021 | 0.0623 | 2.42E-04 | 0.835 |
| 9 | 106664063 | ABCA1 | rs2249891 | A/G | 0.14 | 0.13 | 0.61(0.45, 0.85) | 2.7(1.26, 5.94) | 0.0003 | 0.0126 | 0.0656 | 3.40E-04 | 0.235 |
| 16 | 16159501 | ABCC6 | rs150468 | A/C | 0.13 | 0.19 | 0.70 (0.53, 0.92) | 0.34 (0.15, 0.73) | 0.0017 | 0.0206 | 0.1840 | 0.0500 | 0.469 |
| 10 | 16945506 | CUBN | rs7893395 | C/T | 0.17 | 0.23 | 0.58 (0.45, 0.76) | 0.77 (0.39, 1.55) | 0.0003 | 0.0246 | 0.0658 | 0.0010 | 0.029 |
| 1 | 159461772 | APOA2 | rs3813627 | G/T | 0.33 | 0.34 | 1.52 (1.18, 1.97) | 1.08 (0.73, 1.60) | 0.0045 | 0.0324 | 0.3119 | 0.1051 | 0.065 |
| 1 | 167865878 | SELP | rs732314 | G/A | 0.49 | 0.47 | 1.71 (1.28, 2.30) | 1.43 (1.01, 2.01) | 0.0017 | 0.0376 | 0.1840 | 0.1695 | 0.082 |
| 19 | 50147428 | APOC4 | rs10413089 | T/C | 0.19 | 0.19 | 0.68 (0.52, 0.88) | 1.15 (0.59, 2.23) | 0.0109 | 0.0425 | 0.3900 | 0.0792 | 0.282 |
| 16 | 16188277 | ABCC6 | rs212077 | C/G | 0.14 | 0.20 | 0.72 (0.55, 0.93) | 0.38 (0.18, 0.81) | 0.0036 | 0.0443 | 0.2899 | 0.1555 | 0.818 |
Statistical significance defined as a multiple testing-adjusted P value (PAdj) < 0.05 (adjusted for testing multiple SNPs in a gene). 2df, two degrees of freedom; ABC, ATP-binding cassette transporter; Apo, apolipoprotein; BMI, body mass index; CETP, cholesteryl ester transfer protein; CUBN, cubilin; FOS, Framingham Offspring Study; FDR, false discovery rate; HWE, Hardy Weinberg Equilibrium; LIPC, hepatic lipase; OR, odds ratio; SBP, systolic blood pressure; RXRA, retinoid X receptor alpha; SELP, P-selectin; SNP, single-nucleotide polymorphism; VA-HIT, Veterans Affairs HDL Intervention Trial.
OR comparing heterozygote genotype to the major allele homozygote genotype (95% CI).
OR comparing the minor allele homozygote genotype to the major allele homozygote genotype (95% CI).
P value for a 2df genotypic test of association between the SNP and case status, adjusting for age, PC1-PC4, BMI, SBP, diabetes status, smoking status, and alcohol use.
P value adjusted for multiple testing within the gene for a 2df genotypic test of association between the SNP and case status, adjusting for age, PC1-4, BMI, SBP, diabetes status, smoking status, and alcohol use.
FDR P value for the observed 2df genotypic test of association between the SNP and case status, adjusting for age, PC1-4, BMI, SBP, diabetes status, smoking status, and alcohol use based on the 60 candidate genes.
P value for a 2df genotypic test of association between the SNP and case status, adjusting for age, PC1-4, BMI, SBP, diabetes status, smoking status, alcohol use, HDL-C, triglycerides, and total cholesterol.
P value for test of HWE in control subjects.
In addition to comparing CHD cases to all control subjects, we conducted three subgroup analyses, in attempt to determine if the SNP associations with low HDL-C/CHD were acting through the HDL pathway or via other mechanisms. We compared 1) cases (n = 699) to controls with a HDL-C ≥ 40 mg/dl (n = 435); 2) cases to controls with a HDL-C < 40 mg/dl (n = 270); and 3) controls with a HDL-C < 40 mg/dl to controls with a HDL-C ≥ 40 mg/dl. Subgroup analysis was only conducted for SNPs that reached the significance threshold in the primary analysis. The results of the subgroup analyses are provided in Tables 3–5. All SNP associations identified in the primary analysis remained significant when comparing cases to controls having a HDL-C ≥ 40 mg/dl.
TABLE 3.
Comparison of cases (n = 699) to controls with HDL-C ≥ 40 mg/dl (n = 435)
| SNP | Gene | ORheta | ORhomb | P2dfc |
|---|---|---|---|---|
| rs4775065 | LIPC | 2.29 (1.70, 3.09) | 1.32 (0.64, 2.71) | 2.75E-07 |
| rs5882 | CETP | 0.62 (0.46, 0.86) | 0.33 (0.20, 0.54) | 2.02E-05 |
| rs11185660 | RXRA | 0.86 (0.63, 1.18) | 0.27 (0.11, 0.68) | 0.0170 |
| rs2249891 | ABCA1 | 0.57 (0.39, 0.82) | 2.12 (0.92, 4.92) | 0.0013 |
| rs150468 | ABCC6 | 0.62 (0.45, 0.85) | 0.28 (0.12, 0.65) | 0.0004 |
| rs7893395 | CUBN | 0.62 (0.45, 0.84) | 0.69 (0.31, 1.53) | 0.0076 |
| rs3813627 | APOA2 | 1.74 (1.29, 2.35) | 1.05 (0.67, 1.65) | 0.0009 |
| rs732314 | SELP | 1.90 (1.35, 2.67) | 1.76 (1.18, 2.65) | 0.0007 |
| rs10413089 | APOC4 | 0.64 (0.47, 0.88) | 1.03 (0.49, 2.17) | 0.0175 |
| rs212077 | ABCC6 | 0.59 (0.44, 0.80) | 0.33 (0.14, 0.77) | 0.0003 |
2df, two degrees of freedom; ABC, ATP-binding cassette transporter; Apo, apolipoprotein; BMI, body mass index; CETP, cholesteryl ester transfer protein; CI, confidence interval; CUBN, cubilin; HET, heterozygote; HOM, homozygote; LIPC, hepatic lipase; OR, odds ratio; PC, principal component; RXRA, retinoid X receptor alpha; SBP, systolic blood pressure; SELP, P-selectin; SNP, single-nucleotide polymorphism.
OR comparing heterozygote genotype to the major allele homozygote genotype (95% CI).
OR comparing the minor allele homozygote genotype to the major allele homozygote genotype (95%CI).
P value for a 2df genotypic test of association between the SNP and case status, adjusting for age, PC1–4, BMI, SBP, diabetes status, smoking status, and alcohol use.
TABLE 4.
Comparison of cases (n = 699) to controls with HDL-C < 40 mg/dl (n = 270)
| SNP | Gene | ORheta | ORhomb | P2dfc |
|---|---|---|---|---|
| rs4775065 | LIPC | 2.38 (1.70, 3.34) | 1.86 (0.80, 4.35) | 2.83E-06 |
| rs5882 | CETP | 0.66 (0.46, 0.95) | 0.49 (0.28, 0.86) | 0.0147 |
| rs11185660 | RXRA | 0.59 (0.42, 0.83) | 0.18 (0.07, 0.49) | 0.0001 |
| rs2249891 | ABCA1 | 0.71 (0.47, 1.09) | 5.88 (1.31, 26.38) | 0.0166 |
| rs150468 | ABCC6 | 0.80 (0.55, 1.14) | 0.38 (0.14, 1.02) | 0.0921 |
| rs7893395 | CUBN | 0.52 (0.37, 0.74) | 0.93 (0.35, 2.48) | 0.0010 |
| rs3813627 | APOA2 | 1.38 (0.98, 1.93) | 1.20 (0.70, 2.04) | 0.1737 |
| rs732314 | SELP | 1.51 (1.02, 2.24) | 0.99 (0.64, 1.55) | 0.0403 |
| rs10413089 | APOC4 | 0.73 (0.52, 1.04) | 1.70 (0.63, 4.56) | 0.0976 |
| rs212077 | ABCC6 | 0.91 (0.63, 1.30) | 0.39 (0.16, 0.98) | 0.1254 |
2df, two degrees of freedom; ABC, ATP-binding cassette transporter; Apo, apolipoprotein; BMI, body mass index; CETP, cholesteryl ester transfer protein; CUBN, cubilin; HET, heterozygote; HOM, homozygote; LIPC, hepatic lipase; OR, odds ratio; PC, principal component; RXRA, retinoid X receptor alpha; SBP, systolic blood pressure; SELP, P-selectin; SNP, single-nucleotide polymorphism.
OR comparing heterozygote genotype to the major allele homozygote genotype (95% CI).
OR comparing the minor allele homozygote genotype to the major allele homozygote genotype (95% CI).
P value for a 2df genotypic test of association between the SNP and case status, adjusting for age, PC1–4, BMI, SBP, diabetes status, smoking status, and alcohol use.
TABLE 5.
Comparison of controls with HDL-C < 40 mg/dl (n = 270) to controls with HDL-C ≥ 40 mg/dl (n = 435)
| SNP | Gene | ORheta | ORhomb | P2dfc |
|---|---|---|---|---|
| rs4775065 | LIPC | 0.96 (0.68, 1.35) | 0.82 (0.35, 1.89) | 0.8860 |
| rs5882 | CETP | 0.86 (0.58, 1.26) | 0.75 (0.44, 1.29) | 0.5329 |
| rs11185660 | RXRA | 1.71 (1.22, 2.41) | 1.07 (0.51, 2.25) | 0.0079 |
| rs2249891 | ABCA1 | 0.77 (0.51, 1.15) | 0.32 (0.07, 1.49) | 0.1697 |
| rs150468 | ABCC6 | 0.79 (0.55, 1.14) | 0.54 (0.23, 1.26) | 0.2002 |
| rs7893395 | CUBN | 1.16 (0.83, 1.63) | 0.80 (0.31, 2.12) | 0.5868 |
| rs3813627 | APOA2 | 1.19 (0.84, 1.68) | 0.88 (0.53, 1.48) | 0.4414 |
| rs732314 | SELP | 1.05 (0.71, 1.54) | 1.44 (0.92, 2.25) | 0.2050 |
| rs10413089 | APOC4 | 1.00 (0.71, 1.40) | 0.77 (0.28, 2.13) | 0.8768 |
| rs212077 | ABCC6 | 0.72 (0.50, 1.02) | 0.79 (0.35, 1.77) | 0.1718 |
2df, two degrees of freedom; ABC, ATP-binding cassette transporter; Apo, apolipoprotein; BMI, body mass index; CETP, cholesteryl ester transfer protein; CUBN, cubilin; HET, heterozygote; HOM, homozygote; LIPC, hepatic lipase; OR, odds ratio; PC, principal component; RXRA, retinoid X receptor alpha; SBP, systolic blood pressure; SELP, P-selectin; SNP, single-nucleotide polymorphism.
OR comparing heterozygote genotype to the major allele homozygote genotype (95% CI).
OR comparing the minor allele homozygote genotype to the major allele homozygote genotype (95% CI).
P value for a 2df genotypic test of association between the SNP and case status, adjusting for age, PC1–4, BMI, SBP, diabetes status, smoking status, and alcohol use.
A 0.10 difference in minor allele frequency between cases and controls was observed for SNP rs4775065 in LIPC, with the minor allele having a detrimental effect. Relative to the major allele homozygous genotype, an increased odds ratio (OR) for low HDL-C/CHD was seen in heterozygotes (OR = 2.36; 95% CI: 1.83-3.05), while an OR of 1.54 (95% CI: 0.82-2.89) was observed in minor allele homozygotes. The association remained significant after adjustment for plasma lipid levels (P = 1.15E-06). When controls with a HDL ≥ 40 mg/dl were compared with controls with a HDL-C < 40 mg/dl, we did not find rs4775065 to be significant (P = 0.886). However, in comparing cases to controls with low HDL-C, we observed an increase in the odds ratio, indicating that our top SNP in LIPC may not be acting through the HDL pathway to modify CHD risk. Likewise, the RXRA SNP (rs11185660), which is significantly associated with HDL-C level (P = 0.0079), does not appear to modify CHD risk through the HDL pathway but influences both CHD and HDL-C levels.
For rs732314 in SELP, the minor allele also had a detrimental effect. An OR of 1.71 (95% CI: 1.28-2.30) for low HDL-C/CHD was seen for heterozygous carriers of the minor allele versus major allele homozygotes, while an OR of 1.43 (95% CI: 1.01-2.01) was observed in minor allele homozygotes. The effect was enhanced when cases were compared with controls with high HDL-C (ORhet = 1.9; ORhom = 1.76) and diminished when cases were compared with controls with low HDL-C (ORhet = 1.52; ORhom = 0.99, respectively). These data suggest that rs732314 in SELP may be influencing CHD via the HDL pathway. After adjustment for lipid levels, the association between case status and rs732314 was not significant (P = 0.1695) nor was the association comparing controls with low HDL-C to controls with high HDL-C (P = 0.205). Similarly, patterns in associations for SNPs in CETP (rs5882) and ABCC6 (rs150468 and rs212077) indicate that these SNPs may also influence CHD risk through their effects on HDL.
For the SNPs in ABCA1 (rs2249891), CUBN (rs7893395), APOA2 (rs3813627), and APOC4 (rs10413089), the influence of HDL-C on CHD status is less clear. The associations observed between case status and ABCA1 and APOC4 indicate a heterozygote genotype advantage, while the association with APOA2 indicates a heterozygote disadvantage. In silico functional predictions (32) were seen for the SNPs in APOA2 and APOC4, indicating that these SNPs may regulate transcription by affecting transcription factor binding sites. However, as these findings may be due to gene-gene, allelic, and/or gene-environment interactions, further biological support is required to confirm these results.
Two SNPs in ABCC6, rs150468 and rs212077, reached our significance threshold. In the controls, the linkage disequilibrium (LD) measure (D′) between the two SNPs is 0.9 and the r2 is 0.77, indicating that these two SNPs are capturing the same variant associated with case status. The LD for these SNPs in cases is similar to that seen in controls. A model was fit jointly with both ABCC6 SNPs, and it was found that neither SNP was significant. We encountered colinearity issues when both SNPs were fit in the model, with a variance inflation factor (VIF) of 3.5. Logistic regression values with a VIF greater than 2.5 indicate colinearity. Therefore, a haplotype analysis of the two ABCC6 SNPs (rs150468:rs212077) was performed. We found that the haplotype with alleles C G (frequency of 16%) was significantly different from the haplotype with alleles A C (frequency of 81%) (P value = 8.12E-05). The ABCC6 haplotype A C had a 56% increase risk of CHD/low HDL-C compared with haplotype C G.
DISCUSSION
Nearly half of patients with CHD have low HDL-C as their primary lipid abnormality. Strong evidence exists for the role of genetics in the determination of HDL-C levels, with approximately 50% of the variation in HDL-C levels in humans genetically determined (11). However, HDL-C is a complex genetic trait, which involves the action of multiple genes that interact with each other and with environmental factors. Therefore, we examined 60 candidate genes with roles in HDL metabolism, insulin resistance, and/or inflammation to identify allelic variants associated with susceptibility to low HDL-C and CHD. Candidate gene studies are cost-effective, with little loss of power for detecting common variants (33). Moreover, a candidate gene approach has inherent biological plausibility for the associations uncovered.
The majority of susceptibility alleles for the low HDL-C/CHD trait identified in our study were located in genes with established roles in HDL metabolism. For instance, we identified susceptibility alleles for low HDL-C and/or CHD risk in the genes encoding LIPC (rs4775065), CETP (rs5882), ABCA1 (rs2249891), CUBN (rs7893395), and APOA2 (rs38136627). The strongest association with case status involved a variant in the LIPC gene (rs4775065), with cases having a minor allele frequency of 0.33 compared with 0.23 for controls. LIPC has consistently been associated with HDL-C in genome-wide studies of lipid traits (34–39) and has also been implicated as a susceptibility locus for CHD (40). The lack of a significant association between LIPC variants and HDL-C in the present study may be due to the limited sample sizes in our subgroup analyses, the dichotomizing of HDL-C levels, or the fact that LIPC may be influencing CHD risk via a nonHDL-related mechanism.
We also observed a significant association between case status and rs5882, a nonsynonymous SNP (I405V) in the CETP gene. The minor allele of this variant has been associated with reduced CETP activity, increased lipoprotein particle size, and exceptional longevity in Ashkenazi Jewish probands (41). Consistent with this finding, the minor allele had a protective effect in the present study and appears to be influencing CHD through via its effects on HDL. The effect was enhanced when cases were compared with controls with high HDL-C (ORhet = 0.62; ORhom = 0.33) and diminished when cases were compared with controls with low HDL-C (ORhet = 0.66; ORhom = 0.49).
Included among the novel findings of this study are the identification of susceptibility alleles for low HDL-C/CHD in the genes encoding CUBN (rs7893395) and RXRA (rs11185660), with the minor allele having a protective effect in each case. The cubilin gene encodes a high affinity receptor for endocytosis of HDL-C and lipid-poor apoA-I (42), while RXRA has been implicated in altering the risk for Alzheimer's disease through its effects on cholesterol metabolism (43). In silico functional predictions (32) were not found with the associated CUBN (rs7893395) or RXRA (rs11185660) SNPs or with SNPs in linkage disequilibrium (>0.7) with the associated SNPs.
Although genetic variation in P-selectin (SELP) has previously been associated with atherosclerotic risk in European-American adults from the Coronary Artery Risk Development in Young Adults (CARDIA) Study (44), we provide new information that SELP may influence CHD risk through its effects on HDL-C. When cases with low HDL-C and CHD were compared with controls with HDL-C < 40 mg/dl, the association between SELP and case status diminished (P = 0.0403). Specifically, the odds ratio for the comparison of minor allele homozygotes to major allele homozygotes was reduced from 1.43 (Table 2) to 0.99 (Table 4) when cases were compared with all controls versus controls with HDL-C < 40 mg/dl, respectively. Conversely, when we compared cases to controls with HDL-C ≥ 40 mg/dl, the association was enhanced (ORhom = 1.9, ORhet = 1.76, P = 0.0007). Finally, when we compared controls having a HDL-C < 40 mg/dl to controls having a HDL-C ≥ 40 mg/dl (Table 5), no association between HDL status and the SNP in SELP was observed (P = 0.2050). Since the effect increased when low HDL-C/CHD cases were compared with healthy controls (HDL ≥ 40 mg/dl), this suggests that the variant in SELP may be associated with CHD via HDL or that having the minor allele for provides a higher risk of having both CHD and low HDL-C. It is also important to note that the SELP SNP associated with increased risk for case status in our study (rs732314) is in strong linkage disequilibrium with rs2235302, a SNP shown to be significantly associated with both soluble P-selectin and carotid intima-media thickness among European-Americans in CARDIA (44). Finally, in silico analysis demonstrated a functional prediction (transcription factor binding site) for rs732314 in SELP, suggesting that this SNP is functionally relevant.
To assess whether any of the top risk loci identified in the present study may be related to survival, we determined whether the genotype distribution of the reported SNPs varied by decade of age. We found no evidence of a survival bias in our study. Therefore, the SNPs identified in this study do not appear to be related to survival time but, rather, are likely associated with the development of the combined trait of low HDL-C/CHD. Given that the inability to evaluate survival time is a limitation of our study, further research is required to confirm this finding.
There are additional limitations to our study. The reported novel findings are preliminary in nature. To fully confirm our findings, the results need to be replicated in independent samples and validated through experimentation. Although we adjusted for potential confounding variables in our analyses, we cannot account for potential differences in unmeasured exposures between the cohorts. Furthermore, this study is based on the common variant-common disease hypothesis, which does not allow us to evaluate the contribution of rare alleles to low HDL-C/CHD risk (45). Finally, while we have replicated the findings of some earlier studies (LIPC, CETP, ABCA1, APOA2, ABCC6, and APOC4), other findings were not replicated in the current study (i.e., APOA5, LPL, LCAT, LIPG, and PON1). APOA5 and LPL have been associated with HDL-C in European and multi-ethnic samples using the Illumina cardiovascular disease (CVD) BeadChip (45–47). The lack of replication may be due to the relatively modest sample size of the present study or to the distinctiveness of the cohort studied.
The VA-HIT cohort, composed of men with low HDL-C, normal LDL-C, and established CHD, affords a unique opportunity to identify genetic variants associated with CHD and low HDL-C. With this cohort, we used a candidate gene approach to identify novel allelic variants in the genes encoding CUBN and RXRA that are associated with susceptibility to the low HDL-C/CHD trait, and we provide new information that genetic variation in SELP may influence CHD risk through its effects on HDL-C. We also replicated data from previous studies through the identification of susceptibility alleles for low HDL-C and/or CHD risk in the genes encoding LIPC, CETP, ABCA1, and ABCC6. The findings of the present study may help to identify individuals at increased risk for the development of CHD.
Supplementary Material
Footnotes
Abbreviations:
- ABC
- ATP-binding cassette transporter
- AIM
- ancestry informative marker
- Apo
- apolipoprotein
- AUC
- area under the curve
- BMI
- body mass index
- CARDIA
- Coronary Artery Risk Development in Young Adults (study)
- CETP
- cholesteryl ester transfer protein
- CHD
- coronary heart disease
- CUBN
- cubilin
- FDR
- false discovery rate
- FOS
- Framingham Offspring Study
- HWE
- Hardy Weinberg Equilibrium
- LIPC
- hepatic lipase
- MAF
- minor allele frequency
- OR
- odds ratio
- PC
- principal component
- PCA
- principal components analysis
- RXRA
- retinoid X receptor alpha
- SBP
- systolic blood pressure
- SELP
- P-selectin
- SNP
- single-nucleotide polymorphism
- VA-HIT
- Veterans Affairs HDL Intervention Trial
This work was supported by National Institutes of Health Grant R01-HL-60935 (EJS and MEB) and the National Institutes of Health, National Heart, Lung and Blood Institute Contract N01-HC-25195 (Framingham Heart Study). The Veterans Affairs HDL Intervention Trial (VA-HIT) was supported by the Department of Veterans Affairs, Office of Research and Development, Cooperative Studies Program. The Broad Institute Center for Genotyping and Analysis is supported by the National Institutes of Health, National Center for Research Resources Grant U54 RR0-20278. A portion of this research used the Linux Cluster for Genetic Analysis (LinGA-II) funded by the Robert Dawson Evans Endowment of the Department of Medicine at Boston University School of Medicine and Boston Medical Center. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two tables.
REFERENCES
- 1.Miller G. J., Miller N. E. 1975. Plasma-high-density-lipoprotein concentration and development of ischaemic heart-disease. Lancet. 1: 16–19. [DOI] [PubMed] [Google Scholar]
- 2.Gordon D. J., Probstfield J. L., Garrison R. J., Neaton J. D., Castelli W. P., Knoke J. D., Jacobs D. R., Jr., Bangdiwala S., Tyroler H. A. 1989. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 79: 8–15. [DOI] [PubMed] [Google Scholar]
- 3.Stampfer M. J., Sacks F. M., Salvini S., Willett W. C., Hennekens C. H. 1991. A prospective study of cholesterol, apolipoproteins, and the risk of myocardial infarction. N. Engl. J. Med. 325: 373–381. [DOI] [PubMed] [Google Scholar]
- 4.Goldbourt U., Yaari S., Medalie J. H. 1997. Isolated low HDL cholesterol as a risk factor for coronary heart disease mortality. A 21-year follow-up of 8000 men. Arterioscler. Thromb. Vasc. Biol. 17: 107–113. [DOI] [PubMed] [Google Scholar]
- 5.Genest J. J., McNamara J. R., Salem D. N., Schaefer E. J. 1991. Prevalence of risk factors in men with premature coronary artery disease. Am. J. Cardiol. 67: 1185–1189. [DOI] [PubMed] [Google Scholar]
- 6.Lamarche B., Despres J. P., Moorjani S., Cantin B., Dagenais G. R., Lupien P. J. 1996. Triglycerides and HDL-cholesterol as risk factors for ischemic heart disease. Results from the Quebec cardiovascular study. Atherosclerosis. 119: 235–245. [DOI] [PubMed] [Google Scholar]
- 7.Criqui M. H., Wallace R. B., Heiss G., Mishkel M., Schonfeld G., Jones G. T. 1980. Cigarette smoking and plasma high-density lipoprotein cholesterol. The Lipid Research Clinics Program Prevalence Study. Circulation. 62: IV70–IV76. [PubMed] [Google Scholar]
- 8.Haffner S. M., Applebaum-Bowden D., Wahl P. W., Hoover J. J., Warnick G. R., Albers J. J., Hazzard W. R. 1985. Epidemiological correlates of high density lipoprotein subfractions, apolipoproteins A-I, A-II, and D, and lecithin cholesterol acyltransferase. Effects of smoking, alcohol, and adiposity. Arteriosclerosis. 5: 169–177. [DOI] [PubMed] [Google Scholar]
- 9.Velez-Carrasco W., Lichtenstein A. H., Welty F. K., Li Z., Lamon-Fava S., Dolnikowski G. G., Schaefer E. J. 1999. Dietary restriction of saturated fat and cholesterol decreases HDL ApoA-I secretion. Arterioscler. Thromb. Vasc. Biol. 19: 918–924. [DOI] [PubMed] [Google Scholar]
- 10.Ford E. S., Giles W. H., Dietz W. H. 2002. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 287: 356–359. [DOI] [PubMed] [Google Scholar]
- 11.Heller D. A., de Faire U., Pedersen N. L., Dahlen G., McClearn G. E. 1993. Genetic and environmental influences on serum lipid levels in twins. N. Engl. J. Med. 328: 1150–1156. [DOI] [PubMed] [Google Scholar]
- 12.Brousseau M. E., Bodzioch M., Schaefer E. J., Goldkamp A. L., Kielar D., Probst M., Ordovas J. M., Aslanidis C., Lackner K. J., Bloomfield Rubins H., et al. 2001. Common variants in the gene encoding ATP-binding cassette transporter 1 in men with low HDL cholesterol levels and coronary heart disease. Atherosclerosis. 154: 607–611. [DOI] [PubMed] [Google Scholar]
- 13.Brousseau M. E., O'Connor J. J., Jr., Ordovas J. M., Collins D., Otvos J. D., Massov T., McNamara J. R., Rubins H. B., Robins S. J., Schaefer E. J. 2002. Cholesteryl ester transfer protein TaqI B2B2 genotype is associated with higher HDL cholesterol levels and lower risk of coronary heart disease end points in men with HDL deficiency: Veterans Affairs HDL Cholesterol Intervention Trial. Arterioscler. Thromb. Vasc. Biol. 22: 1148–1154. [DOI] [PubMed] [Google Scholar]
- 14.Brousseau M. E., Goldkamp A. L., Collins D., Demissie S., Connolly A. C., Cupples L. A., Ordovas J. M., Bloomfield H. E., Robins S. J., Schaefer E. J. 2004. Polymorphisms in the gene encoding lipoprotein lipase in men with low HDL-C and coronary heart disease: the Veterans Affairs HDL Intervention Trial. J. Lipid Res. 45: 1885–1891. [DOI] [PubMed] [Google Scholar]
- 15.Rubins H. B., Robins S. J., Iwane M. K., Boden W. E., Elam M. B., Fye C. L., Gordon D. J., Schaefer E. J., Schectman G., Wittes J. T. 1993. Rationale and design of the Department of Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial (HIT) for secondary prevention of coronary artery disease in men with low high-density lipoprotein cholesterol and desirable low-density lipoprotein cholesterol. Am. J. Cardiol. 71: 45–52. [DOI] [PubMed] [Google Scholar]
- 16.Feinleib M., Kannel W. B., Garrison R. J., McNamara P. M., Castelli W. P. 1975. The Framingham Offspring Study. Design and preliminary data. Prev. Med. 4: 518–525. [DOI] [PubMed] [Google Scholar]
- 17.McNamara J. R., Schaefer E. J. 1987. Automated enzymatic standardized lipid analyses for plasma and lipoprotein fractions. Clin. Chim. Acta. 166: 1–8. [DOI] [PubMed] [Google Scholar]
- 18.Warnick G. R., Benderson J., Albers J. J. 1982. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin. Chem. 28: 1379–1388. [PubMed] [Google Scholar]
- 19.Friedewald W. T., Levy R. I., Fredrickson D. S. 1972. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18: 499–502. [PubMed] [Google Scholar]
- 20.Carlson C. S., Eberle M. A., Rieder M. J., Yi Q., Kruglyak L., Nickerson D. A. 2004. Selecting a maximally informative set of single-nucleotide polymorphisms for association analyses using linkage disequilibrium. Am. J. Hum. Genet. 74: 106–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dawber T. R., Kannel W. B., Lyell L. P. 1963. An approach to longitudinal studies in a community: the Framingham Study. Ann. N. Y. Acad. Sci. 107: 539–556. [DOI] [PubMed] [Google Scholar]
- 22.Smith M. W., Patterson N., Lautenberger J. A., Truelove A. L., McDonald G. J., Waliszewska A., Kessing B. D., Malasky M. J., Scafe C., Le E., et al. 2004. A high-density admixture map for disease gene discovery in African Americans. Am. J. Hum. Genet. 74: 1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bauchet M., McEvoy B., Pearson L. N., Quillen E. E., Sarkisian T., Hovhannesyan K., Deka R., Bradley D. G., Shriver M. D. 2007. Measuring European population stratification with microarray genotype data. Am. J. Hum. Genet. 80: 948–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patterson N., Price A. L., Reich D. 2006. Population structure and eigenanalysis. PLoS Genet. 2: e190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Price A. L., Patterson N. J., Plenge R. M., Weinblatt M. E., Shadick N. A., Reich D. 2006. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 38: 904–909. [DOI] [PubMed] [Google Scholar]
- 26.Zhu X., Li S., Cooper R. S., Elston R. C. 2008. A unified association analysis approach for family and unrelated samples correcting for stratification. Am. J. Hum. Genet. 82: 352–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell C. D., Ogburn E. L., Lunetta K. L., Lyon H. N., Freedman M. L., Groop L. C., Altshuler D., Ardlie K. G., Hirschhorn J. N. 2005. Demonstrating stratification in a European American population. Nat. Genet. 37: 868–872. [DOI] [PubMed] [Google Scholar]
- 28.Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M. A., Bender D., Maller J., Sklar P., de Bakker P. I., Daly M. J., et al. 2007. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81: 559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lettre G., Lange C., Hirschhorn J. N. 2007. Genetic model testing and statistical power in population-based association studies of quantitative traits. Genet. Epidemiol. 31: 358–362. [DOI] [PubMed] [Google Scholar]
- 30.Cheverud J. M. 2001. A simple correction for multiple comparisons in interval mapping genome scans. Heredity. 87: 52–58. [DOI] [PubMed] [Google Scholar]
- 31.Benjamini Y., Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. 57: 289–300. [Google Scholar]
- 32.Xu Z., Taylor J. A. 2009. SNPinfo: integrating GWAS and candidate gene information into functional SNP selection for genetic association studies. Nucleic Acids Res. 37: W600–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Bakker P. I., Yelensky R., Pe'er I., Gabriel S. B., Daly M. J., Altshuler D. 2005. Efficiency and power in genetic association studies. Nat. Genet. 37: 1217–1223. [DOI] [PubMed] [Google Scholar]
- 34.Chasman D. I., Pare G., Zee R. Y., Parker A. N., Cook N. R., Buring J. E., Kwiatkowski D. J., Rose L. M., Smith J. D., Williams P. T., et al. 2008. Genetic loci associated with plasma concentration of low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, apolipoprotein A1, and apolipoprotein B among 6382 white women in genome-wide analysis with replication. Circ. Cardiovasc. Genet. 1: 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willer C. J., Sanna S., Jackson A. U., Scuteri A., Bonnycastle L. L., Clarke R., Heath S. C., Timpson N. J., Najjar S. S., Stringham H. M., et al. 2008. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat. Genet. 40: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kathiresan S., Melander O., Guiducci C., Surti A., Burtt N. P., Rieder M. J., Cooper G. M., Roos C., Voight B. F., Havulinna A. S., et al. 2008. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat. Genet. 40: 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kathiresan S., Willer C. J., Peloso G. M., Demissie S., Musunuru K., Schadt E. E., Kaplan L., Bennett D., Li Y., Tanaka T., et al. 2009. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat. Genet. 41: 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sabatti C., Service S. K., Hartikainen A. L., Pouta A., Ripatti S., Brodsky J., Jones C. G., Zaitlen N. A., Varilo T., Kaakinen M., et al. 2009. Genome-wide association analysis of metabolic traits in a birth cohort from a founder population. Nat. Genet. 41: 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aulchenko Y. S., Ripatti S., Lindqvist I., Boomsma D., Heid I. M., Pramstaller P. P., Penninx B. W., Janssens A. C., Wilson J. F., Spector T., et al. 2009. Loci influencing lipid levels and coronary heart disease risk in 16 European population cohorts. Nat. Genet. 41: 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zambon A., Deeb S. S., Pauletto P., Crepaldi G., Brunzell J. D. 2003. Hepatic lipase: a marker for cardiovascular disease risk and response to therapy. Curr. Opin. Lipidol. 14: 179–189. [DOI] [PubMed] [Google Scholar]
- 41.Barzilai N., Atzmon G., Schechter C., Schaefer E. J., Cupples A. L., Lipton R., Cheng S., Shuldiner A. R. 2003. Unique lipoprotein phenotype and genotype associated with exceptional longevity. JAMA. 290: 2030–2040. [DOI] [PubMed] [Google Scholar]
- 42.Moestrup S. K., Kozyraki R. 2000. Cubilin, a high-density lipoprotein receptor. Curr. Opin. Lipidol. 11: 133–140. [DOI] [PubMed] [Google Scholar]
- 43.Kolsch H., Lutjohann D., Jessen F., Popp J., Hentschel F., Kelemen P., Friedrichs S., Maier T. A., Heun R. 2009. RXRA gene variations influence Alzheimer's disease risk and cholesterol metabolism. J. Cell. Mol. Med. 13: 589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reiner A. P., Carlson C. S., Thyagarajan B., Rieder M. J., Polak J. F., Siscovick D. S., Nickerson D. A., Jacobs D. R., Jr., Gross M. D. 2008. Soluble P-selectin, SELP polymorphisms, and atherosclerotic risk in European-American and African-African young adults: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Arterioscler. Thromb. Vasc. Biol. 28: 1549–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Talmud P. J., Drenos F., Shah S., Shah T., Palmen J., Verzilli C., Gaunt T. R., Pallas J., Lovering R., Li K., et al. 2009. Gene-centric association signals for lipids and apolipoproteins identified via the HumanCVD BeadChip. Am. J. Hum. Genet. 85: 628–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lanktree M. B., Anand S. S., Yusuf S., Hegele R. A. 2009. Replication of genetic associations with plasma lipoprotein traits in a multiethnic sample. J. Lipid Res. 50: 1487–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keating B. J., Tischfield S., Murray S. S., Bhangale T., Price T. S., Glessner J. T., Galver L., Barrett J. C., Grant S. F., Farlow D. N., et al. 2008. Concept, design and implementation of a cardiovascular gene-centric 50 k SNP array for large-scale genomic association studies. PLoS ONE. 3: e3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.