Abstract
Previous studies indicate that methyl-β-cyclodextrin (meβ-CD) can greatly enhance translocation of long-chain phospholipids from vesicles to cells in culture, which is very useful when studying, e.g., phospholipid metabolism and trafficking. However, the parameters affecting the transfer have not been systematically studied. Therefore, we studied the relevant parameters including meβ-CD and vesicle concentration, incubation time, phospholipid structure, and cell type. Because meβ-CD can extract cholesterol and other lipids from cells, thereby potentially altering cell growth or viability, these issues were studied as well. The results show that efficient incorporation of phospholipid species with hydrophobicity similar to that of natural species can be obtained without significantly compromising cell growth or viability. Cellular content of phosphatidyl-serine, -ethanolamine, and -choline could be increased dramatically, i.e., 400, 125, and 25%, respectively. Depletion of cellular cholesterol could be prevented or alleviated by inclusion of the proper amount of cholesterol in the donor vesicles. In summary, meβ-CD mediates efficient transfer of long-chain (phospho) lipids from vesicles to cells without significantly compromising their growth or viability. This lays a basis for detailed studies of phospholipid metabolism and trafficking as well as enables extensive manipulation of cellular phospholipid composition, which is particularly useful when investigating mechanisms underlying phospholipid homeostasis.
Keywords: membrane, metabolism, remodeling, homeostasis, traffic, heavy-isotope, electrospray ionization-mass spectrometry
Traditionally, phospholipid (PL) metabolism has been studied by incubating cells with radiolabeled precursors followed by chromatographic analysis of the labeled species (1, 2). More recently, stable isotope-labeled phospholipid precursors, such as D9-choline, have been used to study PL metabolism because their metabolism can be easily monitored by employing head group-specific mass spectrometric detection (3, 4). However, even this approach has its limitations, especially when studying complex phenomena such as phospholipid acyl chain remodeling in mammalian cells. This is because numerous species become labeled during the pulse, which makes it very difficult to trace the remodeling pathways and kinetics of individual species (5). One remedy is to use labeled exogenous phospholipids as precursors because only a single labeled phospholipid species is present initially (5–7). However, introduction of exogenous phospholipids similar to natural species requires the use of carriers due to their hydrophobicity. In principle, phospholipid exchange/transfer proteins are ideal for this purpose, but unfortunately, they do not work efficiently with intact cells and consequently, impractical amounts of proteins are needed (8). Alternatively, cyclodextrins can be used as carriers. Cyclodextrins form soluble complexes with various lipids in vitro (9) and enhance translocation of hydrophobic phospholipids from lipid vesicles to cultured cells (5, 10, 11). However, cyclodextrins can also extract lipids and possibly other molecules from cells (12, 13), thus potentially compromising cell growth or viability. Accordingly, we studied these issues in detail and were able to establish conditions under which highly efficient introduction of phospholipids with hydrophobicity similar to that of natural species could be achieved without significantly compromising cell growth or viability.
MATERIALS AND METHODS
Lipids and other chemicals
Culture media and reagents, DNA quantification kit (Quant-iTTM ds DNA ASSAY Kit BR), and Alamar Blue were obtained from Invitrogen; cholesterol and other unlabeled lipids from Avanti Polar Lipids (Alabaster, AL), D9-choline, D315N-serine and D4-ethanolamine from CIL (Andover, MA); and methyl-β-cyclodextrin (meβ-CD), phospholipase D (Streptomyces sp.), hydroxylamine, methyl arachidonyl fluorophosphonate (MAFP), and Trypan Blue from Sigma. All solvents (HPLC-grade) were obtained from Merck. The phosphatidylethanolamine (PE) and phosphatidylserine (PS) species with a deuterium-labeled head group were synthesized from corresponding phosphatidylcholine (PC) species and D4-ethanolamine or D315N-serine, respectively, using phospholipase D-mediated transphosphatidylation as described previously for unlabeled lipids (14), except that the reaction volume was reduced 5-fold. The D9-PC species were synthesized from corresponding PEs by methylation with D3-methyl iodide (15). The lipids were purified by normal-phase HPLC (16). Their purity was confirmed by mass spectrometry and their concentrations were determined using phosphate analysis (17).
Cell culture
Baby hamster kidney (BHK21) and HeLa cells were grown as previously (5, 18). Human skin fibroblasts were grown in RPMI medium containing 15% FBS, 200 U/ml penicillin, 200 µg/ml streptomycin, 2 mM L-glutamine, and 0.1 mM unessential amino acids. Chinese hamster ovary cells were cultured in Ham F-12 medium containing 10% FBS, 200 U/ml penicillin, and 200 µg/ml streptomycin.
Preparation of donor vesicles
Palmitoyl-oleoyl-phosphatidylcholine (POPC), cholesterol, and heavy isotope-labeled PC, PE, or PS (in varying ratios as specified for each experiment under Results) were mixed in chloroform/methanol (9:1) and the solvent was evaporated under a nitrogen stream followed by high vacuum for 1 h. PBS was added and the sample was probe-sonicated for 3 × 2 min with 30 s intervals. The sample was centrifuged for 5 min at 3000 g to pellet any undispersed lipid and probe particles. The small unilamellar vesicles in the supernatant were used as phospholipid donors.
Introduction of exogenous phospholipid to cells
Cells grown to ∼80% confluency on 6 cm dishes were washed once with DMEM. small unilamellar vesicles, and meβ-CD in 2 ml of DMEM were added and the cells were incubated at 37 °C. After washing three times with DMEM, the cells were chased in DMEM for 0–24 h, washed with PBS, scraped into 0.25M sucrose, and moved to silanized screw-cap tubes. The lipids were then extracted (19). A cocktail of internal standards was added at the one-phase stage of extraction. After evaporation of the solvents, the lipids were dissolved in methanol/chloroform (2:1, v/v) and stored at −20 °C. A trace amount (0.1 mol%) of 22:0/22:0 PC, which is too hydrophobic to be transferred by meβ-CD, was included in the donor vesicles to estimate their adherence to cells. Only negligible amounts of 22:0/22:0-PC were detected in cells after the incubation, thus indicating that adhered donor vesicles do not significantly contribute to cell-associated exogenous lipid.
Mass spectrometry and data analysis
After addition of aqueous NH3 (4%), the sample was infused at 6 μl/min to the ESI source of a Micromass Quattro Micro triple-quadrupole mass spectrometer (Waters) operated as previously (20). The heavy isotope-labeled D9-PC, D4-PE, and D315N-PS species and unlabeled PC, PE, PS, SM, and phosphatidylinositol species were selectively detected using head group-specific precursor or neutral-loss scanning (3, 5). The species were identified and quantified using the LIMSA software (21).
Assessment of cell viability
The cells were incubated in DMEM containing donor vesicles and meβ-CD for 1 h on 12-well plates, washed three times with DMEM, and incubated in 500 µl of DMEM for 2 h. The cells were then washed once with DMEM and incubated in 500 µl DMEM containing 10% of Alamar Blue for 3 h at 37°C. Then 200 µl of the medium was moved to a 96-well plate and fluorescence intensity was measured using a Cary Eclipse fluorescence spectrophotometer (Varian) with the excitation and emission wavelengths set to 530 and 590 nm, respectively. Control samples included: i) cells incubated in DMEM, ii) cells killed with 70% ETOH, iii) cells killed with 5 mM CuSO4, and iv) DMEM. To determine the number of cells remaining on the dishes, the cells were detached with Trypsin-EDTA and counted with a hemocytometer.
To assess cell intactness based on Trypan Blue exclusion, the cells were detached with Trypsin-EDTA and centrifuged at 500 g for 5 min. Trypan Blue (0.4%) in PBS was then added and the stained cells were counted using a hemocytometer.
Quantification of DNA
Cells were scraped into 0.25 M sucrose, pelleted by centrifugation for 10 min at 1500 g and lysed by incubating for 5 min at 100°C in a buffer consisting of 0.1% SDS, 10 mM Tris-HCl, 1 mM EDTA; pH 8.0. DNA was quantified using the Quant-iT dsDNA Broad-Range Assay Kit (Molecular Probes) according to manufacturer's instructions.
Other assays
Published methods were used to quantify cholesterol (22) and protein (23).
RESULTS
Effect of meβ-CD concentration and other parameters on incorporation of phospholipids of varying hydrophobicity to cells
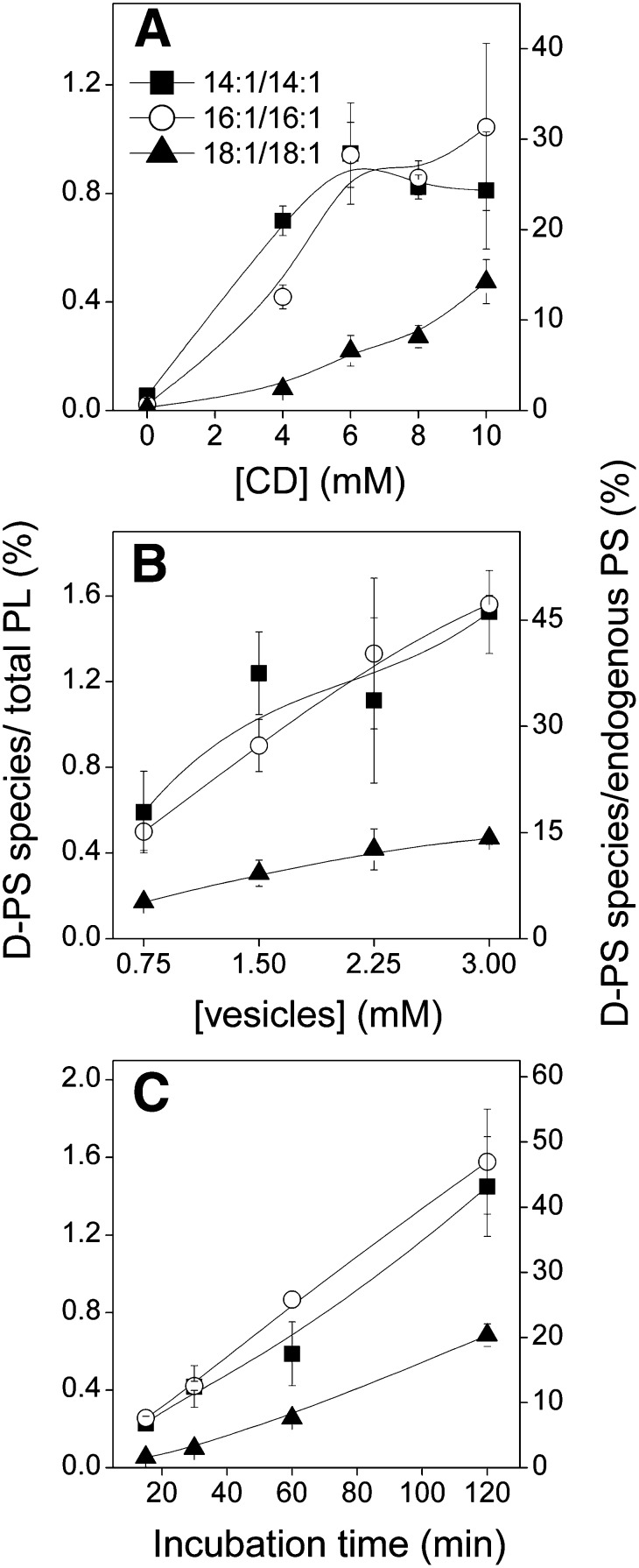
To study the effect of meβ-CD concentration on transfer of different PS species, BHK cells were incubated with 1.5 mM donor vesicles containing cholesterol, POPC, and labeled 14:1/14:1-, 16:1/16:1-, and 18:1/18:1-PS (30:27:1:1:1 mol/mol) in the presence of 0–10 mM meβ-CD for 1 h. As shown in Fig. 1A, transfer of all three PS species increased steadily with increasing meβ-CD concentration. The transfer of the 14:1/14:1 species leveled off above 6 mM meβ-CD, whereas transfer of the two other species increased up to at least 10 mM. The maximal amounts of 14:1/14:1-, 16:1/16:1-, and 18:1/18:1-PS transferred to cells under these conditions were 29 ± 5, 46 ± 6, and 12 ± 2% (n = 8) relative to endogenous PS, respectively. Together, the exogenous PS species thus comprised ∼87% of total PS (endogenous + exogenous) in the cells.
Fig. 1.
Effect of meβ-CD or donor vesicle concentration and incubation time on cellular uptake of exogenous PS. A: BHK cells were incubated for 1 h with 1.5 mM donor vesicles containing cholesterol, POPC, and labeled 14:1/14:1- (squares), 16:1/16:1- (circles), and 18:1/18:1-PS (triangles) (30:27:1:1:1, mol/mol) and indicated concentration of meβ-CD. B: BHK cells were incubated for 1 h in the presence of 8 mM meβ-CD and indicated concentration of the vesicles described in A. C: BHK cells were incubated with vesicles and meβ-CD as in A for the indicated times. In each experiment, 1.5 mM hydroxylamine and 25 μM MAFP were included to prevent decarboxylation and inhibit acyl chain remodeling of exogenous PS, respectively. Symbols as indicated in A. The amount of PS incorporated is shown as the percentage of the total phospholipid (left Y-axis) or relative to endogenous PS (right Y-axis). Data are means of three (A) and two (B and C) independent experiments ± SD.
We next studied the effect of donor vesicle concentration (Fig. 1B). BHK cells were incubated for 1 h in the presence of 8 mM meβ-CD and varying concentrations (0.75–3 mM) of donor vesicles of the composition indicated above. Transfer of each PS species to cells increased approximately linearly with donor vesicle concentration and at the donor total lipid concentration of 3 mM, the amount of 14:1/14:1-, 16:1/16:1-, and 18:1/18:1-PS transferred to cells corresponded to 32 ± 3, 37 ± 2, and 10 ± 1% (n = 6) of endogenous PS, respectively. Together, the exogenous PS species thus comprised ∼79% of total PS in the cells.
We next investigated the effect of incubation time on transfer of PS to cells using donor vesicles (1.5 mM) of the same composition as above and 8 mM meβ-CD (Fig. 1C). Again, the transfer of each of the three PS species to cells increased nearly linearly with time, and at 2 h, the amount of exogenous 14:1/14:1-, 16:1/16:1-, and 18:1/18:1-PS transferred to cells was 28 ± 6, 43 ± 6, and 14 ± 1% (n = 6) relative to endogenous PS, respectively. Together the exogenous PS species thus comprised ∼85% of total PS in the cells.
Some variability in transfer of phospholipids to cells under seemingly identical conditions was observed (cf. Fig. 1A–C). The reasons for this are unclear, but could relate to minor unavoidable variations in the average size of the donor vesicles between experiments. It is known that lipid efflux from a bilayer, which directly correlates with spontaneous and meβ-CD-mediated transfer, is very sensitive to vesicles size (24). Also the composition of the donor vesicles, which influences lipid packing, can have a significant effect on the rate of transfer (see below).
Effect of the polar head group
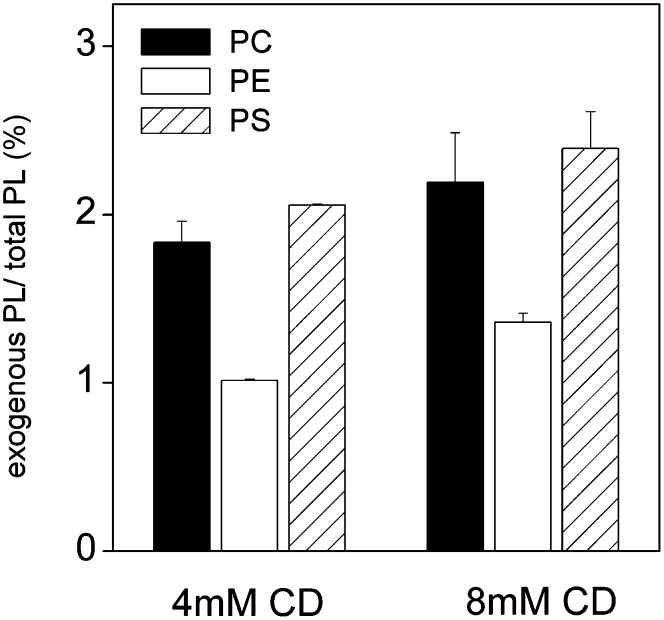
The effect of the phospholipid polar head group on transfer of exogenous phospholipids was studied by incubating BHK cells for 1 h with donor vesicles (0.5 mM) containing cholesterol, POPC, and labeled 14:0/14:0-PC, -PE, or -PS (10:9:1 mol/mol) in the presence of 4 or 8 mM meβ-CD (Fig. 2). Efficient transfer of all three lipids was observed, albeit incorporation of PE was significantly lower than that of PS or PC. However, no consistent head group dependency was observed when incorporation of di-14:1, di-14:0-, and di-18:1-PE or -PS species to different cell types was investigated (see below). Tests with four different molecular species of PC, PE, or PS (i.e., 14:1/14:1, 14:0/16:0, 16:0/18:1, and 18:0/20:4) in HeLa cells indicated that meβ-CD-mediated transfer of phospholipids does not depend significantly on the structure of the head group, but rather on the hydrophobicity (i.e., length and unsaturation) of the acyl chains (data not shown; however, see below). These data are consistent with previous studies indicating that differences in cyclodextrin-mediated transfer of PLs with a different head group but identical acyl chains can be explained by their different propensities to efflux from the bilayer (10).
Fig. 2.
Effect of polar head group on transfer of exogenous phospholipids to cells. BHK cells were incubated for 1 h with 0.5 mM donor vesicles consisting of cholesterol, POPC, and either labeled 14:0/14:0-PC (black bars), -PE (white bars), or -PS (striped bars) (10:9:1 mol/mol) and indicated concentrations of meβ-CD. The amount of incorporated exogenous phospholipid is shown as the percentage of total phospholipid. The data are means of two parallel samples.
Effect of donor vesicle composition
We next studied the effect of donor vesicle composition on meβ-CD-mediated transfer of phospholipids by incubating BHK cells with vesicles composed of 50 mol% cholesterol and varying proportions of POPC and deuteriated di-14:1-PC, -PE, or -PS. Transfer of these lipids to cells increased in proportion to their concentration in the donor vesicles, at least up to 25 mol% (data not shown; however, see Fig. 4). Vesicles with higher content of these lipids were not studied, because extensive reduction of the POPC content would compromise the incorporation of high amounts of cholesterol in the donor vesicles (25) and thus probably lead to depletion of cellular cholesterol.
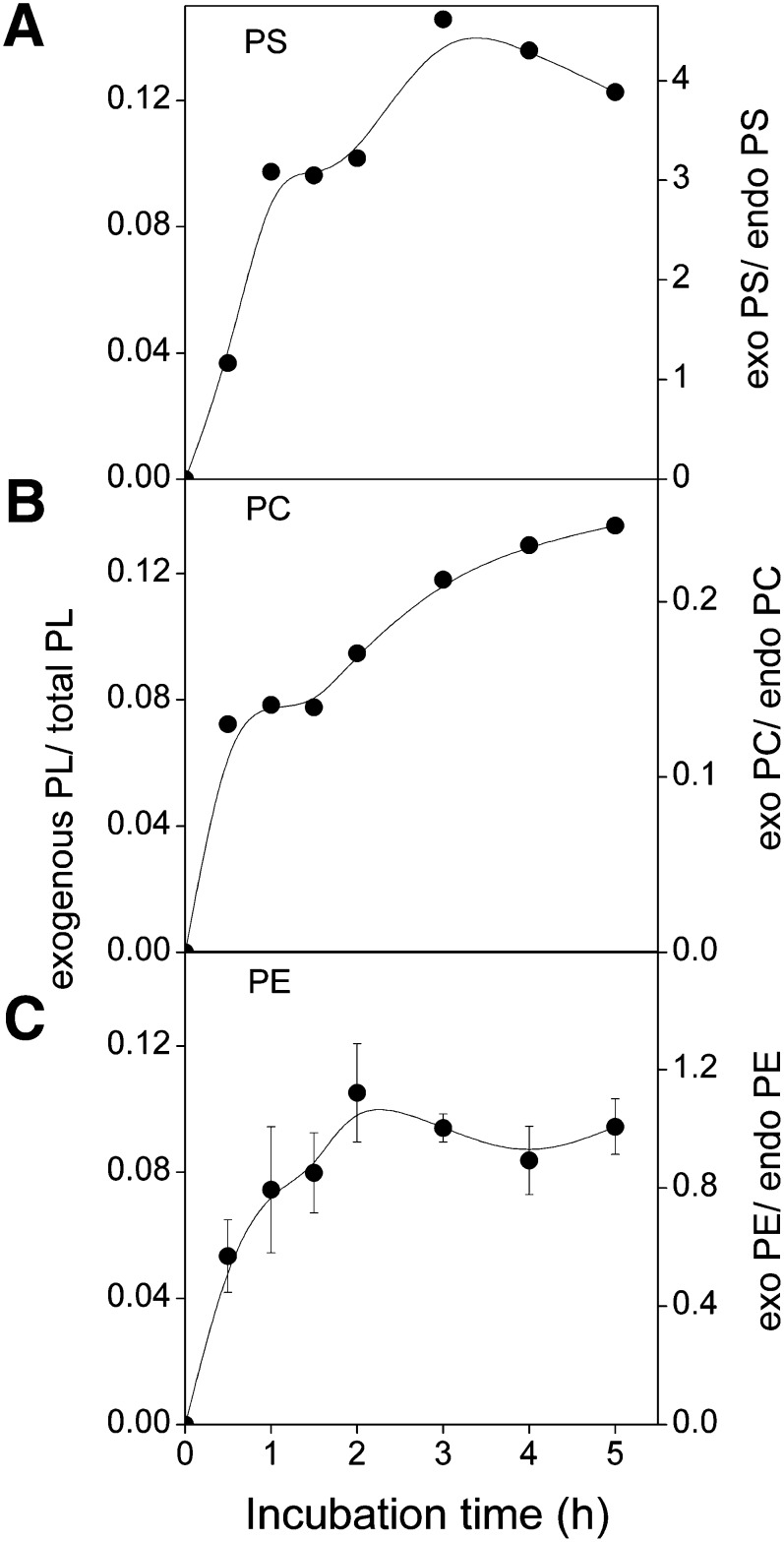
Fig. 4.
Loading BHK cells with exogenous 14:1/14:1-PS, -PC or -PE. BHK21 cells were incubated with donor vesicles (0.5 mM total lipid) containing cholesterol, POPC, and labeled 14:1/14:1-PS (A), -PC (B), or -PE (C) (2:1:1 mol/mol) and 2 mM meβ-CD for indicated times. The amount of incorporated exogenous phospholipid is shown as the percentage of total phospholipid (left Y-axis) or relative to endogenous lipid of the corresponding class (right Y-axis).
We also studied whether replacement of POPC by di-18:0-, di-20:1-, or di-22:1-PC, which are more hydrophobic and thus less efficiently transferred by the cyclodextrin than POPC (5), would prevent the transfer of the matrix phospholipid to cells. Although this was indeed the case, we also found less efficient transfer of di-14:1-PC, -PE, or -PS from such donors (data not shown), possibly because of tighter lateral packing of the bilayer containing these phospholipids.
Effect of cell line
To compare incorporation of exogenous phospholipids to different cell lines, BHK, CHO, HeLa, or HF cells were incubated for 1 h with 1.5 mM donor vesicles containing cholesterol, POPC, and labeled 14:0/14:0-, 14:1/14:1-, and 18:1/18:1-PE or -PS (30:27:1:1:1 mol/mol) in the presence of 8 mM meβ-CD. As expected, transfer of both PE and PS decreased markedly with increasing acyl chain length (hydrophobicity) with each cell line. The different PE species transferred similarly to CHO, HeLa, and HF cells, but less efficiently to BHK cells (Fig. 3A). On the other hand, incorporation of the 14:0/14:0- and 14:1/14:1- PS species to CHO and HF was significantly more efficient than to BHK and HeLa cells (Fig. 3B).
Fig. 3.
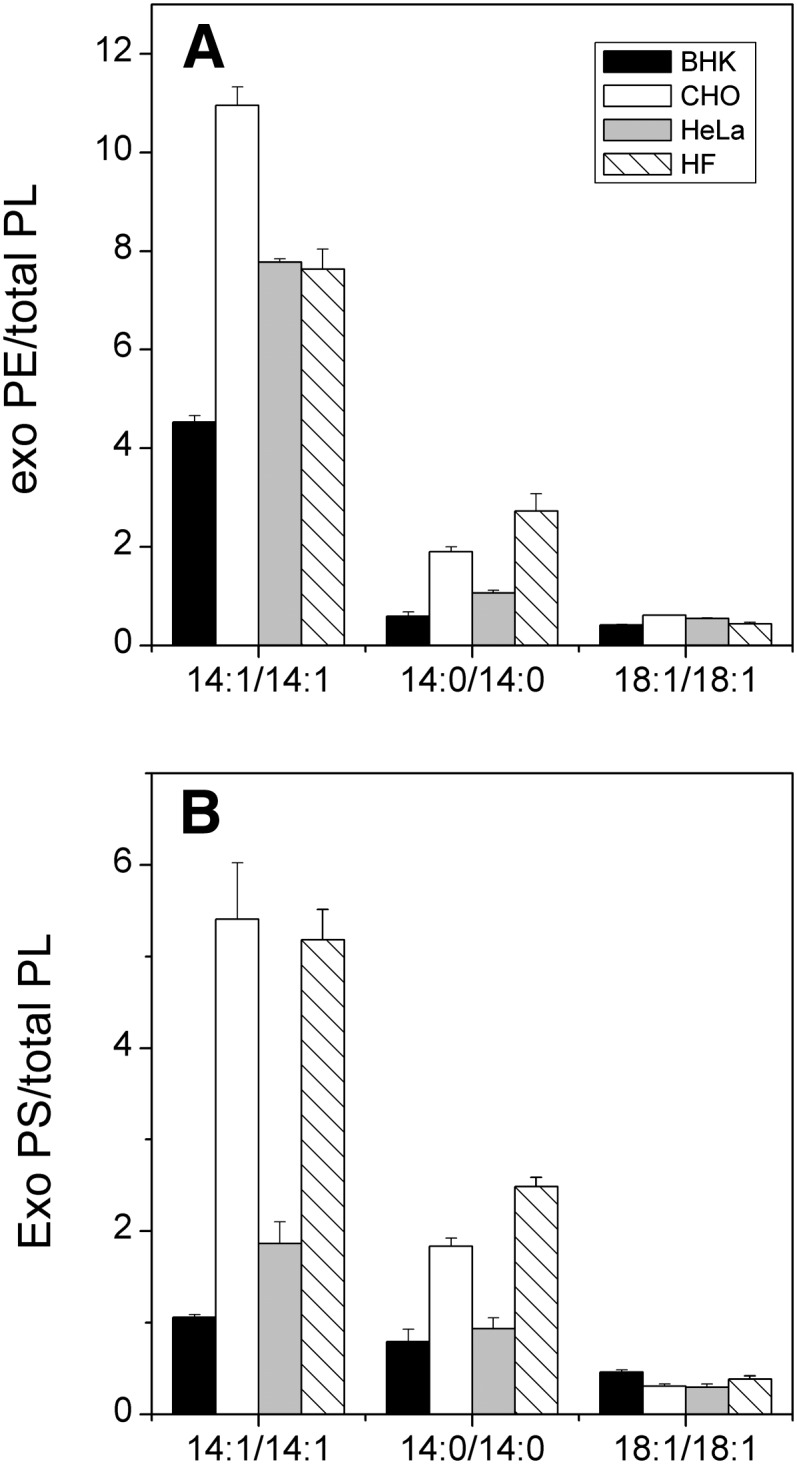
Uptake of exogenous PE and PS into BHK, CHO, HeLa, and HF cells. Cells were incubated for 1 h in the presence of vesicles (1.5 mM total lipid) consisting of cholesterol, POPC, and labeled 14:0/14:0-, 14:1/14:1-, and 18:1/18:1-PE (A) or PS (B) species (30:27:1:1:1, mol/mol) in the presence of 8 mM meβ-CD. Hydroxylamine (1.5 mM) and MAFP (25 μM) were included in the medium to prevent decarboxylation of exogenous PS or to inhibit acyl chain remodeling of PE and PS, respectively. The amount of incorporated exogenous phospholipid is shown as the percentage of total phospholipid. The data are means of two parallel samples.
Loading of cells with exogenous phospholipids
When studying, e.g., phospholipid homeostasis, it would be highly useful if one could significantly increase the concentration of a particular lipid species or class in cells. Thus, to determine how much exogenous phospholipid can be loaded to cells, we incubated BHK or HeLa cells for up to 5 h with 0.5 mM of donor vesicles containing cholesterol, POPC, and 14:1/14:1-PE, -PS, or -PC (2:1:1 mol/mol) and 2 mM meβ-CD. Depending on the species, the transfer increased up to 2–5 h and then leveled off (Fig. 4). The amounts of 14:1/14:1-PS, -PE, and -PC transferred to BHK cells corresponded to 440%, 125%, and 25% of the corresponding endogenous class, respectively, and 14.5%, 10.5%, and 12.5% of the total endogenous phospholipid, respectively. Consistent with these data, the phospholipid to protein ratio (nmol/mg) of the cells increased from 128 ± 7 to 157 ± 11 (n = 8), i.e., 23%. In an analogous experiment, exogenous PC taken up by HeLa cells comprised nearly 60% of total cellular PC or ∼30% of total phospholipid (data not shown). These results show that the relative amount of a phospholipid class can be greatly increased using meβ-CD-mediated transfer of phospholipids from donor vesicles.
Effects on cellular content of other lipids
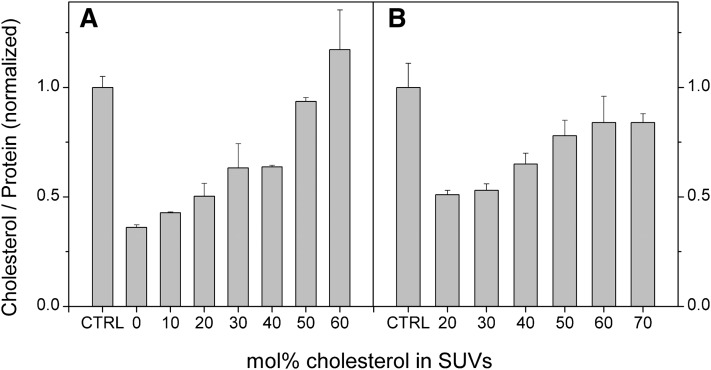
Meβ-CD is known to extract cholesterol efficiently from cells (26, 27). In principle, this could be prevented by incorporating a proper concentration of cholesterol in the donor vesicles (28). Accordingly, we incubated BHK cells with donor vesicles containing different amounts of cholesterol in presence of 4 or 8 mM meβ-CD for 1 h. After washing, the cholesterol and phospholipid contents of the cells were analyzed. As shown in Fig. 5A, ∼75% cellular cholesterol was depleted when the donor vesicles were devoid of cholesterol and the concentration of meβ-CD was 4 mM. Addition of cholesterol to donor vesicles reduced the depletion and, at 50 mol%, no change in the cellular cholesterol content was observed, probably because the efflux of cholesterol from cells equaled its flux in the opposite direction. The results obtained with 8 mM meβ-CD were generally similar, but even inclusion of 70 mol% of cholesterol in the donor vesicle preparation did not fully prevent depletion of cellular cholesterol (Fig. 5B). Notably, however, modest depletion of cellular cholesterol observed has only a minor effect on cell viability and growth (see below).
Fig. 5.
Effect of donor vesicle cholesterol content on cellular cholesterol content. BHK cells were incubated for 1 h with 0.5 mM vesicles composed of POPC and the indicated amounts of cholesterol in the presence of 4 mM (A) or 8 mM (B) meβ-CD. Cells were washed, chased in DMEM for 0.5 h, washed with PBS, and their protein and cholesterol contents were determined. CTRL, untreated cells. Data are means of three parallel samples ± SD.
To study how the incubation of cells with donor vesicles and meβ-CD modifies the cellular phospholipid composition, BHK cells were incubated for 1 h with 0.5 mM donor vesicles (labeled PS or PE/POPC/cholesterol, 1:9:10) and 8 mM meβ-CD. Mass spectrometric analysis showed that the POPC content of the cells increased ∼50%, corresponding to a 4% increase in total cellular PC. Also, an ∼30% decrease of total cellular SM was seen, but the relative abundances of the different SM species were not altered. After a chase in DMEM, the cellular content of POPC, most likely originating from the donor vesicles, as well as the total PC were practically normalized in 7 h, whereas the SM content returned to normal after 24 h (data not shown). No significant changes in the total cellular content of PE, PS, or PI were observed (data not shown). During the chase, a decrease in the abundance of polyunsaturated PL species, such as 18:0/20:4 and 18:1/20:4 PE, was observed, but this could be fully prevented by inclusion of FBS in the medium (data not shown).
Effects on cell growth and viability
We next tested whether incubation with vesicles and meβ-CD compromises cell viability or growth. Cell viability was assessed based on Alamar Blue oxidation or Trypan Blue exclusion after 1 h incubation with donor vesicles at 0–10 mM meβ-CD. Cell viability was not significantly compromised by the incubation based on Alamar Blue oxidation (Fig. 6) or Trypan Blue exclusion (data not shown). However, some detachment of treated cells compared with control cells was observed.
Fig. 6.
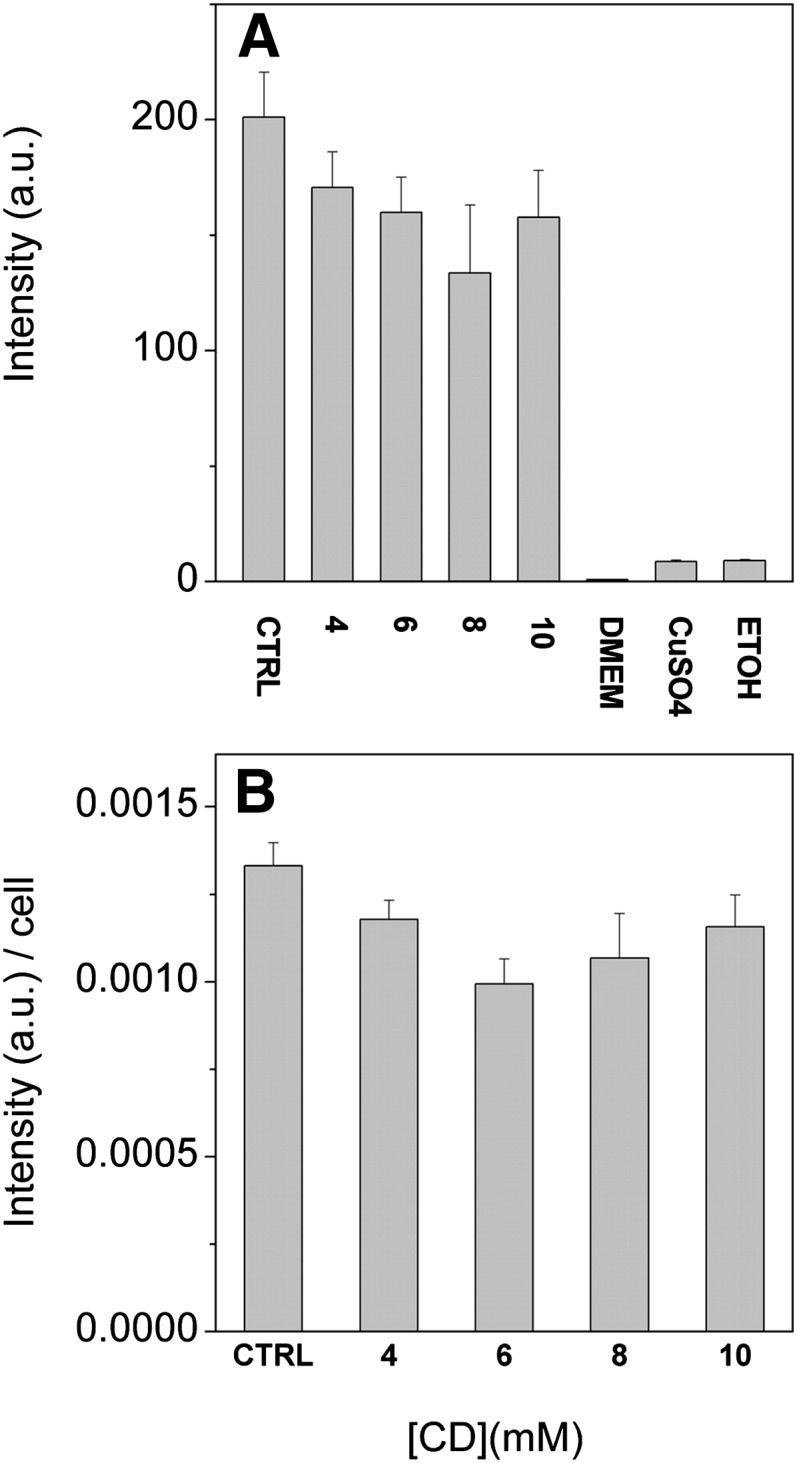
Effect of incubation with donor vesicles and meβ-CD on cell viability. BHK21 cells were incubated for 1 h with 0.5 mM donor vesicles composed of cholesterol, POPC, and 14:1/14:1-PS (10:9:1, mol/mol) in the presence of indicated concentrations of meβ-CD. The cells were then washed and chased for 2 h in DMEM. Alamar Blue was added and the cells were incubated for 3 h. The cells were then counted, an aliquot of the incubation media was collected, and fluorescence was measured as specified in Materials and Methods. CTRL, untreated cells; DMEM, media containing 10% Alamar Blue, CuSO4, cells killed with CuSO4, ETOH, cells killed with ethanol. A: Total fluorescence intensity and (B) fluorescence intensity divided by cell number. Data are means of two independent experiments with three parallel samples ± SD.
Cell growth was assessed by determining the protein and DNA contents of the cells after incubation with donor vesicles and 8 mM meβ-CD for 1 h and after a 24 h chase in DMEM with or without 10% FBS (Fig. 7). Both the protein and DNA contents of the treated cells were essentially identical to those of control cells after the introduction period as well as after the 24 h chase, independent of whether serum was present or not.
Fig. 7.
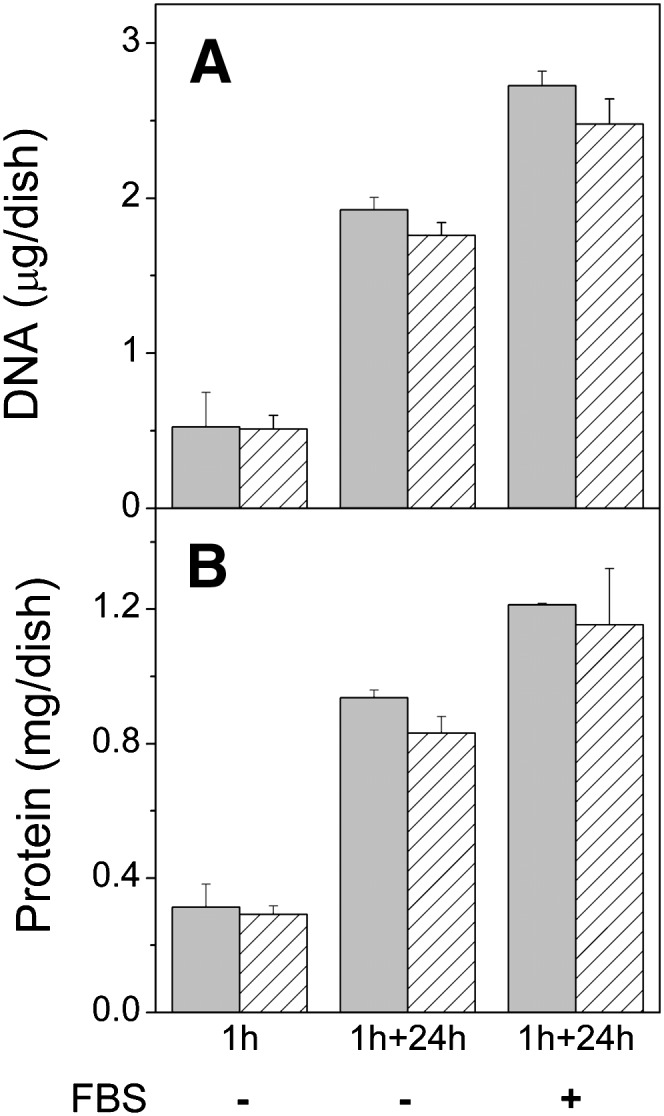
Effect of incubation with vesicles and meβ-CD on cell growth. BHK21 cells were incubated for 1 h in the presence of 8 mM meβ-CD and 0.5 mM donor vesicles composed of cholesterol, POPC, and 14:1/14:1-PS (10:9:1 mol/mol). Cells were then washed and chased in DMEM for 0 h or 24 h in absence (-) or presence (+) of 10% FBS. DNA (A) and protein (B) contents of the cells were then determined. Gray bars are cells incubated with vesicles and meβ-CD, striped bars, untreated cells. Data are means of three parallel samples ± SD. Parallel results were obtained in experiments with somewhat different incubation and chase times.
Decarboxylation of exogenous PS
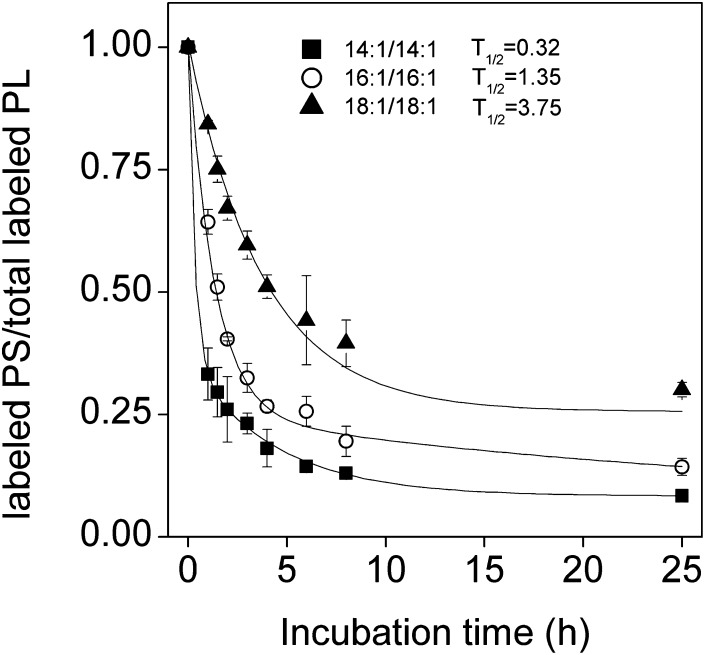
As an implementation of the method, we studied how the acyl chains of a PS molecule affect its translocation from the plasma membrane to mitochondria. The kinetics of this process can be conveniently determined by monitoring decarboxylation of PS to PE because i) PS decarboxylase is located exclusively in the mitochondria in mammalian cells and ii) transfer to mitochondria rather than decarboxylation therein is rate limiting (11, 29). Accordingly, we incubated BHK cells with vesicles containing a heavy isotope-labeled PS species and then determined the kinetics of formation of labeled PE with mass spectrometry. As shown in Fig. 8, the rate of decarboxylation of 14:1/14:1-PS was very rapid and over 70% of it was converted to PE during the 1 h introduction period, thus showing that this PS species translocates to mitochondria very rapidly. The rate of decarboxylation of the other PS species with longer acyl chains was significantly slower, thus indicating their slower transfer to mitochondria. The rate of PS transfer to mitochondria was inversely proportional to the molecular hydrophobicity (Fig. 8), which has important implications regarding the mechanisms of PS translocation, as well as the maintenance of a high PS content in the inner leaflet of the plasma membrane (see Discussion).
Fig. 8.
Decarboxylation of exogenous PS species. BHK cells were incubated for 1 h with 0.5 mM donor vesicles composed of cholesterol, POPC, and labeled 14:1/14:1- (squares), 16:1/16:1- (circles), or 18:1/18:1-PS (triangles) (10:9:1, mol/mol) in the presence of 8 mM meβ-CD. Cells were then washed and chased in DMEM. The curves represent fits of a first order exponential decay model to the data. The half-times of decarboxylation of the PS species are indicated after the respective symbols. Data are means of three independent experiments ± SD.
DISCUSSION
It is desirable to efficiently introduce labeled or unlabeled (phospho) lipids to cells in order to study their metabolism, trafficking, and homeostasis. However, the very low solubility of natural (hydrophobic) phospholipids in aqueous media presents a major obstacle here. Although phospholipid exchange/transfer proteins seem ideal tools to circumvent this obstacle, they do not work efficiently with intact cells and, consequently, impractical amounts of protein are needed (8, 30). Fusion of lipid vesicles containing a virus receptor with the plasma membrane has been used to introduce lipids to infected cells (31) but has complications and has thus been rarely used. Lysophospholipids are readily taken up by cells and can be acylated to intact phospholipids therein (32), but this approach does not allow introduction of a specific molecular species to cells because the reacylation process is not selective regarding the acyl chain added.
Previous studies have shown that certain cyclodextrins can be used to transfer hydrophobic lipids to cells (5, 10, 11), but the parameters affecting transfer efficiency and the possible deleterious effects on cells have not been systematically studied. In the present study, we studied these issues in detail and the key result was that with most phospholipids the amount transferred to cells increased systematically with increasing i) donor vesicle concentration, ii) time, and iii) meβ-CD concentration. However, the transfer of the less hydrophobic species, such as Di-14:1-PS, decreased at higher meβ-CD concentrations. This is not unexpected, because the affinity of phospholipids for cyclodextrin (relative to that for the bilayer) has been shown to increase with decreasing hydrophobicity (9). Thus, if the concentration of meβ-CD is too high, it binds much of the phospholipid rather than transfers it to cells. Accordingly, the optimal cyclodextrin concentration depends on the hydrophobicity of the lipid to be transferred. With the most hydrophobic species, the optimum is necessarily a compromise between transfer efficiency and cell viability.
It is well established that incubation of cells with meβ-CD or similar CDs can deplete a major fraction of their free cholesterol (33), which can severely compromise their viability. Here, we showed that such depletion can be markedly alleviated or even fully prevented by including ∼50 mol% in the donor vesicles. However, the mole fraction of cholesterol needed in the donor vesicles to prevent depletion of cellular cholesterol may depend on several parameters including donor vesicle composition and concentration (unpublished observations). In principle, phospholipid/cyclodextrin complexes could be prepared and used to introduce exogenous phospholipid to cells. However, full complexation of phospholipids requires such a high concentration (>100 mM) of cyclodextrin (9) that most of the cellular cholesterol and a significant fraction of phospholipids would be most likely depleted, thus killing the cells.
Beside cholesterol, meβ-CD has also been shown to extract other lipids from cells (12, 34). In the present study, depletion of up to 30% of total cellular SM was observed. However, analogously to cholesterol, depletion of cellular SM should be preventable by including proper amounts of SM in the donor vesicles. Beside the lipid to be transferred, we also found a small (4%) increase in cellular PC, probably due to transfer of POPC from the donor vesicles. However, the cellular contents of both PC and SM were essentially normalized during a 24 h chase.
Previously, exposure of HaCaT cells to meβ-CD was shown to cause i) a leakage of lactate dehydrogenase, ii) an increase in caspase-3/7 activity, iii) a decrease of ROS concentration, and iv) an increase in interleukin 6 and 8 (35). In addition, inhibitory effects, such as inhibition of cell growth and cell cycle arrest, have been reported for various cell types (36, 37). Such effects might be due to disturbed lipid composition (e.g., cholesterol depletion), or due to interaction of meβ-CD with other cellular components. Typically, the inhibitory effects were observed after an exposure to meβ-CD for several hours. In our experiments, the time of exposure was relatively short (typically ≤1 h) and cholesterol depletion was prevented, which may explain why the viability and growth of the cells were not significantly affected.
Applications
The present method should be very useful when studying various aspects of phospholipid metabolism. For instance, many phospholipids are remodeled after their synthesis de novo, i.e., one or both of their original acyl chains are replaced by others (38). This process involves A-type phospholipases as well as acyl transferases, but the identities of the enzymes involved are not clear. Recent data show that the A-type phospholipases involved in phospholipid remodeling are highly selective as indicated by, for example, acyl positional isomers of PS or PE being remodeled at very different rates (5). Such studies were made feasible by the use of meβ-CD-mediated transfer of exogenous heavy isotope-labeled phospholipids to cells. Notably, the traditional approach relying on soluble phospholipid precursors (such as serine, ethanolamine, or choline) cannot provide equally detailed information on remodeling pathways and kinetics due to initial labeling of a multitude of molecular species (5). This would also be the problem if lysolipids are used as precursors, as the reacylation process is not specific for the acyl chain to be added. Exogenous phospholipids containing truncated acyl chains, such as 6:0 or 8:0 (6, 7, 39) do not suffer from this problem but have other potential problems. First, unlike natural phospholipid species, they translocate very rapidly between organelles and may thus be metabolized differently from their natural counterparts. Second, they could be rapidly degraded by homeostatic phospholipases, which tend to recognize and degrade lipids with unnatural acyl chains (5). Yet due to their very short chains, they may not mix properly with existing lipids, thus potentially compromising structure and function of cellular membranes.
Whereas it is well established that cells maintain the lipid compositions of their organelle membranes within close limits (40), it is not understood how such an accurate regulation is accomplished (41). A useful approach to study this is to perturb cellular lipid composition by loading the cells with exogenous phospholipids using meβ-CD-mediated transport. The alternative approach based on overexpression of rate-limiting synthetic enzymes allows less efficient loading due to rapid degradation of the phospholipid produced in excess (42, 43). This approach is also less specific because the synthesis of numerous phospholipid molecular species and their precursors are simultaneously upregulated. The use of lysolipid precursors suffers from the same problem.
Transport of PS to mitochondria
We have previously shown that transfer of pyrene-labeled PS species from the plasma membrane to mitochondria is inversely proportional to molecular hydrophobicity, which in the case of phospholipids mainly depends on acyl chain length and unsaturation and less on the polar head group structure (11). Because the biological relevance of data obtained with lipids containing a bulky reporter group can be questioned, here we introduced deuterium-labeled PS species to BHK cells and then monitored by mass spectrometry the conversion of these PS species to PE. We found that the rate of decarboxylation and thus the rate of transfer, of these PS species was inversely proportional to their molecular hydrophobicity (Fig. 8). This is consistent with a model suggesting that spontaneous lipid transfer, in which efflux from the donor membrane is the rate limiting step (44), is the main mechanism of PS transfer from the plasma membrane to mitochondria. However, transfer of phospholipids by noncarrier lipid transfer proteins such as non-specific lipid transfer protein (nsLTP) is also inversely proportional to lipid hydrophobicity (45) and must thus be considered as an alternative or parallel mechanism. However, adequate molecular hydrophobicity of PS is required to maintain a high concentration of this phospholipid in the inner leaflet of the plasma membrane (11, 46).
In conclusion, incubation with phospholipid vesicles and meβ-CD allows efficient introduction of intact long-chain phospholipids to cultured cells without significantly compromising their growth or viability and should thus be very useful when studying intracellular phospholipid metabolism and trafficking.
Acknowledgments
The authors thank Tarja Grundström for expert technical assistance and Kati Hokynar and Krishna Batchu for critical reading of the manuscript.
Footnotes
Abbreviations:
- MAFP
- methyl arachidonyl fluorophosphonate
- meβ-CD
- methyl-β-cyclodextrin
- PC
- phosphatidylcholine
- PE
- phosphatidylethanolamine
- PL
- phospholipid
- POPC
- palmitoyl-oleoyl-phosphatidylcholine
- PS
- phosphatidylserine
Funding by Finnish Academy, Sigrid Juselius Foundation, Magnus Ehrnrooth Foundation, University of Helsinki Funds, Finska Läkaresällskapet, Nylands Nation, Svenska Kulturfondet, Orion Farmos, Emil Aaltonen Foundation, and Jenny and Antti Wihuri Funds is acknowledged.
REFERENCES
- 1.Samborski R. W., Ridgway N. D., Vance D. E. 1990. Evidence that only newly made phosphatidylethanolamine is methylated to phosphatidylcholine and that phosphatidylethanolamine is not significantly deacylated-reacylated in rat hepatocytes. J. Biol. Chem. 265: 18322–18329. [PubMed] [Google Scholar]
- 2.Tijburg L. B., Samborski R. W., Vance D. E. 1991. Evidence that remodeling of the fatty acids of phosphatidylcholine is regulated in isolated rat hepatocytes and involves both the sn-1 and sn-2 positions. Biochim. Biophys. Acta. 1085: 184–190. [DOI] [PubMed] [Google Scholar]
- 3.DeLong C. J., Shen Y. J., Thomas M. J., Cui Z. 1999. Molecular distinction of phosphatidylcholine synthesis between the CDP-choline pathway and phosphatidylethanolamine methylation pathway. J. Biol. Chem. 274: 29683–29688. [DOI] [PubMed] [Google Scholar]
- 4.Postle A. D., Hunt A. N. 2009. Dynamic lipidomics with stable isotope labelling. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 877: 2716–2721. [DOI] [PubMed] [Google Scholar]
- 5.Kainu V., Hermansson M., Somerharju P. 2008. Electrospray ionization mass spectrometry and exogenous heavy isotope-labeled lipid species provide detailed information on aminophospholipid acyl chain remodeling. J. Biol. Chem. 283: 3676–3687. [DOI] [PubMed] [Google Scholar]
- 6.Kol M. A., Kuster D. W., Boumann H. A., de Cock H., Heck A. J., de Kruijff B., de Kroon A. I. 2004. Uptake and remodeling of exogenous phosphatidylethanolamine in E. coli. Biochim. Biophys. Acta. 1636: 205–212. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka K., Fukuda R., Ono Y., Eguchi H., Nagasawa S., Nakatani Y., Watanabe H., Nakanishi H., Taguchi R., Ohta A. 2008. Incorporation and remodeling of extracellular phosphatidylcholine with short acyl residues in Saccharomyces cerevisiae. Biochim. Biophys. Acta. 1781: 391–399. [DOI] [PubMed] [Google Scholar]
- 8.van Meer G., Lange L. G., op den Kamp J. A., van Deenen L. L. 1980. Protein-stimulated exchange of phosphatidylcholine between intact erythrocytes and various membrane systems. Biochim. Biophys. Acta. 598: 173–177. [DOI] [PubMed] [Google Scholar]
- 9.Tanhuanpää K., Cheng K. H., Anttonen K., Virtanen J. A., Somerharju P. 2001. Characteristics of pyrene phospholipid/gamma-cyclodextrin complex. Biophys. J. 81: 1501–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanhuanpää K., Somerharju P. 1999. Gamma-cyclodextrins greatly enhance translocation of hydrophobic fluorescent phospholipids from vesicles to cells in culture. Importance of molecular hydrophobicity in phospholipid trafficking studies. J. Biol. Chem. 274: 35359–35366. [DOI] [PubMed] [Google Scholar]
- 11.Heikinheimo L., Somerharju P. 2002. Translocation of pyrene-labeled phosphatidylserine from the plasma membrane to mitochondria diminishes systematically with molecular hydrophobicity: implications on the maintenance of high phosphatidylserine content in the inner leaflet of the plasma membrane. Biochim. Biophys. Acta. 1591: 75–85. [DOI] [PubMed] [Google Scholar]
- 12.Kilsdonk E. P., Yancey P. G., Stoudt G. W., Bangerter F. W., Johnson W. J., Phillips M. C., Rothblat G. H. 1995. Cellular cholesterol efflux mediated by cyclodextrins. J. Biol. Chem. 270: 17250–17256. [DOI] [PubMed] [Google Scholar]
- 13.Ohtani Y., Irie T., Uekama K., Fukunaga K., Pitha J. 1989. Differential effects of alpha-, beta- and gamma-cyclodextrins on human erythrocytes. Eur. J. Biochem. 186: 17–22. [DOI] [PubMed] [Google Scholar]
- 14.Käkelä R., Somerharju P., Tyynelä J. 2003. Analysis of phospholipid molecular species in brains from patients with infantile and juvenile neuronal-ceroid lipofuscinosis using liquid chromatography-electrospray ionization mass spectrometry. J. Neurochem. 84: 1051–1065. [DOI] [PubMed] [Google Scholar]
- 15.Patel K. M., Morrisett J. D., Sparrow J. T. 1979. A convenient synthesis of phosphatidylcholines: acylation of sn-glycero-3-phosphocholine with fatty acid anhydride and 4-pyrrolidinopyridine. J. Lipid Res. 20: 674–677. [PubMed] [Google Scholar]
- 16.Silversand C., Haux C. 1997. Improved high-performance liquid chromatographic method for the separation and quantification of lipid classes: application to fish lipids. J. Chromatogr. B Biomed. Sci. Appl. 703: 7–14. [DOI] [PubMed] [Google Scholar]
- 17.Bartlett E. M., Lewis D. H. 1970. Spectrophotometric determination of phosphate esters in the presence and absence of orthophosphate. Anal. Biochem. 36: 159–167. [DOI] [PubMed] [Google Scholar]
- 18.Heikinheimo L., Somerharju P. 1998. Preferential decarboxylation of hydrophilic phosphatidylserine species in cultured cells. Implications on the mechanism of transport to mitochondria and cellular aminophospholipid species compositions. J. Biol. Chem. 273: 3327–3335. [DOI] [PubMed] [Google Scholar]
- 19.Folch J., Lees M., Sloane Stanley G. H. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226: 497–509. [PubMed] [Google Scholar]
- 20.Hermansson M., Uphoff A., Kakela R., Somerharju P. 2005. Automated quantitative analysis of complex lipidomes by liquid chromatography/mass spectrometry. Anal. Chem. 77: 2166–2175. [DOI] [PubMed] [Google Scholar]
- 21.Haimi P., Uphoff A., Hermansson M., Somerharju P. 2006. Software tools for analysis of mass spectrometric lipidome data. Anal. Chem. 78: 8324–8331. [DOI] [PubMed] [Google Scholar]
- 22.Gamble W., Vaughan M., Kruth H. S., Avigan J. 1978. Procedure for determination of free and total cholesterol in micro- or nanogram amounts suitable for studies with cultured cells. J. Lipid Res. 19: 1068–1070. [PubMed] [Google Scholar]
- 23.Storrie B., Madden E. A. 1990. Isolation of subcellular organelles. Methods Enzymol. 182: 203–225. [DOI] [PubMed] [Google Scholar]
- 24.Thomas P. D., Poznansky M. J. 1989. Curvature and composition-dependent lipid asymmetry in phosphatidylcholine vesicles containing phosphatidylethanolamine and gangliosides. Biochim. Biophys. Acta. 978: 85–90. [DOI] [PubMed] [Google Scholar]
- 25.Marsh D. 2010. Liquid-ordered phases induced by cholesterol: a compendium of binary phase diagrams. Biochim. Biophys. Acta. 1798: 688–699. [DOI] [PubMed] [Google Scholar]
- 26.Keller P., Simons K. 1998. Cholesterol is required for surface transport of influenza virus hemagglutinin. J. Cell Biol. 140: 1357–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lange Y., Ye J., Rigney M., Steck T. L. 1999. Regulation of endoplasmic reticulum cholesterol by plasma membrane cholesterol. J. Lipid Res. 40: 2264–2270. [PubMed] [Google Scholar]
- 28.Klein U., Gimpl G., Fahrenholz F. 1995. Alteration of the myometrial plasma membrane cholesterol content with beta-cyclodextrin modulates the binding affinity of the oxytocin receptor. Biochemistry. 34: 13784–13793. [DOI] [PubMed] [Google Scholar]
- 29.Voelker D. R. 1985. Disruption of phosphatidylserine translocation to the mitochondria in baby hamster kidney cells. J. Biol. Chem. 260: 14671–14676. [PubMed] [Google Scholar]
- 30.Child P., Myher J. J., Kuypers F. A., Op den Kamp J. A., Kuksis A., Van Deenen L. L. 1985. Acyl selectivity in the transfer of molecular species of phosphatidylcholines from human erythrocytes. Biochim. Biophys. Acta. 812: 321–332. [DOI] [PubMed] [Google Scholar]
- 31.van Meer G., Simons K. 1983. An efficient method for introducing defined lipids into the plasma membrane of mammalian cells. J. Cell Biol. 97: 1365–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Besterman J. M., Domanico P. L. 1992. Association and metabolism of exogenously-derived lysophosphatidylcholine by cultured mammalian cells: kinetics and mechanisms. Biochemistry. 31: 2046–2056. [DOI] [PubMed] [Google Scholar]
- 33.Zidovetzki R., Levitan I. 2007. Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim. Biophys. Acta. 1768: 1311–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fukasawa M., Nishijima M., Itabe H., Takano T., Hanada K. 2000. Reduction of sphingomyelin level without accumulation of ceramide in Chinese hamster ovary cells affects detergent-resistant membrane domains and enhances cellular cholesterol efflux to methyl-beta -cyclodextrin. J. Biol. Chem. 275: 34028–34034. [DOI] [PubMed] [Google Scholar]
- 35.Hipler U. C., Schonfelder U., Hipler C., Elsner P. 2007. Influence of cyclodextrins on the proliferation of HaCaT keratinocytes in vitro. J. Biomed. Mater. Res. A. 83: 70–79. [DOI] [PubMed] [Google Scholar]
- 36.Choi Y. A., Chin B. R., Rhee D. H., Choi H. G., Chang H. W., Kim J. H., Baek S. H. 2004. Methyl-beta-cyclodextrin inhibits cell growth and cell cycle arrest via a prostaglandin E(2) independent pathway. Exp. Mol. Med. 36: 78–84. [DOI] [PubMed] [Google Scholar]
- 37.Grosse P. Y., Bressolle F., Pinguet F. 1997. Methyl-beta-cyclodextrin in HL-60 parental and multidrug-resistant cancer cell lines: effect on the cytotoxic activity and intracellular accumulation of doxorubicin. Cancer Chemother. Pharmacol. 40: 489–494. [DOI] [PubMed] [Google Scholar]
- 38.Lands W. E. 1960. Metabolism of glycerolipids. 2. The enzymatic acylation of lysolecithin. J. Biol. Chem. 235: 2233–2237. [PubMed] [Google Scholar]
- 39.Deng L., Fukuda R., Kakihara T., Narita K., Ohta A. 2010. Incorporation and remodeling of phosphatidylethanolamine containing short acyl residues in yeast. Biochim. Biophys. Acta. 1801: 635–645. [DOI] [PubMed] [Google Scholar]
- 40.van Meer G. 2005. Cellular lipidomics. EMBO J. 24: 3159–3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Somerharju P., Virtanen J. A., Cheng K. H., Hermansson M. 2009. The superlattice model of lateral organization of membranes and its implications on membrane lipid homeostasis. Biochim. Biophys. Acta. 1788: 12–23. [DOI] [PubMed] [Google Scholar]
- 42.Baburina I., Jackowski S. 1999. Cellular responses to excess phospholipid. J. Biol. Chem. 274: 9400–9408. [DOI] [PubMed] [Google Scholar]
- 43.Walkey C. J., Kalmar G. B., Cornell R. B. 1994. Overexpression of rat liver CTP:phosphocholine cytidylyltransferase accelerates phosphatidylcholine synthesis and degradation. J. Biol. Chem. 269: 5742–5749. [PubMed] [Google Scholar]
- 44.Jones J. D., Thompson T. E. 1990. Mechanism of spontaneous, concentration-dependent phospholipid transfer between bilayers. Biochemistry. 29: 1593–1600. [DOI] [PubMed] [Google Scholar]
- 45.van Amerongen A., Demel R. A., Westerman J., Wirtz K. W. 1989. Transfer of cholesterol and oxysterol derivatives by the nonspecific lipid transfer protein (sterol carrier protein 2): a study on its mode of action. Biochim. Biophys. Acta. 1004: 36–43. [DOI] [PubMed] [Google Scholar]
- 46.Vance J. E., Steenbergen R. 2005. Metabolism and functions of phosphatidylserine. Prog. Lipid Res. 44: 207–234. [DOI] [PubMed] [Google Scholar]