Abstract
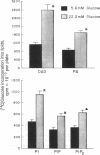
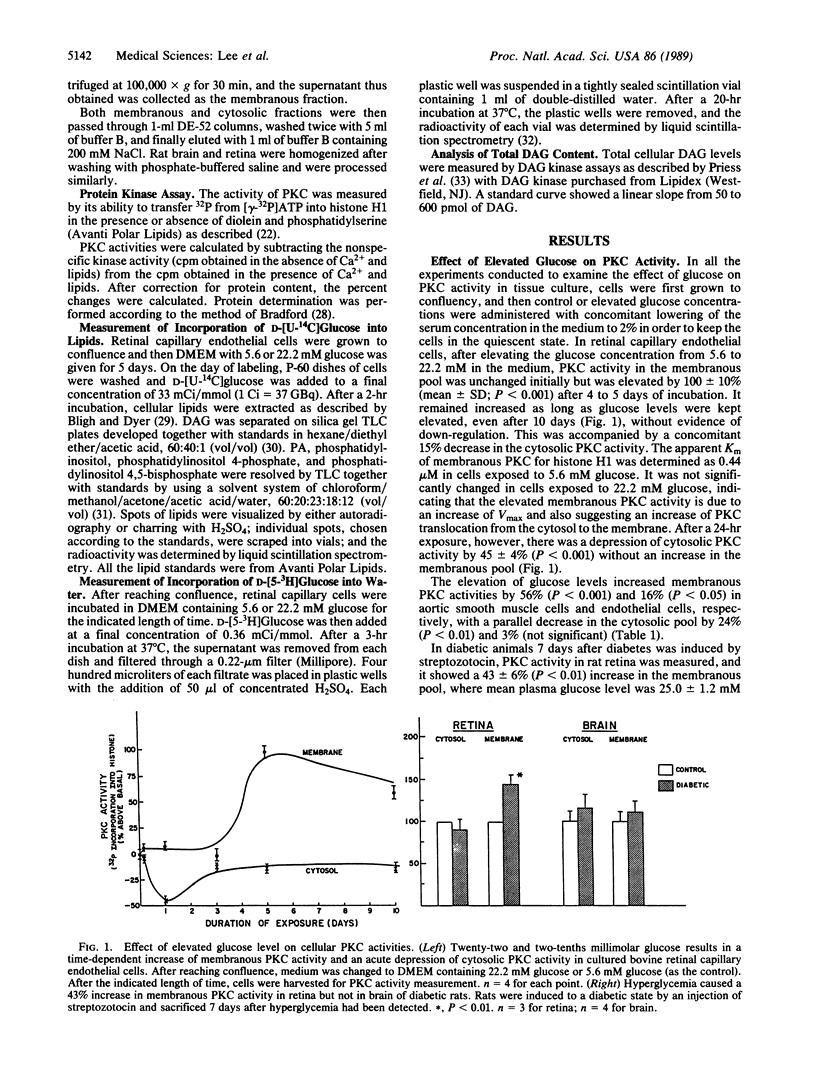
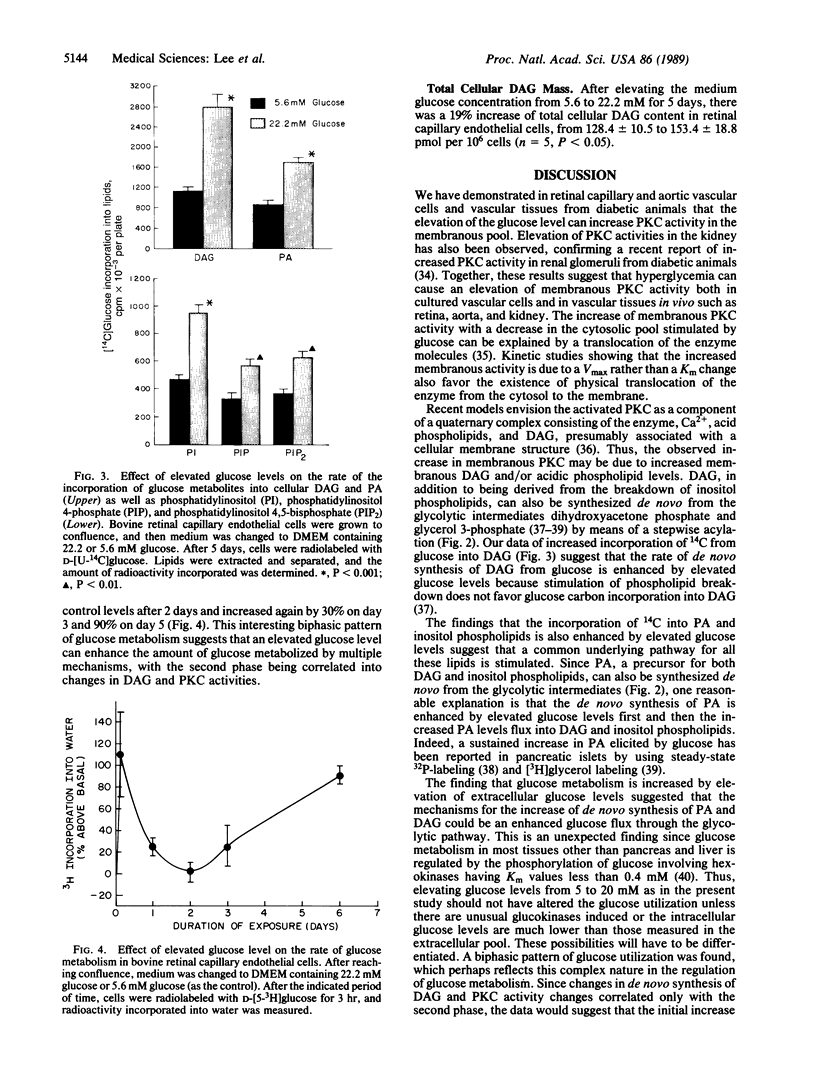
Hyperglycemia is believed to be the major cause of diabetic vascular complications involving both microvessels and arteries as in the retina, renal glomeruli, and aorta. It is unclear by which mechanism hyperglycemia is altering the metabolism and functions of vascular cells, although changes in nonenzymatic protein glycosylation and increases in cellular sorbitol levels have been postulated to be involved. Previously, we have reported that the elevation of extracellular glucose levels with cultured bovine retinal capillary endothelial cells causes an increase in protein kinase C (PKC) activity of the membranous pool with a parallel decrease in the cytosol without alteration of its total activity. Now we demonstrate that the mechanism for the activation of PKC is due to an enhanced de novo synthesis of diacylglycerol as indicated by a 2-fold increase of [14C]diacylglycerol labeling from [14C]glucose. The elevated diacylglycerol de novo synthesis is secondarily due to increased formation of precursors derived from glucose metabolism; this formation is enhanced by hyperglycemia as substantiated by elevated [3H]glucose conversion into water. This effect of hyperglycemia on PKC is also observed in cultured aortic smooth muscle and endothelial cells and the retina and kidney of diabetic rats, but not in the brain. Since PKC in vascular cells has been shown to modulate hormone receptor turnover, neovascularization in vitro, and cell growth, we propose that this mechanism of enhancing the membranous PKC activities by hyperglycemia plays an important role in the development of diabetic vascular complications.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews W. V., Conn P. M. Measurement of inositol phospholipid metabolites by one-dimensional thin-layer chromatography. Methods Enzymol. 1987;141:156–168. doi: 10.1016/0076-6879(87)41064-1. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Baraban J. M., Gould R. J., Peroutka S. J., Snyder S. H. Phorbol ester effects on neurotransmission: interaction with neurotransmitters and calcium in smooth muscle. Proc Natl Acad Sci U S A. 1985 Jan;82(2):604–607. doi: 10.1073/pnas.82.2.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berne C. The metabolism of lipids in mouse pancreatic islets. The biosynthesis of triacylglycerols and phospholipids. Biochem J. 1975 Dec;152(3):667–673. doi: 10.1042/bj1520667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best L., Malaisse W. J. Enhanced de novo synthesis of phosphatidic acid and phosphatidylinositol in rat pancreatic islets exposed to nutrient or neurotransmitter stimuli. Arch Biochem Biophys. 1984 Oct;234(1):253–257. doi: 10.1016/0003-9861(84)90347-3. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brownlee M., Cerami A., Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988 May 19;318(20):1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Gabbay K. H., Gallop P. M. The glycosylation of hemoglobin: relevance to diabetes mellitus. Science. 1978 Apr 7;200(4337):21–27. doi: 10.1126/science.635569. [DOI] [PubMed] [Google Scholar]
- Danthuluri N. R., Deth R. C. Phorbol ester-induced contraction of arterial smooth muscle and inhibition of alpha-adrenergic response. Biochem Biophys Res Commun. 1984 Dec 28;125(3):1103–1109. doi: 10.1016/0006-291x(84)91397-4. [DOI] [PubMed] [Google Scholar]
- Dunlop M. E., Larkins R. G. Pancreatic islets synthesize phospholipids de novo from glucose via acyl-dihydroxyacetone phosphate. Biochem Biophys Res Commun. 1985 Oct 30;132(2):467–473. doi: 10.1016/0006-291x(85)91157-x. [DOI] [PubMed] [Google Scholar]
- Farese R. V., DiMarco P. E., Barnes D. E., Sabir M. A., Larson R. E., Davis J. S., Morrison A. D. Rapid glucose-dependent increases in phosphatidic acid and phosphoinositides in rat pancreatic islets. Endocrinology. 1986 Apr;118(4):1498–1503. doi: 10.1210/endo-118-4-1498. [DOI] [PubMed] [Google Scholar]
- Flaherty M., Chojkier M. Selective inhibition of collagen synthesis by the Ca2+ ionophore A23187 in cultured human fibroblasts. J Biol Chem. 1986 Sep 15;261(26):12060–12065. [PubMed] [Google Scholar]
- Forder J., Scriabine A., Rasmussen H. Plasma membrane calcium flux, protein kinase C activation and smooth muscle contraction. J Pharmacol Exp Ther. 1985 Nov;235(2):267–273. [PubMed] [Google Scholar]
- Ganong B. R., Loomis C. R., Hannun Y. A., Bell R. M. Specificity and mechanism of protein kinase C activation by sn-1,2-diacylglycerols. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1184–1188. doi: 10.1073/pnas.83.5.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A., Sima A. A. Sorbitol, phosphoinositides, and sodium-potassium-ATPase in the pathogenesis of diabetic complications. N Engl J Med. 1987 Mar 5;316(10):599–606. doi: 10.1056/NEJM198703053161007. [DOI] [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A. Sodium- and energy-dependent uptake of myo-inositol by rabbit peripheral nerve. Competitive inhibition by glucose and lack of an insulin effect. J Clin Invest. 1982 Nov;70(5):1009–1018. doi: 10.1172/JCI110688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossbard L., Schimke R. T. Multiple hexokinases of rat tissues. Purification and comparison of soluble forms. J Biol Chem. 1966 Aug 10;241(15):3546–3560. [PubMed] [Google Scholar]
- Hachiya H. L., Takayama S., White M. F., King G. L. Regulation of insulin receptor internalization in vascular endothelial cells by insulin and phorbol ester. J Biol Chem. 1987 May 5;262(13):6417–6424. [PubMed] [Google Scholar]
- Halban P. A., Wollheim C. B., Blondel B., Renold A. E. Long-term exposure of isolated pancreatic islets to mannoheptulose: evidence for insulin degradation in the beta cell. Biochem Pharmacol. 1980 Oct 1;29(19):2625–2633. doi: 10.1016/0006-2952(80)90077-5. [DOI] [PubMed] [Google Scholar]
- Hokin L. E. Receptors and phosphoinositide-generated second messengers. Annu Rev Biochem. 1985;54:205–235. doi: 10.1146/annurev.bi.54.070185.001225. [DOI] [PubMed] [Google Scholar]
- Jiang M. J., Morgan K. G. Intracellular calcium levels in phorbol ester-induced contractions of vascular muscle. Am J Physiol. 1987 Dec;253(6 Pt 2):H1365–H1371. doi: 10.1152/ajpheart.1987.253.6.H1365. [DOI] [PubMed] [Google Scholar]
- Kariya K. I., Kawahara Y., Tsuda T., Fukuzaki H., Takai Y. Possible involvement of protein kinase C in platelet-derived growth factor-stimulated DNA synthesis in vascular smooth muscle cells. Atherosclerosis. 1987 Feb;63(2-3):251–255. doi: 10.1016/0021-9150(87)90128-6. [DOI] [PubMed] [Google Scholar]
- Kariya K., Fukumoto Y., Tsuda T., Kawahara Y., Fukuzaki H., Yamamoto T., Takai Y. Inhibition of DNA synthesis by phorbol esters through protein kinase C in cultured rabbit aortic smooth muscle cells. FEBS Lett. 1987 Jun 8;217(1):69–73. doi: 10.1016/0014-5793(87)81245-0. [DOI] [PubMed] [Google Scholar]
- King G. L., Goodman A. D., Buzney S., Moses A., Kahn C. R. Receptors and growth-promoting effects of insulin and insulinlike growth factors on cells from bovine retinal capillaries and aorta. J Clin Invest. 1985 Mar;75(3):1028–1036. doi: 10.1172/JCI111764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok C. F., Goldstein B. J., Muller-Wieland D., Lee T. S., Kahn C. R., King G. L. Identification of persistent defects in insulin receptor structure and function capillary endothelial cells from diabetic rats. J Clin Invest. 1989 Jan;83(1):127–136. doi: 10.1172/JCI113848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacal J. C., de la Peña P., Moscat J., Garcia-Barreno P., Anderson P. S., Aaronson S. A. Rapid stimulation of diacylglycerol production in Xenopus oocytes by microinjection of H-ras p21. Science. 1987 Oct 23;238(4826):533–536. doi: 10.1126/science.2821623. [DOI] [PubMed] [Google Scholar]
- Lee T. S., MacGregor L. C., Fluharty S. J., King G. L. Differential regulation of protein kinase C and (Na,K)-adenosine triphosphatase activities by elevated glucose levels in retinal capillary endothelial cells. J Clin Invest. 1989 Jan;83(1):90–94. doi: 10.1172/JCI113889. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Limas C. J., Limas C. Phorbol ester- and diacylglycerol-mediated desensitization of cardiac beta-adrenergic receptors. Circ Res. 1985 Sep;57(3):443–449. doi: 10.1161/01.res.57.3.443. [DOI] [PubMed] [Google Scholar]
- May W. S., Jr, Sahyoun N., Wolf M., Cuatrecasas P. Role of intracellular calcium mobilization in the regulation of protein kinase C-mediated membrane processes. Nature. 1985 Oct 10;317(6037):549–551. doi: 10.1038/317549a0. [DOI] [PubMed] [Google Scholar]
- Montesano R., Orci L. Tumor-promoting phorbol esters induce angiogenesis in vitro. Cell. 1985 Sep;42(2):469–477. doi: 10.1016/0092-8674(85)90104-7. [DOI] [PubMed] [Google Scholar]
- Nabika T., Nara Y., Yamori Y., Lovenberg W., Endo J. Angiotensin II and phorbol ester enhance isoproterenol- and vasoactive intestinal peptide (VIP)-induced cyclic AMP accumulation in vascular smooth muscle cells. Biochem Biophys Res Commun. 1985 Aug 30;131(1):30–36. doi: 10.1016/0006-291x(85)91765-6. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Okumura K., Akiyama N., Hashimoto H., Ogawa K., Satake T. Alteration of 1,2-diacylglycerol content in myocardium from diabetic rats. Diabetes. 1988 Sep;37(9):1168–1172. doi: 10.2337/diab.37.9.1168. [DOI] [PubMed] [Google Scholar]
- Peter-Riesch B., Fathi M., Schlegel W., Wollheim C. B. Glucose and carbachol generate 1,2-diacylglycerols by different mechanisms in pancreatic islets. J Clin Invest. 1988 Apr;81(4):1154–1161. doi: 10.1172/JCI113430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss J. E., Loomis C. R., Bell R. M., Niedel J. E. Quantitative measurement of sn-1,2-diacylglycerols. Methods Enzymol. 1987;141:294–300. doi: 10.1016/0076-6879(87)41077-x. [DOI] [PubMed] [Google Scholar]
- Rand L. I. Recent advances in diabetic retinopathy. Am J Med. 1981 Mar;70(3):595–602. doi: 10.1016/0002-9343(81)90581-7. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Forder J., Kojima I., Scriabine A. TPA-induced contraction of isolated rabbit vascular smooth muscle. Biochem Biophys Res Commun. 1984 Jul 31;122(2):776–784. doi: 10.1016/s0006-291x(84)80101-1. [DOI] [PubMed] [Google Scholar]