Abstract
Background
Glomerular filtration rate (GFR) is a heritable trait, and hyperfiltration (GFR increment in remnant nephrons) may accelerate renal functional decline in chronic kidney disease (CKD). Mesangial and vascular smooth myocytes control GFR by contraction, dependent on voltage-gated Ca2+ influx, which is controlled by the regulatory β1-subunit (KCNMB1) of large-conductance heteromeric K+ (‘BK’) channels. KCNMB1 gain-of-function variant Glu65Lys results in generalized vasorelaxation and thus protection against systemic hypertension. Here we asked whether the Glu65Lys variant influences GFR, in the basal state or during progressive renal decline.
Methods
We explored Glu65Lys effects on GFR in three populations spanning two ethnicities and two diseases (hypertension and nephrosclerosis). GFR was either estimated (eGFR from serum creatinine) or directly measured (iothalamate clearance).
Results
The 65Lys variant was relatively common, occurring on ∼5−10% of chromosomes in different biogeographic ancestry groups, and 65Lys carriers exhibited higher eGFR in two primary care populations: extreme BP values in Kaiser clinics (p = 0.029, accounting for ∼0.2% of trait variance), or treated hypertensives in VA clinics (p = 0.017, accounting for ∼0.9% of trait variance). In blacks with progressive renal disease (NIDDK AASK), 65Lys carriers displayed a steeper slope in GFR chronic decline (p = 0.030, accounting for ∼0.4% of trait variance), and Glu65Lys genotype also predicted time of onset of renal failure (log rank p = 0.019).
Conclusions
Common KCNMB1 gain-of-function variant Glu65Lys influences GFR, and 65Lys carriers exhibit not only elevated baseline GFR, but also more rapid GFR decline (and consequent development of renal failure) in CKD. The results suggest that profiling patients at Glu65Lys can assist in gauging renal prognosis as well as selection of rational therapy in hypertension with progressive renal disease.
Key Words: Glomerular filtration rate, KCNMB1, Hypertensive nephrosclerosis, African-American Study of Kidney Disease and Hypertension
Introduction
Glomerular filtration rate (GFR) is a central element of renal function; judicious measurement of GFR allows timely detection of early renal impairment to track the course of established renal disease. Paradoxically, hyperfiltration (elevated GFR in ‘remnant’ nephrons) may accelerate progression of chronic kidney disease (CKD). GFR displays substantial genetic control in twin [1] and family studies [2], with heritability (h2) approaching ∼75–80% of trait variance [1], though the specific genes or even the number of contributing loci remain elusive. End-stage renal disease (ESRD) also displays familial aggregation [3,4], and there is substantial interest in the potential impact of GFR-determining on progressive CKD. However, familial linkage (co-segregation) analyses of the GFR trait have yielded inconsistent results [2,5,6,7,8], and genome-wide association (GWA) studies in >20,000 individuals have identified variants accounting for only <1% of population GFR variance [9]. Thus, the search for genes influencing GFR continues.
Ca2+-activated K+ channels of large conductance (known as ‘Maxi-K’, ‘Big-K’, ‘BK’, or ‘Slo-K’ channels) are widely expressed across mammalian tissues, where they influence a variety of physiological processes, including smooth muscle tone, neurosecretion, and hearing. At the molecular level, the heteromeric BK channel is formed by an ion-conducting (ion-pore) α-subunit in non-covalent contact with a trans-membrane regulatory β-subunit. A prominent form of the heteromeric BK channel in vascular smooth myocytes is an α1 ion pore (KCNMA1) regulated by/interacting with a β1-subunit (KCNMB1). In excitable cells, plasma membrane depolarization occurs by opening of voltage-gated cation (Na+ and Ca2+) channels; plasma membrane re-polarization then proceeds by subsequent opening of voltage-gated K+ channels, such as BK. The β1-subunit has a ‘negative feedback’ effect on BK ion fluxes, increasing channel sensitivity to cytoplasmic Ca2+ [10,11]. Heteromeric KCNMA1/KCNMB1 BK channels are expressed in vascular smooth myocytes and mesangial cells [12], and the role of BK in regulation of GFR has been explored using a Kcnmb1-null mouse model [12], in which Kcnmb1(–/–) mice display blunted GFR response to volume expansion [13].
The KCNMB1 gene has been systematically explored for genetic variation [14], as well as the consequences of such variation. A single nucleotide substitution (G352A) in exon-3 of KCNMB1 creates a Glu65Lys substitution in the extracellular loop, resulting in a gain-of-function (enhanced K+ efflux) mutation [15] that is associated with a diminished prevalence of hypertension.
Based on the mesangial expression of KCNMB1[12], coupled with the electrophysiological and clinical/BP consequences of the Glu65Lys variant [15], we questioned whether this variant might influence GFR as well as its chronic decline in progressive renal disease. In this study, we explored the role of the Glu65Lys variant on GFR in three population samples, spanning two biogeographic ancestries (European and African) and two diseases (hypertension and CKD).
Methods
Subjects
Primary Care (Kaiser) Population. A population-based cohort with extreme blood pressures consisted of 516 male and 636 female white subjects. These participants were selected based on DBP in the upper or lower most extreme (5th) percentiles of DBP distribution in 25,599 males and 27,479 females in a primary care practice at Kaiser-Permanente of Southern California [16]. Individuals in the upper DBP percentiles were diagnosed as hypertensive, and 48% consumed antihypertensive medications; they were age-matched to individuals with DBP values in the lower extreme 5th percentiles. Serum creatinine was ≤1.5 mg/dl. GFR was estimated (eGFR) by the NIDDK MDRD formula: eGFR = (186) · (serum creatinine − 1.154) · (age − 0.203) · (1.21 if black) · (0.742 if female) (table 1a), as well as by the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) algorithm [17]. Since the Kaiser study was observational, proteinuria data were not systematically obtained.
Table 1.
Subject characteristics
| a Characteristics of the primary care (Kaiser) population | |||
|---|---|---|---|
| Lower BP | Higher BP | All | |
| Male/female, n | 251/349 | 265/287 | 516/636 |
| Age, years | 56.7 ± 0.6 | 59.9 ± 0.4 | 58.3 ± 0.4 |
| SBP, mm Hg | 107.8 ± 0.6 | 154.5 ± 0.6 | 129.9 ± 0.8 |
| DBP, mm Hg | 56.3 ± 0.1 | 99.2 ± 0.2 | 76.9 ± 0.6 |
| GFR, ml/min/1.73 m2 | 74.3 ± 0.6 | 74.1 ± 0.7 | 74.1 ± 0.5 |
| Biogeographic ancestry | all white/European | ||
| b Characteristics of the primary care (VA) hypertension population | ||
|---|---|---|
| GFR ml/min/1.73 m2 | ||
| Demographic | ||
| Male/female, n | 530/17(96.996/3.1%) | |
| Biogeographic ancestry | all white/European | |
| Age, years | 66.3 ± 0.5 | |
| SBP, mm Hg | 140.1 ± 0.8 | |
| DBP, mm Hg | 75.4 ± 0.5 | |
| GFR, ml/min/1.73 m2 | 71.8 ± 1.0 | |
| Antihypertensive drugs, % on each class at entry | ||
| ACEI | 54.7 | 74.5 ± 1.1 |
| ARB | 7.3 | 73.7 ± 3.1 |
| α1-Antagonist | 18.3 | 72.8 ± 2.0 |
| α2-Agonist | 5.4 | 75.4 ± 3.6 |
| β-Antagonist | 43.5 | 74.2 ± 1.3 |
| Calcium channel antagonist | 30.6 | 72.0 ± 1.5 |
| Diuretic | 46.9 | 74.3 ± 1.2 |
| None | 9.9 | 71.6 ± 2.6 |
| Other(s) | 26.4 | 73.1 ± 1.6 |
| Number of antihypertensive drugs, % at entry | ||
| 0 | 9.9 | 71.6 ± 2.6 |
| 1 | 20.1 | 73.8 ± 1.9 |
| 2 | 27.4 | 74.0 ± 1.6 |
| 3 | 22.2 | 74.8 ± 1.7 |
| 4 | 13.6 | 77.8 ± 2.3 |
| 5 | 5.6 | 66.4 ± 3.4 |
| 6 | 1.2 | 59.4 ± 8.6 |
| 7 | 0.1 | 62.9 ± 24.3 |
| c Characteristics of the genotyped hypertensive nephrosclerosis (NIDDK AASK) cohort | |
|---|---|
| Demographic | |
| Male/female, n | 466/310 |
| Biogeographic ancestry | all African-American |
| Age, years | 66.6 ± 0.4 |
| Blood pressure goal, usual/low | 391/385 |
| Antihypertensive drugs, randomized to: ramipril/metoprolol/amlodipine | 325/304/147 |
| Physiological | |
| Baseline GFR, ml/min/1.73 m2 | 46.7 ± 0.5 |
| Urine protein/creatinine, g/g | 0.29 ± 0.17 |
| GFR slope, ml/min/1.73 m2/year | −1.93 ± 0.83 |
Because of multiple medications in some individuals, the total equals >100% in Antihypertensive drugs'. Since each individual was tallied once, the total equals 100% in 'Number of antihypertensive drugs'.
Primary Care (VA) Hypertension Population. 530 males and 17 females were recruited from the primary care outpatient clinics at the VA San Diego Healthcare System [http://www.sandiego.va.gov/]. The ancestry was white (European, by self-identification), diagnosed as hypertension. 90.1% of the subjects were treated with antihypertensive drugs, with 47.5% on 1–2 medications and the rest on additional (from 3–7) medications. GFR was calculated by the NIDDK-MDRD formula, as given above (table 1b) and also calculated with the CKD-EPI algorithm [17]. Since the VA study was observational, proteinuria data were not systematically obtained.
CKD (NIDDK AASK) Population. The NIDDK AASK (African-American Study of Kidney Disease and Hypertension) trial of non-diabetic CKD has been described previously [18,19]. Briefly, participants were self-identified African-Americans with hypertension who were aged 18–70 years with a GFR between 20 and 65 ml/min/1.73 m2, urinary protein/creatinine ratio <2.5 g/g, and no other identified causes of renal insufficiency. Based on a 3 × 2 factorial design, participants were randomized to either a usual mean arterial pressure goal of 102−107 mm Hg or to a lower mean arterial pressure goal of ≤92 mm Hg, and to treatment with 1 of 3 antihypertensive drugs: β-adrenergic antagonist metoprolol, 50–200 mg/day; ACE inhibitor ramipril, 2.5–10 mg/day; or dihydropyridine calcium channel antagonist amlodipine, 5–10 mg/day. GFR was assessed by renal clearance of [125I]-iothalamate at baseline twice, then at 3, 6, and every 6 months thereafter. Serum and urinary levels of creatinine and protein were measured by a central laboratory at 6-month intervals. In the AASK trial, despite exclusion of subjects with substantial proteinuria (urinary protein/creatinine ratio <2.5 g/g), initial/entry protein excretion rate correlated inversely with baseline GFR, and predicted accelerated chronic decline of GFR (AASK JAMA 2001 and 2002). At the conclusion of the randomized portion of the study, AASK subjects who had not yet reached ESRD were invited to enroll in a cohort study for up to 10 years after initial enrollment [20].
The analysis is based on the rate of change in GFR (GFR slope). The GFR slope was determined separately during the first 3 months following randomization (acute slope) and after 3 months (chronic slope). The chronic slope was used as outcome in this study. The acute and chronic phases were distinguished because previous studies indicated that the AASK interventions have acute effects on GFR that may differ from their long-term effects on disease progression [18,21]. GFR slope for each individual was calculated from measured GFRs by linear mixed-effects model.
Subjects as well as biological samples for DNA were ascertained with informed written consent and IRB approval.
Genotyping
The KCNMB1 Glu65Lys (E65K, rs11739136, C/T) sequence was obtained from the dbSNP database [http://www.ncbi.nih.nlm.gov/SNP]. Genomic DNA was obtained from blood, or from buccal cells (Gentra Puregene Buccal Cell Kit [http://www1.qiagen.com/], yielding ∼10–20 μg/individual), and typed using a matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry system developed by Sequenom according to a published protocol [22], or by Pyrosequencing [23]. Reproducibility of diploid genotypes was verified with blinded replicate samples, indicating 98.8% concordance.
Statistics
GFR and Progressive Decline in GFR. Since the frequency of minor allele homozygosity (Lys/Lys) is quite low (∼0.25%) in European and African populations, meaningful numbers of Lys/Lys subjects were not available for statistical tests; we therefore combined Glu/Lys heterozygotes and Lys/Lys homozygotes for marker-on-trait analyses, thus effectively adopting a dominant model for 65Lys effects. Statistical analyses were performed with SPSS version 17. ANOVA with covariate (age, sex) adjustment, determined gene effects on GFR in the VA and Kaiser populations. Two-way ANOVA probed the interactions of gene-by-sex, gene-by-drug or, gene-by-BP status on GFR in the Kaiser population. In AASK, one-way ANOVA and linear regression determined the significance of genotype effects on baseline GFR and chronic (after 3 months on drug) GFR decline slope (ml/min/1.73 m2/year). Kaplan-Meier survival analysis investigated gene effects on the progression towards renal failure (composite endpoint: doubling of serum creatinine, or development of ESRD requiring dialysis), based on 10-year follow-up data in the NIDDK AASK population. To quantify the effect of a particular genetic variant on a renal trait, and normalize such values across traits, we estimated the coefficient of determination (adjusted R2) in general linear models (ANOVA or regression), in the presence or absence of the genotype; the difference in adjusted R2 (full model minus model without genotype) represents the contribution of the genotype to the trait; multiplication by 100 yields the coefficient of determination as a % of trait variance, where variance = (standard deviation) [2].
Exact (Permutation) Tests. In an exact test, a reference distribution is obtained by calculating all possible values of the test statistic under rearrangements of the labels on the observed data points. Resampling was performed with ‘Resampling Stats Excel add-in version 3.2’ [http://www.resample.com]. GFR decline rate values were independently permutated 1,000 times to generate an empirically derived distribution of test statistics. The observed test statistics were compared with the empirically derived distributions of test statistics to derive p values.
Population Admixture. African-Americans represent an admixed population with genetic contributions from both African and European biogeographic origins [24]. In order to confirm that AASK individuals with or without trait-associated genotypes were of comparable overall genetic background, and the observed associations were not simply an artifact of differential admixture between higher and lower GFR decline rate, GAMOVA (Generalized Analysis of Molecular Variance) [25] was used to test for and quantify the relationship between the overall genetic background of the subjects and quantitative phenotype GFR decline rate (chronic GFR slope), with an IBS (identity-by-state) distance matrix based on genotypes at 126 bi-allelic markers. GAMOVA thus assesses genetic background diversity using a genetic similarity matrix constructed from the allelic profiles of individuals under study.
Results
KCNMB1 Glu65Lys Allele Frequencies across Populations
The frequency of Glu65Lys variation was probed in 1,064 individuals (i.e. 2,128 chromosomes) arising from four different biogeographic ancestry groups (table 2). The minor (Lys) allele was found in each ancestry group. Frequencies differed across groups (χ2 = 18.4, p = 0.005), with the highest frequencies in subjects of East Asian or Hispanic ancestry (at ∼9–10%), and the lowest in subjects of European or African ancestry (at ∼5–6%). Diploid genotypes were in Hardy-Weinberg equilibrium in each population group. The frequency of minor allele homozygosity (Lys/Lys diploid genotype) is quite low (∼0.25%) in European and African populations, precluding meaningful numbers of subjects for statistical analysis; we therefore combined Glu/Lys heterozygotes and Lys/Lys homozygotes for marker-on-trait analyses, thus effectively adopting a dominant model for 65Lys effects.
Table 2.
KCNMB1 variant Glu65Lys: diploid genotype and allele frequencies across four biogeographic ancestry groups (ancestry determined by self-identification)
| Biogeographic ancestry | Individuals n | Chromo-somes (2n) | Glu65Lys diploid genotype |
Allele, A |
HWE p value | |||
|---|---|---|---|---|---|---|---|---|
| Glu/Glu | Glu/Lys | Lys/Lys | Glu65 | 65Lys | ||||
| White | 452 | 904 | 398 | 54 | 0 | 94.0 | 6.0 | 0.177 |
| Black | 342 | 684 | 310 | 32 | 0 | 95.3 | 4.7 | 0.364 |
| Asian | 150 | 300 | 125 | 24 | 1 | 91.3 | 8.7 | 0.896 |
| Hispanic | 120 | 240 | 98 | 20 | 2 | 90.0 | 10.0 | 0.417 |
| Global | 1,064 | 2,128 | 931 | 130 | 3 | 93.6 | 6.4 | 0.490 |
The genotype frequencies differed across populations, χ2 = 18.4, d.f. = 6, p = 0.005.
KCNMB1 Glu65Lys: Effect on eGFR
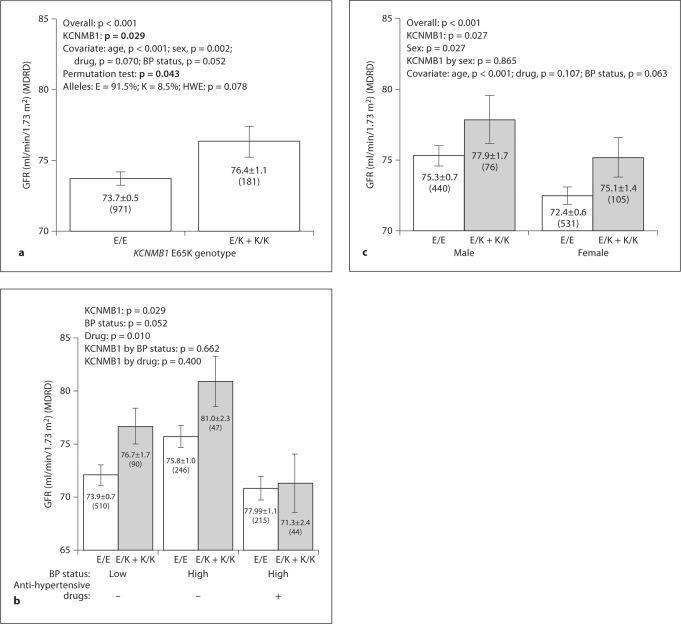
Primary Care Population No. 1 (Kaiser). Here we studied 1,152 individuals from the most extreme (upper and lower 5th percentile) BP values in a primary care population. When we divided the sample into higher versus lower BP groups, BP status itself did not predict eGFR (lower BP at 74.3 ± 0.7 vs. higher BP at 74.1 ± 0.7 ml/min/ 1.73 m2, p = 0.817). The effects of age, sex, antihypertensive drug, BP status and KCNMB1 genotype on eGFR were tested by linear regression; a model combining KCNMB1 genotype, age and sex predicted eGFR (p < 0.001) (table 3), while KCNMB1 genotype itself accounted for ∼0.2% of eGFR variance (p = 0.03). 65Lys allele carriers exhibited higher GFR than major allele homozygotes, by ∼2.7 ml/min/1.73 m2, or ∼3.7% (76.4 ± 1.1 vs. 73.7 ± 0.5 ml/min/1.73 m2, p = 0.017; fig. 1a), and the effect was also captured by permutation test (p = 0.043). After this global analysis, we studied the effects of Glu65Lys in subgroups (fig. 1b): the lower BP group (none on antihypertensive drugs), and higher BP group with or without drug treatment. GFR tended to be higher (p = 0.052) in the elevated BP group, and this effect was blunted by antihypertensive drug treatment (p = 0.01). 65Lys allele carriers displayed higher eGFR in both normotensives and hypertensives (p = 0.029), but the eGFR difference was abrogated by drug treatment (fig. 1b). Males exhibited higher GFR than females (p = 0.027), and 65Lys allele carriers exhibited higher eGFR than Glu/Glu homozygotes in both sexes (p = 0.027), while no gene-by-sex interaction was noted (fig. 1c).
Table 3.
Regression: fractional effects of KCNMB1 variant Glu65Lys on GFR in three independent populations
| Population |
|||
|---|---|---|---|
| Kaiser | VA | AASK | |
| Biogeographic ancestry | white | white | black |
| Source | primary care | primary care | NIDDK nephrosclerosis trial |
| Dependent variable | GFR | GFR | GFR slope |
| Overall MLR model (independent variables) | |||
| Predictors | gene, age, sex | gene, age | gene, initial GFR, sex, urine protein/creatinine |
| F | 80.5 [300.8] | 6.15 [21.26] | 35.3 |
| P | <0.001 [<0.001] | 0.002 [<0.001] | <0.001 |
| Adjusted R2 | 0.17 [0.34] | 0.019 [0.069] | 0.151 |
| Model component (independent variable) p values | |||
| Gene | 0.03 [0.04] | 0.02 [0.02] | 0.04 |
| R2 for gene | 0.2% [0.3%] | 0.9% [1.0%] | 0.4% |
| Age | <0.001 [<0.001] | 0.007 [<0.001] | >0.5 |
| Sex | 0.001 [>0.05] | >0.05 [>0.05] | 0.037 |
| Urine protein/creatinine | N/A | N/A | <0.001 |
| Baseline GFR | dependent | dependent | <0.001 |
GFR was calculated with NIDDK-MDRD formula as well as the CKD-EPI formula. Values in brackets reflect analyses with GFR calculated by the CKD-EPI algorithm.
MLR = Multiple linear regression, by stepwise regression method; Kaiser = primary care (Kaiser) population; VA = primary care (VA) hypertension population; AASK = hypertensive nephrosclerosis (NIDDK AASK) population. GFR: ml/min/1.73 m2; GFR slope: ml/min/1.73 m2/year. N/A = Not available. Values in italics indicate significance.
Fig. 1.
KCNMB1 Glu65Lys: Effect on BP and GFR in a primary care (Kaiser) population. GFR was estimated with the MDRD algorithm and mean ±1 SEM is shown. Numbers in parentheses (n) indicate the n for that observation. a Effects presented overall. The gene effect on GFR was determined by one-way ANOVA. b Role of BP status (higher vs. lower) and antihypertensive drug treatment. GFR was presented in 3 subgroups, high BP with/without antihypertensive medicines and low BP group. c Role of gene and sex. Effects were determined by two-way ANOVA.
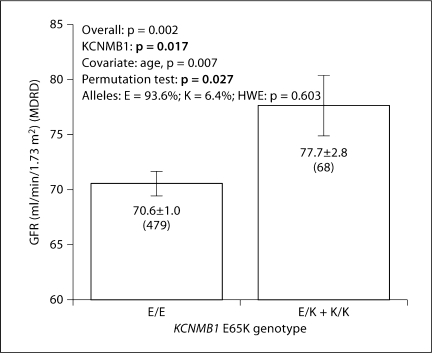
Primary Care Hypertension Population No. 2 (VA). All individuals in this population were diagnosed with hypertension, and 90.1% consumed antihypertensive medications (table 1). In this population, effects of sex and BP status could not be explored, since 96.9% were male and all (100%) had a diagnosis of hypertension. By regression, we analyzed the effects of several variables on eGFR: KCNMB1, age, number of drugs, and different drug groups; among these, KCNMB1 and age jointly predicted eGFR (p < 0.002; table 3), and the genotype effect alone accounted for ∼0.9% of trait variance (p < 0.02). 65Lys allele carriers had ∼7 ml/min/1.73 m2 (or ∼10%) higher eGFR than wild-type Glu/Glu homozygotes (77.7 ± 2.8 vs. 70.6 ± 1.0 ml/min/1.73 m2; fig. 2); a permutation test confirmed the difference (p = 0.027). Antihypertensive treatment did not demonstrate an independent effect on GFR, perhaps because only 9.9% were untreated.
Fig. 2.
KCNMB1 Glu65Lys effect on GFR in a primary care (VA) hypertension population. GFR was estimated by the MDRD algorithm. The gene effect on GFR was determined by one-way ANOVA. Numbers in parentheses (n) indicate the n for that observation.
Since renal function was not diminished in the majority of individuals in the primary care populations No. 1 (Kaiser) and No. 2 (VA), eGFR was also evaluated by the newer CKD-EPI formula [17]. Results were essentially unchanged from those obtained by MDRD-eGFR (table 3, CKD-EPI-eGFR values in brackets; table 4).
Table 4.
KCNMB1 Glu65Lys (E65K) effects on GFR in primary care populations: eGFR calculated by the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) algorithm
| Population |
||
|---|---|---|
| Kaiser | VA | |
| Biogeographic ancestry | white | white |
| Source | primary care | primary care |
| Dependent variable | GFR (CKD-EPI) | GFR (CKD-EPI) |
| One-way AN OVA | ||
| Overall | p < 0.001 | p < 0.001 |
| Gene | p = 0.046 | p = 0.020 |
| Covariates | ||
| BP status | p = 0.054 | all with hypertension |
| Age | p < 0.001 | p < 0.001 |
| Drug | p = 0.056 | p = 0.382 |
| Sex | p = 0.221 | p = 0.890 |
| GFR, ml/min/1.73 m2 | ||
| KCNMB1: E/E | 74.8 ± 0.4 (n = 971) | 66.9 ± 0.9 (n = 479) |
| KCNMB1: E/K+K/K | 77.0 ± 1.0 (n= 181) | 73.1 ± 2.4 (n = 68) |
Values in italics indicate sigificance.
KCNMB1 Glu65Lys: Effect on GFR Decline in CKD (NIDDK AASK)
Baseline GFR. Participants in the AASK trial [19] had hypertension with progressive nephrosclerosis, and at baseline 97% were already taking antihypertensive drugs. Glu65Lys did not predict baseline/entry GFR (F = 0.363, p = 0.574) in this group with limited GFR range, though entry GFR did vary by urine protein/creatinine ratio and sex, being lower in subjects with greater proteinuria, and higher in males versus females.
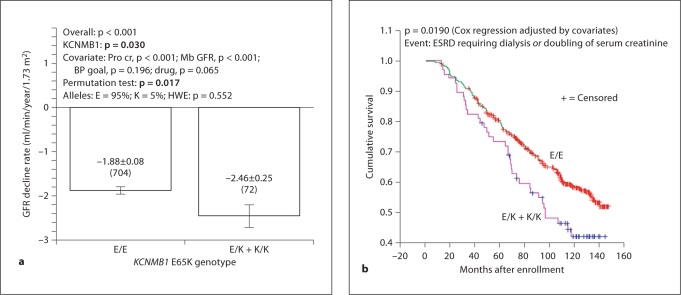
Chronic GFR Decline Rate. The AASK trial was designed to determine factors contributing to progression of hypertensive nephrosclerosis, so here we tested whether KCNMB1 genetic variation influenced chronic GFR decline rate (by serial iothalamate clearance). A regression model including KCNMB1 Glu65Lys, urine protein/creatinine ratio, initial/baseline GFR, and sex predicted chronic GFR slope (p < 0.001; table 3), and KCNMB1 itself accounted for ∼0.4% of trait variance (p = 0.04). 65Lys (minor) allele carriers displayed ∼31% faster GFR decline rate than Glu/Glu (major allele) homozygotes (−2.46 ± 0.25 vs. −1.88 ± 0.08 ml/min/1.73 m2/year, p = 0.030; fig. 3a); the effect was confirmed by permutation test (p = 0.017).
Fig. 3.
KCNMB1 Glu65Lys effect on GFR decline in non-diabetic CKD (NIDDK AASK). a Effect on chronic GFR slope in the genotyped cohort. GFR was determined by [125I]-iothalamate clearance. Chronic GFR slope refers to changes after 3 months on randomized study drug. The significance of the gene effect was determined with one-way ANOVA. b Progression to renal failure: survival analysis. The composite endpoint for Kaplan-Meier analysis was either ESRD requiring dialysis, or doubling of serum creatinine. Cox regression p = 0.019; covariates tested included gender, years of hypertension, SBP, DBP, baseline GFR, uric acid, baseline urine protein/creatinine, BP treatment goal, and drug group.
Progression to Renal Failure during 10-Year Follow-Up. After conclusion of the AASK randomized trial, the AASK cohort was then followed for 10 years after initial enrollment [20]. Kaplan-Meier analysis indicated that the 65Lys allele, which is associated with more rapid decline of GFR, also predicted truncated renal survival as compared to Glu/Glu homozygotes (fig. 3b; Cox regression p = 0.019); by 10 years, terminal event-free renal survival had declined to only ∼41% in 65Lys carriers, as compared to ∼59% in Glu/Glu homozygotes.
Genetic Admixture. The possibility that GFR slope differed as a polygenic function of degree of global genetic admixture was approached by comparison of genotype frequencies at widely dispersed markers, since admixture is a genome-wide phenomenon [26]. 126 bi-allelic markers were genotyped in subjects with the chronic GFR slope phenotype. GAMOVA (see Methods) [25] indicated that individuals with similar predictor variables (GFR slope) are not genetically closer to each other than expected by chance (p = 0.4516). Therefore, the allele frequency differences that we found at KCNMB1 cannot be attributed to differential admixture between the higher and lower GFR decline rate groups.
Discussion
Overview: GFR and Ion Channels
GFR, a direct indicator of renal function in CKD, is a heritable trait [1,2], though the responsible genes remain largely undetermined. Paradoxically, hyperfiltration (elevated GFR in ‘remnant’ nephrons) may accelerate progression of CKD. GFR is influenced by the pressure differential between afferent and efferent arterioles [27,28,29], as well as factors that regulate glomerular capillary surface area [30,31], such as mesangial cells. Glomerular mesangial cells, responding much like smooth muscle cells to vasoactive hormones [32,33,34], affect GFR by changing capillary surface area as well as redirecting capillary flow through mesangial loops.
Regulation of contraction by excitable vascular smooth myocytes and mesangial cells is dependent on voltage-gated Na+ (and hence Ca2+) influx. In response to contractile stimulation, cation channels depolarize the plasma membrane and thereby activate voltage-gated calcium channels, inducing contraction [35]. Depolarization then sets in motion the reciprocal process of re-polarization, via K+ efflux.
Physiological Role of KCNMB1
A crucial element of this re-polarization ‘feedback’ response involves activation/opening of the heteromeric plasma membrane ‘BK’ K+ channel, a large-conductance, Ca2+-responsive channel widely expressed on vascular myocytes. Re-polarization of the plasma membrane then terminates voltage-gated Ca2+ influx. The heteromeric BK channel has two subunits: a pore-forming/K+-translocating α-subunit (typically the α1-subunit KCNMA1), complexed with a membrane-spanning regulatory β-subunit (typically the β1-subunit KCNMB1). The KCNMB1 subunit, expressed in smooth muscle and mesangial cells, increases Ca2+ sensitivity of the BK channel [10,11], increasing the efficiency of the negative-feedback mechanism, hence determining contraction as well as basal tone [10,11,36,37,38].
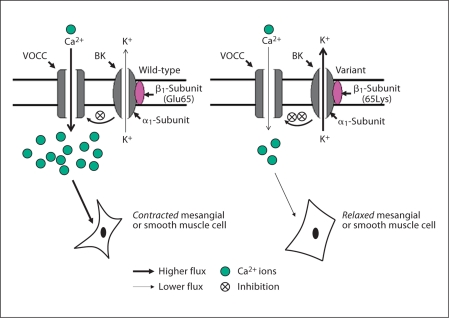
Although there are several genetic variants distributed across exon-3, ∼11-kbp KCNMB1 locus on chromosome 5q34 [14], one variant has clearly documented functional consequences for K+ ion fluxes [15]: amino acid substitution Glu65Lys (G352A) of exon-3, within the extracellular loop domain of the regulatory/β-subunit. Glu65Lys has been associated with hypertension, in that the 65Lys variant seems to protect against development of hypertension [15]. Functional analyses of KCNMB1 65Lys channel currents reveal a gain-of-function mutant, with enhanced outward K+ fluxes through the α-subunit and increased sensitivity to cytoplasmic Ca2+[15]; a more efficient BK-β1 channel would be predicted to yield an enhanced feedback mechanism, leading to a reduced Ca2+ entry via voltage-dependent Ca2+ channels, resulting in vascular smooth muscle and mesangial cell relaxation. Thus the BK channel plays a role much like an endogenous calcium channel blocker, and in this sense the gain-of-function 65Lys allele achieves more effective calcium entry blockade than the wild-type Glu65 allele (fig. 4).
Fig. 4.
KCNMB1 variant Glu65Lys: hypothesis for the effect on GFR. The gain-of-function KCNMB1 65Lys variant permits greater K+ efflux through KCNMA1, and thus more efficiently impedes Ca2+ entry through voltage operated Ca2+ channels (VOCC). Diminished cytosolic Ca2+ results in relaxation of mesangial or vascular smooth muscle cells, thereby increasing GFR.
Effects of Ca2+ Channels and KCNMB1 Genetic Variation on GFR
Calcium channel antagonist effects to increase GFR have been reported in both animal models and human subjects [39]. In the AASK trial, GFR acutely increased during the first 3 months after initiation of the amlodipine limb, but subsequently displayed enhanced decline [40]. By contrast, angiotensin-converting enzyme inhibitors appeared to be more effective than β-blockers or dihydropyridine calcium channel blockers in slowing GFR decline, though there was no additional benefit to slowing progression with more intensive BP lowering. Since KCNMB1 variant 65Lys acts to reduce voltage-gated Ca2+ influx [15], its actions resemble in some respects those of a calcium channel antagonist. Thus, an increase in GFR, with more rapid GFR decline in the face of hypertension, may not be unexpected in subjects carrying the 65Lys allele.
Here we found initially that 65Lys carriers had higher eGFR in two independent primary care populations (Kaiser and VA), each including patients with hypertension. Then, in a population with progressive hypertensive renal disease (NIDDK AASK), we found that the 65Lys allele accelerated long-term renal decline (GFR slope).
Previous studies suggested that the action of calcium channel antagonists to increase GFR might be dependent upon elevated basal renal vascular tone: the drugs acutely elevate GFR in patients with hypertension [40], while in animals the increase in GFR is most apparent during states of increased basal renal vascular tone [39]. Here we found that one population sample equally composed of normotensives and hypertensives (Kaiser) exhibited the eGFR effect, as did an exclusively hypertensive sample (VA) with pharmacological control of BP (average SBP/DBP ∼140/∼76 mm Hg, in ∼90% of treated individuals). Thus, the 65Lys variant may act in even in early stages of nephropathy, without substantial BP elevation.
In progressive renal disease (fig. 3), Glu65Lys influenced not only GFR decline slope, but also timing of arrival of renal failure (defined as arrival of ESRD requiring dialysis, or doubling of serum creatinine): since glomerular hyperfiltration is a risk factor for accelerated renal decline [41], the coordinate effects of 65Lys on GFR (fig. 1, 2) and progressive renal disease (fig. 3) are mechanistically consistent. Glu65Lys did not predict baseline GFR in AASK, perhaps a reflection of the rather narrow GFR entry range (20–65 ml/min/1.73 m2) specified by the AASK enrollment criteria [19], or the multiple vasoactive medications consumed by the AASK subjects even at initial enrollment.
The Literature in Context: Genes and GFR
Here we used a classical, hypothesis-testing experimental approach, proceeding from candidate gene to complex trait, by taking advantage of previous knowledge of vascular and sympathetic physiology and pathology. By contrast, hypothesis-free approaches, such as GWA studies present the theoretical advantage of discovering previously unsuspected contributory loci. Indeed, meta-analyses of four population-based cohorts in the CHARGE (Cohorts for Heart and Aging Research in Genomic Epidemiology) consortium recently discovered novel, replicable effects of at least 3 genetic loci (UMOD, SHROOM3, GATM-SPATA5L1) on eGFR and CKD. However, these loci account for only ∼0.7% of population GFR variance. Since twin and family studies indicate that up to ∼75–80% of BP variance is heritable [1], a substantial discrepancy arises, which has been referred to as the ‘missing heritability problem’ of GWA studies [42], a problem arising for most (if not all) complex traits, including not only GFR but also BP, height and body mass index [43]. What accounts for this discrepancy, or the ‘underperformance’ of GWA for complex traits? A number of explanations have been proposed, including contributions of rare variants [44] (whose effects are not readily detected by the high-minor-allele-frequency SNPs on GWA chips), incomplete linkage disequilibrium between marker and disease loci, the likelihood that individual SNP effects may be too small to pass stringent significance testing (as low as p < 5 × 10−8 for genome-wide multiple tests) [45], gene-by-gene interactions (epistasis), or gene-by-environment interactions. Of note, even here we estimate that KCNMB1 variant Glu65Lys accounts for only ∼0.2–0.9% of GFR trait variance in the three groups studied (table 2). Newer strategies, perhaps encompassing extensive resequencing across physiological pathway loci [43] may finally yield a comprehensive view of the relative roles of heredity and environment in shaping the very complex renal traits of GFR and CKD.
Conclusions and Perspectives
Here we found that KCNMB1 common variant Glu65Lys influences GFR across multiple populations of different clinical characteristics and biogeographic ancestries. The specificity and reproducibility of such effects were documented by replication, alternative statistical approaches, including multivariable tests (e.g., multiple linear regression), exact (permutation) tests, interaction effects, and admixture analyses. Finally, the practical significance of the GFR findings was confirmed by prediction of development of renal failure in subjects with progressive renal disease (fig. 3).
Figure 4 illustrates a hypothetical schema integrating our observations in light of the electrophysiology of the BK channel, to yield a framework for future mechanistic studies of the role of KCNMB1 genetic variation in control of renal function. Since the minor allele frequency of Glu65Lys is substantial in worldwide populations (table 1), ranging from ∼5 to 10%, our observations might be of importance to large numbers of individuals at risk of nephropathy; in this respect, it will be of high interest to extend our observations into populations such as Asians and Hispanics, where 65Lys allele frequencies of ∼9–10% would predict that upwards of ∼18–20% of the population might be susceptible to the hyperfiltration effects we describe.
Acknowledgements
We appreciate the assistance of the UCSD General Clinical Research Center (NIH RR00827), the UCSD Comprehensive Research Center in Health Disparities (CRCHD, NIH MD000220), the UAB/UCSD O'Brien Kidney Disease Research Center (NIH DK079337), and an International Society of Nephrology fellowship to Yuqing Chen. Supported by National Institutes of Health, Department of Veterans Affairs, International Society of Nephrology.
References
- 1.Rao F, Wessel J, Wen G, Zhang L, Rana BK, Kennedy BP, Greenwood TA, Salem RM, Chen Y, Khandrika S, Hamilton BA, Smith DW, Holstein-Rathlou NH, Ziegler MG, Schork NJ, O'Connor DT. Renal albumin excretion: twin studies identify influences of heredity, environment, and adrenergic pathway polymorphism. Hypertension. 2007;49:1015–1031. doi: 10.1161/HYPERTENSIONAHA.106.081679. [DOI] [PubMed] [Google Scholar]
- 2.Fox CS, Yang Q, Cupples LA, Guo CY, Larson MG, Leip EP, Wilson PW, Levy D. Genomewide linkage analysis to serum creatinine, GFR, and creatinine clearance in a community-based population: the Framingham Heart Study. J Am Soc Nephrol. 2004;15:2457–2461. doi: 10.1097/01.ASN.0000135972.13396.6F. [DOI] [PubMed] [Google Scholar]
- 3.Freedman BI, Spray BJ, Tuttle AB, Buckalew VM., Jr The familial risk of end-stage renal disease in African-Americans. Am J Kidney Dis. 1993;21:387–393. doi: 10.1016/s0272-6386(12)80266-6. [DOI] [PubMed] [Google Scholar]
- 4.Lei HH, Perneger TV, Klag MJ, Whelton PK, Coresh J. Familial aggregation of renal disease in a population-based case-control study. J Am Soc Nephrol. 1998;9:1270–1276. doi: 10.1681/ASN.V971270. [DOI] [PubMed] [Google Scholar]
- 5.Cohn DH, Shohat T, Yahav M, Ilan T, Rechavi G, King L, Shohat M. A locus for an autosomal dominant form of progressive renal failure and hypertension at chromosome 1q21. Am J Hum Genet. 2000;67:647–651. doi: 10.1086/303051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeWan AT, Arnett DK, Atwood LD, Province MA, Lewis CE, Hunt SC, Eckfeldt J. A genome scan for renal function among hypertensives: the Hypergen Study. Am J Hum Genet. 2001;68:136–144. doi: 10.1086/316927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freedman BI, Rich SS, Yu H, Roh BH, Bowden DW. Linkage heterogeneity of end-stage renal disease on human chromosome 10. Kidney Int. 2002;62:770–774. doi: 10.1046/j.1523-1755.2002.00534.x. [DOI] [PubMed] [Google Scholar]
- 8.Hunt SC, Hasstedt SJ, Coon H, Camp NJ, Cawthon RM, Wu LL, Hopkins PN. Linkage of creatinine clearance to chromosome 10 in Utah pedigrees replicates a locus for end-stage renal disease in humans and renal failure in the fawn-hooded rat. Kidney Int. 2002;62:1143–1148. doi: 10.1111/j.1523-1755.2002.kid557.x. [DOI] [PubMed] [Google Scholar]
- 9.Kottgen A, Glazer NL, Dehghan A, Hwang SJ, Katz R, Li M, Yang Q, Gudnason V, Launer LJ, Harris TB, Smith AV, Arking DE, Astor BC, Boerwinkle E, Ehret GB, Ruczinski I, Scharpf RB, Ida Chen YD, de Boer IH, Haritunians T, Lumley T, Sarnak M, Siscovick D, Benjamin EJ, Levy D, Upadhyay A, Aulchenko YS, Hofman A, Rivadeneira F, Uitterlinden AG, van Duijn CM, Chasman DI, Pare G, Ridker PM, Kao WH, Witteman JC, Coresh J, Shlipak MG, Fox CS. Multiple loci associated with indices of renal function and chronic kidney disease. Nat Genet. 2009;41:712–717. doi: 10.1038/ng.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McManus OB, Helms LM, Pallanck L, Ganetzky B, Swanson R, Leonard RJ. Functional role of the β-subunit of high conductance calcium-activated potassium channels. Neuron. 1995;14:645–650. doi: 10.1016/0896-6273(95)90321-6. [DOI] [PubMed] [Google Scholar]
- 11.Orio P, Rojas P, Ferreira G, Latorre R. New disguises for an old channel: Maxik channel β-subunits. News Physiol Sci. 2002;17:156–161. doi: 10.1152/nips.01387.2002. [DOI] [PubMed] [Google Scholar]
- 12.Ma R, Pluznick JL, Sansom SC. Ion channels in mesangial cells: function, malfunction, or fiction. Physiology (Bethesda) 2005;20:102–111. doi: 10.1152/physiol.00050.2004. [DOI] [PubMed] [Google Scholar]
- 13.Pluznick JL, Wei P, Carmines PK, Sansom SC. Renal fluid and electrolyte handling in BKCa-β1–/– mice. Am J Physiol Renal Physiol. 2003;284:F1274–F1279. doi: 10.1152/ajprenal.00010.2003. [DOI] [PubMed] [Google Scholar]
- 14.Gong Y, Beitelshees AL, Wessel J, Langaee TY, Schork NJ, Johnson JA. Single nucleotide polymorphism discovery and haplotype analysis of Ca2+-dependent K+ channel β1-subunit. Pharmacogenet Genomics. 2007;17:267–275. doi: 10.1097/FPC.0b013e3280105235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandez-Fernandez JM, Tomas M, Vazquez E, Orio P, Latorre R, Senti M, Marrugat J, Valverde MA. Gain-of-function mutation in the KCNMB1 potassium channel subunit is associated with low prevalence of diastolic hypertension. J Clin Invest. 2004;113:1032–1039. doi: 10.1172/JCI20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rana BK, Insel PA, Payne SH, Abel K, Beutler E, Ziegler MG, Schork NJ, O'Connor DT. Population-based sample reveals gene-gender interactions in blood pressure in white Americans. Hypertension. 2007;49:96–106. doi: 10.1161/01.HYP.0000252029.35106.67. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agodoa LY, Appel L, Bakris GL, Beck G, Bourgoignie J, Briggs JP, Charleston J, Cheek D, Cleveland W, Douglas JG, Douglas M, Dowie D, Faulkner M, Gabriel A, Gassman J, Greene T, Hall Y, Hebert L, Hiremath L, Jamerson K, Johnson CJ, Kopple J, Kusek J, Lash J, Lea J, Lewis JB, Lipkowitz M, Massry S, Middleton J, Miller ER, 3rd, Norris K, O'Connor D, Ojo A, Phillips RA, Pogue V, Rahman M, Randall OS, Rostand S, Schulman G, Smith W, Thornley-Brown D, Tisher CC, Toto RD, Wright JT, Jr, Xu S. Effect of ramipril vs. amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA. 2001;285:2719–2728. doi: 10.1001/jama.285.21.2719. [DOI] [PubMed] [Google Scholar]
- 19.Wright JT, Jr, Kusek JW, Toto RD, Lee JY, Agodoa LY, Kirk KA, Randall OS, Glassock R. Design and baseline characteristics of participants in the African-American Study of Kidney Disease and Hypertension (AASK) pilot study. Control Clin Trials. 1996;17:3S–16S. doi: 10.1016/s0197-2456(96)00081-5. [DOI] [PubMed] [Google Scholar]
- 20.Appel LJ, Middleton J, Miller ER, 3rd, Lipkowitz M, Norris K, Agodoa LY, Bakris G, Douglas JG, Charleston J, Gassman J, Greene T, Jamerson K, Kusek JW, Lewis JA, Phillips RA, Rostand SG, Wright JT. The rationale and design of the AASK cohort study. J Am Soc Nephrol. 2003;14:S166–S172. doi: 10.1097/01.asn.0000070081.15137.c0. [DOI] [PubMed] [Google Scholar]
- 21.Hall WD, Kusek JW, Kirk KA, Appel LJ, Schulman G, Agodoa LY, Glassock R, Grim C, Randall OS, Massry SG. Short-term effects of blood pressure control and antihypertensive drug regimen on glomerular filtration rate: the African-American Study of Kidney Disease and Hypertension Pilot Study. Am J Kidney Dis. 1997;29:720–728. doi: 10.1016/s0272-6386(97)90125-6. [DOI] [PubMed] [Google Scholar]
- 22.Buetow KH, Edmonson M, MacDonald R, Clifford R, Yip P, Kelley J, Little DP, Strausberg R, Koester H, Cantor CR, Braun A. High-throughput development and characterization of a genomewide collection of gene-based single nucleotide polymorphism markers by chip-based matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Proc Natl Acad Sci USA. 2001;98:581–584. doi: 10.1073/pnas.021506298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmadian A, Ehn M, Hober S. Pyrosequencing: history, biochemistry and future. Clin Chim Acta. 2006;363:83–94. doi: 10.1016/j.cccn.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 24.McKeigue PM, Carpenter JR, Parra EJ, Shriver MD. Estimation of admixture and detection of linkage in admixed populations by a bayesian approach: application to African-American populations. Ann Hum Genet. 2000;64:171–186. doi: 10.1017/S0003480000008022. [DOI] [PubMed] [Google Scholar]
- 25.Nievergelt CM, Libiger O, Schork NJ. Generalized analysis of molecular variance. PLoS Genet. 2007;3:e51. doi: 10.1371/journal.pgen.0030051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pritchard JK, Rosenberg NA. Use of unlinked genetic markers to detect population stratification in association studies. Am J Hum Genet. 1999;65:220–228. doi: 10.1086/302449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baylis C, Deen WM, Myers BD, Brenner BM. Effects of some vasodilator drugs on transcapillary fluid exchange in renal cortex. Am J Physiol. 1976;230:1148–1158. doi: 10.1152/ajplegacy.1976.230.4.1148. [DOI] [PubMed] [Google Scholar]
- 28.Brenner BM, Troy JL, Daugharty TM. The dynamics of glomerular ultrafiltration in the rat. J Clin Invest. 1971;50:1776–1780. doi: 10.1172/JCI106667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Myers BD, Deen WM, Brenner BM. Effects of norepinephrine and angiotensin II on the determinants of glomerular ultrafiltration and proximal tubule fluid reabsorption in the rat. Circ Res. 1975;37:101–110. doi: 10.1161/01.res.37.1.101. [DOI] [PubMed] [Google Scholar]
- 30.Inkyo-Hayasaka K, Sakai T, Kobayashi N, Shirato I, Tomino Y. Three-dimensional analysis of the whole mesangium in the rat. Kidney Int. 1996;50:672–683. doi: 10.1038/ki.1996.364. [DOI] [PubMed] [Google Scholar]
- 31.Kriz W, Elger M, Mundel P, Lemley KV. Structure-stabilizing forces in the glomerular tuft. J Am Soc Nephrol. 1995;5:1731–1739. doi: 10.1681/ASN.V5101731. [DOI] [PubMed] [Google Scholar]
- 32.Ausiello DA, Kreisberg JI, Roy C, Karnovsky MJ. Contraction of cultured rat glomerular cells of apparent mesangial origin after stimulation with angiotensin II and arginine vasopressin. J Clin Invest. 1980;65:754–760. doi: 10.1172/JCI109723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sansom SC, Stockand JD. Physiological role of large, Ca2+-activated K+ channels in human glomerular mesangial cells. Clin Exp Pharmacol Physiol. 1996;23:76–82. doi: 10.1111/j.1440-1681.1996.tb03066.x. [DOI] [PubMed] [Google Scholar]
- 34.Venkatachalam MA, Kreisberg JI. Agonist-induced isotonic contraction of cultured mesangial cells after multiple passage. Am J Physiol. 1985;249:C48–C55. doi: 10.1152/ajpcell.1985.249.1.C48. [DOI] [PubMed] [Google Scholar]
- 35.Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am J Physiol. 2000;278:C235–256. doi: 10.1152/ajpcell.2000.278.2.C235. [DOI] [PubMed] [Google Scholar]
- 36.Knaus HG, Folander K, Garcia-Calvo M, Garcia ML, Kaczorowski GJ, Smith M, Swanson R. Primary sequence and immunological characterization of β-subunit of high conductance Ca2+-activated K+ channel from smooth muscle. J Biol Chem. 1994;269:17274–17278. [PubMed] [Google Scholar]
- 37.Meera P, Wallner M, Jiang Z, Toro L. A calcium switch for the functional coupling between α (hslo) and β subunits (Kv, cabeta) of maxi K channels. FEBS Lett. 1996;385:127–128. doi: 10.1016/0014-5793(96)83884-1. [DOI] [PubMed] [Google Scholar]
- 38.Pluger S, Faulhaber J, Furstenau M, Lohn M, Waldschutz R, Gollasch M, Haller H, Luft FC, Ehmke H, Pongs O. Mice with disrupted BK channel β1-subunit gene feature abnormal Ca2+ spark/STOC coupling and elevated blood pressure. Circ Res. 2000;87:E53–E60. doi: 10.1161/01.res.87.11.e53. [DOI] [PubMed] [Google Scholar]
- 39.Carmines PK, Mitchell KD, Navar LG. Effects of calcium antagonists on renal hemodynamics and glomerular function. Kidney Int Suppl. 1992;36:S43–S48. [PubMed] [Google Scholar]
- 40.Wright JT, Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP, Rostand SG. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 41.Hostetter TH. Hyperfiltration and glomerulosclerosis. Semin Nephrol. 2003;23:194–199. doi: 10.1053/anep.2003.50017. [DOI] [PubMed] [Google Scholar]
- 42.Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, McCarthy MI, Ramos EM, Cardon LR, Chakravarti A, Cho JH, Guttmacher AE, Kong A, Kruglyak L, Mardis E, Rotimi CN, Slatkin M, Valle D, Whittemore AS, Boehnke M, Clark AG, Eichler EE, Gibson G, Haines JL, Mackay TF, McCarroll SA, Visscher PM. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang K, Weder AB, Eskin E, O'Connor DT. Genome-wide case/control studies in hypertension: only the ‘tip of the iceberg’. J Hypertens. 2010;28:1115–1123. doi: 10.1097/HJH.0b013e328337f6bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bodmer W, Bonilla C. Common and rare variants in multifactorial susceptibility to common diseases. Nat Genet. 2008;40:695–701. doi: 10.1038/ng.f.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang J, Benyamin B, McEvoy BP, Gordon S, Henders AK, Nyholt DR, Madden PA, Heath AC, Martin NG, Montgomery GW, Goddard ME, Visscher PM. Common SNPs explain a large proportion of the heritability for human height. Nat Genet. 2010;42:565–569. doi: 10.1038/ng.608. [DOI] [PMC free article] [PubMed] [Google Scholar]