Abstract
Background/Aims
There are few studies that evaluate the clinical outcomes of individuals with non-amnestic mild cognitive impairment (MCI). The purpose of this study was to evaluate baseline predictors of clinical progression after 2 years for patients with dysexecutive MCI (dMCI), a single-domain non-amnestic MCI subgroup.
Methods
We evaluated clinical progression in a sample of 31 older adults with dMCI. Clinical progression was defined as a worsening on the Clinical Dementia Rating sum of boxes at the 2-year visit, whereas patients were classified as stable if the score did not worsen over 2 years. We compared baseline brain MRI, neuropsychological tests, and health risk factors.
Results
Twelve individuals with dMCI progressed clinically, and 19 individuals remained stable over 2 years. Compared to the stable dMCI patients, the dMCI patients who progressed showed brain atrophy in the bilateral insula and left lateral temporal lobe on MRI. dMCI patients who progressed were also older, had lower baseline performance on category fluency and a spatial location task, and reported fewer dysexecutive symptoms. Health risk factors, except hypertension, did not differ between groups.
Conclusion
The results suggest that dMCI patients who progress relatively quickly over 2 years may have unique clinical and brain MRI features.
Key Words: Executive function, Non-amnestic mild cognitive impairment, Dysexecutive mild cognitive impairment
Introduction
Mild cognitive impairment (MCI) in older adults reflects a decline in cognition that is not sufficient to meet the criteria for dementia. MCI is a clinically heterogeneous syndrome, and current models suggest that the MCI syndrome is comprised of 4 broad subgroups: amnestic (single and multiple domain) and non-amnestic (single and multiple domain) [1]. The majority of early studies focused on amnestic MCI and progression to Alzheimer's disease (AD). However, there is increasing interest in the clinical outcomes of non-amnestic MCI. Non-amnestic single-domain MCI refers to patients who have an impairment in a single cognitive domain other than memory (e.g. executive, language, visuospatial), whereas non-amnestic multiple-domain MCI refers to impairment in 2 or more non-memory domains.
Prevalence estimates for non-amnestic MCI range from 17 to 38% of all MCI patients [2,3]. There is considerable interest in the clinical outcome, and early studies suggested that non-amnestic MCI patients were most likely to convert to non-AD dementias [1]. In 1 early study, Busse et al. [4] found that 14% of non-amnestic MCI patients converted to AD and 10% converted to non-AD dementias; however, 24% died, and 32% improved to normal. Other studies report that 13–27% of non-amnestic MCI patients convert to AD [3,5], but few autopsy studies have been done. Another study found that single-domain non-amnestic MCI patients had higher rates of death but less frequent conversion to dementia when compared to amnestic MCI [6]. The non-amnestic MCI literature is confounded by the fact that most studies lump all types of non-amnestic MCI patients into 1 group, which often includes both single- and multiple-domain non-amnestic MCI patients. It is likely that the different non-amnestic single-domain MCI subgroups (e.g. dysexecutive, language, visuospatial) will have distinct clinical features and different risks for converting to dementia [7,8]. It is, therefore, important to subdivide single-domain non-amnestic MCI groups to improve clinical differentiation.
The purpose of this study was to evaluate baseline predictors of clinical progression after 2 years for patients with dysexecutive MCI (dMCI), a single-domain non-amnestic MCI subgroup. Our primary aim was to compare baseline behavioral/medical risk factors and brain MRI between the dMCI patients who worsened and those who remained stable on the informant-based Clinical Dementia Rating (CDR) scale, one measure of clinical progression. We hypothesized that the dMCI patients who progressed would have worse clinical characteristics and more brain atrophy than those who remained stable.
Methods
Participants
The participants were recruited prospectively for a study about dMCI [7,8], and were initially referred from the University of California San Francisco (UCSF) Memory and Aging clinic or from a community screening clinic (after responding to a newspaper advertisement about cognitive decline in any domain). The study was approved by the UCSF Committee on Human Research. The clinic and community participants had identical evaluations. Participants were diagnosed with dMCI after a detailed history, physical and neurological examination, a 1-hour neuropsychological screening [9], and a study partner interview that included the CDR [10] to assess clinical severity and the Neuropsychiatric Inventory [11] to evaluate behavior. Study partners had regular contact and had known the participant for at least 10 years. Age at onset was estimated from the history obtained from both the patient and study partner, as were the first and current symptoms. Screening for depression was done using the 30-item Geriatric Depression Scale [12] (self-report) and an interview with the study partner.
Clinical diagnosis was determined by consensus involving the neurologist, neuropsychologist, and nurse using the diagnostic information described above. Patients were classified as dMCI [7] based on clinical judgment of relatively focal executive dysfunction, including cognitive scores at or below the 10th percentile of control performance on at least 1 of 4 screening tests of executive function [9] (table 1); they also had to score within the normal range (within 1 SD from the norm) on tests of memory [9,13], language, and visuospatial skills [9,14] (table 1). The Wechsler Adult Intelligence Scale III vocabulary subtest [15] was used as an estimate of premorbid cognitive ability. The 3 modified tests were shortened versions [9] of classic tests.
Table 1.
Baseline neuropsychological results for déclin ers versus stable dMCI
| Group 1 (decliners) | Group 2 (stable) | F statistic | P value | |
|---|---|---|---|---|
| Global | ||||
| MMSE (max. 30) | 28.8 ± 1.4 | 29.3 ± 1.1 | 0.17 | 0.36 |
| Memory | ||||
| CVLT long-delay free recall (max. 16) | 9.0 ± 2.6 | 10.1 ± 3.4 | 1.56 | 0.67 |
| CVLT hits (max. 16) | 14.5 ± 1.2 | 13.9 ± 2.4 | 0.06 | 0.80 |
| Modified Rey-Osterrieth figure recall (max. 17) | 10.0 ± 2.7 | 12.9 ± 2.3 | 3.73 | 0.06 |
| Executive function | ||||
| Modified Trail-Making Test B (max. 120 s) | 41.4 ± 28.3 | 39.9 ± 20.2 | 1.19 | 0.29 |
| Modified Stroop interference (number correct in 1 min) | 41.6 ± 11.9 | 47.7 ± 12.6 | 0.98 | 0.33 |
| Letter fluency (D words in 1 min) | 13.9 ± 5.8 | 14.5 ± 4.6 | 0.01 | 0.93 |
| Abstractions (max. 6) | 4.6 ± 1.4 | 4.6 ± 1.7 | 0.12 | 0.74 |
| Visuospatial | ||||
| Copy of modified Rey-Osterrieth figure (max. 17) | 15.4 ± 1.1 | 16 ± 1.2 | 0.88 | 0.36 |
| VOSP number location (max. 10) | 8.5 ± 1.8 | 9.4 ± 0.8 | 6.22 | 0.02∗ |
| Language | ||||
| Modified Boston Naming Test (max. 15) | 13.9 ± 1.1 | 14.0 ± 1.2 | 0.35 | 0.56 |
| Category fluency (animals in 1 min) | 15.9 ± 3.6 | 19.7 ± 3.7 | 4.75 | 0.04∗ |
| Syntax comprehension (max. 5) | 4.6 ± 0.5 | 4.8 ± 0.4 | 0.94 | 0.34 |
| Other | ||||
| Calculations (max. 5) | 4.6 ± 0.7 | 4.8 ± 0.5 | 2.05 | 0.16 |
| Geriatric Depression Scale (max. 30) | 4.0 ± 4.6 | 5.5 ± 5.8 | 0.01 | 0.94 |
Figures presented as means ± SD. CVLT = California Verbal Learning Test; VOSP = Visual Object and Space Perception Battery.
p < 0.05, ANCOVA controlling for age.
We selected the 10th percentile to be intermediate between the more liberal 1 SD and the more stringent 1.5 SD cut-off, particularly because screening tests for executive function have not been studied as commonly as screening tests of memory. Although there is considerable debate regarding the optimal psychometric cut-off scores [2,16], participants in the current study were classified using a combination of clinical judgment and psychometric scores.
Participants were excluded if they met criteria for dementia (DSM-IV) [17], had a history of a neurological disorder, current psychiatric illness, substance abuse, head trauma with loss of consciousness >10 min, severe sensory deficits, or were taking medications that affect cognition. In addition, participants were excluded for significant vascular lesions on brain MRI, defined as a Longstreth [18] grade ≥4 (out of 8). The 1.5-tesla MRI of the brain was completed within 3 months of the diagnostic evaluation.
Baseline Behavioral and Functional Measures
We obtained baseline informant measures to assess behavior and instrumental activities of daily living (IADLs). The Dysexecutive Questionnaire (DEX) [19] is a 20-item questionnaire that was used to assess the frequency of dysexecutive symptoms in everyday living (e.g. distractibility, impulsivity, difficulty planning) on a 4-point scale (‘never’ to ‘very often’). Higher scores reflect a higher frequency and/or severity of dysexecutive symptoms. We administered the DEX to both the study partner and participant. We computed a DEX difference score (patient rating minus informant rating) as a measure of participant insight into the presence of dysexecutive symptoms. A negative difference score suggests that the informant reported more symptoms than the participant, whereas a positive score suggests that the participant reported more symptoms. The Functional Activities Questionnaire (FAQ) [20] is a 10-item questionnaire about daily function and was used to assess IADLs. Higher scores suggest more difficulty with completing IADLs.
Baseline Health Risk Factors
To evaluate possible baseline differences in health factors, we recorded the presence or absence of the following medical conditions using the National Alzheimer's Disease Coordinating Center's Uniform Data Set [21]. The presence or absence of these conditions (current or past history) was based on the neurologist's judgment, informant report, and medical records. The cardiovascular risk factors included: (1) heart attack/cardiac arrest, (2) atrial fibrillation, (3) angioplasty/endarterectomy/stent, (4) cardiac bypass procedures, and (5) congestive heart failure. In addition, a modified Hachinski ischemic score [22] was determined (range = 0–12); higher scores reflect more cardiovascular risk factors. The presence or absence of the following medical conditions was also queried: (1) hypertension, (2) hypercholesterolemia, (3) diabetes, (4) B12 deficiency, (5) thyroid disease, and (6) incontinence (urinary or bowel). We report the percentage of patients in each group who have each of these health risk factors.
Longitudinal Assessment and Determination of Clinical Progression
The clinical evaluation described above was repeated annually. We included 31 patients with 2-year clinical data and a baseline MRI. Excluded patients comprised: 1 who died after 1 year, 2 who dropped out due to distance, and 2 who refused MRI. The mean interval between baseline and the 2-year follow-up visit was 23.6 months (SD = 2.6).
The CDR [10] was used to categorize patients as stable or clinically progressing at the 2-year follow-up visit. It is a widely used outcome measure that assesses 6 areas of function: memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care (rated from 0 to 3, with higher scores representing more impairment). Because the global CDR score is computed using an algorithm that weights the memory domain, we used the CDR sum of boxes (CDR-SB) score that calculates all 6 of the individual CDR item scores (range = 0–18). Although the CDR was designed to assess memory and AD, executive dysfunction can affect everyday function assessed by several CDR items, (e.g. judgment and problem solving, community affairs, and orientation) [23]. The CDR was administered by a trained clinician according to the Alzheimer's Disease Centers’ procedures [21,24].
In the current study, clinical progression (‘decliners’) was operationally defined as a worsening (higher score) on the CDR-SB score at the 2-year visit when compared to the baseline visit. In contrast, patients were classified as ‘stable’ if the CDR-SB score did not worsen over 2 years.
Statistical Methods for Clinical Data
A t test with p < 0.05 was used to evaluate possible group differences in demographic variables. Because of a group difference in age, we used a 1-way analysis of covariance (ANCOVA) controlling for age to compare the neuropsychological tests and tests of function and behavior. A χ2 test was used to assess categorical data. We used the Statistical Package for the Social Sciences (SPSS) 17.0 to conduct the statistical analysis.
Brain MRI and Voxel-Based Morphometry
MR Image Acquisition
Images were collected on a Siemens Vision 1.5-tesla MRI scanner (Siemens, Iselin, N.J., USA). T1-weighted 3-dimensional magnetization-prepared rapid acquisition gradient-echo images were acquired (TI/TR/TE = 300/9.7/4 ms; flip angle = 15°; FOV 256 × 256 mm2 with 1.0 × 1.0 mm2 inplane resolution; 154 partitions with 1.5-mm slice thickness).
Imaging Data Analysis
Voxel-based morphometry (VBM) analysis was performed on the T1-weighted images using SPM5 software (Wellcome Department of Imaging Neuroscience, University College London, UK, www.fil.ion.ucl.ac.uk) implemented within Matlab 7 (MathWorks, Natick, Mass., USA). SPM5 uses a unified segmentation process in which image registration, tissue classification, and bias correction are combined making the need to perform ‘optimized VBM’ unnecessary [25]. Further, in SPM5, prior probability maps that are relevant to tissue segmentation are warped to the individual brains, eliminating the need for a study-specific template [26]. All images were normalized, modulated, and segmented in MNI (Montreal Neurological Institute) stereotactic space using the default ICBM template. We applied an isotropic Gaussian smoothing kernel of 12 mm FWHM to minimize individual anatomical variability and reduce the chance of false positives [27]. All images were reviewed prior to statistical analysis to ensure quality of the segmentation process.
The preprocessed images were passed up to voxel-wise statistical comparison. We investigated whole-brain differences in patterns of gray matter atrophy between the decliners and stable dMCI patients using SPM5. We conducted a multiple regression analysis with age, gender, and intracranial volume as nuisance variables. Group differences were assessed using a 1-sample t test at the threshold of p < 0.05, family-wise error rate (FWE)-corrected.
Results
Demographic and Baseline Neuropsychological Screening
Twelve dMCI patients were classified as ‘decliners’, and 19 were classified as ‘stable’. Table 2 summarizes the demographics. The decliners were older, but the groups did not differ on education. After controlling for age, there was a trend for the dMCI decliners to have lower baseline CDR-SB scores; however, this difference was not statistically significant, and the range of scores on the baseline CDR-SB was similar for both groups. As expected, the decliners had higher CDR-SB scores than the stable group after 2 years.
Table 2.
Demographics by group
| Group 1 (decliners) | Group 2 (stable) | P value | |
|---|---|---|---|
| n (males/females) | 12 (7/5) | 19 (11/8) | NA |
| Mean age at baseline, years | 68.3 ± 7.0 | 60.4 ± 6.9 | 0.005∗ |
| Age at onset, years | 65.2 ± 7.6 | 56.3 ± 7.3 | 0.004∗ |
| Mean education, years | 17.8 + 2.1 | 16.6 + 2.3 | 0.16 |
| WAIS-III vocabulary, scaled score | 13.3 ± 2.3 | 12.7 + 2.1 | 0.45 |
| Baseline CDR-SB (max. 18)1 | 0.9 ± 0.9 | 1.4+ 1.0 | 0.31 |
| Two-year CDR-SB (max. 18)1 | 2.8 ± 1.1 | 0.7 ± 0.7 | 0.002∗ |
Figures presented as means ± SD.
p < 0.05 (t test). WAIS-III = Wechsler Adult Intelligence Scale III.
ANCOVA controlling for age.
Table 1 summarizes the baseline neuropsychological test results used for diagnosis. The dMCI patients who progressed clinically had lower scores on animal fluency and a spatial location test. There was also a non-significant trend for the decliners to score lower than the stable dMCI patients on the test of visual memory. There were no differences on the remaining baseline neuropsychological scores.
Baseline Behavioral and IADL Measures
There were no baseline differences on the FAQ and informant-rated DEX (table 3). On the self-report DEX, however, the stable dMCI patients reported almost twice as many dysexecutive symptoms when compared to the decliners. When considering the DEX difference score as a measure of insight into the presence of dysexecutive symptoms, the dMCI patients who declined had underestimated the presence of their dysexecutive symptoms, while the dMCI patients who remained stable overestimated the presence of their dysexecutive symptoms.
Table 3.
Summary of baseline functional and behavioral measures
| Group 1 (decliners) | Group 2 (stable) | P value | |
|---|---|---|---|
| IADL | |||
| FAQ | 1.7 ± 2.9 | 1.6 ± 1.5 | 0.84 |
| Behavior | |||
| DEX-informant score | 14.7 ± 12.7 | 10.9 ± 9.3 | 0.42 |
| DEX-subject score | 11.7 ± 6.6 | 21.9 ± 15.3 | 0.04∗ |
| DEX-difference score | −2.5 ± 15.0 | 11.0 ± 18.0 | 0.04∗ |
Figures presented as means ± SD.
p < 0.05 (t test).
Baseline Health Risk Factors
The dMCI patients who progressed over 2 years had a higher baseline prevalence of hypertension (55 vs. 11%) when compared to those who did not progress (χ2 test, p = 0.02) (table 4). There were no differences in the other baseline health risk factors, including the Hachinski score.
Table 4.
Baseline cardiovascular risk factors
| Group 1 (decliners) | Group 2 (stable) | P value | |
|---|---|---|---|
| Modified Hachinski score (max. 12) | 1.1 (0.9) | 0.6 (1.0) | 0.17 |
| Hypertension, % | 55 | 11 | 0.02∗ |
| Hypercholesteremia, % | 73 | 53 | 0.25 |
| Atrial fibrillation, % | 18 | 0 | 0.13 |
| Diabetes, % | 9 | 5 | 0.61 |
| B12 Deficiency, % | 9 | 10 | 0.42 |
| Thyroid disease, % | 0 | 5 | 0.44 |
| Heart attack / cardiac arrest, % | 0 | 0 | NA |
| Incontinence, % | 0 | 0 | NA |
Pearson χ2 test, two-tailed, p < 0.05.
Diagnostic Classification after 2 Years
Of the 12 dMCI patients who worsened on the CDR, 2 converted to dementia (probable dementia with Lewy bodies) [28], 10 retained a clinical diagnosis of MCI, and none reverted to normal. As expected, none of the stable dMCI patients converted to dementia over 2 years; 17 retained a clinical diagnosis of MCI, and 2 reverted to normal.
VBM Results
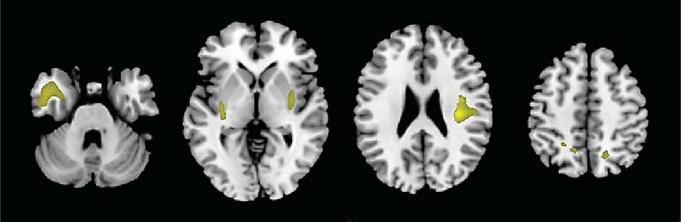
Compared to the stable dMCI group, the dMCI patients who declined had less gray matter volume in the bilateral insula and left anterior temporal lobe (p < 0.05, FWE-corrected; fig. 1). There was a non-significant trend for decliners to have smaller volumes in the right precuneus (p = 0.06, FWE-corrected).
Fig. 1.
Regions of less gray matter volume in dMCI patients who progress clinically compared with dMCI patients who remain stable. Compared to the stable dMCI group, the dMCI patients who declined had less gray matter volume in the bilateral insula and left anterior temporal lobe (p < 0.05, FWE-corrected). Results are shown at p < 0.001, uncorrected for display purposes.
Discussion
Overall, dMCI patients who worsened on the CDR-SB after 2 years had more atrophy in the bilateral insula and left lateral temporal lobe on baseline MRI when compared to the stable dMCI patients. There was also a trend for smaller baseline precuneus volume in the dMCI patients who declined. The decliners were also older, had lower baseline performance on category fluency and a visuospatial task, and reported fewer dysexecutive symptoms than their study partner when compared with stable dMCI patients. With the exception of hypertension that was more prevalent in the decliners, no other cardiovascular or health risk factors differed between the groups. These results suggest that dMCI patients who decline clinically over 2 years may have unique brain MRI and clinical features.
The finding that the overall distribution of baseline MRI volumetric differences was more posterior than anterior in the dMCI patients who declined was somewhat unexpected. A previous study comparing controls and dMCI patients found more atrophy in the left prefrontal cortex [7]. However, atrophy in the lateral temporal lobe and insula in dMCI patients who decline may provide hints about the clinical trajectory. For example, patients with dementia with Lewy bodies can have volume loss in bilateral temporal lobes, frontal lobes and insula when compared to controls [29,30]. Medial temporal lobe atrophy may be highly predictive of AD [31], but atrophy in other posterior regions may be less specific for AD. Several studies using VBM suggest that amnestic MCI patients who convert to AD have atrophy in the precuneus, posterior cingulate and temporoparietal areas [32,33,34].
After controlling for age, the dMCI patients who declined over 2 years had lower baseline scores on tests of category fluency and spatial location with a trend for lower scores on visual memory. All MCI patients in this study met criteria for dMCI at baseline and did not have clinical features of amnestic MCI. Interestingly, the decliners did not have significantly lower baseline scores on executive function when compared to the stable dMCI patients, supported by the fact that both groups had a similar degree of executive dysfunction at baseline. The groups were also comparable on global measures of cognition (MMSE) and IADLs (FAQ), providing further support that the decliners were not more impaired than the stable dMCI patients at baseline. Low score on category fluency is a commonly studied predictor of AD [35,36], and low scores on visuospatial tasks can be associated with other neurodegenerative diseases, such as dementia with Lewy bodies, corticobasal degeneration, and frontotemporal dementia [37,38,39,40]. Thus, the dMCI patients with lower scores on tests of visuospatial skills are likely to progress clinically and may have an underlying neurodegenerative disease.
Although there were no differences in the number of informant-rated dysexecutive symptoms (DEX-informant), the dMCI patients who progressed clinically reported fewer dysexecutive symptoms than their informants (DEX-difference score), when compared with stable dMCI patients. Decreased awareness of cognitive symptoms (e.g. anosognosia) has been reported in individuals with MCI and has been linked to midline cortical structures [41] and frontal cortex [42]. It is possible that a discrepancy between informant and patient reports is a more sensitive predictor of clinical progression than the absolute number of dysexecutive symptoms.
Over 2 years, 2 of the stable dMCI patients reverted to normal. Indeed, some of the participants in the stable group may remain healthy and not have a neurodegenerative disease. Several studies found that approximately 25% of non-amnestic MCI patients revert to normal [5]. It is also possible that dMCI patients who remain stable over the first 2 years may have a longer disease course. A recent meta-analysis [43] found that the annual conversion rate to dementia for non-amnestic MCI patients was 4.1%, whereas the annual conversion rate for amnestic MCI was 11.7%. It is also important to keep in mind that the mean age of the dMCI patients in this study was 64 years of age, which is younger than reported in most studies of MCI and may also affect progression rates. Isolated executive dysfunction can be a prodromal stage for several neurodegenerative diseases, including AD, dementia with Lewy bodies, progressive supranuclear palsy, corticobasal degeneration, vascular dementia, frontotemporal dementia, and Parkinson disease [44]. Whitwell et al. [45] found MRI atrophy in the basal forebrain and hypothalamus in 9 dMCI patients compared to controls. Three of these dMCI patients converted to dementia with Lewy bodies, and another 3 to AD. We previously reported AD neuropathology with an atypical distribution in a non-demented participant with isolated executive dysfunction [46]. Recent studies link executive dysfunction with cardiovascular disease and prodromal vascular dementia [47,48]. However, the dMCI patients with evidence of significant vascular disease on brain MRI were excluded from our study, and the 2 dMCI groups only differ on the prevalence of hypertension but not other cardiovascular and medical risk factors.
The current study has several limitations. First, the sample size is relatively small, and the null finding for differences in the majority of the baseline neuropsychological tests may be an underestimate. However, a focus on non-amnestic MCI is relatively recent, and larger sample sizes are currently being recruited. Longitudinal studies are currently needed to determine the diagnostic outcome of the dMCI patients. Comparisons with other MCI subgroups would also be useful.
Acknowledgements
This study was supported by NIH NIA R01-AG022538, R01-AG010897, P50-AG0300601, K23-NS408855, K01-AG03417501 and John D. French Foundation. We are grateful for the participation of the participants and their families.
References
- 1.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Backman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Duijn C, Visser P, Petersen RC. Mild cognitive impairment – Beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 2.Busse A, Hensel A, Guhne U, Angermeyer MC, Riedel-Heller SG. Mild cognitive impairment: long-term course of four clinical subtypes. Neurology. 2006;67:2176–2185. doi: 10.1212/01.wnl.0000249117.23318.e1. [DOI] [PubMed] [Google Scholar]
- 3.Palmer K, Backman L, Winblad B, Fratiglioni L. Mild cognitive impairment in the general population: occurrence and progression to Alzheimer disease. Am J Geriatr Psychiatry. 2008;16:603–611. doi: 10.1097/JGP.0b013e3181753a64. [DOI] [PubMed] [Google Scholar]
- 4.Busse A, Bischkopf J, Riedel-Heller SG, Angermeyer MC. Mild cognitive impairment: prevalence and incidence according to different diagnostic criteria. Results of the Leipzig Longitudinal Study of the Aged (LEILA75+) Br J Psychiatry. 2003;182:449–454. [PubMed] [Google Scholar]
- 5.Fischer P, Jungwirth S, Zehetmayer S, Weissgram S, Hoenigschnabl S, Gelpi E, Krampla W, Tragl KH. Conversion from subtypes of mild cognitive impairment to Alzheimer dementia. Neurology. 2007;68:288–291. doi: 10.1212/01.wnl.0000252358.03285.9d. [DOI] [PubMed] [Google Scholar]
- 6.Yaffe K, Petersen RC, Lindquist K, Kramer J, Miller B. Subtype of mild cognitive impairment and progression to dementia and death. Dement Geriatr Cogn Disord. 2006;22:312–319. doi: 10.1159/000095427. [DOI] [PubMed] [Google Scholar]
- 7.Pa J, Boxer A, Chao LL, Gazzaley A, Freeman K, Kramer J, Miller BL, Weiner MW, Neuhaus J, Johnson JK. Clinical-neuroimaging characteristics of dysexecutive mild cognitive impairment. Ann Neurol. 2009;65:414–423. doi: 10.1002/ana.21591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 9.Kramer JH, Jurik J, Sha SJ, Rankin KP, Rosen HJ, Johnson JK, Miller BL. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol. 2003;16:211–218. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Berg L. Clinical Dementia Rating Scale (CDR) Psychopharmacol Bull. 1988;24:637–639. [PubMed] [Google Scholar]
- 11.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 12.Yesavage JA, Brink TL, Rolse TL, Lum O, Huang V, Adey M, Leiter VO. Development and validity of a Geriatric Depression Scale: a preliminary report. J Psychiatr Res. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 13.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. San Antonio: The Psychological Corporation; 2000. [Google Scholar]
- 14.Warrington EK, James M. The Visual Object and Space Perception Battery. Bury St Edmunds: Thames Valley Test Company; 1991. [Google Scholar]
- 15.Wechsler D. Wechsler Adult Intelligence Scale. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- 16.Jak AJ, Bangen KJ, Wierenga CE, Delano-Wood L, Corey-Bloom J, Bondi MW. Contributions of neuropsychology and neuroimaging to understanding clinical subtypes of mild cognitive impairment. Int Rev Neurobiol. 2009;84:81–103. doi: 10.1016/S0074-7742(09)00405-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. ed 4. Washington: American Psychiatric Association; 1994. [Google Scholar]
- 18.Longstreth WT, Jr, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, Enright PL, O'Leary D, Fried L. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- 19.Burgess PW, Alderman N, Evans J, Emslie H, Wilson BA. The ecological validity of tests of executive function. J Int Neuropsychol Soc. 1998;4:547–558. doi: 10.1017/s1355617798466037. [DOI] [PubMed] [Google Scholar]
- 20.Pfeffer RI, Kurosaki TT, Harrah CH, Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 21.Beekly DL, Ramos EM, Lee WW, Deitrich WD, Jacka ME, Wu J, Hubbard JL, Koepsell TD, Morris JC, Kukull WA. The National Alzheimer's Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis Assoc Disord. 2007;21:249–258. doi: 10.1097/WAD.0b013e318142774e. [DOI] [PubMed] [Google Scholar]
- 22.Rosen WG, Terry RD, Fuld PA, Katzman R, Peck A. Pathological verification of ischaemic score in differentiation of the dementias. Ann Neurol. 1980;7:486–488. doi: 10.1002/ana.410070516. [DOI] [PubMed] [Google Scholar]
- 23.Torralva T, Roca M, Gleichgerrcht E, Lopez P, Manes F. INECO Frontal Screening (IFS): a brief, sensitive, and specific tool to assess executive functions in dementia. J Int Neuropsychol Soc. 2009;15:777–786. doi: 10.1017/S1355617709990415. [DOI] [PubMed] [Google Scholar]
- 24.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules [see comments] Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 25.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 26.Rusch N, Spoletini I, Wilke M, Bria P, Di Paola M, Di Iulio F, Martinotti G, Caltagirone C, Spalletta G. Prefrontal-thalamic-cerebellar gray matter networks and executive functioning in schizophrenia. Schizophr Res. 2007;93:79–89. doi: 10.1016/j.schres.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 27.Salmond CH, Ashburner J, Vargha-Khadem F, Connelly A, Gadian DG, Friston KJ. Distributional assumptions in voxel-based morphometry. Neuroimage. 2002;17:1027–1030. [PubMed] [Google Scholar]
- 28.McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 29.Burton EJ, Karas G, Paling SM, Barber R, Williams ED, Ballard CG, McKeith IG, Scheltens P, Barkhof F, O'Brien JT. Patterns of cerebral atrophy in dementia with Lewy bodies using voxel-based morphometry. Neuroimage. 2002;17:618–630. [PubMed] [Google Scholar]
- 30.Risacher SL, Saykin AJ, West JD, Shen L, Firpi HA, McDonald BC. Baseline MRI predictors of conversion from MCI to probable AD in the ADNI cohort. Curr Alzheimer Res. 2009;6:347–361. doi: 10.2174/156720509788929273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burton EJ, Barber R, Mukaetova-Ladinska EB, Robson J, Perry RH, Jaros E, Kalaria RN, O'Brien JT. Medial temporal lobe atrophy on MRI differentiates Alzheimer's disease from dementia with Lewy bodies and vascular cognitive impairment: a prospective study with pathological verification of diagnosis. Brain. 2009;132:195–203. doi: 10.1093/brain/awn298. [DOI] [PubMed] [Google Scholar]
- 32.Karas G, Sluimer J, Goekoop R, van der Flier W, Rombouts SA, Vrenken H, Scheltens P, Fox N, Barkhof F. Amnestic mild cognitive impairment: structural MR imaging findings predictive of conversion to Alzheimer disease. AJNR Am J Neuroradiol. 2008;29:944–949. doi: 10.3174/ajnr.A0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamalainen A, Tervo S, Grau-Olivares M, Niskanen E, Pennanen C, Huuskonen J, Kivipelto M, Hanninen T, Tapiola M, Vanhanen M, Hallikainen M, Helkala EL, Nissinen A, Vanninen R, Soininen H. Voxel-based morphometry to detect brain atrophy in progressive mild cognitive impairment. Neuroimage. 2007;37:1122–1131. doi: 10.1016/j.neuroimage.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 34.Chetelat G, Landeau B, Eustache F, Mezenge F, Viader F, de la Sayette V, Desgranges B, Baron JC. Using voxel-based morphometry to map the structural changes associated with rapid conversion in MCI: a longitudinal MRI study. Neuroimage. 2005;27:934–946. doi: 10.1016/j.neuroimage.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 35.Albert MS, Moss MB, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. J Int Neuropsychol Soc. 2001;7:631–639. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]
- 36.Backman L, Jones S, Berger AK, Laukka EJ, Small BJ. Cognitive impairment in preclinical Alzheimer's disease: a meta-analysis. Neuropsychology. 2005;19:520–531. doi: 10.1037/0894-4105.19.4.520. [DOI] [PubMed] [Google Scholar]
- 37.Lambon Ralph MA, Powell J, Howard D, Whitworth AB, Garrard P, Hodges JR. Semantic memory is impaired in both dementia with Lewy bodies and dementia of Alzheimer's type: a comparative neuropsychological study and literature review. J Neurol Neurosurg Psychiatry. 2001;70:149–156. doi: 10.1136/jnnp.70.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rascovsky K, Salmon DP, Ho GJ, Galasko D, Peavy GM, Hansen LA, Thal LJ. Cognitive profiles differ in autopsy-confirmed frontotemporal dementia and AD. Neurology. 2002;58:1801–1808. doi: 10.1212/wnl.58.12.1801. [DOI] [PubMed] [Google Scholar]
- 39.Bak TH, Crawford LM, Hearn VC, Mathuranath PS, Hodges JR. Subcortical dementia revisited: similarities and differences in cognitive function between progressive supranuclear palsy (PSP), corticobasal degeneration (CBD) and multiple system atrophy (MSA) Neurocase. 2005;11:268–273. doi: 10.1080/13554790590962997. [DOI] [PubMed] [Google Scholar]
- 40.Molano J, Boeve B, Ferman T, Smith G, Parisi J, Dickson D, Knopman D, Graff-Radford N, Geda Y, Lucas J, Kantarci K, Shiung M, Jack C, Silber M, Pankratz VS, Petersen R: Mild cognitive impairment associated with limbic and neocortical Lewy body disease: a clinicopathological study. Brain, 133:540–556. [DOI] [PMC free article] [PubMed]
- 41.Ries ML, Jabbar BM, Schmitz TW, Trivedi MA, Gleason CE, Carlsson CM, Rowley HA, Asthana S, Johnson SC. Anosognosia in mild cognitive impairment: relationship to activation of cortical midline structures involved in self-appraisal. J Int Neuropsychol Soc. 2007;13:450–461. doi: 10.1017/S1355617707070488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vogel A, Hasselbalch SG, Gade A, Ziebell M, Waldemar G. Cognitive and functional neuroimaging correlate for anosognosia in mild cognitive impairment and Alzheimer's disease. Int J Geriatr Psychiatry. 2005;20:238–246. doi: 10.1002/gps.1272. [DOI] [PubMed] [Google Scholar]
- 43.Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia – Meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand. 2009;119:252–265. doi: 10.1111/j.1600-0447.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- 44.Johnson JK. Mild cognitive impairment subgroups. In: Miller BL, Boeve BF, editors. The Behavioral Neurology of Dementia. Cambridge: Cambridge University Press; 2009. [Google Scholar]
- 45.Whitwell JL, Petersen RC, Negash S, Weigand SD, Kantarci K, Ivnik RJ, Knopman DS, Boeve BF, Smith GE, Jack CR., Jr. Patterns of atrophy differ among specific subtypes of mild cognitive impairment. Arch Neurol. 2007;64:1130–1138. doi: 10.1001/archneur.64.8.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson JK, Vogt BA, Kim R, Cotman CW, Head E. Isolated executive impairment and associated frontal neuropathology. Dement Geriatr Cogn Disord. 2004;17:360–367. doi: 10.1159/000078183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ravaglia G, Forti P, Maioli F, Martelli M, Servadei L, Brunetti N, Pantieri G, Mariani E. Conversion of mild cognitive impairment to dementia: predictive role of mild cognitive impairment subtypes and vascular risk factors. Dement Geriatr Cogn Disord. 2006;21:51–58. doi: 10.1159/000089515. [DOI] [PubMed] [Google Scholar]
- 48.Meyer JS, Xu G, Thornby J, Chowdhury MH, Quach M. Is mild cognitive impairment prodromal for vascular dementia like Alzheimer's disease? Stroke. 2002;33:1981–1985. doi: 10.1161/01.str.0000024432.34557.10. [DOI] [PubMed] [Google Scholar]