Abstract
Background
Pulmonary arterial hypertension (PAH) is characterized by sustained elevation of pulmonary vascular resistance resulting from endothelial and smooth muscle cell dysfunction and collagen deposition in pulmonary vascular walls. In this study, we investigated the role of the adenosine A2A receptor (A2AR) in the development of PAH by determining the effect of genetic inactivation of A2ARs on pulmonary vascular remodeling in mice.
Methods and Results
We characterized hemodynamic, histological and ultrastructural changes in pulmonary vascular remodeling in A2AR knockout (KO) mice compared with their wild-type (WT) littermates after exposure to normoxia and hypoxic conditions. After exposure to normoxia, compared to WT mice, A2AR KO mice displayed: (1) increased right ventricular systolic pressures and an elevated ratio of the right ventricle over left ventricle plus septum (Fulton index), (2) increased wall area and thickness as well as enhanced smooth muscle actin immunoreactivity in pulmonary resistance vessels, (3) increased proliferating cell nuclear antigen-positive cells in pulmonary resistance vessels and (4) increased smooth muscle cells hypertrophy and collagen deposition in the adventitia of pulmonary arteriole walls as revealed by electron microscope. By contrast, histological analysis revealed no features of hypertensive nephropathy in A2AR KO mice and there was no significant difference in systemic blood pressure, and left ventricular masses among the 3 genotypes. Furthermore, following chronic exposure to hypoxia, A2AR KO mice exhibited exacerbated elevation in right ventricular systolic pressure, hypertrophy of pulmonary resistance vessels and increased cell proliferation in pulmonary resistance vessels, compared to WT littermates. Thus, genetic inactivation of A2ARs selectively produced PAH and associated increased smooth muscle proliferation and collagen deposition.
Conclusions
Extracellular adenosine acting at A2ARs represents an important regulatory mechanism to control the development of PAH and pulmonary vascular remodeling.
Key Words: Adenosine A2A receptor, Collagen hyperplasia, Pulmonary arterial hypertension, Pulmonary vascular remodeling, Smooth muscle hypertrophy
Introduction
Pulmonary arterial hypertension (PAH) develops as a primary disease with an unclear underlying cause or occurs as a complication of various pathological conditions (such as chronic exposure to hypoxia at high altitude) [1]. Pulmonary vascular remodeling with abnormal sclerosis/stenosis of distal pulmonary arteries produces progressive elevation of pulmonary vascular resistance and pulmonary arterial pressure, followed by impaired right ventricular output. The morphological changes consist of common hypertrophy of the tunica media, proliferative lesions such as intimal thickening or plexiform lesions and in situthrombosis [2]. The expansion of the tunica media in pulmonary arteries in PAH occurs by a combination of hypertrophy and hyperplasia. Hypertrophy of medial smooth muscle cells (SMCs) is a characteristic pathological feature of PAH that involves muscularized arteries and precapillary vessels. In addition, the collagen deposition in the pulmonary vascular wall is another important characteristic of pulmonary vascular remodeling. The increase in collagen expression and disordered proteolysis of extracellular matrix likely contributes to pathological pulmonary arterial structural remodeling and loss of vasoreactivity [3]. Under certain conditions, adventitial fibroblasts are activated and proliferate and upregulate the expression of collagen and extracellular matrix proteins. Thus, the development of PAH involves a complex interplay of multiple genetic, environmental and hormonal abnormalities, leading to abnormal pulmonary vascular remodeling involving endothelial cells, smooth muscle and fibroblasts. However, the physiological regulator of PAH development remains to be determined.
Adenosine is an important intermediate of purine and energy (ATP) metabolisms, and is ubiquitously distributed throughout the body. Endogenous adenosine levels vary from tissue to tissue from 10 to 100 nM, with adenosine levels being higher in the pulmonary than the systemic circulation [4]. For example, SMCs synthesize a substantial amount of adenosine [5]. Importantly, extracellular adenosine levels increased markedly in response to hypoxia [6]. However, patients with PAH have lower plasma adenosine levels in the pulmonary circulation compared to control subjects, indicating a possible deficiency in adenosine signaling in PAH [4]. Adenosine acting through multiple G protein-coupled receptors, namely A1, A2A, A2B and A3, exert important regulatory effects on various physiological and pathophysiological processes, including suppression of immune response and inflammation, tissue protection, vasodilatation and angiogenesis, neuromodulation and sleep promotion [7]. Immunohistochemical analysis of lung parenchyma demonstrated A2A receptor (A2AR) expression in bronchiolar and alveolar epithelial cells, SMCs localized in bronchiolar and vessel walls, and endothelial cells in pulmonary arterials [8]. On the other hand, the A2B receptor was expressed mainly in mast cells and macrophages and the A1 receptor was expressed only in a few alveolar macrophages [8].
Several lines of experimental evidence suggest that adenosine may be a potential endogenous regulator of PAH development by regulating SMC growth and collagen synthesis and by maintaining vascular homeostasis in systemic circulation systems. First, adenosine acting at A2AR is a powerful vasodilator for systemic arterial pressure with possible similar effects on pulmonary arterials [9]. Importantly, this effect does not require a functional endothelium [10], since adenosine can induce significant pulmonary vasodilation under conditions of severe nitric oxide deficiency and endothelial dysfunction, that is, conditions seen in severe PAH [11]. Second, adenosine acting at A2AR is also a powerful stimulator of angiogenesis and is responsible for up to 50–70% of hypoxia-induced angiogenesis under certain pathological conditions [12]. Third, exogenous adenosine inhibited SMC growth induced by fetal calf serum [13]. Fourth, adenosine may contribute to the abnormal synthesis and deposition of collagen observed in vascular-occlusive disorders associated with hypertension, atherosclerosis and restenosis [2,14]. For instance, adenosine acting through the cAMP pathway inhibits collagen synthesis and hypertrophy of vascular SMCs and cardiac fibroblasts [15]. Based on these findings, we hypothesized that adenosine acting at A2AR regulates the pulmonary vascular remodeling (particularly SMCs and fibroblasts) and contributes to the development of PAH.
In this study, we critically evaluated the contribution of A2AR to the development of PAH using A2AR knockout (KO) mice. Development of the A2AR KO mice, particularly the A2AR KO mice in the congenic C57BL/6 background to reduce potential genetic background effect [16,17], circumvents intrinsic limitations of partial specificity of A2AR pharmacologic agents and provides a unique opportunity to investigate A2AR in pulmonary vascular remodeling [18]. Using the A2AR KO mice, we provided the first experiment evidence at hemodynamic, histological and ultrastructural levels that genetic deletion of the A2AR confers a selective hypertensive and hypertrophyic phenotypes of pulmonary circulation in A2AR KO mice.
Materials and Methods
Generation and Genotyping of A2AR KO Mice
The A2AR KO mice were developed by Chen et al. [16] and described previously. Congenic A2AR KO mice in a C57BL/6 background were generated by backcrossing A2AR KO mice in mixed 129-Steel × C57BL/6 background to C57BL/6 mice for >10 generations [18], and used for this study. Heterozygous crossbreeding was used to generate homozygous A2AR KO and wild-type (WT) mice, all from the same breeding pairs. The A2AR KO and WT mice at the age of 14–16 weeks were used for this study. The genotypes of the mice were determined by PCR analysis using the 3 primer sets targeting to the neocassettes and the adjacent A2AR genes as described previously [18,19]. A total of 80 arterioles from 20 sections/20 mice were analyzed by an investigator who was blinded to the genotypes.
Experimental Model of Pulmonary Hypertension after Chronic Exposure to Hypoxia
A2AR KO and WT mice were exposed to chronic hypoxia (9–11% O2) in a sealed but ventilated chamber as described previously [20]. The hypoxic environment was established by flushing the chamber with a mixture of room air and nitrogen, and the gas was recirculated. The chamber environment was monitored using an oxygen analyzer. Carbon dioxide was removed by soda lime granules. Normoxic mice, both A2AR KO and WT, were kept in a similar chamber flushed with normoxic gas (21% oxygen) in the same room. After 2 weeks of exposure, mice were measured for the right ventricular systolic pressure (RVSP), and lungs were processed for morphological and histological examination.
Hemodynamic Measurements of RVSP
Mice were anesthetized with intraperitoneal sodium pentobarbital (85 mg/kg). After dissection to expose the right jugular vein, a 1.4F Millar Mikro-Tip pressure catheter (Millar Instruments) was inserted into the vein and advanced to the right ventricle (RV). The catheter was connected to a transducer unit interfaced with a signal amplifier and recorder (Powerlab Systems; AD Instruments). Following a 5-min stabilization period, RVSP and heart rate were recorded. We also monitored systemic blood pressure (SBP) by catheterization via the carotid artery. The investigator who was blinded to the genotypes performed all measurements.
Assessment of RV Hypertrophy Morphometrical Analysis of Pulmonary and Renal Arteries
The RV was dissected from the left ventricle plus septum and each component was weighed separately. The Fulton index (RV/left ventricle plus septum) was calculated as previously described [18]. All measurements were also made by an investigator who was blinded to the genotype.
Pulmonary and renal arteries were perfused with 4% paraformaldehyde via a cannula at a perfusion pressure of 20 cm H2O. The left lungs were fixed in 4% paraformaldehyde, and then dehydrated in increasing grade of ethanol (30, 50, 70, 80, 95 and 100%). After delipidation with xylene, the lungs were embedded in paraffin and cut into 3–4 transverse sections. Lung sections (4 μm) were stained with hematoxylin and eosin (HE) and with the Elastica-van Gomori method. Pulmonary vascular remodeling was assessed by measuring the percentage of wall thickness and wall areas of the arteries [21]. Five random fields (with 1–2 arterioles) from one section of each mouse were chosen and arteries/arterioles with diameters between 25 and 100 μm and being associated terminal bronchioli or respiratory bronchioli or alveolar ducts or alveolar walls were analyzed for their wall thickness, wall areas, external diameter and external areas. The wall thickness and wall areas of a total of 131 arteries/arterioles from 26 sections of 26 mice were analyzed using a computer imaging analysis. Wall thickness 1 was measured at one point of the vessel wall and wall thickness 2 at the diametrically opposite point, and the diameter was the shortest one across the center of the vessels. The percentage of wall thickness (% wall thickness) was calculated as [(wall thickness 1) + (wall thickness 2)] × 100/external diameter. External wall areas were measured as wall areas 1 and internal wall areas were measured as wall areas 2. The percentage of wall areas (% wall areas) was calculated as [(wall areas 1) – (wall areas 2)] × 100/wall areas 1. All morphometric analyses were performed by the investigator who was blinded to the genotype.
The sections (4 μm) were stained with HE and Elastica-van Gomori's staining for evidence of any structural abnormalities, such as fibrosis and thickening of the alveolar septa, or for any evidence of edema that may have occurred secondary to left ventricular dysfunction.
Detection of Expression of A2AR, α-Smooth Muscle Actin and Proliferating Cell Nuclear Antigen in Pulmonary and Renal Arteries by Immunohistochemistry
A2AR expression in pulmonary arteries of WT and A2AR KO mice was detected by immunofluorescence immunohistochemistry using A2AR antibody conjugated with FITC secondary antibody. Briefly, the frozen sections of lung tissues were acetone fixed for 10 min, followed by incubation on individual slides for 30 min at 37°C with rabbit anti-mouse A2AR antibody (Santa Cruz; 1:300). After washing 3 times with PBS and incubating with FITC-conjugated goat anti-rabbit secondary antibody for 30 min at 37°C, the sections were washed in a similar manner and A2AR-positive cells were viewed under fluorescence microscopy at 200× magnification. Total cells/nuclei in the same section were stained with 4′-6-diamidino-2-phenylindole (DAPI).
The sections were immunostained with monoclonal antibody to α-smooth muscle actin (α-SMA), a marker for SMCs (Chemicon; 1:500 dilutions) to demonstrate the expression of SMCs. Quantitative immunohistochemical assessments were performed as previously reported [22]. The integrated optical density, which relates to immunohistochemical staining intensity and area, was calculated in the vessel wall of pulmonary arteries and afferent glomerular arteries.
The lung sections were also immunostained with antibodies against proliferating cell nuclear antigen (PCNA; Boster Biological Technology; 1:100 dilutions) to determine cell proliferation in pulmonary arterial walls (arterioles 25–100 μm in diameter). PCNA, a 37-kDa molecular weight protein, was originally identified as an antigen that is expressed in the nuclei of cells during the DNA synthesis phase of the cell cycle [23]. The cells positive for PCNA staining were brown in nuclei. We counted the numbers of positive cells and all the cells in the arterial walls. The percentage of positive cell number was calculated as positive cells/all cells.
Ultrastructural Examination of Pulmonary Arteries
Lung tissues of WT and A2AR KO mice (n = 3 per group) were collected proximal to hilus pulmonis and immersed immediately in 3% glutaral solution in 0.2 M phosphate buffer. The tissues were processed further for transmission electron microscopy examination by postfixation with 2% osmium tetroxide followed by dehydration in acetone series and embedding. Grids were stained with 2% uranyl acetate followed by lead citrate. Ultrastructural images of at least 1 arteriole per sample were recorded and photographed on a Hitachi H-7500 electron microscope (Hitachi). The ultrastructural features and appearance of pulmonary vascular walls for each group were evaluated by transmission electron microscopy.
Statistical Analysis
Data are expressed as mean ± SEM. The comparison among 3 genotypes was analyzed by one-way ANOVA, followed by post hoc comparison with LSD test (equal variances assumed) or Dunnett's test (equal variances not assumed). Two-way ANOVA was used to analyze the effect of genotypes (WT vs. KO) and oxygen condition (normoxia vs. hypoxia) and their interaction. A value of p < 0.05 is accepted as statistically significant.
Results
Expression of A2AR in Pulmonary Arteries
To better characterize the role of A2AR in the development of pulmonary hypertension in mice, we first determined the expression pattern of A2AR in small and large pulmonary arterials of WT mice by fluorescence immunohistochemistry, and confirmed the specificity of the A2AR immunoreactivity using A2AR KO mice. A2AR-positive cells were detected in both small pulmonary arteries (fig. 1a) and large pulmonary arteries (fig. 1b) of WT mice lung sections. Notably, most of these cells were detected in the tunica intima and tunica adventitia of pulmonary artery, indicating that their cell type may be endothelia cells and fibroblasts. Importantly, in both small pulmonary arteries (fig. 1c) and large pulmonary arteries (fig. 1d) of A2AR KO mice, there was no A2AR-positive cell in lung sections, validating the specificity of A2AR immunoreactivity by fluorescence immunohistochemistry.
Fig. 1.
Expression of A2AR in small and large pulmonary arteries in WT and A2AR KO mice by fluorescence immunohistochemistry. A2AR immunoreactivity was determined in lung sections for WT and A2AR KO mice by fluorescence immunohistochemistry as described in Materials and Methods. Lung sections were co-stained with DAPI for total cells. a–d A2AR-positive cells in lung sections. a1–d1 Nucleoli stained by DAPI. White arrow = pulmonary artery; red arrow = A2AR-positive cells in pulmonary artery; blue arrow = A2AR-positive cells in not pulmonary artery. ×200.
A2AR KO Mice Exhibited Elevation in RVSP and Hypertrophy of RV
At the age of 14–16 weeks, the mean RVSP in WT mice was 28.20 ± 1.86 mm Hg, consistent with the measurement in the previous studies using C57BL/6 mice. Interestingly, the RVSP in A2AR KO mice was 40.86 ± 2.80 mm Hg, increasing by 44.89% compared to the WT littermates (p < 0.01; fig. 2a). Figure 2b shows the trace recording of RVSP in 3 genotypes, with the RVSP in A2AR KO markedly higher than that of A2AR+/– and A2AR KO mice. In contrast to enhanced RVSP, there was no significant difference in mean SBP among 3 genotypes (WT mice 80.09 ± 11.79 mm Hg; A2AR KO mice 94.46 ± 13.73 mm Hg). The difference in heart rate was not significant among 3 genotypes (WT mice 330.80 ± 61.16 bpm; A2AR+/– mice 276.25 ± 35.62 bpm; A2AR KO mice 326.71 ± 21.50 bpm). Thus, genetic deletion of A2AR selectively produces PAH without affecting systemic circulation or heart rate.
Fig. 2.
A2AR KO mice exhibited elevation in RVSP and hypertrophy of RV. RVSP and hypertrophy measurements were made in adult A2AR KO and WT littermates as described in Materials and Methods. a Representative pictures of RVSP waves in WT and A2AR KO mice. b Mean RVSP (mm Hg) in WT (n = 5) and A2AR KO (n = 7) mice. ∗∗ p < 0.01, A2AR KO vs. WT, one-way ANOVA, Dunnett's post hoc test. c Fulton index in WT and A2AR KO mice. WT, n = 16; A2AR KO, n = 18. ∗ p < 0.05, A2AR KO vs. WT, one-way ANOVA, LSD test.
To seek additional evidence in support of PAH phenotype, we also measured the mean Fulton index in the mice of 3 genotypes. The Fulton index was significantly higher in A2AR KO mice compared to WT mice (mean ± SE: 26.44 ± 0.63 vs. 24.06 ± 1.02%; fig. 2c, ∗ p < 0.05, one way ANOVA followed by LSD test). Thus, A2AR KO mice displayed hypertrophic RVs, consistent with a PAH phenotype.
A2AR KO Mice Exhibited Hypertrophy of Pulmonary Resistance Vessels with Increased Wall Thickness and Area
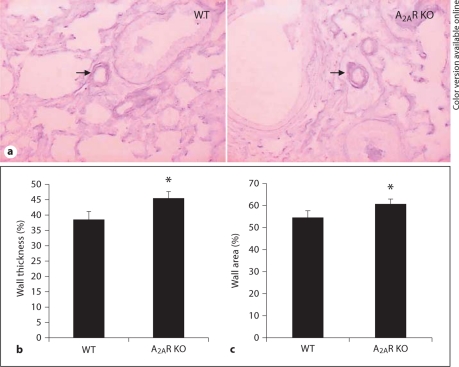
Histological analysis revealed no pulmonary edema secondary to left ventricular dysfunction among the 3 genotypes. Moreover, lung sections with HE staining revealed no abnormalities such as infiltration of inflammatory cells, edema, fibrosis, hypercellularity or alteration/thickening of the alveolar septa in all genotypes. Thus, the PAH phenotype in A2AR KO is not due to enhanced inflammation of lungs. To evaluate vascular remodeling of pulmonary resistance vessels, we determined the percentage of wall thickness and areas in pulmonary resistance vessels (25–100 μm in diameter) in A2AR KO mice and their WT littermates. As you can see from figure 3a and b, the mean percentage of wall thickness in the A2AR KO mice (mean ± SE: 45.34 ± 2.08%) was significantly higher than that of the WT mice (38.28 ± 2.91%). The mean percentage of wall thickness of the A2AR+/– mice (42.93 ± 2.33%) displayed the intermediate value between the A2AR KO and WT mice, indicating the gene-dose effect. Consistent with the wall thickness, the mean percentage of wall areas in A2AR KO mice (60.83 ± 2.06%) was also significantly higher than in the WT mice (54.51 ± 3.30%; fig. 3c). Together, these results strongly suggest that hypertrophy of the pulmonary arterials is a result of PAH in A2AR KO mice.
Fig. 3.
A2AR KO mice exhibited hypertrophy of pulmonary resistance vessels with increased wall thickness and area. Pulmonary vascular wall thickness and wall areas in A2AR KO mice and WT littermates were assessed as described in Materials and Methods. a Representative photomicrograph of pulmonary vascular remodeling in lung. ×200. b Percentage of wall thickness. c Wall areas in WT and A2AR KO mice. ∗ p < 0.05, A2AR KO vs. WT, one-way ANOVA, LSD test, n = 9.
A2AR KO Mice Exhibited Increased Expression of α-SMA and PCNA in Pulmonary Resistance Vessels
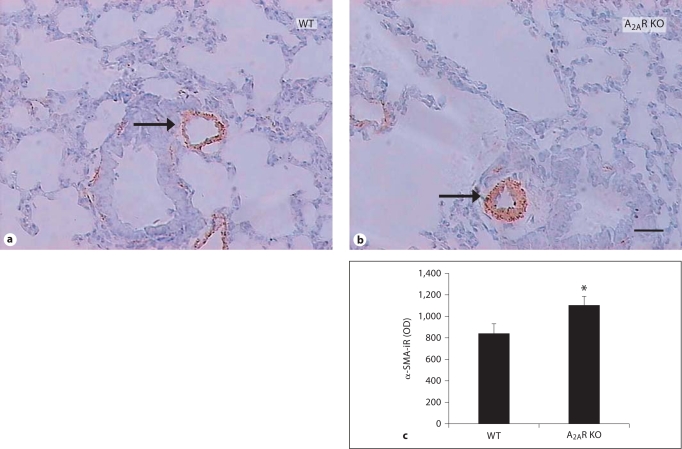
To determine the cellular basis for the increased thickness and area of pulmonary resistance vessels, we evaluated the hyperplastic smooth muscularization in lung sections using an antibody against α-SMA (fig. 4). Small pulmonary arterials (25–100 μm in diameter) were intensively stained with α-SMA immunoreactivity. Importantly, integrated optical density value of α-SMA in A2AR KO (mean ± SE: 1,099.30 ± 87.03) was significantly higher than in WT mice (840.00 ± 86.94; fig. 4). Thus, increased thickness and area of pulmonary resistance vessels in A2AR KO mice are associated with enhanced expression or proliferation of SMCs. Furthermore, we determined cell proliferation in lung sections containing pulmonary resistance vessels. Increased PCNA+ cells were detected in all major cell types across entire pulmonary arterial walls of WT mice, including smooth muscle, endothelium cell and fibroblasts (fig. 5). Importantly, the percentage of PCNA+ cells in A2AR KO and A2AR+/– were significantly higher than in the WT mice (A2AR KO 30.04 ± 1.75%, WT 22.92 ± 2.20%). Increased cell proliferation in A2AR KO mice was observed in cell types across entire pulmonary arteries.
Fig. 4.
A2AR KO mice exhibited increased expression of α-SMA in pulmonary resistance vessels. Expression of α-SMA in pulmonary resistance vessels was determined by immunohistochemistry as described in Materials and Methods. a, b Representative photomicrographs of α-SMA staining in lung of WT and A2AR KO mice. ×200. c Quantitative analysis of optical density (OD) value of α-SMA immunoreactivity in WT, A2AR+/– and A2AR KO mice. ∗ p < 0.05, A2AR KO vs. WT, one-way ANOVA, LSD test, n = 10–11. Scale bar = 100 μm.
Fig. 5.
A2AR KO mice exhibited increased PCNA-positive cells in pulmonary resistance vessels. Expression of α-SMA in pulmonary resistance vessels was determined by immunohistochemistry as described in Materials and Methods. a, b Representative photomicrographs of PCNA staining in lung. ×200. c Quantitative analysis of PCNA-positive cells in lung sections of WT andA2AR KO mice. ∗ p < 0.05, A2AR KO vs. WT, one-way ANOVA, LSD test, n = 6–7.
A2AR KO Mice Exhibited Increased Activation and Proliferation of Endothelium Cells and Smooth Muscle as well as Enhanced Hyperplasis of Fibroblasts of Pulmonary Arteries at Ultrastructure Level
Compared with WT mice, the pulmonary arteries of the A2AR KO mice showed swelling and hypertrophy of endothelial (fig. 6a) and SMCs (fig. 6b), with abundance of cytoplasms and of increased intracytoplasmic vesicles. The endothelium contained more Weibel-Palade bodies in A2AR KO mice than WT mice. In the cytoplasm of SMCs, there were numerous filaments and dense bodies. It indicated the activation of endothelial cells and SMCs. Furthermore, while the internal and external elastic laminae were unbroken in the 3 genotypes, more fibroblasts (fig. 6c) were seen outside the external elastic laminae of A2AR KO mice than WT mice. There were also more clustered collagen fibers deposited in adventitia pulmonary arterial walls in A2AR KO mice than WT mice. These ultrastructural changes are indicative of hyperplasia of fibroblasts. Together, ultrastructural analysis reveals increased activation and proliferation of endothelium cells and SMCs, hypertrophy of fibroblast and the increased deposition of collagen fibers in A2AR KO mice, all contributing to the pulmonary vascular remodeling.
Fig. 6.
A2AR KO mice exhibited increased activation and proliferation of endothelium cells and smooth muscle as well as enhanced hyperplasis of fibroblasts of pulmonary arteries at ultrastructure level. Transmission electron micrographs of mouse pulmonary arteries: endothelial cell in pulmonary artery endomembrane layer (a), SMC in pulmonary artery tunica media layer (b) and collagen in pulmonary artery wall (c). Red arrow = cells in pulmonary artery (color only in online version); blue arrow = deposited collagen fibers; yellow arrow = Weibel-Palade bodies in endothelial cell. a ×12,000. b, c ×10,000.
A2AR KO Mice Displayed No Histological Features of Hypertensive Nephropathy
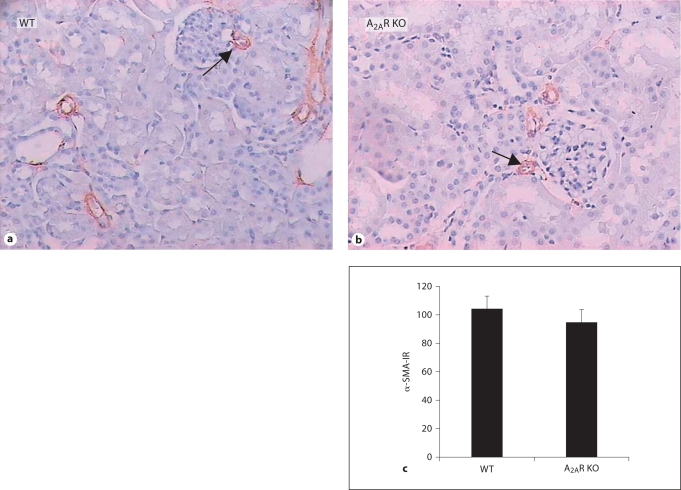
We also evaluated histological changes in renal arteries in A2AR KO, A2AR+/– and WT mice (fig. 7). Capillary membranes of afferent glomerular arteriole were of normal thickness in the 3 genotypes. There is neither a hyaline nor a nodular-sclerotic appearance in lobules of the glomeruli in the mice of the 3 genotypes. α-SMA immunoreactivity in A2AR KO, A2AR+/– and WT mice were indistinguishable (fig. 7). Furthermore, no tubular atrophy, interstitial fibrosis or an inflammatory component were detected in renal sections of the 3 genotypes (fig. 7). Thus, there were no features of hypertension detected in the kidneys of the 3 genotypes. Specifically, histological features indicative of hypertensive nephropathy (for example, intimal fibroelastosis, hyaline arteriosclerosis and fibrinoid necrosis) were not seen in A2AR KO and WT mice.
Fig. 7.
A2AR KO mice displayed no histological features of hypertensive nephropathy. Expression of α-SMA in renal arteries was determined by immunohistochemistry as described in Materials and Methods. a, b Representative photomicrographs of α-SMA staining in kidney. ×200. c Quantitative analysis of α-SMA-immunoreactivity (optical density) in WT and A2AR KO mice. WT (n = 5), A2AR KO (n = 7). p > 0.05, one-way ANOVA, LSD test.
Following Chronic Exposure to Hypoxia, A2AR KO Mice Exhibited Exacerbated Elevation in RVSP, Hypertrophy of Pulmonary Resistance Vessels and Increased Cell Proliferation in Pulmonary Resistance Vessels, Compared to WT Littermates
Lastly, we investigated whether A2AR gene deletion develops more severe PAH in a model of pulmonary arterial hypertension after exposure to hypoxia. A separate set of A2AR KO and WT littermates was exposed to a hypoxic chamber containing 10% oxygen in normobaric pressure for 2 weeks. Their RVSP were measured and histological changes of the lungs in these mice were examined. After exposure to hypoxia, both WT and A2AR KO mice increased RVSP compared to their corresponding groups exposed to normoxia (21% oxygen; fig. 8a. Interestingly, the increase in RVSP in A2AR KO mice compared to WT in normoxia was comparable to the increase in WT mice after exposure to hypoxia compared to WT mice in normoxia. Consistent with these hemodynamic changes, the mean percentage of wall area in WT and A2AR KO mice after exposure to hypoxia was significantly higher than that of their corresponding genotypes after exposure to normoxia (fig. 8b). Similarly, the increase in the wall area in A2AR KO mice compared to WT in normoxia was comparable to the increase in WT mice after exposure to hypoxia compared to WT mice in normoxia. However, two-way ANOVA analysis indicates that there was no interaction between genotype (WT vs. KO) and oxygen condition (normoxia vs. hypoxia). These results indicate that pathological pulmonary hypertension by genetic deletion of A2AR in normoxia was equivalent to the increase in pulmonary hypertension induced by exposing WT mice to hypoxia for 2 weeks. The increase in pulmonary hypertension in A2AR KO mice under hypoxic condition seems to be an additive (not synergistic) effect of an A2AR KO effect under normal oxygen and a hypoxic effect in WT mice.
Fig. 8.
Following chronic exposure to hypoxia, A2AR KO mice exhibited exacerbated elevation in RVSP, wall areas and PCNA+ cells in pulmonary resistance vessels compared to WT littermates. A separate set of A2AR KO and WT littermates was exposed to a hypoxic chamber containing 10% oxygen or normoxia (21% oxygen) for 2 weeks. Their RVSP (a), pulmonary vessel wall areas (b) and PCNA+ cells (c) were measured as described in Materials and Methods. + p < 0.05, KO-normoxia vs. WT-normoxia; ∗ p < 0.001, WT-hypoxia vs. WT-normoxia; ° p < 0.001, KO-hypoxia vs. KO-normoxia, two-way ANOVA, LSD post hoc test (a, b) or two-way ANOVA, post hoc Dunnett's test (c).
Furthermore, we also examined the effect of A2AR inactivation on cell proliferation (that is PCNA+ cells) in WT and A2AR KO mice after exposure to hypoxia. As expected, both A2AR KO and WT mice exhibited increased expression of PCNA in pulmonary resistance vessels after exposure to hypoxia (fig. 8c). Of note, the increase in WT mice exposure to hypoxia was clearly larger than the increase in A2AR KO mice compared to WT littermates under normal oxygen. However, two-way ANOVA analysis indicates that there was no interaction between the genotype and oxygen condition. The increase in PCNA+ cells in A2AR KO mice under hypoxic condition seems to be an additive (not synergistic) effect of an A2AR KO effect under normal oxygen and a hypoxic effect in WT mice.
Discussions
Using the A2AR KO model, we have provided the first evidence for the critical contribution of A2AR to the development of PAH in mice. At the postnatal age of 14–16 weeks, A2AR KO mice exhibited characteristics of hemodynamic, histological and ultrastructural changes of PAH: (1) increased RVSP and the elevated Fulton index, as a result of increased right ventricular mass; (2) increased wall area and thickness and enhanced α-SMA immunoreactivity in pulmonary resistance vessels; (3) increased cellular proliferation in pulmonary resistance vessels as evident by increased PCNA-positive cells; (4) increased activation and hypertrophy SMCs and endothelium as well as collagen deposition in the adventitia of pulmonary arterial walls as revealed by electron microscope examination; (5) following chronic exposure to hypoxia, A2AR KO mice exhibited exacerbated elevation in RVSP, hypertrophy of pulmonary resistance vessels and increased cell proliferation in pulmonary resistance vessels, compared to WT littermates.
Spontaneous PAH and altered remodeling of pulmonary arterials at hemodynamic, histological and ultrastructual levels are supported by anatomical localization of A2ARs primarily in the vasculature to mediate vasodilation [9], and by functional demonstration that activation of A2AR in endothelium cells mediates adenosine-induced vasodilation [9]. Adenosine can exert profound hypotensive effects by direct vasodilation [24], by stimulating nitric oxide release from vascular endothelial cells [25], and by attenuating the sympathetic nervous system [26], and by inhibiting the renin angiotensin system [27]. Thus, adenosine has been shown to elicit vascular smooth muscle relaxation in the pulmonary circulation and hence has been proposed for the therapy of clinical and experimental pulmonary hypertension [28]. Our finding further argues that the adenosine effect is likely to be mediated by A2AR in pulmonary vessels.
By contrast, hemodynamic and histological analyses revealed no features of hypertensive nephropathy in both genotypes and there was no significant difference in SBP, and left ventricular masses between A2AR KO and WT mice. Furthermore, there was no histological feature of hypertensive nephropathy and the renal vasculature was indistinguishable among the 3 genotypes. This A2AR KO phenotype is consistent with the pharmacological finding that intravenous infusion of adenosine decreases pulmonary arterial pressure without obvious change of systemic mean artery pressure [28]. This selective increase in pulmonary arterial hypertension is likely attributed to the fact that the normal pulmonary vasculature is a low-pressure system with less than one tenth the resistance to flow observed in the systemic vascular bed [27], thus particular sensitive to adenosine-mediated hemodynamic and remodeling changes. While the hypotensive effect of A2AR activation on SBP has been reproducibly demonstrated, pharmacological and genetic inactivation of A2AR has produced mixed results on SBP. For example, chronic consumption of caffeine, a nonselective adenosine antagonist, has been associated with increased blood pressure in several epidemiological studies [28,29]. However, many other epidemiological studies show that hypertensive effects were evident only at relatively high dose of caffeine (>250 mg/day) [30] and no association between regular caffeine consumption (<200 mg/kg) and hypertension was found [31,32]. On the other hand, hypertensive phenotype was reported in A2AR KO mice in CD1 strain (an outbreed strain) [17], but not in pure 129Sv or congenic C57BL/6 background or mixed 129Sv × C57BL/6 background [18,19,33].
Our finding of the lack of evidence for renal hypertensive phenotype and of indistinguishable mean SBP in the A2AR KO and WT mice further substantiate the conclusion of the lack of A2AR inactivation on SBP. The lack of renal damage caused by sustained systemic hypertension and the absence of the pulmonary edema secondary to left ventricular dysfunction in A2AR KO mice suggest that left ventricular dysfunction and the increased resistance in systemic vascular bed are not the cause of PAH in A2AR KO mice.
PAH phenotype was observed in the mature adult A2AR KO mice (14–16 weeks old) so that the developmental effects of A2AR gene deletion on the early (P3–P8, the rapid alveolar proliferation) and late postnatal (that is P42, increasing numbers of alveoli formed by growth processes) [34] were manifested at this mature adult age. It would be extremely interesting to examine the A2AR KO and WT littermates at early as well as later development stages. By defining hemodynamic and histological changes in these mice at early postnatal age (for example after 2–4 weeks), we may distinguish the pulmonary vascular remodeling as triggering factor of PAH or secondary response to PAH. On the other hand, characterization of hemodynamic and histological changes in more matured or aged mice (12- to 14-month-old mice) may uncover whether there is a progressive development of PAH and associated pathological changes in aged mice.
The exact mechanism underlying the development of PAH in A2AR KO mice is not clear. Three major mechanisms including vasoconstriction, remodeling of pulmonary vessels wall and thrombosis are proposed to play critical roles in the development of PAH. Adenosine exerts several anti-vaso-occlusive effects, including inhibition of platelet aggregation [17,35], diminished neutrophil adhesion to vascular endothelial cells and attenuation of neutrophil-induced endothelial cell damage [36]. However, the lack of the abnormalities such as infiltration of inflammatory cells, edema, fibrosis, hypercellularity or alteration/thickening of the alveolar septa in A2AR KO mice strongly argues against the notion that pulmonary vaso-occulsion is unlikely the major contributor to the PAH phenotype in A2AR KO mice. Furthermore, it was reported that variants of the genes for the A1, A2A and A2B receptors do not appear to alter susceptibility to the disease of essential (systemic) hypertensives [37]. However, pulmonary artery vasoconstriction greatly contributes to a sustained elevation of pulmonary vascular resistance and pulmonary arterial pressure in patients with pulmonary hypertension [6]. It remains to be determined whether genetic variants or deletion of the A2AR gene affect pulmonary vascular responsiveness, which might lead to vasoconstriction with pulmonary hypertension and progressive remodeling.
This abnormal pulmonary vascular remodeling contributes to the development of PAH in A2AR KO mice. The pathological feature of PAH is characterized by an abnormal proliferation of endothelium and SMC, hypertrophy of SMC and abundant extracellular matrix deposition [38]. In support of this notion, we found that A2AR KO mice displayed cells hyperplasia in pulmonary artery walls with increased immunoreactivity of α-SMA, as well as cell proliferation with increased PCNA+ cells in lung sections across entire pulmonary resistance vessels, indicating involvement of endothelium, smooth muscle and fibroblasts. Moreover, the enhanced activation and proliferation of endothelium cell and smooth muscle, and more importantly increased multiplication of collagen fiber and activation of fibroblasts were observed at ultrastructural level in pulmonary artery walls of A2AR KO mice compared to the WT littermates. These observations strongly suggested that A2ARs might affect pulmonary vascular remodeling, leading to PAH. Further experiments are warranted to dissect out the mechanism that A2ARs modulates the hyperplasia, proliferation and activation of various cells and the deposition of extracellular matrix in pulmonary artery walls.
Genetic mutations of BMP are found in approximately 30% of idiopathic PAH and 15% sporadic PAH. Gene deletion of vasoactive intestinal peptide [39] and a GTP-cyclohydrolase 1 [40] have been reported to develop moderate PAH with enhanced pulmonary vascular remodeling. This report represents the new example by which a single gene deletion produces selective PAH without affecting SBP. This finding invites epidemiological investigation into possible genetic association of variants of the A2AR gene and PAH pathogenesis. Since the pulmonary vascular alteration in A2AR KO mice closely resembles those pathologies of moderately severe idiopathic PAH, demonstration of the critical role of A2AR in the development of PAH has important clinical implications for the treatment of PAH. Adenosine is a potent pulmonary vasodilator that has been used in therapy for clinical and experimental pulmonary hypertension [28]. Development of PAH in the absence of A2AR suggests that the selective activation of A2ARs may be an important therapeutic strategy for the treatment of PAH.
Acknowledgements
The authors would like to thank Drs. S.-S. Dai and Y.-G. Zhou for their help with immunohistochemistry of A2AR in lung sections. This work was supported by grants from the Nature Science Foundation of Zhejiang Province (Y 2080503), Development Funding of Zhejiang Key Subject (Pharmacology and Biochemical Pharmaceutics), Wenzhou Medical College Key Research Project (No. 30328015), China, and from the US public health grants (NIH; NS41083 and NS48995).
References
- 1.Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:13S–24S. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 2.Dorfmuller P, Humbert M, Capron F, Muller KM. Pathology and aspects of pathogenesis in pulmonary arterial hypertension. Sarcoidosis Vasc Diffuse Lung Dis. 2003;20:9–19. [PubMed] [Google Scholar]
- 3.Shifren A, Durmowicz AG, Knutsen RH, Faury G, Mecham RP. Elastin insufficiency predisposes to elevated pulmonary circulatory pressures through changes in elastic artery structure. J Appl Physiol. 2008;105:1610–1619. doi: 10.1152/japplphysiol.90563.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saadjian AY, Paganelli F, Gaubert ML, Levy S, Guieu RP. Adenosine plasma concentration in pulmonary hypertension. Cardiovasc Res. 1999;43:228–236. doi: 10.1016/s0008-6363(99)00059-0. [DOI] [PubMed] [Google Scholar]
- 5.Dubey RK, Gillespie DG, Mi Z, Suzuki F, Jackson EK. Smooth muscle cell-derived adenosine inhibits cell growth. Hypertension. 1996;27:766–773. doi: 10.1161/01.hyp.27.3.766. [DOI] [PubMed] [Google Scholar]
- 6.Moudgil R, Michelakis ED, Archer SL. Hypoxic pulmonary vasoconstriction. J Appl Physiol. 2005;98:390–403. doi: 10.1152/japplphysiol.00733.2004. [DOI] [PubMed] [Google Scholar]
- 7.Fredholm B, Chen JF, Masino SA, Vaugeois JM. Actions of adeosine at its receptors in the CNS: Insights from knckouts and drugs. Annu Rev Pharmacol Toxicol. 2005:45. doi: 10.1146/annurev.pharmtox.45.120403.095731. [DOI] [PubMed] [Google Scholar]
- 8.Varani K, Caramori G, Vincenzi F, Adcock I, Casolari P, Leung E, Maclennan S, Gessi S, Morello S, Barnes PJ, Ito K, Chung KF, Cavallesco G, Azzena G, Papi A, Borea PA. Alteration of adenosine receptors in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173:398–406. doi: 10.1164/rccm.200506-869OC. [DOI] [PubMed] [Google Scholar]
- 9.Schindler CW, Karcz-Kubicha M, Thorndike EB, Muller CE, Tella SR, Ferre S, Goldberg SR. Role of central and peripheral adenosine receptors in the cardiovascular responses to intraperitoneal injections of adenosine A1 and A2A subtype receptor agonists. Br J Pharmacol. 2005;144:642–650. doi: 10.1038/sj.bjp.0706043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Kashef H, Elmazar MM, Al-Shabanah OA, Al-Bekairi AM. Effect of adenosine on pulmonary circulation of rabbits. Gen Pharmacol. 1999;32:307–313. doi: 10.1016/s0306-3623(98)00184-0. [DOI] [PubMed] [Google Scholar]
- 11.Tofovic SP, Jackson EK, Rafikova O. Adenosine deaminase-adenosine pathway in hemolysis-associated pulmonary hypertension. Med Hypothes. 2009;72:713–719. doi: 10.1016/j.mehy.2008.12.043. [DOI] [PubMed] [Google Scholar]
- 12.Adair TH. Growth regulation of the vascular system: an emerging role for adenosine. Am J Physiol Regul Integr Comp Physiol. 2005;289:R283–R296. doi: 10.1152/ajpregu.00840.2004. [DOI] [PubMed] [Google Scholar]
- 13.Dubey RK, Gillespie DG, Mi Z, Rosselli M, Keller PJ, Jackon EK. Estradiol inhibits smooth muscle cell growth in part by activating the cAMP-adenosine pathway. Hypertension. 2000;35:262–266. doi: 10.1161/01.hyp.35.1.262. [DOI] [PubMed] [Google Scholar]
- 14.Dorfmuller P, Humbert M, Perros F, Sanchez O, Simonneau G, Muller KM, Capron F. Fibrous remodeling of the pulmonary venous system in pulmonary arterial hypertension associated with connective tissue diseases. Hum Pathol. 2007;38:893–902. doi: 10.1016/j.humpath.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 15.Dubey RK, Gillespie DG, Shue H, Jackson EK. A(2B) receptors mediate antimitogenesis in vascular smooth muscle cells. Hypertension. 2000;35:267–272. doi: 10.1161/01.hyp.35.1.267. [DOI] [PubMed] [Google Scholar]
- 16.Chen JF, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, Moskowitz MA, Fink JS, Schwarzschild MA. A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci. 1999;19:9192–9200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ledent C, Vaugeois JM, Schiffmann SN, Pedrazzini T, El Yacoubi M, Vanderhaeghen JJ, Costentin J, Heath JK, Vassart G, Parmentier M. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature. 1997;388:674–678. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- 18.Yu L, Huang Z, Mariani J, Wang Y, Moskowitz M, Chen JF. Selective inactivation or reconstitution of adenosine A2A receptors in bone marrow cells reveals their significant contribution to the development of ischemic brain injury. Nat Med. 2004;10:1081–1087. doi: 10.1038/nm1103. [DOI] [PubMed] [Google Scholar]
- 19.Day YJ, Huang L, McDuffie MJ, Rosin DL, Ye H, Chen JF, Schwarzschild MA, Fink JS, Linden J, Okusa MD. Renal protection from ischemia mediated by A2A adenosine receptors on bone marrow-derived cells. J Clin Invest. 2003;112:883–891. doi: 10.1172/JCI15483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savale L, Tu L, Rideau D, Izziki M, Maitre B, Adnot S, Eddahibi S. Impact of interleukin-6 on hypoxia-induced pulmonary hypertension and lung inflammation in mice. Resp Res. 2009;10:6. doi: 10.1186/1465-9921-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu L, Quinn DA, Garg HG, Hales CA. Cyclin-dependent kinase inhibitor p27Kip1, but not p21WAF1/Cip1, is required for inhibition of hypoxia-induced pulmonary hypertension and remodeling by heparin in mice. Circ Res. 2005;97:937–945. doi: 10.1161/01.RES.0000188211.83193.1a. [DOI] [PubMed] [Google Scholar]
- 22.Rondelet B, Kerbaul F, Motte S, van Beneden R, Remmelink M, Brimioulle S, McEntee K, Wauthy P, Salmon I, Ketelslegers JM, Naeije R. Bosentan for the prevention of overcirculation-induced experimental pulmonary arterial hypertension. Circulation. 2003;107:1329–1335. doi: 10.1161/01.cir.0000053443.27512.33. [DOI] [PubMed] [Google Scholar]
- 23.Hall PA, Levison DA, Woods AL, Yu CC, Kellock DB, Watkins JA, Barnes DM, Gillett CE, Camplejohn R, Dover R, et al. Proliferating cell nuclear antigen (PCNA) immunolocalization in paraffin sections: an index of cell proliferation with evidence of deregulated expression in some neoplasms. J Pathol. 1990;162:285–294. doi: 10.1002/path.1711620403. [DOI] [PubMed] [Google Scholar]
- 24.Sato A, Terata K, Miura H, Toyama K, Loberiza FR, Jr, Hatoum OA, Saito T, Sakuma I, Gutterman DD. Mechanism of vasodilation to adenosine in coronary arterioles from patients with heart disease. Am J Physiol Heart Circ Physiol. 2005;288:H1633–H1640. doi: 10.1152/ajpheart.00575.2004. [DOI] [PubMed] [Google Scholar]
- 25.Konduri GG, Mital S. Adenosine and ATP cause nitric oxide-dependent pulmonary vasodilation in fetal lambs. Biol Neonate. 2000;78:220–229. doi: 10.1159/000014274. [DOI] [PubMed] [Google Scholar]
- 26.Ichinose TK, O'Leary DS, Scislo TJ. Activation of NTS A2a adenosine receptors differentially resets baroreflex control of renal vs. adrenal sympathetic nerve activity. Am J Physio Heart Circ Physiol. 2009;296:H1058–H1068. doi: 10.1152/ajpheart.00906.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai EY, Martinka P, Fahling M, Mrowka R, Steege A, Gericke A, Sendeski M, Persson PB, Persson AE, Patzak A. Adenosine restores angiotensin II-induced contractions by receptor-independent enhancement of calcium seneitivity in renal arterioles. Circ Res. 2006;99:1117–1124. doi: 10.1161/01.RES.0000249530.85542.d4. [DOI] [PubMed] [Google Scholar]
- 28.McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation. 2006;114:1417–1431. doi: 10.1161/CIRCULATIONAHA.104.503540. [DOI] [PubMed] [Google Scholar]
- 29.Winkelmayer WC, Stampfer MJ, Willett WC, Curhan GC. Habitual caffeine intake and the risk of hypertension in women. JAMA. 2005;294:2330–2335. doi: 10.1001/jama.294.18.2330. [DOI] [PubMed] [Google Scholar]
- 30.Hartley TR, Sung BH, Pincomb GA, Whitsett TL, Wilson MF, Lovallo WR. Hypertension risk status and effect of caffeine on blood pressure. Hypertension. 2000;36:137–141. doi: 10.1161/01.hyp.36.1.137. [DOI] [PubMed] [Google Scholar]
- 31.Geleijnse JM. Habitual coffee consumption and blood pressur: an epidemiological perspective. Vasc Health Risk Manag. 2008;4:963–970. doi: 10.2147/vhrm.s3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lancaster T, Muir J, Silagy C. The effects of coffee on serum lipids and blood pressure in a UK population. J R Soc Med. 1994;87:506–507. doi: 10.1177/014107689408700905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen JF, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, Moskowitz MA, Fink JS, Schwarzschild MA. A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci. 1999;19:9192–9200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Massaro GD, Massaro D, Chambon P. Retinoic acid receptor-α regulates pulmonary alveolus formation in mice after, but not during, perinatal period. Am J Physiol Lung Cell Mol Physiol. 2003;284:L431–L433. doi: 10.1152/ajplung.00245.2002. [DOI] [PubMed] [Google Scholar]
- 35.Takeuchi T, Yoneyama Y, Suzuki S, Sawa R, Otsubo Y, Araki T. Regulation of platelet aggregation in vitro by plasma adenosine in preeclampsia. Gynecol Obstet Invest. 2001;51:36–39. doi: 10.1159/000052888. [DOI] [PubMed] [Google Scholar]
- 36.Mazar J, Rogachev B, Shaked G, Ziv NY, Czeiger D, Chaimovitz C, Zlotnik M, Mukmenev I, Byk G, Douvdevani A. Involvement of adenosine in the antiinflammatory action of ketamine. Anesthesiology. 2005;102:1174–1181. doi: 10.1097/00000542-200506000-00017. [DOI] [PubMed] [Google Scholar]
- 37.Wright K, Tajouri L, Lea RA, Ovcaric M, Heux S, Morin F, Bey W, Headrick JP, Griffiths LR. The role of adenosine-related genes variants in susceptibility to essential hypertension. J Hypertens. 2004;22:1519–1522. doi: 10.1097/01.hjh.0000133723.16947.6d. [DOI] [PubMed] [Google Scholar]
- 38.Tuder RM, Marecki JC, Richter A, Fijalkowska I, Flores S. Pathology of pulmonary hypertension. Clin Chest Med. 2007;28:23–42. doi: 10.1016/j.ccm.2006.11.010. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Said SI, Hamidi SA, Dickman KG, Szema AM, Lyubsky S, Lin RZ, Jiang YP, Chen JJ, Waschek JA, Kort S. Moderate pulmonary arterial hypertension in male mice lacking the vasoactive intestinal peptide gene. Circulation. 2007;115:1260–1268. doi: 10.1161/CIRCULATIONAHA.106.681718. [DOI] [PubMed] [Google Scholar]
- 40.Nandi M, Miller A, Stidwill R, Jacques TS, Lam AA, Haworth S, Heales S, Vallance P. Pulmonary hypertension in a GTP-cyclohydrolase 1-deficient mouse. Circulation. 2005;111:2086–2090. doi: 10.1161/01.CIR.0000163268.32638.F4. [DOI] [PubMed] [Google Scholar]