Abstract
This study identified structural changes in the caudate nucleus in offspring of mothers who drank moderate levels of alcohol during pregnancy. In addition, the effect of duration of alcohol use during pregnancy was assessed. Young adults were recruited from the Maternal Health Practices and Child Development Project. Three groups were evaluated: prenatal alcohol exposure (PAE) during all three trimesters (3T), PAE during the first trimester only (1T), and controls with no PAE (0T). Magnetic resonance images were processed using the automated labeling pathway technique. Volume was measured as the number (gray + white) and relative percentage (caudate count/whole brain count × 100) of voxels. Asymmetry was calculated by subtracting the caudate volume on the left from the right and dividing by the total (L−R/L+R). Data analyses controlled for gender, handedness, and prenatal tobacco and marijuana exposures. There were no significant differences between the groups for whole brain, left, or right volumes. There was a dose-response effect across the three exposure groups both in terms of magnitude and direction of asymmetry. In the 3T group, the left caudate was larger relative to the right caudate compared to the 0T group. The average magnitude of caudate asymmetry for the 1T group was intermediate between the 0T and 3T groups. Subtle anatomical changes in the caudate are detected at the moderate end of the spectrum of prenatal alcohol exposure.
Keywords: prenatal alcohol exposure, prenatal tobacco exposure, prenatal marijuana exposure, morphometry, structural MRI, asymmetry
1. Introduction
Heavy maternal alcohol use during pregnancy may lead to Fetal Alcohol Syndrome (FAS), which is diagnosed when a child has growth deficits, specific facial dysmorphology, and cognitive impairment. However, not all fetuses who are exposed to alcohol prenatally have all of the characteristics required for a diagnosis of FAS. Fetal alcohol spectrum disorder (FASD) is a term used to describe individuals who experience the negative effects of prenatal alcohol exposure, of which FAS is at one end of the spectrum.
A recent CDC study showed that while only 1.9% of pregnant women reported frequent drinking (7 or more drinks/week), 10.1% reported any use at lower levels (MMWR, 2004). Earlier studies have also noted that the majority of the women who drink during pregnancy are light to moderate users who quit or decrease their consumption of alcohol by mid-pregnancy (Day et al., 1993; Ebrahim et al., 1998). The effects of exposures to low and moderate levels of alcohol have not been well explored, particularly with respect to brain function. Given that the relation between prenatal alcohol exposure and outcomes appears, in general, to be a dose-response association (Day et al., 2002; Richardson et al., 2002; Willford et al., 2004), it is expected that the effects of PAE on brain structure and cognitive function should be observed at lower levels of exposure.
The cognitive effects of FAS/FASD among children with high levels of alcohol exposure include deficits in learning and memory (Kaemingk et al., 2003; Mattson et al., 1996; Mattson & Roebuck, 2002; Pei et al., 2008; Richardson et al., 2002; Roebuck-Spencer & Mattson, 2004; Willford et al., 2004), attention (Burden et al., 2005; Lei et al., 2004; Mattson et al., 2006; Streissguth et al., 1994), and executive functions (Connor et al., 2000; Kodituwakku et al., 2001; Mattson et al., 1999; McGee et al., 2008; Noland et al., 2003; Vaurio et al., 2008). Altered brain structure and function have also been found. PAE-related structural defects have been linked to the impairments in cognitive behavior among more heavily exposed offspring (Sowell et al., 2001; Sowell et al., 2008a; Sowell et al., 2008b).
Previous studies indicate the caudate nucleus may be vulnerable to the effects of prenatal alcohol exposure. Cortese and colleagues (2006) showed the ratio of n-acetyl-aspartate/creatinine, an indicator of neuronal function, was abnormally elevated in the left caudate of FAS and FASD subjects compared to controls. A functional neuroimaging study (Fryer et al., 2007), using a go/no-go task as a measure of response inhibition, showed heavy PAE was associated with decreased activation in the right caudate. Clark et al. (2000) found that compared to controls, the alcohol-exposed group showed significant reductions in regional metabolic rates and bilateral disruption in the head of the caudate. Thus, heavy PAE is consistently associated with functional deficits in the caudate.
The findings are also consistent with respect to caudate volume among individuals with high levels of exposure. Although structural imaging studies have found a reduction in caudate volume in subjects with FAS (Archibald et al., 2001; Astley et al., 2009; Mattson et al., 1992, 1994, 1996) one study did not (Clark et al., 2000). Among individuals with FASD, one study with a small sample found a reduction in caudate volume (Mattson et al., 1994), while a second study with a larger sample size found a marginally significant reduction in caudate volume (Archibald et al., 2001). Recently, Astley et al. (2009) showed that the mean relative volume of the caudate was reduced by 12–14% in individuals in two groups: FAS/partial FAS and Static Encephalopathy/Alcohol Exposed. Clark et al. (2000) reported that a group of young adults with FAS with a mean IQ of 80.2 did not differ in structural volume of the caudate, but did show a significant difference in standardized metabolic rates in the head and body of the caudate compared to controls.
Changes in brain structure can be evaluated by studying regional asymmetry, the degree to which the right and left sides of a brain structure differ in size. Increases in regional asymmetries suggest abnormal brain development and deviant patterns of functional organization, which can result in altered regional specialization and impaired information processing (Hynd et al., 1995; Watkins et al., 2001). Atypical asymmetries have also been used to identify the neuroanatomical basis of developmental disorders (Crow, 1990; Filipek, 1995; Hendren et al., 2000; Petty, 1999; Sharma et al., 1999).
Symmetry is determined by the number of neurons located in the left and right sides of a brain structure and the density of interhemispheric connections between the two sides of a structure. While brain asymmetries are normal due to the functional specialization of the hemispheres, increases or decreases in regional asymmetry may reflect abnormal structural and functional development. The magnitude, rather than the direction, of asymmetry is the key predictive variable. Smaller size reflects fewer neurons and a reduced density of interhemispheric connections (Galaburda et al., 1990; Rosen et al., 1992). Developmentally, the reduced number of neurons is a result of increased unilateral programmed cell death and increased axonal pruning (Galaburda et al., 1990). Events occurring early in corticogenesis, during the period of progenitor cell proliferation and/or cell death, affect the formation of asymmetric cortical areas (Rosen, 1996).
Prior studies that have reported PAE-related structural and functional differences in the caudate have relied primarily on heavily exposed subjects with or without FAS. Earlier studies also did not examine the effects of PAE in combination with prenatal tobacco or prenatal marijuana exposure. The purpose of the current study is to assess the effects of light to moderate PAE on caudate volume and asymmetry. We compared the offspring of women who drank only during the first trimester to offspring of women who drank throughout pregnancy, and to the offspring of women who did not drink at all during pregnancy. We hypothesize: (i) the effects of light to moderate PAE will be detectable on measures of caudate volume and asymmetry; (ii) PAE will affect caudate volume and asymmetry as the magnitude (dose and duration) of exposure increases. That is, offspring of women who drank throughout pregnancy will have the largest effects, compared to offspring of women who only drank alcohol during the first trimester, who should be intermediate, and the offspring of non-drinking controls who show the least effect. (iii) prenatal exposures to tobacco and marijuana will not significantly modify the primary effects of PAE on caudate volume and asymmetry.
2. Methods
2.1 Participants
Subjects were recruited from the Maternal Health Practices and Child Development (MHPCD) Project, a longitudinal study of the effects of prenatal substance exposure on developmental outcomes (n= 585). A subsample of subjects (n=45) was recruited to participate in this MRI study based on their PAE. Subjects were required to be 18–22 years of age and to have an IQ >70. None of these young adults has been diagnosed with FAS. Three groups of subjects were recruited: offspring whose mothers did not drink during pregnancy (0T group, n = 20), offspring whose mothers drank during the first trimester only (1T group, n = 15), and offspring whose mothers drank throughout pregnancy (3T group, n = 10). Four additional subjects, one in the 3T and 3 in the 1T group, were scanned but their data were not used due to equipment failure or excessive motion in the scanner.
2.2. Measures of Prenatal Substance Exposure
The mothers were recruited in their fourth prenatal month. The minimum, usual, and maximum quantity and frequency of alcohol and marijuana were assessed at the end of each trimester. At the first interview, the mothers were asked about their substance use prior to pregnancy and in the first trimester. Using a calendar, the women were asked when they conceived, when they recognized they were pregnant, and when their pregnancy was confirmed. For each of these time periods, the women were asked (for both alcohol and marijuana), whether they “drank alcohol more like they reported prior to pregnancy” or “more like they reported for the first trimester”. The calendar data were then used to compute a weighted average of use from conception to recognition of pregnancy, from recognition to confirmation, and from confirmation to the end of the first trimester (Day & Robles, 1989). This weighted average allows us to accurately evaluate the actual use in the first trimester prior to the recognition of the pregnancy. Marijuana use was assessed in a similar fashion. For the second and third trimesters, assessment was across the entire trimester. The quantity and frequency of tobacco, cocaine, and illicit drugs were also measured at each trimester. Assessors were unaware of the substance use of the mother during pregnancy and in the postpartum.
Prenatal alcohol use was expressed as an average daily volume (ADV) in drinks per day. The usual, maximum, and minimum quantity and frequency were assessed for wine, beer, liquor, and wine and beer coolers. A standard drink was defined as 12 oz of beer, 1.5 oz of 72-proof distilled spirits, or 4 oz of wine. ADV is a summary measure of the total amount of alcohol consumed, averaged to represent the number of drinks per day, adjusting for the number of days per month. Prenatal marijuana use was expressed as average daily joints (ADJ). For each trimester of pregnancy, tobacco use was reported as number of packs per day and converted to average number of cigarettes smoked per day (ADC).
2.3. Brain MRI Evaluation
MRI images were collected at UPMC Presbyterian Hospital at the Magnetic Resonance Research Center on a 3.0 T Signa whole body scanner (GE Medical Systems, Milwaukee) with a standard head coil. Structural images were obtained using a 3D volume spoiled gradient-echo (SPGR) pulse sequence acquired with 1.5 mm slices in the axial plane. Acquisition parameters included: TE=50 msec, TR = 2500 msec, 256 × 192 acquisition matrix, field of view (FOV) = 24×18 cm2 and 124 slices with a 1.5 mm gap.
2.3.1. Automated Labeling Technique
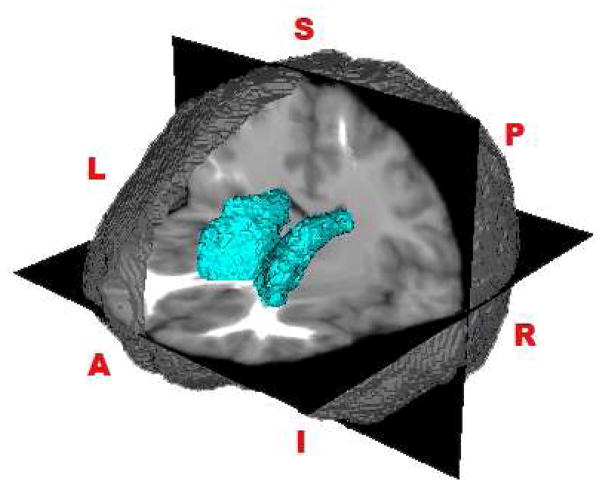
Data were processed using the Automated Labeling Pathway (ALP). The steps in processing included the following: (i) brain stripping (Brain Extraction Tool, BET, Smith, 2002), (ii) regional growth segmentation (RGS, Wu et al., 2005), and (iii) multi-tracer (Woods, 2003) to remove unwanted tissue surrounding the brain, including neck muscles and eyeballs. Stripped brains were compared to the original to verify the stripping quality and to ensure no brain tissue was removed. The MNI (Montreal Neurological Institute) brain (Collins et al., 1998), a common reference brain, was then aligned with the subject’s SPGR image. Over 200 regions of interest (ROIs) (Damasio and Damasio, 1989; Tzourio-Mazoyer et al., 2002) traced on the MNI template brain were transferred to each subject’s SPGR (spoiled gradient recalled) space using a series of automated warpings followed by segmentation. The reference brain was registered to each subject’s SPGR using a 12 parameter linear affine algorithm followed by a fully-deformable registration implemented in ITK (www.itk.org). For further details of the atlas-based segmentation method, see Wu et al. (2006). Registration of the MNI transformed the atlas brain to fit the individual’s brain instead of stretching or warping the individual’s brain onto the atlas brain. The automated labeling technique provided region-specific voxel counts of gray and white matter, and cerebrospinal fluid. Voxel counts in the right and left caudate were considered. An example of the segmented caudate from a subject in the 3T group can be viewed in Figure 1.
Figure 1.
Example of the right and left segmented caudate nucleus from a subject in the 3T group. (L=Left, R = Right, S = Superior, I = Inferior, A = Anterior, P = Posterior).
Accurate brain region identification is critical for both within subject and across group comparisons. The ALP method achieves a significantly greater volumetric overlap with the original structures compared with alternative registration methods (Wu et al., 2006) such as statistical parametric mapping (SPM, 1995), and Automated Image Registration (AIR, 1998). Brain regions are mapped using the atlas brain and are then shaped to fit the subject’s brain to obtain an accurate map of the brain regions. In addition, ALP eliminates human subjectivity because its segmentation is automated.
2.3.2. Volume and Asymmetry Measures
The number of voxels (gray + white) within the caudate was used as a measure of caudate volume(caudate count). To control for individual differences in total brain volume, relative caudate percentages were also evaluated. Caudate voxel percentages were calculated as the number of voxels in each ROI divided by the total brain volume (gray + white) for each individual and multiplied by 100 (caudate percentage). The caudate asymmetry variable was calculated by subtracting the left caudate volume from the right caudate volume and dividing by the sum of both sides (L−R/L+R) (Medina et al., 2007). Zero represents perfect symmetry between the two hemispheres: Values greater than 0 reflect a rightward bias (R>L) and values less than 0 reflect a leftward bias (L>R).
2.4. Statistical Analysis
Descriptive data are summarized as means and standard errors. Box-and-whisker plots provide a visual display of the distribution of the data. Statistical inferences were based on the results from a univariate general linear model (ANOVA). Key dependent variables were caudate asymmetry and measures of regional and overall brain volume. Independent variables included PAE group, gender, handedness, and measures of PTE and PME. Continuous variables were assessed for normality using probability plots and the Anderson- Darling test (Stephens 1974). Gender, handedness, and age are associated with anatomical asymmetry and were evaluated between the groups in this study (Toga and Thompson, 2003). The results of the analyses were assessed for second-order interactions between exposure variables (PAE, PTE, and PME) and adjusted means are provided for the final model. All pairwise group analyses used Fisher’s least significant difference (LSD) procedure for post-hoc comparisons (Steel and Torrie, 1980).
3. Results
3.1. Sample Characteristics
Sample characteristics are presented in Table 1. Subjects in this study were predominantly female. (0T = 70%, 1T = 67%, and 3T = 80%, p = .78). The mean age for each group of subjects participating in this study was 21 years (range 20–22, p = .77). All of the participants were right handed with the exception of 4 subjects in the 0T group (p = .26). Disease processes that affect blood supply to the brain can interact with brain asymmetries or exacerbate their functional effects (Toga and Thompson, 2003). None of the subjects participating in this study had a previous history of blood pressure problems, stroke, or heart attack.
Table 1.
Socio-Demographic Characteristics of Study Subjects
| Socio-Demographic Factors | Prenatal Alcohol Exposure (PAE) | Overall P-Value | ||
|---|---|---|---|---|
| 0T (n=20) | 1T (n=15) | 3T (n=10) | ||
| Mean Age (SD) | 20.9 (.52) | 21.0 (.68) | 20.9 (.62) | 0.77 |
| Race (White/Black%) | 60/40 | 60/40 | 90/10 | 0.21 |
| Sex (Female/Male%) | 70/30 | 67/33 | 80/20 | 0.76 |
| Full-Scale IQ* (Mean, SD) | 86.8 (9.6) | 83.9 (11.5) | 83.3 (7.7) | 0.57 |
| Education (<HS/≥HS%) | 40/60 | 57/42 | 44/56 | 0.61 |
| Work Status: | 0.58 | |||
| Unemployed % | 15 | 26 | 30 | |
| Employed % | 65 | 41 | 40 | |
| Student % | 20 | 33 | 30 | |
| Monthly Income: | 0.15 | |||
| <$1000 % | 50 | 79 | 78 | |
| ≥$1000 % | 50 | 21 | 22 | |
At 14 years
3.2. Substance Use
The average daily levels of prenatal alcohol, tobacco, and marijuana exposure throughout pregnancy are shown in Table 2. Although women in each of the three PAE groups also used tobacco and marijuana during pregnancy, the mean levels of exposure to these substances were not significantly different across the PAE groups. The first trimester average daily volume of alcohol did not differ between the 1T and 3T groups.
Table 2.
Mean (±SEM) Prenatal Exposure to Alcohol, Tobacco, and Marijuana by Prenatal Alcohol Exposure Group and Trimester of Pregnancy
| Substance by Trimester | PAE Group | p-value | ||
|---|---|---|---|---|
| 0T | 1T | 3T | ||
| Alcohol (avg drinks/day) | ||||
| 1st trimester | 0 (0) | 1.6 (.38) | 2.47 (.72) | .001a |
| 3rd trimester | 0 (0) | 0 (0) | 2.14 (.41) | .001 |
| Tobacco (avg cigarettes/day) | ||||
| 1st trimester | 8.52 (3.26) | 8.40 (2.69) | 13.05 (5.16) | .653b |
| 3rd timester | 8.80 (3.47) | 8.70 (2.77) | 9.55 (3.89) | .986b |
| Marijuana (avg joints/day) | ||||
| 1st trimester | .63 (.43) | .95 (.40) | .74 (.29) | .846b |
| 3rd trimester | .69 (.48) | .12 (.06) | .93 (.32) | .374b |
First trimester alcohol exposure was not significantly different for the 1T and 3T groups (p = .117).
No statistically significant pairwise comparisons.
3.3. Caudate Volume
Whole brain, left, and right caudate volumes are summarized in Table 3. There were no statistically significant overall or pairwise differences in caudate volumebetween the PAE groups for whole brain, left or right caudates, while controlling for gender and handedness.
Table 3.
Mean Caudate (by side) and Whole Brain Volumes (Voxel Counts) and Standard Errors by Prenatal Alcohol Exposure Groups
| PAE Group | |||
|---|---|---|---|
| 0T (n=20) | 1T (n=15) | 3T (n=10) | |
| Left Caudate | 5943 (145) | 5924 (191) | 5619 (129) |
| Right Caudate | 6642 (194) | 6749 (263) | 6329 (203) |
| Whole Brain | 1156015 (26209) | 1180354 (30329) | 1097938 (31381) |
ANOVA’s control for sex and handedness:
F2,40=.786, p=.46 (all pariwse comparisons non-significant)
F2,40=.443, p=.65 (all pariwse comparisons non-significant)
F2,40=1.52, p=.23 (all pariwse comparisons non-significant)
3.4. Caudate Asymmetry
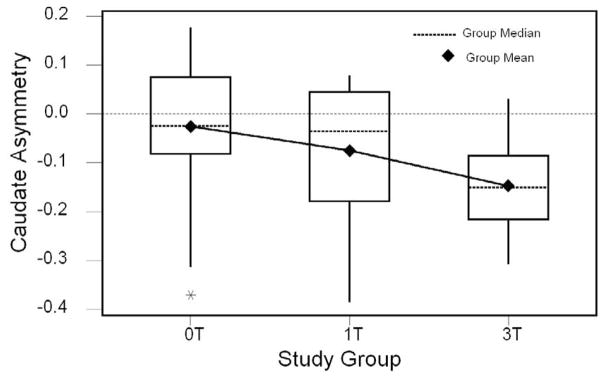
The means and medians for caudate asymmetry for each of the PAE groups are plotted in Figure 2. Observed means and standard errors are presented in Table 5. These data show evidence of a dose-response effect across the three exposure groups in both the magnitude and direction of caudate asymmetry. The 3T group had a statistically significant leftward bias in caudate asymmetry compared to the 0T group (F1,26 = 6.835, p = .018), controlling for gender and handedness. When the 1T group was added to the model, the level of significance became marginally significant (F2,40=2.84, p=.070). While the average magnitude of caudate asymmetry for the 1T group is intermediate between the 0T (p=0.29) and 3T (p=0.20) groups, it is not statistically different from either of these groups.
Figure 2.
Caudate asymmetry by study group: Medians, means and outliers shown.
Table 5.
General Linear Model for the Effects of PAE on Caudate Asymmetry, Controlling for Gender, Handedness, PME, and PTE
| Factor | Df | F-Value | P-Value |
|---|---|---|---|
| Gender | 1 | 0.932 | 0.341 |
| Handedness | 1 | 0.543 | 0.466 |
| PME | 2 | 1.200 | 0.313 |
| PTE | 2 | 0.315 | 0.732 |
| PAE (all groups)* | 2 | 3.276 | 0.049 |
| Error | 36 |
Pairwise: 0T=1T≠3T
3.5. Prenatal Tobacco and Marijuana Exposures
Prenatal tobacco exposure (PTE) was measured by average number of cigarettes per day (average daily cigarettes; ADC) and PME was measured by average number of joints per day (average daily joints, ADJ) throughout pregnancy. Since the PTE and PME variables were not normally distributed (Anderson-Darling test, p<.005), they were introduced into our model as categorical variables. ADJ was categorized as none (n=21), moderate (ADJ>0 and <1, mean=.34, n=12), or heavy (ADJ≥1, mean=2.3, n=8), following criteria used in earlier MHPCD studies (Goldschmidt et al., 2004). Average daily cigarette consumption (PTE) was categorized as none (n=17), moderate (ADC≤10, n=13), or heavy (ADC >10, n=15), using a median split for the active smokers.
The final model in Table 4 summarizes the effects of PAE on caudate asymmetry, controlling for PTE and PME as well as gender and handedness. The only statistically significant factor is PAE, which includes all three exposure groups (0T, 1T, 3T). PTE and PME were not individually associated with caudate asymmetry and none of the two-way interactions between the substance exposure variables (PAE, PTE, PME) was statistically significant. Adjusted means for caudate asymmetry are shown in Table 5. In a post-hoc comparisons, the difference between the 0T to 3T groups was statistically significant (p=.026), while both comparisons to the 1T group (0T p=.29, 3T p=.20) remained non-significant.
Table 4.
Observed and Adjusted Means (SEM) for Caudate Asymmetry by PAE Group
| PAE Group | |||
|---|---|---|---|
| 0T (n=20) | 1T (n=15) | 3T (n=10) | |
| Observed | −.026 (0.32) | −.075 (.036) | −.147 (.021) |
| Adjusteda | −.035 (.045) | −.101 (.054) | −.187 (.061) |
F2,40=.786, p=.46 (all pairwise comparisons non-significant), adjusting for covariates.
4. Discussion
The purpose of this study was to assess the effects of light to moderate prenatal alcohol exposure on two measures of brain structure; volume and asymmetry. The study was organized around three specific hypotheses. The first hypothesis was that the effects of light to moderate PAE would be detectable on measures of caudate volume and asymmetry. This was not confirmed for measures of brain volume: There were no differences in right or left caudate or whole brain volume between the three groups. There was, however, evidence that moderate PAE was significantly associated with differences in asymmetry in the caudate. Specifically, PAE was associated with a statistically significant leftward bias in caudate asymmetry among the continuously exposed (3T) study subjects.
The second hypothesis was that PAE would affect caudate volume and asymmetry as the magnitude of exposure increased among offspring of women who did not drink during pregnancy, the offspring of women who quit drinking after the first trimester, and the offspring of women who drank continuously throughout pregnancy. We demonstrated a significant linear association in the predicted direction (Figure 2, Table 4) that was driven largely by the contrast between the two most extreme exposure groups (0T vs. 3T), while the contrasts with the 1T group remained statistically non-significant.
The third hypothesis was that prenatal exposures to tobacco and marijuana would not significantly affect caudate volume and asymmetry and would not modify the primary effects of PAE on caudate volume and asymmetry. This hypothesis was confirmed. PTE and PME were not associated with caudate asymmetry nor did they interact with PAE to affect the PAE and asymmetry association.
This study and the work of Sowell et al. (2002), both demonstrate that regional asymmetries are a sensitive measure of changes in the structure and organization of the brain. The studies of Sowell have demonstrated this among heavily exposed subjects, while we have now extended this finding to show that the effects of PAE can be detected among those with low and moderate levels of PAE. Regional asymmetries can detect subtle differences in small brain regions that may otherwise be masked as the size or the unit of analysis increases. Regional asymmetries in the absence of measured differences in volume, may be due to changes in the distribution of cells and connections between the right and left caudate, so that the total caudate volume is similar (Galaburda et al., 1990; Rosen et al., 1992).
Anomalies associated with the interconnections within the caudate can adversely affect the larger network of brain regions that underlie complex cognitive behavior. Disruptions in neural circuitry can lead to poor coordination between individual brain regions and ultimately may affect the speed of processing (Kodituwakku 2007). Deficits in executive functions, which require the integration of many brain regions and place high demands on processing speed, are associated with FAS/FASD (Aragon et al., 2008; Burden et al., 2005; Rasmussen, 2005) and have been reported with exposure to low and moderate levels of PAE (Willford et al., under review).
Asymmetry in the caudate, could also develop as an adaptive response to changes in other brain regions. For example, PAE is associated with changes in the corpus callosum (CC) including decreased functional anisotropy in the genu and splenium (Ma et al., 2005; Sowell et al., 2008) and increased variability in the shape and location of the CC (Bookstein et al., 2002a, Bookstein et al., 2002b, Riley et al., 1995). The majority of interhemispheric cortical communication is through the CC, and reduced volume in the CC may result in constraints on the efficiency and speed of processing of information through this region. One adaptation that could occur would be increased reliance on information processing within the caudate (Zheng and Purves, 1995).
Potential limitations of this study warrant discussion. The sample sizes of the 1T and 3T groups were small, which limited the statistical power and the ability to evaluate higher- order interactions. This was especially important for the dose-response analysis (hypothesis 3). In addition, although we found changes in caudate symmetry, these data do not identify the specific location of the changes in the caudate.
The results of this study demonstrate a statistically significant effect of PAE on caudate asymmetry. The anatomical changes occurred at light and moderate levels of prenatal alcohol exposure. This new finding adds to earlier reports of effects on brain volume, chemistry, shape, and symmetry in heavily alcohol-exposed populations. The results suggest possible deficits in the functioning of the caudate after low to moderate PAE, although the current data cannot confirm this. Further work is needed to determine the functional significance of PAE-related increase in caudate asymmetry.
Acknowledgments
The expert technical assistance of Robert Tamburo PhD, Megan Nable, Matthew Zeglen, and Alan Ho is gratefully acknowledged. This work was supported by NIAAA grants AA013981(JA Willford, PI) and AA06666 (NL Day, PI) and NIDA grant 003874 (NL Day, PI).
Footnotes
Conflict of Interest Statement
There are no conflicts of interest for any of the authors of this report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aragón AS, Kalberg WO, Buckley D, Barela-Scott LM, Tabachnick BG, May PA. Neuropsychological study of FASD in a sample of American Indian children: processing simple versus complex information. Alcohol Clin Exp Res. 2008 doi: 10.1111/j.1530-0277.2008.00802.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev Med Child Neurol. 2001;43:148–154. [PubMed] [Google Scholar]
- 3.Bookstein FL, Sampson PD, Connor PD, Streissguth AP. Midline corpus callosum is a neuroanatomical focus of fetal alcohol damage. Anat Rec. 2002a;269:162–174. doi: 10.1002/ar.10110. [DOI] [PubMed] [Google Scholar]
- 4.Bookstein FL, Streissguth AP, Sampson PD, Connor PD, Barr HM. Corpus callosum shape and neuropsychological deficits in adult males with heavy fetal alcohol exposure. Neuroimage. 2002b;15:233–251. doi: 10.1006/nimg.2001.0977. [DOI] [PubMed] [Google Scholar]
- 5.Burden MJ, Jacobson SW, Jacobson JL. Relation of prenatal alcohol exposure to cognitive processing speed and efficiency in childhood. Alcohol Clin Exp Res. 2005;29:1473–1483. doi: 10.1097/01.alc.0000175036.34076.a0. [DOI] [PubMed] [Google Scholar]
- 6.Burden MJ, Jacobson SW, Sokol RJ, Jacobson JL. Effects of prenatal alcohol exposure on attention and working memory at 7.5 years of age. Alcohol Clin Exp Res. 2005;29:443–452. doi: 10.1097/01.alc.0000156125.50577.ec. [DOI] [PubMed] [Google Scholar]
- 7.Clark CM, Li D, Conry J, Conry R, Loock C. Structural and functional brain integrity of fetal alcohol syndrome in nonretarded cases. Pediatrics. 2000;105:1096–1099. doi: 10.1542/peds.105.5.1096. [DOI] [PubMed] [Google Scholar]
- 8.Collins DL, Zijdenbos AP, Kollokian V, Sled JG, Kabani NJ, Holmes CJ, Evans AC. Design and construction of a realistic digital brain phantom. IEEE Trans Med Imaging. 1998;17:463–468. doi: 10.1109/42.712135. [DOI] [PubMed] [Google Scholar]
- 9.Connor PD, Sampson PD, Bookstein FL, Barr HM, Streissguth AP. Direct and indirect effects of prenatal alcohol damage on executive function. Dev Neuropsychol. 2000;18:331–354. doi: 10.1207/S1532694204Connor. [DOI] [PubMed] [Google Scholar]
- 10.Crow TJ. Temporal lobe asymmetries as the key to the etiology of schizophrenia. Schizophr Bull. 1990;16:433–443. doi: 10.1093/schbul/16.3.433. [DOI] [PubMed] [Google Scholar]
- 11.Cortese BM, Moore GJ, Bailey BA, Jacobson SW, Delaney-Black V, Hannigan JH. Magnetic resonance and spectroscopic imaging in prenatal alcohol-exposed children: preliminary findings in the caudate nucleus. Neurotoxicol Teratol. 2006;28:597–606. doi: 10.1016/j.ntt.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Damasio H, Damasio A. Lesion Analysis in Neuropsychology. Oxford University Press; New York, NY: 1989. [Google Scholar]
- 13.Day NL, Cottreau CM, Richardson GA. The epidemiology of alcohol, marijuana, and cocaine use among women of child bearing age and pregnant women. Clin Obstet Gynecol. 1993;36:232–245. doi: 10.1097/00003081-199306000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Day NL, Leech SL, Richardson GA, Cornelius MD, Robles N, Larkby C. Prenatal alcohol exposure predicts discontinued deficits in offspring size at 14 years of age. Alcohol Clin Exp Res. 2002;26:1584–1591. doi: 10.1097/01.ALC.0000034036.75248.D9. [DOI] [PubMed] [Google Scholar]
- 15.Day NL, Robles N. Methodological issues in the measurement of substance use. Ann N Y Acad Sci. 1989;562:8–13. doi: 10.1111/j.1749-6632.1989.tb21002.x. [DOI] [PubMed] [Google Scholar]
- 16.Ebrahim SH, Luman ET, Floyd RL, Murphy CC, Bennett EM, Boyle CA. Alcohol consumption by pregnant women in the United States during 1988–1995. Obstet Gynecol. 1998;92:187–192. doi: 10.1016/s0029-7844(98)00205-1. [DOI] [PubMed] [Google Scholar]
- 17.Filipek PA. Neurobiological correlates of developmental dyslexia: how do dyslexics’ brains differ from those of normal readers? J Child Neurol. 1995;10:S62–S69. doi: 10.1177/08830738950100S113. [DOI] [PubMed] [Google Scholar]
- 18.Fryer SL, Tapert SF, Mattson SN, Paulus MP, Spadoni AD, Riley EP. Prenatal alcohol exposure affects frontal-striatal BOLD response during inhibitory control. Alcohol Clin Exp Res. 2007;31:1415–1424. doi: 10.1111/j.1530-0277.2007.00443.x. [DOI] [PubMed] [Google Scholar]
- 19.Galaburda AM, Rosen GD, Sherman GF. Individual variability in cortical organization: its relationship to brain laterality and implications to function. Neuropsychologia. 1990;28:529–546. doi: 10.1016/0028-3932(90)90032-j. [DOI] [PubMed] [Google Scholar]
- 20.Goldschmidt L, Richardson GA, Cornelius MD, Day NL. Prenatal marijuana and alcohol exposure and academic achievement at age 10. Neurotoxicol Teratol. 2004;26:521–532. doi: 10.1016/j.ntt.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Hendren RL, De Backer I, Pandina GJ. Review of neuroimaging studies of child and asolescent psychiatric disorders from the past 10 years. J Am Acad Child Adolesc Psychiatry. 2000;39:815–828. doi: 10.1097/00004583-200007000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Hynd GW, Hall J, Novey ES, Eliopulos D, Black K, Gonzalez JJ, Edmonds JE, Riccio C, Cohen M. Dyslexia and corpus callosum morphology. Arch Neurol. 1995;52:32–38. doi: 10.1001/archneur.1995.00540250036010. [DOI] [PubMed] [Google Scholar]
- 23.Kaemingk KL, Mulvaney S, Halverson PT. Learning following prenatal alcohol exposure: performance on verbal and visual multitrial tasks. Arch Clin Neuropsychol. 2003;18:33–47. [PubMed] [Google Scholar]
- 24.Kodituwakku PW. Defining the behavioral phenotype in children with fetal alcohol spectrum disorders: a review. Neurosci Biobehav Rev. 2007;31:192–201. doi: 10.1016/j.neubiorev.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 25.Kodituwakku PW, May PA, Clericuzio CL, Weers D. Emotion-related learning in individuals prenatally exposed to alcohol: an investigation of the relation between set shifting, extinction of responses, and behavior. Neuropsychologia. 2001;39:699–708. doi: 10.1016/s0028-3932(01)00002-1. [DOI] [PubMed] [Google Scholar]
- 26.Lee KT, Mattson SN, Riley EP. Classifying children with heavy prenatal alcohol exposure using measures of attention. J Int Neuropsychol. 2004;10:271–277. doi: 10.1017/S1355617704102142. [DOI] [PubMed] [Google Scholar]
- 27.Ma X, Coles CD, Lynch ME, LaConte SM, Zurkiya O, Wang D, Hu X. Evaluation of corpus callosum anisotropy in young adults with fetal alcohol syndrome according to diffusion tensor imaging. Alcohol Clin Exp Res. 2005;29:1214–1222. doi: 10.1097/01.alc.0000171934.22755.6d. [DOI] [PubMed] [Google Scholar]
- 28.Mattson SN, Calarco KE, Lang AR. Focused and shifting attention in children with heavy prenatal alcohol exposure. Neuropsychol. 2006;20:361–369. doi: 10.1037/0894-4105.20.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mattson SN, Goodman AM, Caine C, Delis DC, Riley EP. Executive functioning in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 1999;23:1808–1815. [PubMed] [Google Scholar]
- 30.Mattson SN, Riley EP, Delis DC, Stern C, Jones KL. Verbal learning and memory in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 1996;20:810–816. doi: 10.1111/j.1530-0277.1996.tb05256.x. [DOI] [PubMed] [Google Scholar]
- 31.Mattson SN, Riley EP, Jernigan TL, Ehlers CL, Delis DC, Jones KL. Neuropsychological comparison of alcohol-exposed children with or without physical features of fetal alcohol syndrome. Neuropsychology. 1998;12:146–153. doi: 10.1037//0894-4105.12.1.146. [DOI] [PubMed] [Google Scholar]
- 32.Mattson SN, Riley EP, Jernigan TL, Ehlers CI, Delis DC, Jones KL, Stern C, Johnson KA, Hesselink JR, Bellugi U. Fetal alcohol syndrome: a case report of neuropsychological, MRI and EEG assessment of two children. Alcohol Clin Exp Res. 1992;16:1001–1003. doi: 10.1111/j.1530-0277.1992.tb01909.x. [DOI] [PubMed] [Google Scholar]
- 33.Mattson SN, Riley EP, Jernigan TL, Garcia A, Kaneko WM, Ehlers CI, Jones KL. A decrease in the size of the basal ganglia following prenatal alcohol exposure: A preliminary report. Neurotoxicol Teratol. 1994;16:283–289. doi: 10.1016/0892-0362(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 34.Mattson SN, Riley EP, Sowell ER, Jernigan TL, Sobel DF, Jones KL. A decrease in the size of the basal ganglia in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 1996;20:1088–1093. doi: 10.1111/j.1530-0277.1996.tb01951.x. [DOI] [PubMed] [Google Scholar]
- 35.Mattson SN, Roebuck TM. Acquisition and retention of verbal and nonverbal information in children with heavy prenatal exposure. Alcohol Clin Exp Res. 2002;26:875–882. [PubMed] [Google Scholar]
- 36.McGee CL, Schonfeld AM, Roebuck-Spencer TM, Riley EP, Mattson SN. Children with heavy prenatal alcohol exposure demonstrate deficits on multiple measures of concept formation. Alcohol Clin Exp Res. 2008;32:1388–1397. doi: 10.1111/j.1530-0277.2008.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicol Teratol. 2007;29:141–152. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noland JS, Singer LT, Arendt RE, Minnes S, Short EJ, Bearer CF. Executive functioning in preschool-age children prenatally exposed to alcohol, cocaine, and marijuana. Alcohol Clin Exp Rex. 2003;27:647–656. doi: 10.1097/01.ALC.0000060525.10536.F6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pei JR, Rinaldi CM, Rasmussen C, Massey V, Massey D. Memory patterns of acquisition and retention of verbal and nonverbal information in children with fetal alcohol spectrum disorders. Can J Clin Pharmacol. 2008;15:e44–56. [PubMed] [Google Scholar]
- 40.Petty RG. Structural asymmetries of the human brain and their disturbance in schizophrenia. Schizophr Bull. 1999;25:121–139. doi: 10.1093/oxfordjournals.schbul.a033360. [DOI] [PubMed] [Google Scholar]
- 41.Rasmussen C. Executive functioning and working memory in fetal alcohol spectrum disorder. Alcohol Clin Exp Res. 2005;29:1359–1367. doi: 10.1097/01.alc.0000175040.91007.d0. [DOI] [PubMed] [Google Scholar]
- 42.Richardson GA, Ryan C, Willford J, Day NL, Goldschmidt L. Prenatal alcohol and marijuana exposure: effects on neuropsychological outcomes at 10 years. Neurotoxicol Teratol. 2002;24:309–320. doi: 10.1016/s0892-0362(02)00193-9. [DOI] [PubMed] [Google Scholar]
- 43.Riley EP, Mattson SN, Sowell ER, Jernigan TL, Sobel DG, Jones KL. Abnormalities of the corpus callsoum in children prenatally exposed to alcohol. Alcohol Clin Exp Res. 1995;19:1198–1202. doi: 10.1111/j.1530-0277.1995.tb01600.x. [DOI] [PubMed] [Google Scholar]
- 44.Roebuck-Spencer TM, Mattson SN. Implicit strategy affects learning in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 2004;28:1424–1431. doi: 10.1097/01.alc.0000139826.25247.5b. [DOI] [PubMed] [Google Scholar]
- 45.Rosen G. Cellular, morphometric, ontogenetic and connectional substrates of anatomical asymmetry. Neurosci Biobehav Rev. 1996;20:607–615. doi: 10.1016/0149-7634(95)00073-9. [DOI] [PubMed] [Google Scholar]
- 46.Rosen G, Sherman GF, Galaburda AM. Biological substrates of anatomic asymmetry. Prog Neurobiol. 1992;39:507–515. doi: 10.1016/0301-0082(92)90004-x. [DOI] [PubMed] [Google Scholar]
- 47.Sharma T, Lancaster E, Sigmundsson T, Lewis S, Takei N, Gurling H, Barta P, Pearlson G, Murray R. Lack of normal pattern of cerebral asymmetry in familial schizophrenic patients and their relatives—The Maudsley Family Study. Shizophr Res. 1999;40:111–120. doi: 10.1016/s0920-9964(99)00143-7. [DOI] [PubMed] [Google Scholar]
- 48.Sowell ER, Johnson A, Kan E, Lu LH, Van Horn JD, Toga AW, O’Connor MJ, Bookheimer SY. Mapping white matter integrity and neurobehavioral correlates in children with fetal alcohol spectrum disorders. J Neurosci. 2008a;28:1313–1319. doi: 10.1523/JNEUROSCI.5067-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sowell ER, Mattson SN, Kan E, Thompson PM, Riley EP, Toga AW. Abnormal cortical thickness and brain-behavior correlation patterns in individuals with heavy prenatal alcohol exposure. Cereb Cortex. 2008b;18:136–144. doi: 10.1093/cercor/bhm039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sowell ER, Mattson SN, Thompson PM, Jernigan TL, Riley EP, Toga AW. Mapping callosal morphology and cognitive correlates: effects of heavy prenatal alcohol exposure. Neurology. 2001;57:235–244. doi: 10.1212/wnl.57.2.235. [DOI] [PubMed] [Google Scholar]
- 51.Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, Toga AW. Regional brain shape abnormalities persist into adolescence after heavy prenatal alcohol exposure. Cereb Cortex. 2002;12:856–865. doi: 10.1093/cercor/12.8.856. [DOI] [PubMed] [Google Scholar]
- 52.Steel RGD, Torrie JH. Principals and Procedures of Statistics: A Biometrical Approach. McGraw-Hill; NY: 1980. [Google Scholar]
- 53.Stephens MA. EDF Statistics for Goodness of Fit and Some Comparisons. J Amer Stat Assoc. 1974;69:730–737. [Google Scholar]
- 54.Streissguth AP, Sampson PD, Olson HC, Bookstein FL, Barr HM, Scott M, Feldman J, Mirsky AF. Maternal drinking during pregnancy: Attention and short-term memory in 14-year old offspring – A longitudinal study. Alcohol Clin Exp Res. 1994;18:202–218. doi: 10.1111/j.1530-0277.1994.tb00904.x. [DOI] [PubMed] [Google Scholar]
- 55.Toga AW, Thompson PM. Mapping brain asymmetry. Nat Rev Neurosci. 2003;4:37–48. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- 56.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliet M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 57.Vaurio L, Riley EP, Mattson SN. Differences in executive functioning in children with heavy prenatal alcohol exposure or attention-deficit/hyperactivity disorder. Psychiatry Res. 2008;163:201–212. doi: 10.1017/S1355617708080144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watkins KE, Lerch JP, Zijdenbos A, Collins DL, Neelin P, Taylor J, Worsley KJ, Evans AC. Structural asymmetries in the human brain: a voxel-based statistical analysis of 142 MRI scans. Cereb Cortex. 2001;11:868–877. doi: 10.1093/cercor/11.9.868. [DOI] [PubMed] [Google Scholar]
- 59.Willford JA, Chandler L, Goldschmidt L, Day NL. Effects of prenatal tobacco and/or alcohol exposure on processing, visual-motor coordination, and interhemispheric transfer. doi: 10.1016/j.ntt.2010.06.004. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Willford JA, Richardson GA, Leech SL, Day NL. Attention mediates the relations between prenatal alcohol exposure and executive function. Presented at Neurobehavioral Teratology Society; 2002. [Google Scholar]
- 61.Willford JA, Richardson GA, Leech SL, Day NL. Verbal and visuospatial learning and memory function in children with moderate prenatal alcohol exposure. Alcohol Clin Exp Res. 2004;28:497–507. doi: 10.1097/01.alc.0000117868.97486.2d. [DOI] [PubMed] [Google Scholar]
- 62.Woods RP. Multitracer: a java-based tool for anatomic delineation of grayscale volumetric images. NeuroImage. 2003;19:829–1834. doi: 10.1016/s1053-8119(03)00243-x. [DOI] [PubMed] [Google Scholar]
- 63.Wu M, Carmichael O, Lopez-Garcia P, Carter CS, Aizenstein HJ. Quantitative comparison of AIR, SPM, and the fully deformable model for atlas-based segmentation of functional and structural MR images. Hum Brain Mapp. 2006;27:747–754. doi: 10.1002/hbm.20216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu M, Rosano C, Aizenstein H. A morphological method to improve skull stripping of MR brain images. Paper presented at the Human Brain Mapping Conference; Toronto, CA. 2005. [Google Scholar]
- 65.Zheng D, Purves D. Effects of increased neural activity on brain growth. Proc Natl Acad Sci USA. 1995;92:1802–1806. doi: 10.1073/pnas.92.6.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]