Abstract
There is an urgent need for animal models of autism spectrum disorder (ASD) to understand the underlying pathology and facilitate development and testing of new treatments. The synaptic growth-associated protein-43 (GAP43) has recently been identified as an autism candidate gene of interest. Our previous studies show many brain abnormalities in mice lacking one allele for GAP43 (GAP43 (+/−)) that are consistent with the disordered connectivity theory of ASD. Thus, we hypothesized that GAP43 (+/−) mice would demonstrate at least some autistic-like behaviors.
We found that GAP43 (+/−) mice, relative to wild-type (+/+) littermates, displayed resistance to change, consistent with one of the diagnostic critera for ASD. GAP43 (+/−) mice also displayed stress-induced behavioral withdrawal and anxiety, as seen in many autistic individuals. In addition, both GAP43 (+/−) mice and (+/+) littermates demonstrated low social approach and lack of preference for social novelty, consistent with another diagnostic criterion for ASD. This low sociability is likely due to the mixed C57BL/6J 129S3/SvImJ background.
We conclude that GAP43 deficiency leads to the development of a subset of autistic-like behaviors. Since these behaviors occur in a mouse that displays disordered connectivity, we propose that future anatomical and functional studies in this mouse may help uncover underlying mechanisms for these specific behaviors. Strain-specific low sociability may be advantageous in these studies, creating a more autistic-like environment for study of the GAP43-mediated deficits of resistance to change and vulnerability to stress.
Keywords: Autistic Disorder; Disease models, animal; Behavior, animal; GAP43; Mice, mutant strains; Maze learning; Reversal learning; Anxiety behavior; Social Behavior
INTRODUCTION
Autism spectrum disorder (ASD) involves impaired social interactions, communication deficiencies, and repetitive and stereotyped patterns of behavior (DSM IV). There is no single cause for ASD. Rather, it is an epigenetic phenomenon with numerous paths to a common outcome (Herbert, 2005, Minshew & Williams, 2007, Veenstra-Vanderweele et al., 2004).
One hypothesized path to ASD is abnormal development and function of cortical circuits (Belmonte et al., 2004, Rippon et al., 2007). A central concept of this “disordered connectivity theory” is decreased connections between specialized brain areas, with increased or maintained connections within areas (Just et al., 2004, Rippon et al., 2007). This over connection of local circuit axons could produce excessive excitability, a proposed neurological state in ASD (Rubenstein & Merzenich, 2003). A major contributor to disordered connectivity may be transient brain overgrowth, seen in many autistic children (Courchesne et al., 2001, Herbert, 2005).
Recently, growth-associated protein-43 (GAP43) has been identified as an autism candidate gene in the human gene region 3q13.2-q13.31 (Allen-Brady et al., 2009). Findings from this study, and others (Schellenberg et al., 2006, Szatmari et al., 2007, Trikalinos et al., 2006), suggest that genetic variants in this region may predispose males to broad spectrum ASD. GAP43 is found in growth cones of extending axons in the central nervous system (Meiri et al., 1986, Skene et al., 1986). Its many functions include growth cone navigation, neurite outgrowth, stabilization of axonal branches, neurotransmission and synaptic plasticity ((Denny, 2006).
Mice lacking one allele for GAP43 (GAP43 (+/−) mice) show multiple failures to establish or maintain long-distance cortical connections. These include poorly arborized thalamocortical axons with aberrant pathfinding (Mcilvain et al., 2003) and decreased corpus callosum and hippocampal commissure volumes (Shen et al., 2002). GAP43 (+/−) mice also display transient neonatal enlargement of barrel fields, which is normalized by adulthood (Mcilvain & McCasland, 2006, Mcilvain et al., 2003). Despite this anatomical recovery, adult GAP43 (+/−) mice show enlarged excitatory receptive fields (Dubroff et al., 2006). Taken together, these defects are consistent with both early brain overgrowth and disordered connectivity found in some individuals with ASD.
Mice lacking GAP43 (GAP43 (−/−) mice) also show interesting anatomical and functional deficits (Albright et al., 2007, Donovan et al., 2002, Donovan & Mccasland, 2008, Dubroff et al., 2006, Maier et al., 1999). However, the autistic-like findings of early overgrowth and disordered connectivity in GAP43 (+/−) mice cannot be confirmed in (−/−) mice due to high mortality rates (Maier et al., 1999). GAP43 (−/−) mice also display multiple sensorimotor deficits that would confound tests for autism-like behavior, none of which are observed in GAP43 (+/−) mice (Metz & Schwab, 2004). For these reasons, we focused this study on GAP43 (+/−) mice.
Taken together, neurological abnormalities and human genetic findings suggest that GAP43 (+/−) mice may model at least some aspects of the ASD behavioral phenotype. To determine the utility of this animal model, we performed comprehensive testing for autistic-like behaviors in GAP43 deficient mice.
MATERIALS AND METHODS
Animals
This study investigated 54 GAP43 (+/−) adult mice (3–9 months, male and female) in a mixed C57BL/6J 129S3/SvImJ (B6129S3) background, and 50 (+/+) littermate controls. Mice were generated as previously described (Maier et al., 1999). The initial mutation was bred in two strains - a 129S3/SvImJ strain and the progeny of a seventh-generation backcross into C57BL/6J. Since initial anatomical studies revealed no differences between the two strains (Maier et al., 1999), the lines were subsequently interbred for efficient colony maintenance. The current mixed background is a result of approximately five generations of interbreeding between these two lines. For the social behavior testing, six adult male C57BL/6J mice (B6, 2 months) were purchased from Jackson Laboratory (Bar Harbor, ME) and used as additional controls. Based on the availability of testing equipment, mice were tested at either State University of New York Upstate Medical University (SUNY Upstate) or the University of Texas Southwestern Medical Center (UT Southwestern), as detailed in Table 1.
Table 1.
Overview of behavioral tests performed and significant results. Tests for Group A/B were performed on a rotating schedule based on litter, with only one test being performed at a time. Tests for Group C/D were performed in order presented in table.
| Test | Evaluates | Animal Group | Significant Results |
|---|---|---|---|
| T-maze | Spatial learning Resistance to change |
Group A SUNY Upstate Total N=26 Male (+/+) N = 6; (+/−) N = 5 Female (+/+) N = 6; (+/−) N = 9 |
Learning Phase No differences between (+/−) and (+/+) mice Reversal phase Decreased number to attain reversal criteria in (+/−) mice Increased latency to attain reversal criteria in (+/−) mice Increased arm choice errors in (+/−) mice |
|
Morris Water Maze (MWM) |
Spatial learning Resistance to change |
Group B SUNY Upstate (Group A plus 2 additional litters) Total N=39 Male (+/+) N = 11; (+/−) N = 10 Female (+/+) N = 9; (+/−) N = 9 |
Habituation Phase Increased time spent in outer 25% of pool by (+/−) mice (thigmotaxis) Learning Phase Increased latency to find hidden platform in (+/−) mice in early, but not late, days of training Increased latency to reach learning criteria in (+/−) compared to (+/+) mice Reversal Phase No differences between (+/−) and (+/+) mice |
| Elevated Plus Maze (EPM) | Anxiety | Group B | No differences between (+/−) and (+/+) mice |
| Marble Burying | Repetitive behavior | Group D Total N=33 Male (+/+) N = 10; (+/−) N = 12 Female (+/+) N = 5; (+/−) N = 6 |
No differences between (+/−) and (+/+) mice |
|
Transmission of food preference Social (STFP) Non-social (NSTFP) |
Social communication |
Group B – STFP Group D – NSTFP |
No difference between (+/+) and (+/−) mice No difference in either genotype in ratio of cued food/total food eaten between social and non-social transmission |
|
Porsult Forced Swim Test (FST) |
Stress-induced behavioral withdrawal |
Group B | Decreased swimming/climbing in (+/−) compared to (+/+) Increased swimming/climbing in (+/+) males compared to (+/+) females |
| Social Approach | Social approach | Group C UT Southwestern N=32 Male (+/+) N = 7; (+/−) N = 9 Female (+/+) N = 8; (+/−) N = 8 B6 control group: N= 6 male |
Both (+/+) and (+/−) spent more time in interaction zone with target absent than with target present B6 controls spent more time in interaction zone with target present |
| Juvenile Interaction | Reciprocal social interactions, social memory |
Group C B6 control group |
No significant difference between (+/+) and (+/−) All mice decreased interaction during subsequent interaction |
|
3-Chambered Social Apparatus |
Social approach, object novelty, social novelty |
Group C B6 control group |
Neither (+/+) nor (+/−) mice spent more time with mouse vs. object in sociability trial or with novel vs. familiar mouse in social novelty trial B6 controls spent more time with mouse in sociability trial and more time with novel mouse in social novelty trial |
| Tail Suspension Test (TST) | Stress-induced behavioral withdrawal |
Group C | Decreased struggling in (+/−) compared to (+/+) mice |
| Locomotor Testing | Baseline activity | Group C | No differences between (+/−) and (+/+) mice |
| Light/Dark Test (LDT) | Anxiety | Group C | No differences between (+/−) and (+/+) mice |
| Fear Conditioning | Fear, learning & memory |
Group C | No differences between (+/−) and (+/+) mice |
All animals were handled in accordance with guidelines set forth by the Department of Laboratory Animal Resources at SUNY Upstate. Mice tested at SUNY Upstate were on a 14-hour light cycle (light on at 6 a.m.), and behavior tests were done between 8 a.m. and 4 p.m. after a minimum 30-minute period of habituation in the test room. Mice that were tested at UT Southwestern were shipped from SUNY Upstate at 3–5 months of age, and underwent 5 weeks of quarantine before testing. These mice were on a 12-hour light cycle (light on at 7 a.m.), and behavior tests were done between 8 a.m. and 5 p.m. after a minimum 30-minute period of habituation in the test room. All behavioral testing and scoring was done while blinded to animal genotype.
Behavioral Tasks
Learning and Memory
T-Maze
The T-maze test was performed to evaluate spatial learning and reversal learning, as previously described (Moy et al., 2007). Mice were habituated to the apparatus (15 cm entrance tube, 5 cm in diameter, split into two 35 cm tubes at right angles) during 10 minute trials for 6 days with a food reward (sweetened condensed milk – Wegmans, Rochester, NY) in both arms. Mice were then trained for 10 consecutive trials per day to receive the food reward from one arm of the T-maze (learning phase), with a brief pause between trials to replenish the food, if needed. Once subjects met the learning criteria of 80% correct arm choice for 3 consecutive training days, the food reward was moved into the opposite arm and the test was repeated (reversal phase). Reversal criteria were also 80% correct arm choice for 3 consecutive training days. Both the number of correct arm choices per day and days to attain learning and reversal criteria were measured. If a mouse did not meet learning criteria after 9 training days, it was excluded from the reversal phase. If a mouse did not achieve reversal criteria by day 9, it was assigned a 10 for “days to reach reversal criteria”.
Morris Water Maze (MWM)
The MWM task was performed to evaluate spatial learning and reversal learning, similar to previously described protocols (Crawley, 2004, Moy et al., 2007). A pool of 122 cm diameter, 91.5 cm height was filled with 20 cm of 26±2°C water. Four geometric spatial cues were placed around the walls of the pool in each quadrant (named NE, NW, SE, and SW).
For all mice, an initial probe trial was carried out in the open pool to habituate them to the apparatus, and ensure they were capable of swimming. These sessions were videotaped. Two litters (N=19) were randomly selected for post hoc analysis of swimming behavior to evaluate thigmotaxis (the tendency to stay near the edge of the pool). Traces of swimming were manually prepared, and time spent in the outer 25% of the tank was hand scored.
After habituation, mice were trained to locate a translucent platform of 12 cm diameter, covered by 1 cm of water, at a fixed location (SE quadrant). There were four trials per day, with 10–20 minutes between trials. The latency to find the platform and the number of days to reach learning criteria (average latency to find platform <15 seconds for 3 consecutive training days) were measured. After mice met learning criteria, a probe trial in an open pool was performed. If mice demonstrated spatial bias (≥40% of time in SE quadrant, and at least 10% more time than in any other quadrant), they moved onto the reversal phase. If a mouse did not exhibit bias, training continued. In the reversal phase, the platform was moved to the NW quadrant, and the latency to find the platform and number of days to reach learning criteria were measured. Probe trials for spatial bias were repeated. Each phase lasted for up to 9 days.
Fear Conditioning
Both context- and cue-dependent fear conditioning were completed to assess learning and memory using a contextual conditioning system (Med Associates, St. Albans,VT), as previously described (Powell et al., 2004). The chamber was 9.5in wide × 12in long × 5¾in tall, with clear plastic walls and ceilings and a floor with standard 4.8mm grid rods. The chamber was equipped with a light and a fan. For Trial 1 (training), mice were placed in the chamber with both the light and fan on. After a 2-minute habituation, a 30 second 90-dB tone that coterminated with a 2 second 0.5mA shock was delivered twice, with a 1-min interstimulus interval. Mice remained in the context for 2 minutes prior to return to home cage overnight. After 24 hours, Trial 2 assessed context learning by placing mice into the same context as training but in the absence of any shock. After 3 hours, Trial 3 assessed cue determined learning by placing mice in a novel context for a 3 minute baseline assessment, followed by 3 minute presentation of the 90-dB tone. To create a novel context the box size was reduced by 50%, made from a different type of plastic, scented with vanilla, and the box light and fan were removed. In each of these sessions, freezing behavior was scored manually by two independent observers (within 80% agreement) at 10 second intervals.
Repetitive Behavior
Marble Burying
Marble burying was performed to evaluate repetitive behaviors, as previously described (Thomas et al., 2009). Briefly, clean cages were filled with 4 cm of Sani-chip bedding (P.J. Murphy Forest Products, Montville, NJ). Eighteen 30mm blue marbles were placed in a 3×6 grid on top of the bedding. The number of marbles buried at least 50% by bedding were counted at the end of a 30-minute trial.
Social Behavior
Social Interaction
GAP43 (+/+) and (+/−) mice were evaluated for social interaction with a caged unfamiliar adult mouse, as previously described (Tabuchi et al., 2007). The apparatus was built to the following specifications – an open field (48cm × 48cm) containing a wire mesh cage (6.0cm × 9.5cm), with a defined “interaction zone” (15cm × 30cm) surrounding the wire cage. For trial 1, the wire cage was empty. For trial 2, the wire cage housed a novel adult B6 mouse. For both trials, mice were allowed to freely explore the open field for 5 minutes. Time spent in an “interaction zone” during each trial was quantified using Ethovision 2.3.19 videotracking software (Noldus, Leesburg, VA). The same protocol was followed for B6 controls, except that each session lasted 2.5 minutes (Lagace et al., 2010).
3-Chambered Social Interaction Test
The 3-chambered task was performed, as previously described (Crawley, 2004, Tabuchi et al., 2007). The apparatus was built to the following specifications – three 15 × 29 cm chambers, with empty wire cages in the 2 end chambers. During the habituation trial (10 minutes), mice were allowed to freely explore all three chambers. Next, during the sociability trial (10 minutes), mice were allowed to interact with a same-sex, unfamiliar B6 mouse in the wire cage in one end chamber, versus the still-empty wire cage in the other end chamber. Lastly, during the social novelty trial (10 minutes), the now familiar mouse remained in its cage, while a novel same-sex B6 stranger mouse was placed in the wire cage in the other end chamber. Locations of empty cages and target mouse, as well as novel vs. familiar mouse, were counterbalanced across subjects. Time spent in all three chambers was calculated for each trial, using Ethovision 2.3.19 videotracking software from (Noldus, Leeburg, VA).
Juvenile Interaction
A juvenile interaction paradigm was performed to evaluate social interaction and social memory, as previously described (Tabuchi et al., 2007). All mice were habituated for 15 minutes to dark with red light in testing room in home cage. Test mice were placed in a clean unfamiliar mouse cage with no bedding, with an unfamiliar 4 week old B6 mouse of the same sex for 2 minutes. Three days later, mice were again tested in the same condition, in a novel cage with the same juvenile mouse for a 2-minute trial. Time spent interacting (following, sniffing etc.) with the juvenile was measured.
Social Communication
Social Transmission of Food Preference (STFP)
The STFP test was performed to evaluate social communication, as previously described (McFarlane et al., 2008). Mice were habituated to feeding cup (4cm diameter, 3 cm high, glued in a Petri dish to collect spillage) filled with unflavored ground chow (PMI Nutrition, Brentwood, MO) for 3 days prior to the start of the test. After overnight food deprivation, sex-matched (+/+) adult demonstrator mice were given free access to ground chow, flavored either 1% cinnamon (McCormick, Hunt Valley, MD) or 2% cocoa (Hersey’s, Hersey PA) (split 50/50), for 1 hour. Demonstrator mice were then placed in a fresh cage with observer mice for a 30-minute interaction, and returned to their home cage. Observer mice were then food-deprived overnight. The next day they were placed in a clean cage and given free choice between cued food (flavor consumed by demonstrator) and non-cued food for 1 hour. To control for innate flavor preference, ~50% of the observers were cued to each flavor. After the trial, mice were returned to their homecage and the amount of each food consumed was measured. To control for appetite, a ratio of “cued food eaten/total food eaten” was calculated.
To ensure that we were actually measuring social transmission of food preference, a non-social transmission of food preference (NSTFP) control was performed. The same protocol was followed, with the exception that demonstrator mice were replaced with a novel object (cube built with children’s interlocking blocks – Mega Brands, Montreal QC, Canada). The cued flavored chow was packed between layers of blocks so that the scent could be detected, but the chow would not be accessible for consumption. Again, ~50% of the mice were cued to each flavor to control for innate flavor preference. This control was done to test if exposure to the odorant alone, without the social component, was adequate to convey flavor preference.
Anxiety and Stress-Induced Behavioral Withdrawal
Elevated Plus Maze (EPM)
The EPM was performed to evaluate anxiety, as previously described (Mulder & Pritchett, 2004). The apparatus was built to the following specifications - elevated 32 cm high, 2 open and 2 closed arms each 30 cm long, 2 cm wide, walls on closed arms 17 cm high. Mice were placed in the center of the apparatus and allowed to freely explore for 5 minutes. All sessions were videotaped. Time spent in each arm was measured. Data are presented as ratio of “time spent in open arm/(time spent in open arm + time spent in closed arm)” to control for any locomotor differences.
Light Dark Test (LDT)
The LDT was performed to evaluate anxiety, as previously described (Liu et al., 2007). The apparatus consisted of a light and a dark chamber, each 10 inches by 10 inches, separated by a wall with a small vestibule. The chambers were constructed and automated as described by Gershenfeld and Paul (1997). Briefly, the light chamber was open, transparent, and illuminated at 40 watts. The dark chamber was closed, painted black, and dark. Movements between the two chambers were detected by photocells. Mice were placed into the dark box for a 2-minute habituation. They were then allowed 10 minutes of access to the light box. The latency to scan the light box (head, but <4 paws, in light box), latency to completely emerge from the dark box, and time spent in light box were measured.
Porsult Forced Swim Test (FST)
The FST was performed to evaluate behavioral withdrawal induced by stress, as previously described (Krishnan et al., 2008, Nakasato et al., 2008). Mice were placed in a 1000 ml beaker (Corning Inc. Life Sciences, Lowell, MA), filled with 700 ml of 26±2°C water. After a 2-minute habituation, latency to immobility, time spent immobile (behavioral withdrawal), and time spent swimming/climbing (escape-oriented behavior) were measured for the next 4 minutes. The entire 6-minute session was videotaped.
Tail Suspension Test (TST)
The TST was performed to evaluate stress-induced behavioral withdrawal, as previously described (Liu et al., 2003). During a 6-minute trial, mice were suspended by their tail on an automated TST device (Med Associates Inc, St. Albans, VT). The device’s force transducer used pre-set thresholds to determine time spent immobile (behavioral withdrawal) and time spent struggling (escape-oriented behavior) for each mouse. These measurements were detected and calculated by the computer.
Baseline Activity
Locomotor Testing
Locomotor activity was assessed using photo beams linked to computer data acquisition software (San Diego Instruments, San Diego, CA), as previously described (Tabuchi et al., 2007). Mice were placed for 2 hours in a fresh home cage with minimal bedding. After allowing the mice to habituate for the first hour, horizontal activity and total distance moved was measured for the second hour and averaged into 5-minute bins.
Statistics
All measures are reported as mean ± SEM. The percent attaining reversal data from T-Maze was analyzed using Fisher’s exact probability test. For the FST, EPM, and LDT, data were analyzed using a two-way analysis of variance (anova) with factors of sex and genotype, followed by Tukey post hoc comparisons if a significant F-value was determined. For all other tests, differences between genotypes were analyzed using two-tailed Student’s t-test. All GAP43 (+/+) vs. (+/−) comparisons assumed unequal variances. Paired t-tests were used for within genotype comparisons (e.g. trial 1 vs. trial 2) for social paradigms.
RESULTS
General Appearance
There were no obvious differences in physical appearance between (+/+) and (+/−) littermates, consistent with previous reports (Metz & Schwab, 2004). There was no significant difference in weights between (+/+) and (+/−) mice (Groups B,C) − t69=0.65, p=0.52. Therefore, weight-matched controls were unnecessary. The average weight for (+/+) mice was 34.56±1.49g, and the average weight for (+/−) mice was 33.10±1.66g.
Behavior
Behavioral results are summarized in Table 1, and presented in detail below.
Learning and Memory
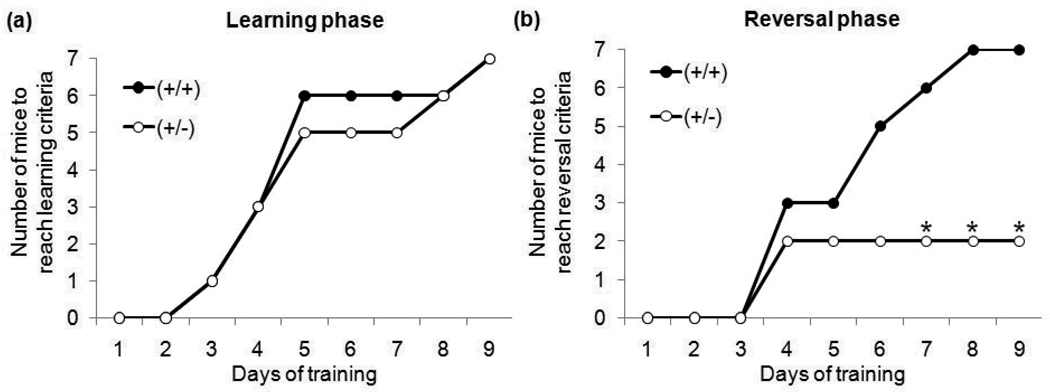
In the learning phase of the T-Maze test, there were no significant differences between GAP43 (+/+) and (+/−) mice in the 1) number of mice that met learning criteria (Figure 1a); 2) latency to reach learning criteria ((+/+) 5.43±0.84 days, (+/−) 5.00±0.72 days); or 3) average number of correct arm choices ((+/+) 8.10±0.70, (+/−) 8.32±0.56). Both genotypes had a ~50% failure in initial task acquisition, with no sex differences. Similar acquisition rates have been reported in other mouse strains (Moy et al., 2007). Only the 14 mice that met criteria in the learning phase were assessed for reversal learning.
Figure 1. Number of mice to reach passing criteria during (a) the learning phase and (b) the reversal phase of the T-maze task.
There was no difference in the number of GAP43 (+/+) and (+/−) mice to attained learning criteria within 9 days of training. However, significantly fewer GAP43 (+/−) than (+/+) mice attained reversal criteria within 9 days of training. (*p≤0.05)
In the reversal phase of the T-Maze test, GAP43 (+/−) mice displayed significantly impaired performance compared to (+/+) mice for 1) number of mice that met reversal criteria (Figure 1b) – Fisher exact probability test: df=1, p≤0.05; 2) latency to reach reversal criteria ((+/+) 5.29±0.57 days, (+/−) 8.29±1.11 days)- t12=2.31, p≤0.05; and 3) average number of correct arm choices ((+/+) 8.10±.039, (+/−) 4.43±1.37) − t12=2.35, p≤0.05.
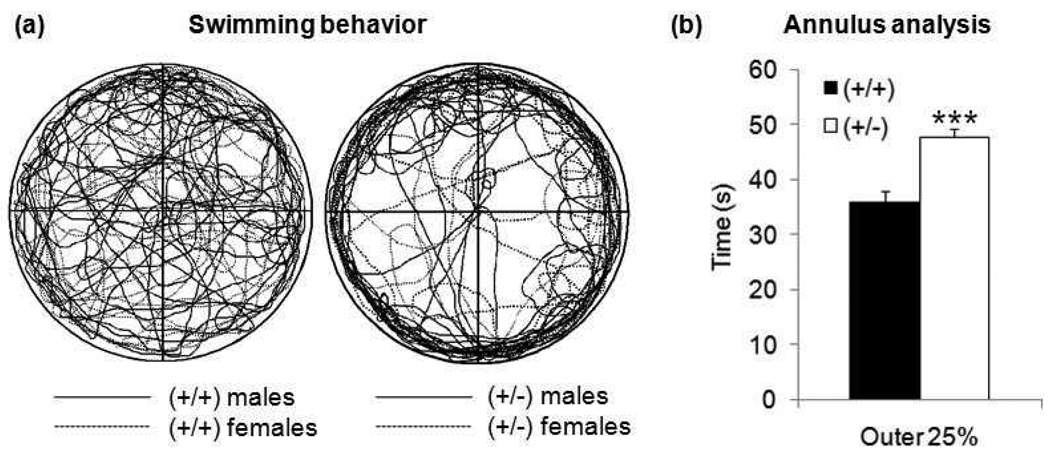
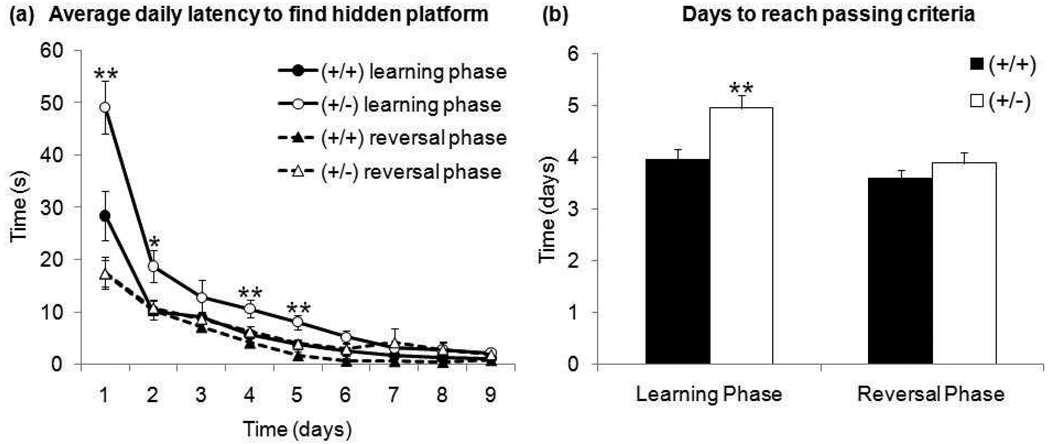
As an additional assessment for spatial and reversal learning, GAP43 (+/+) and (+/−) mice also completed the Morris water maze (MWM). There was no apparent difference in swimming capabilities between genotypes in the initial probe trial in the open pool with no escape platform. However, there was a difference in swimming behavior. GAP43 (+/−) mice displayed increased thigmotaxis (Figure 2a), spending significantly more time than (+/+) mice in the outer 25% of the tank’s diameter (Figure 2b) − t17=4.83, p≤0.001. Considering this behavior, it was not surprising that GAP43 (+/−) mice had significantly increased latencies to find the hidden platform (located toward the center of the pool) during early days of training (Figure 3a)- day 1 t38=3.02, p≤0.01, day 2 t38=2.56, p≤0.05, day 4 t38=2.72, p≤0.01, day 5 t38=2.69, p≤0.01. This increase in latency resulted in GAP43 (+/−) mice taking significantly more days to attain learning criteria than (+/+) mice (Figure 3b) - t38=3.00, p≤0.01.
Figure 2. (a) Qualitative and (b) quantitative analysis of swimming behavior during initial probe trial of MWM.
Composite traces of swimming behavior of all mice showed that (+/−) mice had a strong tendency to swim near the edge of the pool (thigmotaxis). Quantitative annulus analysis revealed that GAP43 (+/−) mice spent significantly more time in the outer 25% of the pool than (+/+) mice. (***p≤0.001)
Figure 3. (a) Average daily latency to find hidden platform and (b) days to reach passing criteria during the learning and reversal phases of MWM.
GAP43 (+/−) mice take significantly longer to locate the hidden platform during early, but not late, training days during the learning phase. GAP43 (+/−) mice also take significantly longer than (+/+) mice to reach passing criteria during the learning phase. However, there is no difference between genotypes in daily latency to find hidden platform or in days to reach passing criteria during the reversal phase. (*p≤0.05,** p≤0.01)
Mice that attained learning criteria showed no genotype difference in percent time spent in correct quadrant during the test probe trial - (+/+) 39.2±2.1%, (+/−) 37.8±1.7%. Five mice – (+/+) 3 females, (+/−) 1 female, 1 male – did not display spatial bias after training, and were excluded from the reversal phase. In the reversal phase, there was no significant genotype difference in latency to find the platform on any day between genotypes (Figure 3a). There was also no difference between genotypes in the number of days to reach reversal criteria (Figure 3b). Mice that attained reversal criteria showed no genotype difference in percent time spent in correct quadrant during the test probe trial - (+/+) 41.3±2.5%, (+/−) 40.7±0.3%. Two male (+/−) mice did not display spatial bias after reversal training.
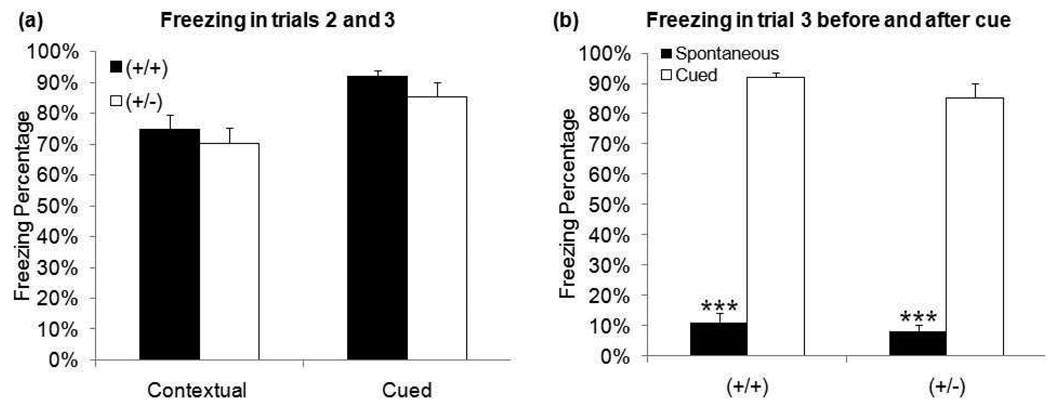
In addition to spatial learning, fear conditioning was tested to assess both context- and cue-dependent memory. Both genotypes demonstrated low levels of unconditioned freezing in Trial 1 (<5%) and spontaneous freezing (freezing before tone presentation) in Trial 3 (<15%). There was no difference between genotypes in either contextual or cued freezing percentage (Figure 4a). Both genotypes displayed a significant increase between spontaneous and cued freezing in Trial 3 (Figure 4b) - (+/+) t32=10.84, p≤0.001, (+/−) t32=8.94, p≤0.001.
Figure 4. Freezing behavior during fear conditioning task.
(a) There is no difference in either contextual (trial 2) or cued (trial 3) freezing between genotypes. (b) Both genotypes displayed significantly less spontaneous freezing than cued freezing in trial 3. (***p≤0.001)
Stress-Induced Behavioral Withdrawal
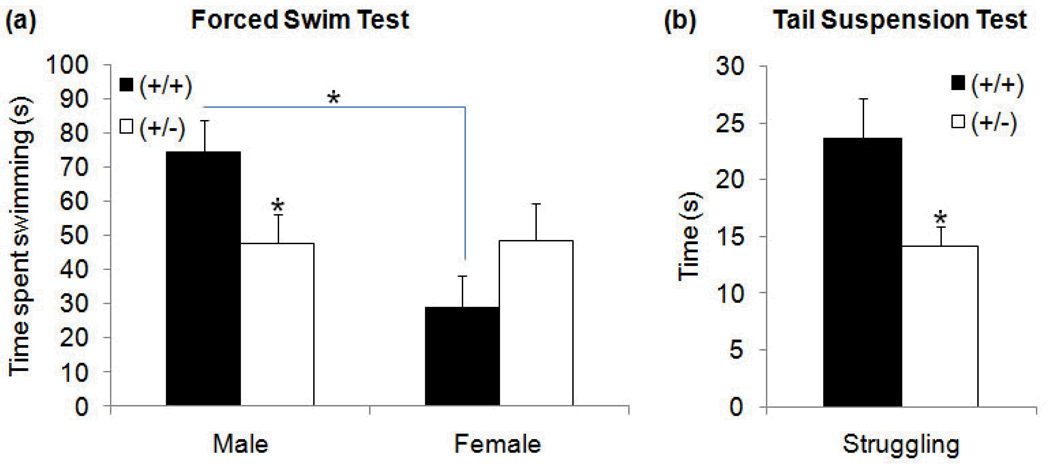
Significant sex × genotype differences in escape oriented (swimming/climbing) behavior were observed during the FST − F1,36=5.67, p≤0.05. Tukey post hoc comparisons revealed two sources of significant difference. GAP43 (+/−) males, but not females, displayed a significant decrease in time spent swimming/climbing compared to (+/+) counterparts (Figure 5a) − p≤0.05. In addition, GAP43 (+/+) males exhibited significantly more swimming/climbing (escape oriented behavior) than (+/+) females (Figure 5a) − p≤0.05. This sex difference was not seen between (+/−) males and females - p=.95. These findings were not associated with any significant differences in latency to immobility (male: (+/+) 26.45±12.12s, (+/−) 18.33±4.58s; female: (+/+) 19.89±12.21s, (+/−) 14.63±5.87s) or time spent immobile (male: (+/+) 92.45±10.09s, (+/−) 119.33±8.17s; female: (+/+) 103.00±12.96s, (+/−) 86.50±14.91s).
Figure 5. Time spent engaged in escape-oriented behavior during (a) FST and (b) TST.
In the FST, GAP43 (+/−) males displayed significantly decreased active swimming than (+/+) males. This difference was not seen between (+/−) and (+/+) females. GAP43 (+/+) mice displayed sex differences, with (+/+) males spending significantly more time engaged in active swimming than (+/+) females. In the TST, GAP43 (+/−) mice displayed significantly decreased struggling compared to (+/+) mice. There were no sex differences in the TST. (*p≤0.05,**p≤0.01)
In the tail-suspension test (TST), GAP43 (+/−) mice demonstrated a significant decrease in time engaged in struggling relative to (+/+) mice (Figure 5b) − t31=2.28, p≤0.05. This occurred in the absence of any significant difference in time spent immobile ((+/+) 274.14±6.60s, (+/−) 286.21±5.12s). There were no sex-specific effects detected in any of the TST measures.
Anxiety
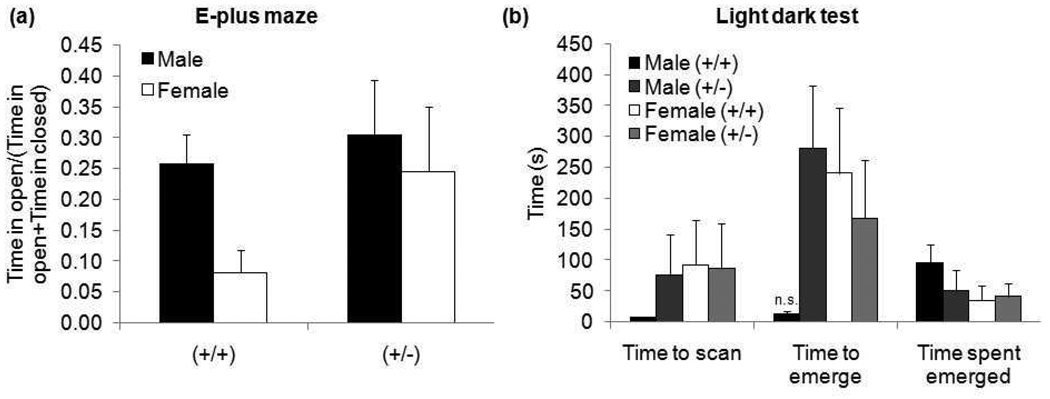
In the elevated plus maze (EPM) task, there appears to be a qualitative difference in performance between (+/+) males and females in the ratio of time in open arm/(time in open + closed arms) (Figure 6a). However, differences between groups were not statistically significant.
Figure 6. Results from (a) EPM and (b) LDT.
EPM data is presented as a ratio of time spent in the open arm to total time spent in all arms to correct for differences in locomotion. There were no significant differences between groups. LDT testing showed a near-significant trend with (+/+) males fully emerging more quickly than other groups. There were no significant differences in the other measures. (n.s. p=0.056)
In the light dark test (LDT), there was a near-significant sex × genotype effect in time to emerge (F1,33=3.94, p=0.056). GAP43 (+/+) males were much faster to emerge than any other group. There were no significant differences in time to scan or time spent fully emerged between groups (Figure 6b).
Social Behavior
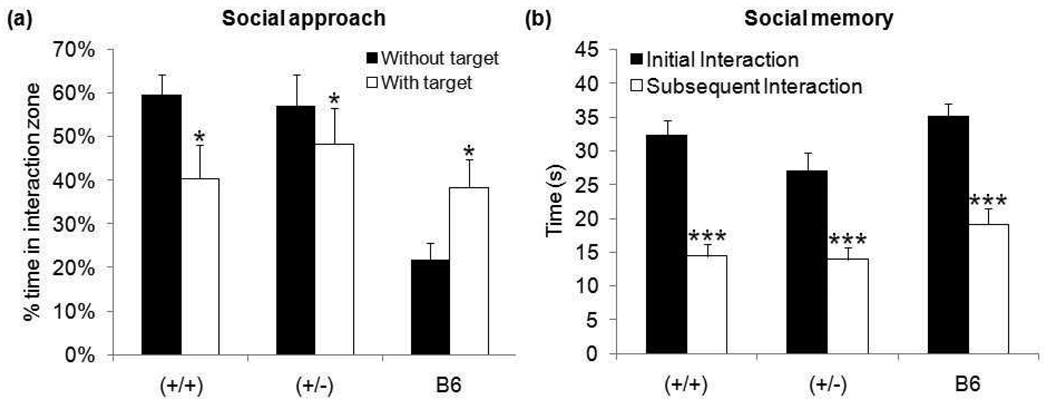
In the social interaction task with adults, B6 controls showed the expected social behavior (Crawley, 2004) by spending significantly more time in the interaction zone with a social target present − t10=2.18, p≤0.05. In contrast, both GAP43 (+/+) and (+/−) mice spent significantly more time in the interaction zone during the habituation phase (empty cage) than they did during the social approach phase (social target in cage) − (+/+) t32=2.37, p≤0.05, (+/−) t32=2.00, p≤0.05 – (Figure 7a). There was no genotype difference on this test in our mixed strain.
Figure 7. Behavioral measures of (a) social approach and (b) social memory.
Both GAP43 (+/+) and (+/−) mice spent a significantly greater percent of time in the interaction zone when the target mouse was absent than when the target mouse was present during the social approach paradigm. In contrast, B6 controls spent a significantly greater percent of time in the interaction zone when the target mouse is present. All mice decreased interaction time upon re-exposure to a juvenile mouse. (*p≤0.05,***p≤0.001)
There was also no significant difference for interaction time with a juvenile between GAP43 (+/+) and (+/−) mice during either initial or subsequent exposure. Both genotypes, as well as B6 controls, displayed a significant decrease in social interaction on second exposure - (+/+) t32=5.82, p≤0.001, (+/−) t32=4.31, p≤0.001, B6 t10=5.44, p≤0.001 (Figure 7b).
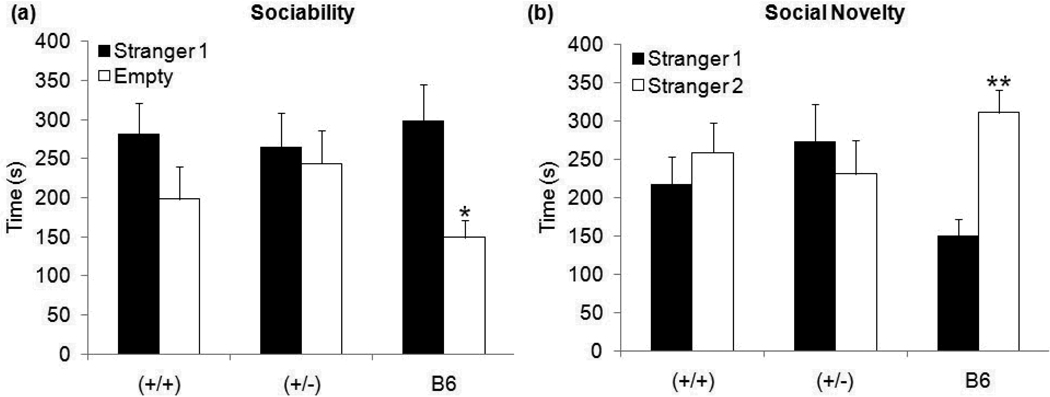
During the sociability trial of 3-chambered social interaction test, B6 controls displayed the expected behavior (Moy et al., 2009) by spending significantly more time with a stranger mouse (labeled as stranger 1) than in chamber with an empty cage − t10=2.82, p≤0.05. In contrast, neither GAP43 (+/+) nor (+/−) mice showed a significant preference for the stranger mouse rather than an inanimate object (Figure 8a). Similarly, in the social novelty trial of the 3-chambered test, B6 controls displayed the expected preference (Moy et al., 2009) by spending significantly more time in the chamber with stranger 1 (now familiar mouse) than in the chamber with stranger 2 (novel mouse) − t10=4.27, p≤0.01. In contrast, once again, neither GAP43 (+/+) nor (+/−) mice showed a significant preference for the novel mouse (Figure 8b). There was no genotype difference in any trial of the 3-chambered social interaction test in our mixed strain.
Figure 8. Time spent in outer chambers during the (a) sociability trial and (b) social novelty trial of the 3-chambered apparatus test.
During the sociability trial, neither GAP43 (+/+) nor (+/−) mice spent more time in the chamber containing stranger 1 than the chamber without a target mouse. In contrast, B6 controls spent significantly more time in the chamber containing stranger 1. Similarly, in the social novelty trial, neither GAP43 (+/+) nor (+/−) mice spent more time in the chamber containing stranger 1 (familiar mouse) than the chamber containing stranger 2 (novel mouse). In contrast, B6 controls spent significantly more time in the chamber containing stranger 2 (novel mouse). (*p≤0.05,**p≤0.01)
These effects occurred in the absence of any significant differences in the analysis of locomotion in GAP43 (+/+) and (+/−) mice, which revealed similar overall distance beam breaks after habituation to a novel home cage - (+/+) 42.94±4.39, (+/−) 57.35±11.73).
Social Communication
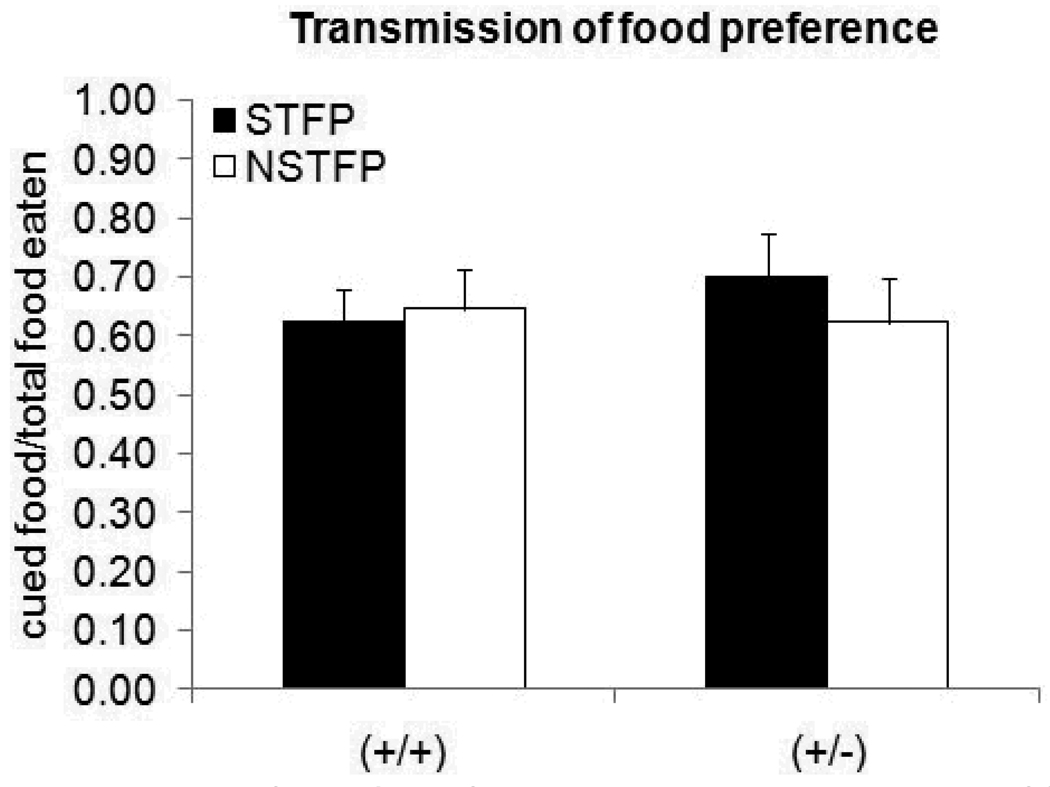
There were no differences in the social transmission of food preference (STFP) task between genotypes. Both GAP43 (+/+) and (+/−) mice had a “cued food eaten/total food eaten” ratio greater than .50, indicating that they consumed more cued than non-cued food. However, both genotypes also had a “cued food eaten/total food eaten” ratio greater than .50 in the non-social transmission of food preference (NSTFP) control task. There was no difference in this ratio in either genotype between the STFP and NSTFP tasks (Figure 9) − (+/+) t33=0,21 p=0.83, (+/−) t30=0.44 p=0.78.
Figure 9. Ratio of cued/total food eaten during transmission of food preference tasks.
There was no difference in the ratio of cued food eaten between genotypes in either the social transmission (STFP) or non-social transmission (NSTFP) tasks.
Repetitive Behavior
In the marble burying task, there was no difference between GAP43 (+/+) and (+/−) mice in the number of marbles buried ((+/+) 10.33±1.52, (+/−) 8.78±1.19).
DISCUSSION
Autism affects an estimated 1% of children in the United States (Kogan et al., 2009, Mulvihill et al., 2009). Animal models are needed to better understand its disease processes and develop new treatment strategies for this growing population. Here, we present findings that a specific subset of autistic-like behaviors is mediated by GAP43 deficiency in our mice.
GAP43 (+/−) mice display resistance to change
Individuals with ASD demonstrate resistance to change and/or repetitive behaviors (DSM-IV). In the T-maze, GAP43 (+/−) mice displayed clear deficits in reversal learning, indicating resistance to change (Crawley, 2004, Moy et al., 2007). However, they did not show this deficit in the MWM. This difference may be due to task/reward variables that more strongly motivate reversal learning in the MWM. The T-maze rewarded correct choices, but did not punish incorrect ones. However, failure in the MWM task led to the aversive outcome of remaining in the water. This prolonged period of immersion was a strong motivation for mice to find the hidden platform, regardless of location. We propose that resistance to change in GAP43 (+/−) mice is overcome in the MWM due to the stronger aversive consequence of failure.
While GAP43 (+/−) mice demonstrated resistance to change, they did not exhibit repetitive behavior, at least as measured by marble burying. However, a diagnosis of autism does not require both behaviors, as they are grouped into one diagnostic domain (DSM-IV).
GAP43 (+/−) mice display increased stress-induced behavioral withdrawal and anxiety
Many autistic individuals show depression and social withdrawal in response to stress (Pearson et al., 2006). Decreased struggling by GAP43 (+/−) mice in both the FST and TST indicate a lack of escape-oriented behavior, suggesting stress-induced behavioral withdrawal (Lucki, 1997, Nakasato et al., 2008). Interestingly, in the FST, this decrease was only observed in GAP43 (+/−) males. These data are especially intriguing because autism is ~4× more common in males (Kogan et al., 2009, Mulvihill et al., 2009). Although GAP43 is an autosomal gene, this sex-specific finding is consistent with the finding that sex steroids modulate GAP43 mRNA levels in some postnatal brain regions (Shughrue & Dorsa, 1993, Shughrue & Dorsa, 1994).
There is no evidence for increased baseline anxiety in GAP43 (+/−) mice from the EPM and LDT tasks. However, when challenged with the stress of being placed in water (Nakasato et al., 2008), increased thigmotaxis in the MWM suggests that GAP43 (+/−) have heightened stress-induced anxiety compared to (+/+) mice. This evidence, along with decreased escape-oriented behaviors in the FST and TST, indicate that GAP43 (+/−) mice, especially males, are less able to cope with stress than (+/+) mice. Increased vulnerability to stress has been shown in many individuals with autism, and may substantially contribute to autistic behaviors (Groden et al., 1994).
Spatial learning is modulated by heightened anxiety in GAP43 (+/−) mice
We evaluated spatial learning in GAP43 (+/−) mice, since deficiencies have been reported in many autistic individuals (Steele et al., 2007). GAP43 (+/−) mice did not display impaired learning compared to (+/+) in T-maze. However, GAP43 (+/−) mice displayed learning delays in the MWM compared to (+/+) littermates. This is probably due to modification of learning by the observed stress-induced anxiety, as mentioned above. We hypothesize that longer search times for (+/−) mice to find the hidden platform on early days of training were caused by initial reluctance to leave the side of the pool due to heightened anxiety. This modulation of behavior by anxiety creates an apparent spatial learning deficit that is probably not due to a cognitive defect.
Both GAP43 (+/−) mice and (+/+) littermates display decreased social interaction
Individuals with autism demonstrate low social interaction and decreased social communication (DSM-IV). Social mice prefer interaction with other mice over inanimate objects, and novel mice over familiar mice (Bolivar et al., 2007, Moy et al., 2009, Moy et al., 2007). While our B6 control group displayed the expected social behavior, neither GAP43 (+/−) nor (+/+) mice displayed high social approach to a stranger or preference for social novelty. This lack of normal social behavior is not due to impaired social memory, since both GAP43 (+/+) and (+/−) demonstrated memory for a juvenile mouse upon re-exposure.
Since both (+/−) and (+/+) demonstrated low sociability, we cannot determine GAP43’s role in social behavior. The observed social deficits are likely due to strain-specific low sociability of the mixed B6129S3 background, as different strains exhibit varying levels of social behavior (Moy et al., 2007). Future backcross studies into the highly social C57BL/6J background will determine whether GAP43 dependent social deficits are being masked by B6129S3 strain-specific low sociability.
In the STFP task, both GAP43 (+/+) and (+/−) mice consumed a greater ratio of cued:non-cued food, indicating intact social communication (Galef & Whiskin, 1995). However, this also occurred when the “demonstrator” was a novel object, as opposed to a stranger mouse. These results suggest that the olfactory cue can be transmitted from an inanimate object, and that the social component is not necessary. In both paradigms, it is likely that decreased consumption of the non-cued food was a result of neophobia. Future pup vocalization studies (Crawley, 2004) are planned to more directly measure if GAP43 deficiency affects social communication.
Altered synaptogenesis may contribute to abnormal GAP43 (+/−) mouse behavior
Taken together, our published studies provide strong evidence for disordered connectivity in GAP43 (+/−) mice (Dubroff et al., 2006, Mcilvain & McCasland, 2006, Mcilvain et al., 2003, Shen et al., 2002). It has been proposed that disordered connectivity is the common aberrant neurological finding resulting from the various insults that lead to the development of ASD (Belmonte et al., 2004, Just et al., 2004, Rippon et al., 2007). This theory proposes that observed early brain overgrowth in autistic individuals (Courchesne et al., 2001, Herbert, 2005) coincides with the peak of synaptogenesis and myelination, preventing formation of proper connections (Rippon et al., 2007). Thus, it is likely that abnormal synaptic development and elimination contribute to the development of autistic behavior (Pfeiffer & Huber, 2009, Zoghbi, 2003).
We propose that abnormal behavior in GAP43 (+/−) mice could be due in part to aberrant synaptogenesis. We have recently shown that these mice display early abnormalities in glutamate receptor composition and dendritic organization in the cortex subsequent to sparse thalamocortical input (Kelly et al., 2010). Interestingly, both deficits are then normalized by compensatory changes. These findings emphasize the importance of complex interplay between pre- and postsynaptic elements for proper development of cortical networks (Kelly et al., 2010).
Further study of altered GAP43 (+/−) synaptic development may reveal novel treatment strategies for the specific autistic-like behaviors observed. Planned experiments will test whether mGluR5 antagonists, which affect NMDA receptor function (Lea et al., 2002), can reduce these abnormal behaviors. This drug treatment has been shown to be effective for reducing repetitive behaviors in the BTBR T+tf/J mouse model of autism (Silverman et al., 2010).
Utilizing the B6129B3 GAP43 (+/−) mouse to study ASD
Since autism is a disorder of human behavior, there can never be a truly “autistic” mouse. Nevertheless, mice with abnormal behaviors that parallel human ASD can serve as valid animal models (Crawley, 2004). However, the behavioral profile of ASD is extremely complex, and likely has multiple underlying causes (Happe et al., 2006). Therefore, modeling and studying all aspects in one mouse model is proving very difficult, and may not be advantageous.
We have isolated specific autistic-like behaviors that can be attributed to GAP43 deficiency, including resistance to change and stress-induced behavioral withdrawal and anxiety. Since these behaviors occur in a mouse that displays disordered connectivity, we propose that future anatomical and functional studies in this mouse may help uncover underlying mechanisms for these specific behaviors. In addition, our mice exhibit strain-specific low sociability, similar to the BTBR T+tf/J mouse model of autism (McFarlane et al., 2008, Moy et al., 2009, Moy et al., 2007). This inherent deficit in sociability can be used to our advantage, creating a more autistic-like environment in which to study these GAP43-mediated behavioral deficits.
ACKNOWLEDGEMENTS
This work was supported by NIH Grant RO1NS40779, Golisano Children’s Hospital/Children’s Miracle Network, SUNY Upstate Intramural Pilot Grant, and NARSAD 2008 Young Investigator Award. We thank Dr. Shveta Malhotra for expert technical assistance with the behavioral testing, Dr. Sandra Mooney for statistical assistance, Ginny Grieb for expert technical assistance and colony maintenance, and Dr. Valerie Bolivar for critical reading of the manuscript.
REFERENCES
- Albright MJ, Weston MC, Inan M, Rosenmund C, Crair MC. Increased Thalamocortical Synaptic Response and Decreased Layer IV Innervation in GAP-43 Knockout Mice. J Neurophysiol. 2007;98:1610–1625. doi: 10.1152/jn.00219.2007. [DOI] [PubMed] [Google Scholar]
- Allen-Brady K, Miller J, Matsunami N, Stevens J, Block H, Farley M, Krasny L, Pingree C, Lainhart J, Leppert M, McMahon WM, Coon H. A high-density SNP genome-wide linkage scan in a large autism extended pedigree. Mol Psychiatry. 2009;14:590–600. doi: 10.1038/mp.2008.14. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. J Neurosci. 2004;24:9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar VJ, Walters SR, Phoenix JL. Assessing autism-like behavior in mice: variations in social interactions among inbred strains. Behav Brain Res. 2007;176:21–26. doi: 10.1016/j.bbr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, Chisum HJ, Moses P, Pierce K, Lord C, Lincoln AJ, Pizzo S, Schreibman L, Haas RH, Akshoomoff NA, Courchesne RY. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Designing mouse behavioral tasks relevant to autistic-like behaviors. Ment Retard Dev Disabil Res Rev. 2004;10:248–258. doi: 10.1002/mrdd.20039. [DOI] [PubMed] [Google Scholar]
- Denny JB. Molecular Mechanisms, Biological Actions, and Neuropharmacology of the Growth-Associated Protein GAP-43. Curr Neuropharmacol. 2006;4:293–304. doi: 10.2174/157015906778520782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan SL, Mamounas LA, Andrews AM, Blue ME, McCasland JS. GAP-43 is critical for normal development of the serotonergic innervation in forebrain. J Neurosci. 2002;22:3543–3552. doi: 10.1523/JNEUROSCI.22-09-03543.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan SL, McCasland JS. GAP-43 is critical for normal targeting of thalamocortical and corticothalamic, but not trigeminothalamic axons in the whisker barrel system. Somatosens Mot Res. 2008;25:33–47. doi: 10.1080/08990220701830696. [DOI] [PubMed] [Google Scholar]
- Dubroff JG, Stevens RT, Hitt J, Hodge CJ, Jr, McCasland JS. Anomalous functional organization of barrel cortex in GAP-43 deficient mice. Neuroimage. 2006;29:1040–1048. doi: 10.1016/j.neuroimage.2005.08.054. [DOI] [PubMed] [Google Scholar]
- Galef BG, Jr, Whiskin EE. Learning socially to eat more of one food than of another. J Comp Psychol. 1995;109:99–101. doi: 10.1037/0735-7036.109.1.99. [DOI] [PubMed] [Google Scholar]
- Gershenfeld HK, Paul SM. Mapping quantitative trait loci for fear-like behaviors in mice. Genomics. 1997;46:1–8. doi: 10.1006/geno.1997.5002. [DOI] [PubMed] [Google Scholar]
- Groden J, Cautela JR, Prince S, Berryman J. The impact of stress and anxiety on individuals with autism and developmental disabilities. In: Schopler E, Mesibov GB, editors. Behavioral issues in autism. New York: Plenum Press; 1994. pp. 177–194. [Google Scholar]
- Happe F, Ronald A, Plomin R. Time to give up on a single explanation for autism. Nat Neurosci. 2006;9:1218–1220. doi: 10.1038/nn1770. [DOI] [PubMed] [Google Scholar]
- Herbert MR. Large brains in autism: the challenge of pervasive abnormality. Neuroscientist. 2005;11:417–440. doi: 10.1177/0091270005278866. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kelly EA, Tremblay ME, McCasland JS, Majewska AK. Postsynaptic Deregulation in GAP-43 Heterozygous Mouse Barrel Cortex. Cereb Cortex. 2010;20:1696–1707. doi: 10.1093/cercor/bhp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan MD, Blumberg SJ, Schieve LA, Boyle CA, Perrin JM, Ghandour RM, Singh GK, Strickland BB, Trevathan E, van Dyck PC. Prevalence of Parent-Reported Diagnosis of Autism Spectrum Disorder Among Children in the US, 2007. Pediatrics. 2009;124:1395–1403. doi: 10.1542/peds.2009-1522. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Graham A, Mazei-Robison MS, Lagace DC, Kim KS, Birnbaum S, Eisch AJ, Han PL, Storm DR, Zachariou V, Nestler EJ. Calcium-sensitive adenylyl cyclases in depression and anxiety: behavioral and biochemical consequences of isoform targeting. Biol Psychiatry. 2008;64:336–343. doi: 10.1016/j.biopsych.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace DC, Donovan MH, DeCarolis NA, Farnbauch LA, Malhotra S, Berton O, Nestler EJ, Krishnan V, Eisch AJ. Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proc Natl Acad Sci U S A. 2010;107:4436–4441. doi: 10.1073/pnas.0910072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea PM, Custer SJ, Vicini S, Faden AI. Neuronal and glial mGluR5 modulation prevents stretch-induced enhancement of NMDA receptor current. Pharmacol Biochem Behav. 2002;73:287–298. doi: 10.1016/s0091-3057(02)00825-0. [DOI] [PubMed] [Google Scholar]
- Liu GX, Cai GQ, Cai YQ, Sheng ZJ, Jiang J, Mei Z, Wang ZG, Guo L, Fei J. Reduced anxiety and depression-like behaviors in mice lacking GABA transporter subtype 1. Neuropsychopharmacology. 2007;32:1531–1539. doi: 10.1038/sj.npp.1301281. [DOI] [PubMed] [Google Scholar]
- Liu X, Peprah D, Gershenfeld HK. Tail-suspension induced hyperthermia: a new measure of stress reactivity. J Psychiatr Res. 2003;37:249–259. doi: 10.1016/s0022-3956(03)00004-9. [DOI] [PubMed] [Google Scholar]
- Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol. 1997;8:523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- Maier DL, Mani S, Donovan SL, Soppet D, Tessarollo L, McCasland JS, Meiri KF. Disrupted cortical map and absence of cortical barrels in growth-associated protein (GAP)-43 knockout mice. Proc Natl Acad Sci U S A. 1999;96:9397–9402. doi: 10.1073/pnas.96.16.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- McIlvain V, McCasland JS. GAP-43 heterozygous mice show delayed barrel patterning, differentiation of radial glia, and downregulation of GAP-43. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:143–157. doi: 10.1002/ar.a.20291. [DOI] [PubMed] [Google Scholar]
- McIlvain VA, Robertson DR, Maimone MM, McCasland JS. Abnormal thalamocortical pathfinding and terminal arbors lead to enlarged barrels in neonatal GAP-43 heterozygous mice. J Comp Neurol. 2003;462:252–264. doi: 10.1002/cne.10725. [DOI] [PubMed] [Google Scholar]
- Meiri KF, Pfenninger KH, Willard MB. Growth-associated protein, GAP-43, a polypeptide that is induced when neurons extend axons, is a component of growth cones and corresponds to pp46, a major polypeptide of a subcellular fraction enriched in growth cones. Proc Natl Acad Sci U S A. 1986;83:3537–3541. doi: 10.1073/pnas.83.10.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz GA, Schwab ME. Behavioral characterization in a comprehensive mouse test battery reveals motor and sensory impairments in growth-associated protein-43 null mutant mice. Neuroscience. 2004;129:563–574. doi: 10.1016/j.neuroscience.2004.07.053. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Williams DL. The new neurobiology of autism: cortex, connectivity, and neuronal organization. Arch Neurol. 2007;64:945–950. doi: 10.1001/archneur.64.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Nonneman RJ, Grossman AW, Murphy DL, D'Ercole AJ, Crawley JN, Magnuson TR, Lauder JM. Social approach in genetically engineered mouse lines relevant to autism. Genes Brain Behav. 2009;8:129–142. doi: 10.1111/j.1601-183X.2008.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder GB, Pritchett K. The elevated plus-maze. Contemp Top Lab Anim Sci. 2004;43:39–40. [PubMed] [Google Scholar]
- Mulvihill B, Wingate M, Kirby RS, Pettygrove S, Cunniff C, Meaney FJ, Miller L, Robinson C, Quintana G, Kaiser MY, Lee LC, Landa R, Newschaffer C, Constantino J, Fitzgerald R, Daniels J, Giarelli E, Pinto-Martin J, Levy SE, Charles J, Nicholas J, Durkin M, Rice C, Baio J, Braun KV, Yeargin-Allsopp M, Hepburn M, Garner N, Mancilla KC, Ratchford A, Castillo Y, Kolotos M, Fitzgerald R, Bell P, Meade R, King L, Arneson C, Washington A, Graham S, Lance R, Grummon N, Plummer L, Jones L, Wojcik J, Doernberg N. Prevalence of autism spectrum disorders – Autism and Developmental Disabilities Monitoring Network, United States, 2006. MMWR Surveill Summ. 2005;58:1–20. [PubMed] [Google Scholar]
- Nakasato A, Nakatani Y, Seki Y, Tsujino N, Umino M, Arita H. Swim stress exaggerates the hyperactive mesocortical dopamine system in a rodent model of autism. Brain Res. 2008;1193:128–135. doi: 10.1016/j.brainres.2007.11.043. [DOI] [PubMed] [Google Scholar]
- Pearson DA, Loveland KA, Lachar D, Lane DM, Reddoch SL, Mansour R, Cleveland LA. A comparison of behavioral and emotional functioning in children and adolescents with Autistic Disorder and PDD-NOS. Child Neuropsychol. 2006;12:321–333. doi: 10.1080/09297040600646847. [DOI] [PubMed] [Google Scholar]
- Pfeiffer BE, Huber KM. The State of Synapses in Fragile X Syndrome. Neuroscientist. 2009;15:549–567. doi: 10.1177/1073858409333075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell CM, Schoch S, Monteggia L, Barrot M, Matos MF, Feldmann N, Sudhof TC, Nestler EJ. The presynaptic active zone protein RIM1alpha is critical for normal learning and memory. Neuron. 2004;42:143–153. doi: 10.1016/s0896-6273(04)00146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippon G, Brock J, Brown C, Boucher J. 2007) Disordered connectivity in the autistic brain: challenges for the "new psychophysiology". Int J Psychophysiol. 2007;63:164–172. doi: 10.1016/j.ijpsycho.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellenberg GD, Dawson G, Sung YJ, Estes A, Munson J, Rosenthal E, Rothstein J, Flodman P, Smith M, Coon H, Leong L, Yu CE, Stodgell C, Rodier PM, Spence MA, Minshew N, McMahon WM, Wijsman EM. Evidence for multiple loci from a genome scan of autism kindreds. Mol Psychiatry. 2006;11:1049–1060. doi: 10.1038/sj.mp.4001874. 1979. [DOI] [PubMed] [Google Scholar]
- Shen Y, Mani S, Donovan SL, Schwob JE, Meiri KF. Growth-associated protein-43 is required for commissural axon guidance in the developing vertebrate nervous system. J Neurosci. 2002;22:239–247. doi: 10.1523/JNEUROSCI.22-01-00239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shughrue PJ, Dorsa DM. Gonadal steroids modulate the growth-associated protein GAP-43 (neuromodulin) mRNA in postnatal rat brain. Brain Res Dev Brain Res. 1993;73:123–132. doi: 10.1016/0165-3806(93)90054-e. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Dorsa DM. The ontogeny of GAP-43 (neuromodulin) mRNA in postnatal rat brain: evidence for a sex dimorphism. J Comp Neurol. 1994;340:174–184. doi: 10.1002/cne.903400204. [DOI] [PubMed] [Google Scholar]
- Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology. 2010;35:976–989. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene JH, Jacobson RD, Snipes GJ, McGuire CB, Norden JJ, Freeman JA. A protein induced during nerve growth (GAP-43) is a major component of growth-cone membranes. Science. 1986;233:783–786. doi: 10.1126/science.3738509. [DOI] [PubMed] [Google Scholar]
- Steele SD, Minshew NJ, Luna B, Sweeney JA. Spatial working memory deficits in autism. J Autism Dev Disord. 2007;37:605–612. doi: 10.1007/s10803-006-0202-2. [DOI] [PubMed] [Google Scholar]
- Szatmari P, Paterson AD, Zwaigenbaum L, Roberts W, Brian J, Liu XQ, Vincent JB, Skaug JL, Thompson AP, Senman L, Feuk L, Qian C, Bryson SE, Jones MB, Marshall CR, Scherer SW, Vieland VJ, Bartlett C, Mangin LV, Goedken R, Segre A, Pericak-Vance MA, Cuccaro ML, Gilbert JR, Wright HH, Abramson RK, Betancur C, Bourgeron T, Gillberg C, Leboyer M, Buxbaum JD, Davis KL, Hollander E, Silverman JM, Hallmayer J, Lotspeich L, Sutcliffe JS, Haines JL, Folstein SE, Piven J, Wassink TH, Sheffield V, Geschwind DH, Bucan M, Brown WT, Cantor RM, Constantino JN, Gilliam TC, Herbert M, Lajonchere C, Ledbetter DH, Lese-Martin C, Miller J, Nelson S, Samango-Sprouse CA, Spence S, State M, Tanzi RE, Coon H, Dawson G, Devlin B, Estes A, Flodman P, Klei L, McMahon WM, Minshew N, Munson J, Korvatska E, Rodier PM, Schellenberg GD, Smith M, Spence MA, Stodgell C, Tepper PG, Wijsman EM, Yu CE, Roge B, Mantoulan C, Wittemeyer K, Poustka A, Felder B, Klauck SM, Schuster C, Poustka F, Bolte S, Feineis-Matthews S, Herbrecht E, Schmotzer G, Tsiantis J, Papanikolaou K, Maestrini E, Bacchelli E, Blasi F, Carone S, Toma C, Van Engeland H, de Jonge M, Kemner C, Koop F, Langemeijer M, et al. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39:319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM, Sudhof TC. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–76. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology (Berl) 2009;204:361–373. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trikalinos TA, Karvouni A, Zintzaras E, Ylisaukko-oja T, Peltonen L, Jarvela I, Ioannidis JP. A heterogeneity-based genome search meta-analysis for autism-spectrum disorders. Mol Psychiatry. 2006;11:29–36. doi: 10.1038/sj.mp.4001750. [DOI] [PubMed] [Google Scholar]
- Veenstra-Vanderweele J, Christian SL, Cook EH., Jr Autism as a paradigmatic complex genetic disorder. Annu Rev Genomics Hum Genet. 2004;5:379–405. doi: 10.1146/annurev.genom.5.061903.180050. [DOI] [PubMed] [Google Scholar]
- Zoghbi HY. Postnatal neurodevelopmental disorders: meeting at the synapse? Science. 2003;302:826–830. doi: 10.1126/science.1089071. [DOI] [PubMed] [Google Scholar]