Abstract
Objective
Population studies have consistently found that shorter sleep durations are associated with obesity and cardiovascular disease, particularly among women. Adiponectin is an adipocyte-derived, anti-inflammatory hormone that is related to cardiovascular disease risk. We hypothesized that sleep restriction would reduce adiponectin levels in healthy young adults.
Methods
74 healthy adults (57% men, 63% African American, mean age 29.9y) completed 2 nights of baseline sleep at 10h time in bed (TIB) per night followed by 5 nights of sleep restricted to 4h TIB per night. An additional 8 participants were randomized to a control group that received 10h TIB per night throughout the study. Plasma adiponectin levels were measured following the second night of baseline sleep and the fifth night of sleep restriction or control sleep.
Results
Sleep restriction resulted in a decrease in plasma adiponectin levels among Caucasian women (Z = −2.19, p = .028), but an increase among African American women (Z = −2.73, p = 0.006). No significant effects of sleep restriction on adiponectin levels were found among men. A 2×2 between group analysis of covariance on adiponectin change scores controlling for BMI confirmed significant interactions between sleep restriction and race/ethnicity [F(1,66) = 13.73, p < 0.001], as well as among sleep restriction, race/ethnicity and sex [F(1,66) = 4.27, p = 0.043)].
Conclusions
Inflammatory responses to sleep loss appear to be moderated by sex and race/ethnicity; observed decreases in adiponectin following sleep restriction may be one avenue by which reduced sleep duration promotes cardiovascular risk in Caucasian women.
Keywords: adiponectin; sleep restriction; sex, race; ethnicity; obesity
1. Introduction
Increasing evidence suggests that short sleep durations are associated with a number of health risks, including obesity [1], atherosclerosis [2], coronary artery disease [3], cardiovascular events [4], and mortality secondary to cardiovascular disease [5, 6]. These associations are particularly important given the trend towards decreasing sleep durations in industrialized countries in recent years [7–9]. Additionally, there are now a growing number of studies documenting relationships between short sleep durations and both heart disease and its precursors among women but not men, including increased risk of hypertension [10], markers of inflammation associated with cardiovascular disease risk [11], and incident myocardial infarction [12].
Adiponectin is a relatively novel anti-inflammatory marker that has garnered interest for its anti-atherogenic and anti-inflammatory properties [13] and is involved in regulation of insulin sensitivity and lipid oxidation [14, 15]. Adiponectin levels are significantly lower in obese, compared to non-obese, individuals [16, 17] and have been found to be inversely related to type 2 diabetes (for review see [18]) and cardiovascular risk in some [19–22], but not all [23], studies. Several epidemiological studies have reported little, if any association, between short sleep durations and adiponectin levels [24–26], and one small experimental study (N=6 men) observed no effect of sleep restriction on adiponectin levels [27].
While there are an increasing number of studies exploring the changes in pro-inflammatory markers related to cardiovascular disease that may result from sleep restriction (e.g., C-reactive protein, IL-6 [28]), there are no systematic studies examining sex- or race- based differences in response to sleep loss among otherwise healthy populations. Women have been included in multiple studies to date; however, small sample sizes have precluded any comparative analyses. Additionally, these studies have largely been conducted in relatively young, Caucasian men [29–31], limiting the generalizations that can be drawn from these findings. Studies examining potential differential vulnerabilities to the effect of shortened sleep are important because they may increase our understanding of documented sex-, as well as ethnicity/race- based, differences in the development of cardiovascular disease [32], among other health risks.
Given that the relationship between reduced sleep duration and cardiovascular disease appears to be more evident in women than men [10–12] and the majority of sleep restriction studies have been conducted in small samples without independent analyses of female participants, adiponectin remains a marker of potential interest. We hypothesized that if sleep restriction is one of the mechanisms underlying the relationship between sleep duration and cardiovascular disease, women may experience a greater change in adiponectin levels than men in response to sleep restriction. Additionally, as most of the previous research demonstrating the pro-inflammatory effects of experimental sleep restriction has been conducted in Caucasian-only samples, or samples that are too small to analyze separately by race/ethnicity, we hypothesized that Caucasian participants may also be more likely to demonstrate decreases in adiponectin levels in response to experimentally restricted sleep.
2. Materials and methods
2.1 Study Population
Participants were recruited from advertisements in Philadelphia area newspapers. Eligibility criteria included being in good health, aged 22–45 years, and having a healthy body mass index (BMI). Prior to enrollment, potential participants underwent a complete medical history and physical screening to rule out hepatitis, cancer, other serious medical conditions and Axis I psychiatric disorders (e.g. major depressive disorder, schizophrenia). Clinical chemistry and urine tests were also performed to ensure that participants were free of active infection and common recreational drugs. Participants were not permitted to be taking any prescription medications. Normal sleep wake rhythms and average sleep duration (i.e., 6.5–8.5 hours per night, with morning wake time between 0600h and 0900h) were also required for enrollment, and were verified by sleep logs and actigraphy for a period of at least 1 week prior to study participation.
2.2 Protocol design
Participants were enrolled into two studies that were identical with respect to participant eligibility, recruitment, and experimental protocol for the data presented in this manuscript. Data used for the current study were collected from an 11-day protocol (N=48) and a 15-day protocol (N=34) that were both conducted in the Sleep and Chronobiology Laboratory at the Hospital of the University of Pennsylvania, and were approved by the University of Pennsylvania Institutional Review Board. There were no significant differences between participants from the two protocols in demographics or baseline adiponectin levels (p values >0.16). All participants remained in the laboratory environment and were kept in constant dim ambient light of <50 lux for the duration of the study and were not permitted visitors. Participants were allowed to ambulate freely in the laboratory and were not confined to their beds throughout the study (excluding assigned sleep periods), but were restricted from exercise and more strenuous activities.
2.2.1 Sleep restriction protocol
N=74 participants (45% female) completed two nights of 10h time in bed (TIB) baseline sleep (B1 and B2) followed by 5 nights of sleep restricted to 4h TIB (SR1 to SR5). In the sleep restriction condition, bed-times were delayed and participants were allowed sleep opportunities between 0400–0800 on SR1–SR5. All sleep-restricted participants received 2 nights of full sleep (10h+ sleep/night) prior to leaving the laboratory. An additional cohort of N=8 control participants were randomized to 10h TIB/night for the duration of the study (sleep opportunity: 2200-0800).
2.2.2 Randomization
Randomization to condition was based on the full study protocols, which included additional nights of variable recovery sleep; as such, randomization through the first seven days of the study produced unequal numbers of sleep restriction and control participants. Sample size was determined based on power calculations for the primary (neurobehavioral) study outcomes. Participants completed the protocol in groups of 4–5 participants; these study ‘runs’ were scheduled and randomly allocated as sleep restriction or control sequences prior to the enrollment of any participants. Participants were enrolled in the next study run feasible following screening by the study coordinator, and remained blind to study condition until after the second night of baseline sleep (i.e., following B2). The biochemist conducting all adiponectin assays was blind to study condition.
2.2.3 Blood sampling paradigm
Blood draws were performed through the antecubital vein between 1030–1200h on the mornings following B2 and SR5. Samples were collected from control participants on equivalent study protocol days. Samples were drawn into 10mL plastic vacutainer tubes spray-coated with sodium heparin, centrifuged, aliquotted, and frozen at −80° C until analysis. They were assayed for adiponectin and a subset of other biomarkers.
2.3 Adiponectin assays
Plasma adiponectin levels were measured by a commercially available radioimmunoassay kit (Millipore, Billerica, MA, USA). While the assay reagents remained consistent across all samples, the quality control standards were changed by the manufacturer over the course of the study; the maximum values for each assay coefficient are presented here. The intra-assay coefficients of variation were 2.20% and 3.84% (for low and high adiponectin concentrations) and inter-assay coefficients of variation 8.20% and 14.90% (for low and high concentration adiponectin standards). The sensitivity limit for all assays was 380 ng/mL, and all samples and standards were assayed in duplicate within the same assay kit.
2.4 Food intake
Participants received three meals per day, plus an optional evening snack on nights when they received sleep opportunities of less than 8h TIB. The timing of food consumption was determined by the study participants; however, breakfast was typically consumed between 0830–1100, lunch between 1230–1600 and dinner between 1830 and 2000. Additional snack food was available ad libitum throughout the study, as were water, juice and caffeine-free soda. Meal choices were selected by participants from a standardized set of options provided by the Metabolic Kitchen of the Hospital of the University of Pennsylvania; chocolate, turkey, bananas, and caffeinated beverages were prohibited, due to potential effects on sleep and alertness.
2.5 Polysomnography
Polysomnographic (PSG) recordings were made using the Sandman Suzanne portable recording system (Puritan Bennett, Ontario Canada). Electroencephalography (EEG) scalp recording electrodes (C3-A2, Fz-A1 and O2-A1 derivations) were worn by the participants for several 24-hour periods during the protocol, including the second night of baseline sleep (B2) and the fifth night of sleep restriction or control sleep (SR5). During recording periods, participants did not shower, and electrodes were replaced every 12 hours. EEG from C3-A2 derivation was scored according to Rechtshaffen and Kales [33] by a trained technician blind to condition. Polysomnography was used to verify sleep/wake times and to rule out any overt sleep disorders (e.g., sleep apnea).
2.6 Statistical Analyses
SPSS Statistical Software, version 16.0 (SPSS Inc, Chicago, IL, USA) was used for statistical analyses. As adiponectin data were not normally distributed, non-parametric analyses were conducted using the total sample in order to assess the effects of five nights of partial sleep restriction on plasma adiponectin levels (B2 to SR5). Mann-Whitney U tests were used for between-group comparisons and Wilcoxon Signed Rank tests were used for within-group comparisons. Comparisons were also conducted within demographic sub-groups to assess potential differential vulnerability to sleep restriction. An additional 2-by-2 between-group analysis of covariance (ANCOVA) was conducted among sleep restricted participants to assess the effect of sex and race/ethnicity on log-transformed adiponectin levels, controlling for BMI.
3. Results
3.1 Participant Characteristics
Data for the current study was collected from August 2004-October 2008. Among the N=95 participants who were randomized to experimental condition, N=13 (14%) had incomplete data (N=10 withdrew for personal reasons or minor physical complaints, such as headache; N=2 had a data point below the lowest adiponectin level that could be reliably detected by assay reagents [verified with standardized samples], N=1 had missing data). Complete data were collected from the remaining N=82 participants (mean age 29.9 years, range 22–45 years; mean body mass index [BMI] 24.6 kg/m2, range 17.7–33.1 kg/m2; sex: 57% men; race/ethnicity: 31% Caucasian, 63% African American, 6% other). Race/ethnicity was considered a variable of interest for the current study due to differences in the rates of cardiovascular disease across these populations and our current focus on effects of sleep restriction on adiponectin, a biological marker of cardiovascular risk. Race/ethnicity was self-identified by participants using the following categories designated by the National Institutes of Health for use in research: ethnicity (Hispanic or Latino, Not Hispanic or Latino, Unknown) and race (American Indian or Alaska Native, Asian, Black or African American, Native Hawaiian or Other Pacific Islander; White, More than one race). A description of each of these categories was provided, e.g., “Black or African American. A person having origins in any of the black racial groups of Africa. Terms such as ‘Haitian’ or ‘Negro’ can be used in addition to ‘Black’ or African American.” Participants were requested to select one ethnicity category and one or more of the race categories. They were also informed that they were not required to provide this information in order to be eligible for participation. Participants with complete data did not differ significantly from the N=13 with incomplete data on any baseline variables (all p values > 0.17; race/ethnicity comparison based on Caucasian and African American participants only). Data from participants with incomplete data are not presented.
3.2 Total sample
Baseline levels of adiponectin were significantly correlated with BMI (rho = −0.31, p = 0.005), but not age (rho = 0.020, p = 0.86). Baseline levels were significantly higher in women compared to men (Z = −4.88, p < 0.001, r = 0.57). There was no baseline difference in adiponectin levels between Caucasian and African American participants (Z = 1.12, p = 0.26, r = −0.13).
Mean adiponectin levels for the sleep restriction participants were 9.81 (±5.80) ug/mL at baseline (B2) and 11.83 (±12.28) ug/mL following five nights of partial sleep restriction (SR5). Adiponectin levels did not change significantly from B2 to SR5 in either the sleep restriction or the control group (Z = −1.22, p = 0.22, r = −0.14; Z = −0.28, p = 0.78, r = −0.10, respectively). There was no significant difference in change in adiponectin from B2 to SR5 between sleep restriction and control groups (Z = −0.56, p = 0.58, r = −0.061).
3.3 Sub-group analyses (within sleep restriction condition only)
3.3.1 Sex
Neither male nor female participants were observed to have a significant change in adiponectin levels from baseline (B2) to the fifth day of sleep restriction (SR5; men: Z = −0.80, p = 0.43, r = −0.12; women: Z = −0.87, p = 0.39, r = −0.15). A between-sex comparison of change scores (B2-SR5) did not reach statistical significance (Z = −0.43, p = 0.67, r = −0.05).
3.3.2 Race/ethnicity
Caucasian participants demonstrated a significant decrease in adiponectin levels following sleep restriction (Z = −2.06, p = 0.040, r = −0.42) and African American participants demonstrated a significant increase (Z = −3.06, p = 0.002, r = −0.36). A between-group comparison of change scores (B2-SR5) by race/ethnicity was also significant (Z = −3.43, p = 0.001, r = −.40).
3.4 Interactions
A 2-by-2 between-group analysis of covariance (ANCOVA) was conducted among sleep restricted participants to assess the effect of sex and race/ethnicity on adiponectin levels, controlling for BMI. Log transformed data were utilized for this ANCOVA to adjust for the non-normally distributed adiponectin data. The independent variables were sex (male, female) and race/ethnicity (Caucasian, African American); the dependent variables were log-transformed B2 and SR5 adiponectin levels. After adjustment for BMI, there was no main effect of sleep restriction [F(1, 66) = 1.76, p = 0.19], with a small to moderate effect size (partial eta squared = 0.026). However, there was a significant two-way interaction between the effect of sleep restriction and race/ethnicity [F(1,66) = 13.73, p < 0.001], with a large effect size (partial eta squared = 0.17). Additionally, there was a significant three-way interaction among sex, race/ethnicity and between sleep restriction [F(1,66) = 4.27, p = 0.043] (partial eta squared = 0.061 [moderate effect size]). There was no significant interaction with sex alone [F(1,66) = 3.48, p = 0.066, partial eta squared = 0.050], and BMI was not a significant covariate [F(1,66) = 2.27, p = 0.14, partial eta squared = 0.033]. When BMI was not used as a covariate, statistically similar relationships were produced from this analysis (data not presented).
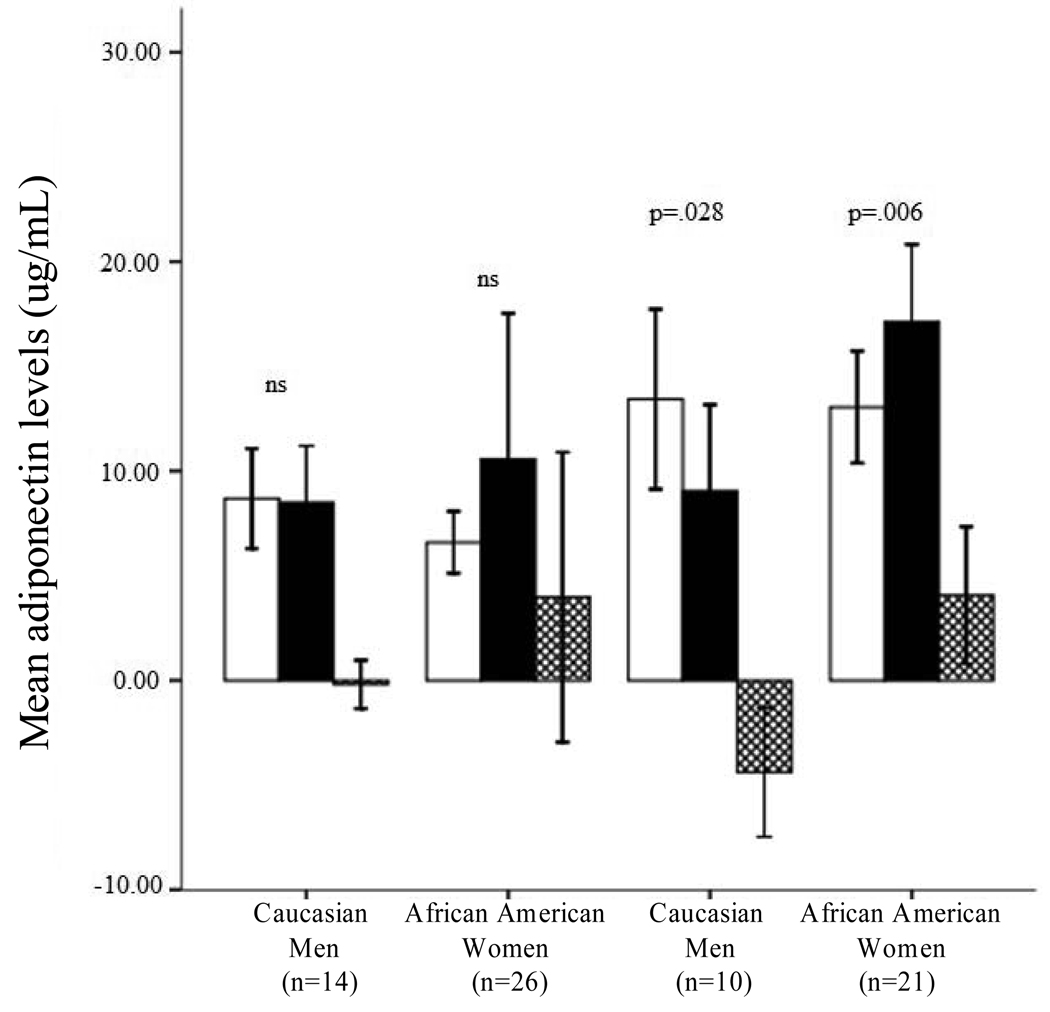
These findings parallel individual within-group comparisons using sex-by-race/ethnicity subgroups (e.g., African American women) to assess changes in adiponectin from B2 to SR5. Comparisons within male-only groups were not significant (African American men: Z = −1.54, p = 0.12, r = −0.30; Caucasian men: Z = −0.41, p = 0.68, r = −0.11). However, Caucasian women (N=10) demonstrated a significant decrease in adiponectin levels in response to sleep restriction (Z = −2.19, p = 0.028, r = −0.69), while African American women (N=21) demonstrated a significant increase in adiponectin levels (Z = −2.73, p = 0.006, r = −0.60) following sleep restriction (Figure 1).
Figure 1. Adiponectin levels among sleep restricted participants.
Mean adiponectin levels at baseline (B2,  ) and following sleep restriction (SR5,
) and following sleep restriction (SR5,  ). Change in adiponectin levels (SR5 minus B2,
). Change in adiponectin levels (SR5 minus B2,  is also presented. Significant differences between B2 and SR5 indicated with p-values. Data from control condition not presented; Wilcoxon signed rank tests conducted. Error bars are +/−2 SE of mean.
is also presented. Significant differences between B2 and SR5 indicated with p-values. Data from control condition not presented; Wilcoxon signed rank tests conducted. Error bars are +/−2 SE of mean.
4. Discussion
This study is the first to document changes in adiponectin, an anti-atherogenic endocrine marker, in response to sleep restriction in healthy adults between 22–45 years of age. While no systematic changes in adiponectin levels were observed in the total sample or for men of either race/ethnicity, there was a significant sex by race/ethnicity interaction in which Caucasian women were found to have a decrease in adiponectin levels in response to sleep restriction and African American women demonstrated an increase. These results partially supported our hypothesis that sleep restriction would result in a decrease in adiponectin, particularly among Caucasian participants. We found no significant main effect of sleep restriction on adiponectin levels among N=41 healthy men, which is consistent with the report by Shea and colleagues [27]. A novel finding in our investigation was that both Caucasian and African American women demonstrated reliable adiponectin responses to sleep restriction, albeit it opposite directions.
The underlying basis of the differential adiponectin responses we observed for sex and racial/ethnic groups is unclear. There is also evidence of sex differences in relationships between both sleep duration and quality with inflammation, with stronger associations among women [11, 34]. In the current study, however, there was a sex by race/ethnicity interaction, such that Caucasian women were observed to have a decrease after sleep restriction in adiponectin, a beneficial anti-inflammatory marker, while African American women were observed to have an increase. While menstrual phase was not aligned for study participation in women, there was no significant effect of self-reported menstrual phase on change in adiponectin level (p > .05), suggesting that this factor was not a significant contributor to the study results. No additional post-hoc comparisons examining relationships between change in adiponectin and other potential variables of interest (e.g., changes in leptin [35], a proxy for childhood socioeconomic status [parental education], depression scores) were significant.
We know of no other evidence for a differential vulnerability to the effects of sleep restriction based on race/ethnicity (e.g., on cognitive performance measures, sleepiness ratings). With respect to amount of habitual sleep duration, two recent studies have shown that African Americans are more likely to report both short and long sleep durations compared to Caucasians [36, 37]; however, given the 6.5–8.5h habitual sleep duration eligibility requirement for the current study, it is unlikely that there was a systematic race/ethnicity-based difference in the magnitude of sleep restriction imposed on participants by this protocol. Race/ethnicity-based differences in some sleep-related variables have been documented, however [e.g., nocturnal dips in blood pressure], and recent evidence suggests that psychosocial factors (e.g., education, marital status, social support) may mediate this relationship [38–40]. These studies raise the possibility of a third-variable (e.g., psychosocial factors) that may also be mediating at least part of the observed relationships relative to sex and race/ethnicity in the current study.
Data regarding the relative secretion of adiponectin from subcutaneous and visceral adipose tissue are currently limited [41]. African American women have been reported to have significantly higher subcutaneous and total fat levels than any other sex-race/ethnicity group, with both African American and Caucasian women having higher levels of visceral fat than men [42]. However, in several studies adjusting for body mass index (or using populations with similar BMIs), African Americans have been observed to have less total fat mass [43] and less intra-abdominal visceral adipose tissue compared to Caucasians [44–48]. Together, these studies suggest that the distribution of adipose tissue, as well as the relative production of adiponectin from these tissue sources, may play a role in the interaction between sex and race/ethnicity in adiponectin response to sleep restriction.
The significant decrease in adiponectin levels in response to sleep restriction among Caucasian women is consistent with other evidence suggesting an inflammation-increasing response to sleep restriction [29, 49, 50] and adds to the growing evidence that short sleep durations are associated with risk for obesity and cardiovascular disease [1, 3], at least among some segments of the population. While there may be an underlying genetic basis for the observed differential adiponectin responses between racial/ethnic groups among women (e.g., in adipose fat distribution), it is also possible that there may be differences in behavioral responses to sleep restriction (e.g., in rates of food consumption, or food consumption preferences) between groups, which may in turn contribute to the different adiponectin levels observed among women in response to sleep restriction.
The direction of the findings, suggesting a protective or beneficial response of increased adiponectin among African American women in response to sleep restriction, does not parallel epidemiological findings that risks for heart disease and stroke are higher among African American compared to Caucasian women [51]. However, paradoxical relationships between adiponectin and cardiovascular disease have been observed; among individuals with extant coronary artery disease a positive (rather than the predicted negative) relationship between adiponectin levels and future cardiac events has been reported [21]. As such, it may be that adiponectin levels serve as a counter-regulatory factor following a physiological challenge [21] and if so, sleep restriction may also trigger a similarly physiologically protective response. Moreover, the severity (acuity/chronicity) of sleep loss and the extent to which it is voluntary versus involuntary, are two among likely many factors that contributor to cardiovascular disease risk, including those that have a more direct effect, such as diet or levels of C-reactive protein [52].
It is a limitation of the current study that exact energy intake and expenditures (i.e., food consumption and physical activity) were not assessed, as it is possible that these factors contributed to the observed systematic sex-based variance in the data. There is mixed evidence about whether food consumption and preferences change with sleep restriction [53, 54], and whether shifts in food intake (e.g., increased consumption of sodium-rich foods) affect adiponectin levels in humans [55–57]; as such, quantifying food intake and examining effects on adiponectin levels will be an important area to explore in future studies. Another methodological limitation was the use of a single blood draw per sampling day. Although adiponectin has not been observed to have a circadian rhythm [27], the methodology utilized in the current study may not have adequately captured any potential changes in adiponectin over a 24h period in response to sleep restriction. Lastly, while a relatively large sample was enrolled in the current study, participants were restricted to healthy adults, limiting the generalizations that can be made regarding the effects of sleep restriction on adiponectin levels across a more representative population-based sample, or one at greater immediate risk for cardiovascular disease.
5. Conclusions
These findings contribute to a developing literature suggesting that people can have markedly different vulnerabilities to the effects of sleep loss on regulatory biology involving both the body (e.g., biological markers [50] and the brain [58]). The current study is the first to document a significant effect of sleep restriction on adiponectin levels among healthy women, suggesting that adiponectin may play a role in the relationship between sleep duration and cardiovascular risk that has previously been observed in this population [10–12]. However, this association does not appear to be consistent across race/ethnicity or sex, a result which is of particular importance because it suggests that research findings from experimental sleep restriction studies in one subgroup may not be generalizable across populations. Future research is needed to further elucidate the relationships between sleep duration and clinically useful biomarkers of health, with an emphasis on differential vulnerability as a function of sex, race/ethnicity and their interactions.
Research Highlights.
Sleep restriction did not change adiponectin levels in the total sample or in men
Adiponectin levels increased following sleep restriction in African American women
Adiponectin decreased in response to sleep restriction among Caucasian women
Acknowledgments
Supported by NIH NR004281, HFP00404, UL1RR024134, F31 AG031352. Adiponectin assays were conducted by the RIA/Biomarker Core Laboratory (PI: Heather Collins, Ph.D.). The authors also thank Marisa Moreta and Lilia Lakhtman for their assistance with data management.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Patel SR, Hu FB. Short sleep duration and weight gain: A systematic review. Obesity (Silver Spring) 2008;16:643–653. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolff B, Volzke H, Schwahn C, Robinson D, Kessler C, John U. Relation of self-reported sleep duration with carotid intima-media thickness in a general population sample. Atherosclerosis. 2008;196:727–732. doi: 10.1016/j.atherosclerosis.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 3.King CR, Knutson KL, Rathouz PJ, Sidney S, Liu K, Lauderdale DS. Short sleep duration and incident coronary artery calcification. Journal of the American Medical Association. 2008;300:2859–2866. doi: 10.1001/jama.2008.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eguchi K, Pickering TG, Schwartz JE, Hoshide S, Ishikawa J, Ishikawa S, et al. Short sleep duration as an independent predictor of cardiovascular events in Japanese patients with hypertension. Archives of Internal Medicine. 2008;168:2225–2231. doi: 10.1001/archinte.168.20.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikehara S, Iso H, Date C, Kikuchi S, Watanabe Y, Wada Y, et al. Association of sleep duration with mortality from cardiovascular disease and other causes for Japanese men and women: the JACC study. Sleep. 2009;32:259–301. doi: 10.1093/sleep/32.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrie JE, Shipley MJ, Cappuccio FP, Brunner EJ, Miller MA, Kumari M, et al. A prospective study of change in sleep duration: Associations with mortality in the Whitehall II Cohort. Sleep. 2007;30:1659–1666. doi: 10.1093/sleep/30.12.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Sleep Foundation. 1997 Sleep in America Poll. Washington, D.C.: National Sleep Foundation; 1998. [Google Scholar]
- 8.National Sleep Foundation. 2005 Sleep in America Poll. Washington, D.C.: National Sleep Foundation; 2006. [Google Scholar]
- 9.Tune GS. Sleep and wakefulness in normal human adults. British Medical Journal. 1968;2:269–271. doi: 10.1136/bmj.2.5600.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cappuccio FP, Stranges S, Kandala NB, Miller MA, Taggart FM, Kumari M, et al. Gender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension. 2007;50:693–700. doi: 10.1161/HYPERTENSIONAHA.107.095471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller MA, Kandala NB, Kivimaki M, Kumari M, Brunner EJ, Lowe GD, et al. Gender differences in the cross-sectional relationships between sleep duration and markers of inflammation: Whitehall II study. Sleep. 2009;37:857–864. [PMC free article] [PubMed] [Google Scholar]
- 12.Meisinger C, Heier M, Lowel H, Schneider A, Doring A. Sleep duration and sleep complaints and risk of myocardial infarction in middle-aged men and women from the general population: the MONICA/KORA Augsburg cohort study. Sleep. 2007;30:1121–1127. doi: 10.1093/sleep/30.9.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldstein BJ, Scalia RG, Ma XL. Protective vascular and myocardial effects of adiponectin. Nature clinical practice. Cardiovascular Medicine. 2009;6:27–35. doi: 10.1038/ncpcardio1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE. Hypoadiponectinemia in obesity and type 2 diabetes: Close association with insulin resistance and hyperinsulinemia. Journal of Clinical Endocrinology and Metabolism. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 15.Yamauchi T, Kamon J, H W, Y T, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nature Medicine. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 16.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochemical and Biophysical Research Communications. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 17.Silha JV, Krsek M, Skrha JV, Sucharda P, Nyomba BLG, Murphy LJ. Plasma resistin, adiponectin and leptin levels in lean and obese subjects: correlations with insulin resistance. European Journal of Endocrinology. 2003;149:331–335. doi: 10.1530/eje.0.1490331. [DOI] [PubMed] [Google Scholar]
- 18.Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: A systematic review and meta-analysis. Journal of the American Medical Association. 2009;302:179–188. doi: 10.1001/jama.2009.976. [DOI] [PubMed] [Google Scholar]
- 19.Maahs DM, Ogden LG, Kinney GL, Wadwa P, Snell-Bergeon JK, Dabelea D, et al. Low plasma adiponectin levels predict progression of coronary artery calcification. Circulation. 2005;111:747–753. doi: 10.1161/01.CIR.0000155251.03724.A5. [DOI] [PubMed] [Google Scholar]
- 20.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. Journal of the American Medical Association. 2004;291:1730–1734. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 21.Schnabel R, Messow CM, Lubos E, Espinola-Klein C, Rupprecht HJ, Bickel C, et al. Association of adiponectin with adverse outcome in coronary artery disease patients: Results from the AtheroGene study. European Heart Journal. 2008;29:649–657. doi: 10.1093/eurheartj/ehn009. [DOI] [PubMed] [Google Scholar]
- 22.Schulze MB, Shai I, Rimm EB, Li T, Rifai N, Hu FB. Adiponectin and future coronary heart events among men with Type 2 diabetes. Diabetes. 2005;54:534–539. doi: 10.2337/diabetes.54.2.534. [DOI] [PubMed] [Google Scholar]
- 23.Lawlor DA, Smith GD, Ebrahim S, Thompson C, Sattar N. Plasma adiponectin levels are associated with insulin resistance but do not predict future risk of coronary heart disease in women. Journal of Clinical Endocrinology and Metabolism. 2005;90:5677–5683. doi: 10.1210/jc.2005-0825. [DOI] [PubMed] [Google Scholar]
- 24.Hitze B, Bosy-Westphal A, Bielfeldt F, Settler U, Plachta-Danielzik S, Pfeuffer M, et al. Determinants and impact of sleep duration in children and adolescents: data of the Kiel Obesity Prevention Study. European Journal of Clinical Nutrition. 2008;63:739–746. doi: 10.1038/ejcn.2008.41. [DOI] [PubMed] [Google Scholar]
- 25.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. Plos Medicine. 2004;1:210–217. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams CJ, Hu FB, Patel SR, Mantzoros CS. Sleep duration and snoring in relation to biomarkers of cardiovascular disease risk among women with Type 2 diabetes. Diabetes Care. 2007;30:1233–1240. doi: 10.2337/dc06-2107. [DOI] [PubMed] [Google Scholar]
- 27.Shea SA, Hilton MF, Orlova C, Ayers RT, Mantzoros CS. Independent circadian and sleep/wake regulation of adipokines and glucose in humans. Journal of Clinical Endocrinology and Metabolism. 2005;90:2537–2544. doi: 10.1210/jc.2004-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Progress in Cardiovascular Diseases. 2009;51:294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. Journal of the American College of Cardiology. 2004;43:678–683. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 30.Spiegel K, Leproult R, L'Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: Relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. Journal of Clinical Endocrinology and Metabolism. 2004;89:5762–5771. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 31.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Annals of Internal Medicine. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 32.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics--2009 update: a reports from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 33.Rechtschaffen A, Kales AE. In: A manual of standardized terminology, techniques and scoring system for sleep stages in human subjects. Rechtschaffen A, Kales A, editors. Washington, D.C.: U.S. Government Printing Office; 1968. [Google Scholar]
- 34.Suarez EC. Self-reported symptoms of sleep disturbance and inflammation, coagulation, insulin resistance and psychosocial distress: Evidence for a gender disparity. Brain, Behavior and Immunity. 2008;22:960–968. doi: 10.1016/j.bbi.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simpson NS, Banks S, Dinges DF. Sleep restriction is associated with increased morning plasma leptin concentrations, especially among women. Biological Research For Nursing. 2010;12:47–53. doi: 10.1177/1099800410366301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hale L, Do DP. Racial differences in self-reports of sleep duration in a population-based study. Sleep. 2007;3:1096–1103. doi: 10.1093/sleep/30.9.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nunes J, Jean-Louis G, Zizi F, Casimir GJ, H vG, Brown CD, et al. Sleep duration among black and white Americans: results of the National Health Interview Survey. Journal of the National Medical Association. 2008;100:317–322. doi: 10.1016/s0027-9684(15)31244-x. [DOI] [PubMed] [Google Scholar]
- 38.Cooper DC, Ziegler MG, Nelesen RA, Dimsdale JE. Racial differences in the impact of social support on nocturnal blood pressure. Psychosomatic Medicine. 2009;71:524–531. doi: 10.1097/PSY.0b013e31819e3a93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spruill TM, W G, Ogedegbe G, Burg M, Schwartz JE, Pickering TG. Socioeconomic and psychosocial factors mediate race differences in nocturnal blood pressure. American Journal of Hypertension. 2009;22:637–642. doi: 10.1038/ajh.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Troxel WM, Buysse DJ, Hall M, Kamarck TW, Strollo PJ, Owens JF, et al. Social interaction, social contacts, and blood pressure dipping in African-Americans and whites. Journal of Hypertension. 2010;28:265–271. doi: 10.1097/HJH.0b013e328333ab01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–2282. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 42.Demerath EW, Sun SS, Rogers N, Lee M, Reed D, Choh AC, et al. Anatomical patterning of visceral adipose tissue: race, sex, and age variation. Obesity (Silver Spring) 2007;15:2984–2993. doi: 10.1038/oby.2007.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rahman M, Temple JR, Brietkopf CR, Berenson AB. Racial differences in body fat distribution among reproductive-aged women. Metabolism: Clinical and Experimental. 2009;58:1329–1337. doi: 10.1016/j.metabol.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carroll JF, Chiapa AL, Rodriquez M, Phelps DR, Cardarelli KM, Vishwanatha JK, et al. Visceral fat, waist circumference, and BMI: Impact of race/ethnicity. Obesity (Silver Spring) 2008;16:600–607. doi: 10.1038/oby.2007.92. [DOI] [PubMed] [Google Scholar]
- 45.Conway JM, Yanovski SZ, Avila NA, Hubbard VS. Visceral adipose tissue differences in black and white women. American Journal of Clinical Nutrition. 1995;61:765–771. doi: 10.1093/ajcn/61.4.765. [DOI] [PubMed] [Google Scholar]
- 46.Kanaley JA, Giannopoulou I, Tillapaugh-Fay G, Nappi JS, Ploutz-Snyder LL. Racial differences in subcutaneous and visceral fat distribution in postmenopausal black and white women. Metabolism. 2003;52:186–191. doi: 10.1053/meta.2003.50024. [DOI] [PubMed] [Google Scholar]
- 47.Lovejoy JC, de la Bretonne JA, Klemperer M, Tulley R. Abdominal fat distribution and metabolic risk factors: effects of race. Metabolism. 1996;45:1119–1124. doi: 10.1016/s0026-0495(96)90011-6. [DOI] [PubMed] [Google Scholar]
- 48.Wee CC, Mukamal KJ, Huang A, Davis RB, McCarthy EP, Mittleman MA. Obesity and C-reactive protein levels among white, black, and Hispanic US adults. Obesity (Silver Spring) 2008;16:875–880. doi: 10.1038/oby.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30:1145–1152. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Banks S, Jones CW, Simpson NS, Dinges DF. Sustained sleep restriction in healthy adults with ad libitum access to food results in weight gain without increased appetite or food cravings [Abstract] Sleep. 2009;32:A128. [Google Scholar]
- 51.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. Heart disease and stroke statistics - 2008 Update. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 52.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. New England Journal of Medicine. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 53.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. American Journal of Clinical Nutrition. 2009;89:1–8. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmid SM, Hallschmid M, Jauch-Chara K, Wilms B, Benedict C, Lehnert H, et al. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. American Journal of Clinical Nutrition. 2009;90:1476–1482. doi: 10.3945/ajcn.2009.27984. [DOI] [PubMed] [Google Scholar]
- 55.Esposito K, Nappo F, Giugliano F, De Palo C, Ciotola M, Barbieri M, et al. Meal modulation of circulating interleukin 18 and adiponectin concentrations in healthy subjects and in patients with type 2 diabetes mellitus. American Journal of Clinical Nutrition. 2003;78:1135–1140. doi: 10.1093/ajcn/78.6.1135. [DOI] [PubMed] [Google Scholar]
- 56.Lely AT, Krikken JA, Bakker SJ, Boomsma F, Dullaart RP, Wolffenbuttel BH, et al. Low dietary sodium and exogenous angiotensin II infusion decrease plasma adiponectin concentrations in healthy men. Journal of Clinical Endocrinology and Metabolism. 2007;92:1821–1826. doi: 10.1210/jc.2006-2092. [DOI] [PubMed] [Google Scholar]
- 57.Nakandakare ER, Charf A, Santos FC, Nunes VS, Ortega K, Lottenberg AM, et al. Dietary salt restriction increases plasma lipoprotein and inflammatory marker concentrations in hypertensive patients. Atherosclerosis. 2008;200:410–416. doi: 10.1016/j.atherosclerosis.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 58.Van Dongen HPA, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: Evidence of trait-like differential vulnerability. Sleep. 2004;27:423–433. [PubMed] [Google Scholar]