Abstract
This study examines relations between children’s salivary interleukin-6 (IL-6) and secretory immunoglobulin A (SIgA) and mental health. Child sex was considered as a moderator of relations. Data were from 329 normally-developing children (M age = 9.85 years; SD = 0.98 years); 67% of children were European American and 33% were African American. Saliva samples were obtained during the afternoon and assayed for SIgA and IL-6. Parents completed questionnaire measures of child internalizing and externalizing symptoms, and children completed self-report measures of anxiety and depression. Structural equation models were fit to the data, and indicated that greater levels of salivary IL-6 and SIgA were associated with adjustment problems more strongly for girls than for boys.
The role of immunology in developmental psychopathology is a critical avenue for research [1], especially given the paucity of studies on children’s immune functioning and mental health. The advent of reliable assays of salivary immune parameters opens the door to such research. Developmental psychopathologists rarely collect blood samples from children because of the technical expertise required and the aversiveness of the blood collection procedure for children, but they commonly collect saliva samples (e.g., for cortisol assay). Salivary measures of immune functioning are of interest due to the emerging field of periodontal medicine. This new paradigm proposes that oral health is a window into systemic health [2]. On the other hand, systemic disease can also affect oral health. Among the many important next steps is for research to characterize the correlates and concomitants of salivary markers of immunity and inflammation. The current study addresses this need by examining mental health correlates of secretory immunoglobulin A (SIgA) and salivary interleukin-6 (IL-6) in a large, ethnically diverse sample of children.
Oral Immunity and Inflammation
Among the least understood characteristics of the immune system for behavioral and developmental scientists is that its component subsystems are highly compartmentalized and localized. For this reason, salivary measures of immunity cannot replace serum or plasma measures, and should not be used to infer status beyond the oral cavity. While blood samples should remain the standard for immunologists, mucosal immunity is important in its own right because the oral, mucosal, and respiratory pathways are the route via which most foreign antigens gain access to the body’s internal tissues and cells [3].
The two major classes of soluble immune-related biomarkers present in oral fluids are immunoglobulins and cytokines. Immunoglobulins are proteins secreted by lymphoid cells (B-cells) that function to discriminate self versus other, selectively bind to foreign antigens (bacteria, fungi, toxins) and facilitate their elimination (i.e., agglutination). In mucosal secretions, the most common immunoglobulin is secretory IgA (SIgA) [4]. SIgA plays a key role as one of the body’s first lines of defense against foreign antigens present in the air or food [5, 6]. Cytokines are protein signaling molecules that lymphoid cells use to amplify or down regulate the inflammatory response. Interleukin-6 (IL-6) initiates and up-regulates inflammation. IL-6 triggers the release of acute phase proteins, which can inhibit the growth of microbial pathogens, regulate inflammatory response, attract immune cells to the site of injury or infection, and stimulate coagulation [7].
Behavior, Stress, Oral Immunity and Inflammation
Contemporary theorists champion the relevance of links between the brain, behavior, and immunity in relation to child health and adjustment [8]. Healthy adults show decreases in SIgA when recollecting depressing life events [9]. Greater depressed mood across a week-long period is associated with lower SIgA across this same period [10]. Other studies have also found that SIgA is lower on days when mood is more negative [11]. The link between negative mood and lower SIgA may be especially pronounced for those suffering from clinical levels of depression [12]. In one of the very few studies that examined relations between SIgA and mental health in children, psychotherapy designed to improve mental health was found to increase SIgA levels among a group of 8-12 year-old children who had experienced frequent upper respiratory tract infections [13].
Higher salivary IL-6 levels is linked to poor mental health. Adolescents with a diagnosis of major depressive disorder have marginally elevated plasma IL-6 [14]. Higher serum IL-6 is associated with greater PTSD symptoms in children and adolescents following an automobile accident [15]. IL-6 has also been linked with aggression and hostility; however, these studies made use of blood samples and it is not known if findings extend to salivary IL-6 [16].
Gaps in Current Research
There are several important gaps in current research. First, most studies have included only small sample sizes, making estimation of effect sizes unreliable. Second, prior work has been almost exclusively limited to adults. The human immune system continues to develop until adolescence, and problems with immune function that emerge in early life may become difficult to address in adulthood [1].
Another gap in research involves the identification of processes involved in links between mucosal immunity and mental health, including moderators of associations. A potential moderator of associations is child sex. Research consistently reports sex differences in immune activity. In comparison to men, women show greater resistance to some infections, exhibit greater cellular and humoral immune response, and suffer from several autoimmune disorders more frequently [17]. The mechanisms involved in the sex dimorphism in immunity are yet to be fully determined. There is evidence that it is mediated by sex steroids [18]. However, some studies report no effects of sex steroids on immune parameters, including serum IL-6 [19]. There may also be direct genetic effects on the immune system [20]. Regardless of the mechanism through which sex dimorphisms in immunity arise, it is important to consider whether sex moderates relations between immunity and health. In one of the very few studies to do so, associations between self-reported health and plasma IL-6 were stronger for women than for men [21].
The Present Study
The present study addresses two research questions: First, are salivary immune parameters (SIgA and Salivary IL-6) related to mental health measures in normally-developing children? This study makes use of a large (N > 300) and diverse (33% African American) sample of healthy community children. Consistent with the adult literature, we hypothesized that lower levels of SIgA but higher levels of salivary IL-6 would be linked to mental health problems. Second, do relations between salivary immune parameters and mental health differ based on child sex? This question is designed to advance process-oriented research in the study of immunology and developmental psychopathology. Based on prior work [21], we hypothesize that relations between salivary immune parameters and mental health will be stronger for girls than for boys.
Method
Participants
Data are from two independent longitudinal studies of the effects of family stress on children’s development. Given the similarity in sample characteristics, the two samples were aggregated for analyses. The first study included normally developing children in the 2nd or 3rd grade and their parents [22]. Families were eligible to participate if two parents were present in the home for at least 2 years, and based on parent report, children were not suffering from acute or chronic physical illness, ADHD, learning disability, or mental retardation. Of those families contacted who qualified for the study, 37% participated, 18% declined participation, and 45% were interested but were not included because the desired cell/sample size had been reached. Saliva samples were collected from children at the second time point (T2), which occurred one year after the first assessment (T1). All participants from T1 were eligible to participate at T2. However, not all families could be reached (e.g., because they moved away) or agreed to participate (e.g., because they were too busy). Of the original 261 families, 217 (83%) were included at T2, and saliva samples were assayed for SIgA for 195 children and were assayed for IL-6 for 179 children. The primary reason for missing data was insufficient saliva for assay.
The second study included children in the 3rd grade and their parents [23]. Eligibility criteria for inclusion were the same as for the first study. Of those families who qualified for the study, 66% participated and 34% declined participation and no families were interested but not included because desired cell/sample size had been reached. As was the case for the first study, saliva samples were collected from children at the second time point (T2), which occurred one year after the first assessment (T1). Again, not all families could be reached or agreed to participate. Of the original 177 families, 135 (76%) were included at T2, and saliva samples were assayed for SIgA for 134 children and were assayed for IL-6 for 128 children.
Thus, the current study includes data from a total of 329 children (from two investigations) whose saliva samples were assayed for SIgA; 307 children also have data for IL-6. Of these 329 children, 177 were girls and 152 were boys. Mean age was 9.85 years (SD = 0.98) and ranged from 7.58 to 11.92 years. Thirty three percent (n = 110) were African-American (AA) and the remainder were European-American (EA). Families represented the complete spectrum of socioeconomic levels based on standard criteria [39], with 24% in levels 1 or 2 (e.g., laborers); 36% in level 3 (skilled workers); and 39% in levels 4 or 5 (professionals).
Pubertal development of the children was assessed via maternal report on the Pubertal Development Scale [24]. This widely-used scale consists of ratings of whether certain characteristics have appeared in the child. These include growth spurt, pubic hair, skin change, facial hair growth for boys, voice change for boys, breast development for girls, and menarche for girls. Characteristics are rated on a 4-point scale: (1) no development; (2) development has barely begun; (3) development was definitely underway; (4) development is already complete. For the total score, items are averaged. In the present study, 74% of children were at level 1, 25% were at level 2, 5 children were at level 3 (< 1%) and 1 child was at level 4 (<1%). Six percent of girls had achieved menarche. Because sex steroids may be involved in sex differences, and puberty is associated with a surge in sex steroids, we also assess whether puberty level serves to moderate relations between immune parameters and mental health in children, and control for pubertal development in primary analyses.
Procedure
Both studies from which data were drawn were conducted with the approval of the Auburn University Internal Review Board. Procedures were identical across the two studies. Families received monetary compensation for their participation. Parents and children visited a university laboratory to complete a variety of measures. After obtaining informed consent from parents and assent from children, whole saliva was collected from the children (M time of day = 1:36 PM; SD = 2 hr 43 min) using a passive drool method [8]. To facilitate saliva production, children were asked by the experimenter to imagine they were chewing their favorite food, to close their eyes and imagine they were eating that food item, and to slowly and gently move their jaws as if chewing that food. The process of moving the jaw as if chewing generates saliva, which is then allowed to pool briefly under the tongue before being gently “drooled” through a short section of a common plastic drinking straw into a collection vial. This approach minimizes the influence of substances used to collect or stimulate saliva flow on immunoassays [25]. Samples were immediately placed on ice and frozen at -20° C. They were stored frozen until batched and shipped on dry ice overnight to Salimetrics labs (State College, PA). Samples were stored frozen at -80° C until assay. On the day of testing, samples were brought to room temperature, centrifuged at 3,000 RPM for 15 minutes, and the clear top-phase of the sample was pipetted into test tubes.
Measures
Determination of salivary biomarkers. Saliva samples were assayed for SIgA using a commercially available indirect enzyme immunoassay without modification to the manufacturer’s recommended protocol (Salimetrics, State College, PA). The assay used 25 μl of saliva per determination, has a lower limit of sensitivity of 2.5 μg/mL, range of standard curve from 2.5 to 600 μg/mL, and average intra- and inter-assay coefficients of variation are 5.6 and 8.8%. In the current study, 22% of samples were assayed in duplicate, and these two assays were almost perfectly correlated (r = .995, p < .001). Duplicates were only run twice where the %CV difference between the first two values was greater than 15%. Because only 22% of children had assays in duplicates, only results from the first SIgA assay were used in statistical analyses.
Samples were assayed for salivary IL-6 in duplicate using a modification of a commercially available enzyme immunoassay protocol (R & D Systems, MN). The test uses 25 μl of saliva per determination, has a lower limit of sensitivity of 0.16 pg/mL, range of standard curve from 0.16 to 10 pg/mL, and average intra- and inter-assay coefficients of variation are 9.3% and 6.2%, respectively. Method accuracy was determined by spike recovery (105.5%) and linearity was determined by serial dilution (95.4%). In the current study, the duplicate assays were run for 100% of samples and values were almost perfectly correlated (r = .998, p < .001). Duplicates were only run twice if the percent CV difference between the first two values was greater than 15%. The results from the two assays were averaged to provide a single measure of salivary IL-6 for statistical analyses.
Children’s Mental Health Problems. Mothers and fathers each completed the Personality Inventory for Children-II (PIC2) [26], which provides measures of children’s internalizing and externalizing symptoms. The Internalizing Symptoms scale includes subscale measures of psychological Distress, social Withdrawal, and Somatic concerns. The Externalizing Symptoms scale includes subscale measures of Impulsivity and Delinquency. Subscale scores were used in analyses for specificity. Items for both scales are rated as true or false, and true responses are summed and converted to T scores. The PIC2 is a widely-used measure and has demonstrated test-retest reliability, interrater reliability, as well as discriminant and construct validity [26, 27]. Mother and father reports were correlated on all subscales (rs = .34 to .57, ps < .001). Therefore, to reduce the number of statistical tests, mother and father reports were averaged to produce one aggregate score for each subscale.
Children also completed the 27 item Children’s Depression Inventory (CDI) [28], which assesses cognitive, emotional, and behavioral symptoms of depression. It is the most widely used measure of depression in childhood and has excellent psychometric properties, including reliability, convergent validity and discriminant validity [29-32]. Finally, children completed the 37 item Revised Children’s Manifest Anxiety Scale (RCMAS) [33]. The RCMAS is another widely used and well-established measure of children’s adjustment [34, 35].
Results
Analysis Plan
Salivary flow rate was computed by dividing the sample volume by time taken to provide the sample. Following guidelines provided by Salimetrics (College Park, PA), measured concentrations were then multiplied by the flow rate to provide a corrected assessment of SIgA and IL-6 for analyses. Following preliminary analyses, path models were fit using AMOS 17.0.0 [36]. Path modeling was chosen for two reasons. First, it enables the use of full information maximum likelihood estimation (FIML) to handle missing data. Options available for correlation and regression (e.g., casewise deletion and pairwise deletion) are likely to lead to bias in parameter estimates unless data are missing completely at random, a difficult assumption to meet [37]. Second, path modeling enables estimation of multiple dependent variables at the same time, reducing the number of analyses and the probability of a type I error.
Initial models were fit where the study in which children participated was included as a covariate and a moderator of relations between predictors of interest and children’s mental health. No significant interactions were found, nor was study (i.e., 1 or 2) related to any of the dependent variables. Thus, study was removed from all subsequent models to preserve parsimony. Separate models were fit for SIgA and IL-6. The centered immune parameter, sex, and the Immune x Sex interaction were the independent variables. Puberty was also included as a covariate. In each model, seven measures of child mental health were considered as dependent variables: Depression, Anxiety, Impulsivity, Delinquency, Distress, Withdrawal, and Somatic Complaints. Models are presented in Figures 1 and 2. Significant interactions were probed using multi-group models, with boys and girls representing the two groups.
Figure 1.
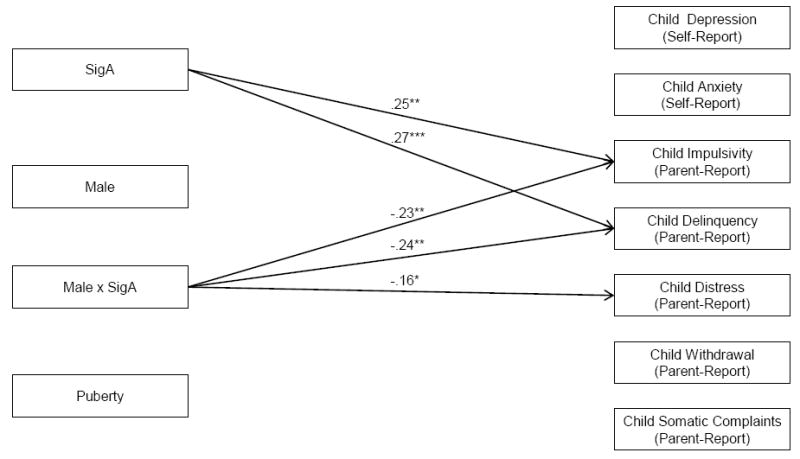
Interactions between SIgA and Child Sex in Prediction of Children’s Mental Health Problems. Note: Standardized path coefficients are presented and are interpreted similarly to regression coefficients; Only significant path coefficients are indicated with arrows; *p < .05; **p < .01; ***p < .001. Models are fully saturated and therefore no fit indices were generated or presented.
Figure 2.
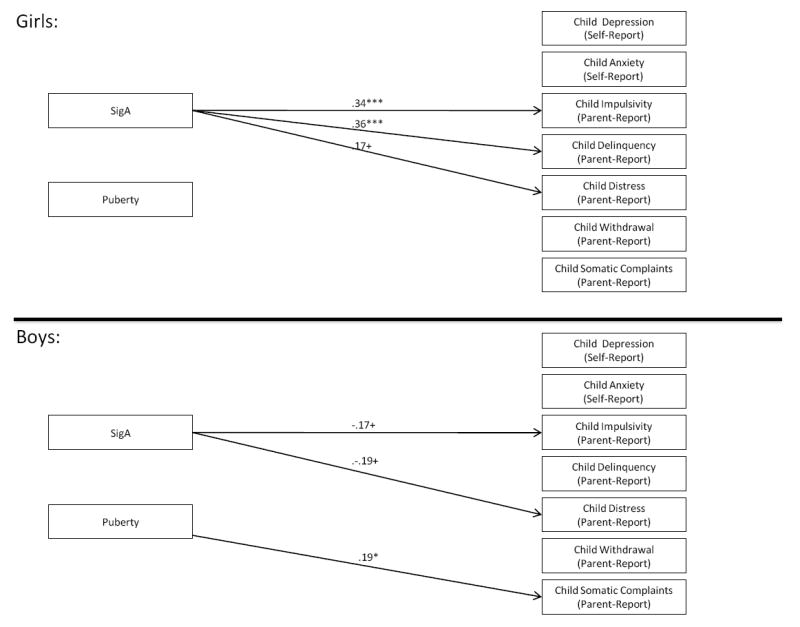
Results from Multigroup Models of SIgA as a Predictor of Children’s Mental Health. Note: Standardized path coefficients are presented and are interpreted similarly to regression coefficients; Only significant path coefficients are indicated with arrows; +p < .10, *p < .05; **p < .01; ***p < .001. Models are fully saturated and therefore no fit indices were generated or presented.
Following similar procedures, subsequent models were then fit substituting pubertal level for sex and determining whether puberty moderates associations between salivary immune parameters and child mental health. Significant interactions would suggest that hormonal surges involved in puberty may account for differences in relations across gender. Models were fully saturated and therefore fit indices are not generated nor reported.
Preliminary analyses
Means, standard deviations, and sex comparisons for study variables are provided in Table 1. Salivary IL-6 was greater in boys than in girls, t(254) = 2.59, p < .05, and girls were further along in pubertal development, t(340) = 7.74, p < .001. No other sex-related effects were observed. Correlations among variables are provided in Table 2. SIgA and salivary IL-6 were positively correlated, r(181) = .36, p < .001. No bivariate correlations between salivary immune parameters and child maladjustment were observed.
Table 1.
Means, Standard Deviations, and Comparisons by Gender
| Variable | Boys | Girls | t | df | ||
|---|---|---|---|---|---|---|
| M | SD | M | SD | |||
| SigA | 1.29 | 28.61 | -1.14 | 32.67 | 0.55 | 195 |
| IL-6 | 2.61 | 21.53 | -2.41 | 5.57 | 2.59* | 254 |
| Child Depression (Self-Report) | 7.43 | 8.09 | 6.39 | 7.68 | 1.23 | 344 |
| Child Anxiety (Self-Report) | 9.96 | 8.43 | 9.74 | 8.48 | 0.23 | 340 |
| Child Impulsivity (Parent-Report) | 49.14 | 8.26 | 49.31 | 7.96 | 0.19 | 348 |
| Child Delinquency (Parent-Report) | 47.54 | 7.54 | 47.97 | 6.71 | 0.57 | 348 |
| Child Distress (Parent-Report) | 49.19 | 9.09 | 48.21 | 7.68 | 1.10 | 348 |
| Child Withdrawal (Parent-Report) | 47.46 | 7.50 | 47.29 | 7.94 | 0.20 | 348 |
| Child Somatic Complaints (Parent-Report) | 47.81 | 6.20 | 47.15 | 6.20 | 0.99 | 348 |
| Child Puberty Level (1 – 4) | 1.45 | .40 | 1.84 | .52 | 7.74*** | 340 |
Note: Salivary immune parameters have been centered and adjusted for flow rate;
p < .05
Table 2.
Bivariate Correlations among Study Variables
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Sig-A | - | ||||||||||
| 2. IL-6 | .36* | - | |||||||||
| 3. Child Depression (Self-Report) | -.07 | .04 | - | ||||||||
| 4. Child Anxiety (Self-Report) | .06 | .04 | .66* | - | |||||||
| 5. Child Impulsivity (Parent-Report) | .09 | .04 | .03 | .08 | - | ||||||
| 6. Child Delinquency (Parent-Report) | .11 | .05 | .03 | .09 | .79* | - | |||||
| 7. Child Distress (Parent-Report) | .01 | .00 | .04 | .07 | .49* | .49* | - | ||||
| 8. Child Withdrawal (Parent-Report) | -.03 | .02 | .06 | .10 | .14* | .12* | .44* | - | |||
| 9. Child Somatic Complaints (Parent-Report) | .03 | .02 | .06 | .12* | .38* | .35* | .60* | .38* | - | ||
| 10. Child Puberty Level (1 – 4) | -.05 | -.06 | -.04 | -.02 | -.06 | -.05 | .03 | -.03 | .07 | - | |
| 11. Study Child Participated In | -.02 | .16* | -.04 | .01 | -.02 | .02 | .06 | .01 | .05 | .24* | - |
p < .05
Relations with SIgA
See Figure 1. Controlling for puberty, SIgA interacted with child sex to predict Impulsivity, β = -.23, p < .01; Delinquency, β = -.24, p < .01; and Distress, β = -.16; p < .05. Multigroup modeling (Figure 2) indicated that higher SIgA was associated with marginally lower Impulsivity for boys, β = -.17, p < .10, but significantly greater Implusivity for girls, β = .34, p < .001. Higher SIgA was unrelated to Delinquency in boys, but was related to significantly greater Delinquency in girls, β = .36, p < .001. Higher SIgA was also linked to marginally lower Distress in boys, β = -.19, p < .10, but marginally higher Distress in girls, β = .17, p < .10.
Next, a model was fit in which puberty was considered as a possible moderator of relations between SIgA and child mental health. This model was identical in form to that shown in Figure 1, with puberty as an interacting variable and sex as the covariate. Only one significant interaction was observed: the puberty x SIgA interaction predicted Delinquency, β = -.16, p < .05. The relation between SIgA and Delinquency is positive but nonsignificant at the mean puberty level, β = .05, p = .47. For below-mean puberty levels, the association grows increasingly positive, while for above-mean puberty levels the association grows negative. Given that females were found to have higher puberty levels, these results are consistent with results of models considering sex as a moderator and suggest the possibility that greater pubertal development among girls may account for why SIgA is related more strongly to girls’ Delinquency. A model including both interactions would not converge, however.
Models of Relations with Salivary IL-6
See Figure 3. Salivary IL-6 interacted with child sex to predict Depression, β = -.52, p < .05, Anxiety, β = -.56, p < .05, Impulsivity, β = -.44, p < .05, Withdrawal, β = -.50, p < .05, and Distress, β = -.47, p < .05. The interaction marginally predicted Delinquency, β = -.37, p < .10 and Somatic Complaints, β = -.41, p < .10. Multigroup modeling (Figure 4) indicated that Salivary IL-6 was unrelated to boys’ mental health. However, higher Salivary IL-6 was related to greater Depression, β = .23, p < .01, Anxiety, β = .25, p < .01, and Impulsivity, β = .17, p < .05, among girls. There were marginal associations between Salivary IL-6 and girls’ Withdrawal, β = .15, p < .10, Distress, β = .15, p < .10, and Delinquency, β = .16, p < .10. Thus, relations between Salivary IL-6 and children’s adjustment were stronger for girls than for boys.
Figure 3.
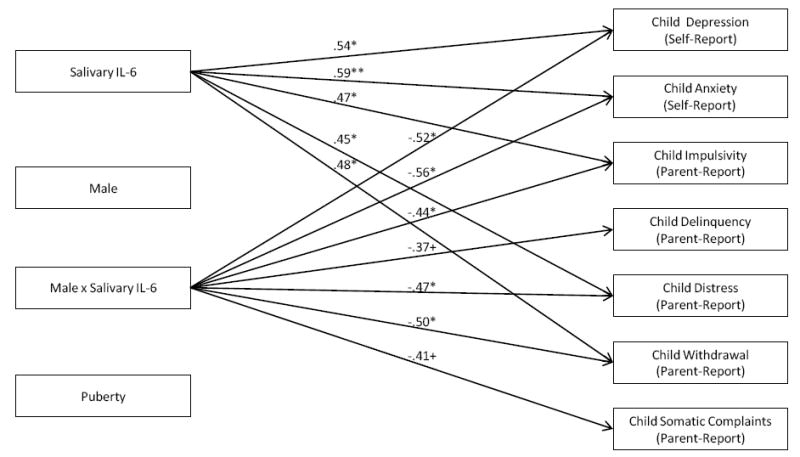
Interactions between IL-6 and Child Sex in Prediction of Children’s Mental Health Problems. Note: Standardized path coefficients are presented and are interpreted similarly to regression coefficients; Only significant path coefficients are indicated with arrows;*p < .05; **p < .01; ***p < .001. Models are fully saturated and therefore no fit indices were generated or presented.
Figure 4.
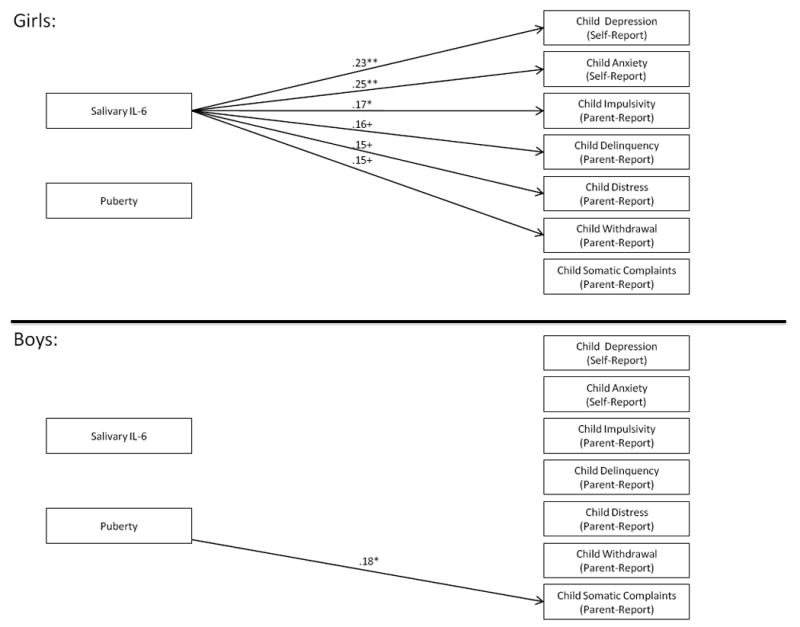
Results from Multigroup Models of Salivary IL-6 as a Predictor of Children’s Mental Health. Note: Standardized path coefficients are presented and are interpreted similarly to regression coefficients; Only significant path coefficients are indicated with arrows; +p < .10, *p < .05; **p < .01; ***p < .001. Models are fully saturated and therefore no fit indices were generated or presented.
Next, a model was fit in which puberty was considered as a possible moderator of relations between Salivary IL-6 and child mental health. This model was identical in form to that shown in Figure 3, with puberty as the interacting variable and sex as the covariate. Only one significant interaction was observed: the puberty x Salivary IL-6 interaction predicted Withdrawal, β = .18, p < .05. The relation between Salivary IL-6 and Withdrawal is positive but nonsignificant at the mean puberty level, β = .09, p = .27. For below-mean puberty levels, the association grows negative, while for above-mean puberty levels the association grows increasingly positive. Given that females were found to have higher puberty levels, these results are consistent with results of models considering sex as a moderator and suggest the possibility that greater pubertal development among girls may account for why SIgA is related more strongly to girls’ Withdrawal. However, a model including both interactions indicated that neither interaction term significantly predicts Withdrawal when controlling for the other.
Discussion
The primary purpose of the current study was to examine relations between salivary immune parameters (SIgA and salivary IL-6) and children’s mental health. Numerous associations were found indicating that greater oral immune activity is associated with internalizing and externalizing symptoms for girls, while some marginal negative associations were found for boys. Specifically, higher levels of SIgA were associated with girls’ greater Impulsivity, Delinquency, and (marginally) emotional Distress. Higher levels of Salivary IL-6 were associated with girls’ greater self-reported Depression and Anxiety, as well as parent-reported Impulsivity and (marginally) social Withdrawal, Emotional Distress, and Delinquency. Higher SIgA was marginally associated with boys’ lower Impulsivity and emotional Distress. This study has broken new ground in demonstrating relations between salivary immune parameters and children’s mental health. It is important to note that the human body is exposed to antigens on a daily basis, primarily via the respiratory and gastrointestinal systems. So while mucosal immune activity may not be representative of immune activity throughout the body and cannot replace serum and plasma assessments of immunity, it is an important dimension of immune health.
It was hypothesized that higher levels of salivary IL-6 would be associated with greater mental health problems, and results are therefore supportive of hypotheses and are especially compelling given that associations were found for both self and parent reports of child mental health. Findings are also consistent with prior research. For example, among healthy adults, higher plasma IL-6 is associated with trait hostility and negative affect [16]. Adolescents with a diagnosis of major depressive disorder have marginally elevated plasma IL-6 [14]. Higher serum IL-6 is associated with greater PTSD symptoms in children and adolescents following an automobile accident [15]. The body of evidence is therefore converging on high levels of IL-6 as a risk factor for psychological problems, possibly due to the results of inflammation [38].
However, it was also hypothesized that lower levels of SIgA would be associated with greater mental health problems. Results of analyses with SIgA suggest the opposite pattern, and these findings are inconsistent with the adult literature. For example, depressed mood or clinical depression has been associated with lower SIgA in several studies of adults [10-12]. There are very few studies of SIgA in children. However, a study of the effects of psychotherapy indicated that it increases chronically ill school-children’s SIgA levels [13]. Discrepancies in results between the current study and prior work may be due to the sample. The current study is one of the first studies to consider a large, healthy community sample of children. Higher levels of SIgA may be more adaptive in children coping with chronic illness as immune activation is required to fight off infection, but less adaptive for healthy children who are not suffering from chronic or acute illness and for whom immune activation may develop into overactivation.
Overactive immune systems can do serious damage to the human body (e.g., autoimmune disorders). Therefore, associations between the brain, endocrine system, and immune system involve inhibitory connections that prevent an unchecked immune response [39]. Where immune parameters such as SIgA remain at high levels, there may be increased risk for adverse effects, including mental health issues. Further, children’s immune systems are undergoing development. High levels of SIgA in childhood may have different implications for well-being than they do in adulthood. Overactivation of the immune system may have more serious consequences for children because their immune systems are not fully equipped for the sophisticated adult response to stress and immune challenge. Obviously these attempted explanations for findings are speculative and additional research with healthy children is needed.
It is interesting that SIgA was primarily associated with girls’ externalizing problems (Implusivity, Delinquency) and was only marginally related to one measure of internalizing problems (Emotional Distress). This was not the case for Salivary IL-6, as there were relations with girls’ Depression, Anxiety, and Impulsivity. Findings need to be replicated and only tentative explanations can be offered. One possibility is that the higher levels of SIgA represented low stress among the girls [40, 41] and such low stress may be related to greater approach behavior, lower inhibition, and reduced fears (potentially including fear of punishment). These characteristics have been associated with impulsivity and delinquency [42, 43]. It should also be noted that relations with SIgA were found only for parent report of child mental health. It is therefore possible that SIgA is not as strongly tied to child mental health as Salivary IL-6 is.
The larger question is why immune parameters that vary from day to day would be related to mental health. It would seem that immune parameters would have to have some degree of stability in order to reliably predict mental health. The present study is not able to provide a definitive answer to this question. However, we propose a few possible explanations: (1) There may be more stability in salivary immune parameters than has been previously thought; (2) Daily variations may represent noise, but the core component of variation is stable; and (3) mental health symptoms are related to biological activity that either directly or indirectly involves the immune system. Considering these possibilities is an exciting direction for the next generation of research on mental health correlates of both systemic and mucosal immunity.
Finally, the current study examined child sex as a moderator of relations. As described above, the majority of relations between mucosal immunity and child mental health were moderated by sex. Significant relations between SIgA and Salivary IL-6 were only observed for girls. It was expected that relations between mucosal immune parameters would be stronger for girls than for boys, and results generally supported this hypothesis. Findings are consistent with prior research [18]. Relations between plasma IL-6 and perceptions of physical health have been found to be stronger for women than for men [21]. The current study extends this research to the context of children, and suggests that relations between mental health and salivary IL-6 and SIgA are stronger for girls than for boys.
The mechanisms underlying the commonly found sex dimorphism in immunity have yet to be fully delineated. One possibility is that the observed sex differences are due to the effects of sex hormones. Sex hormones surge in levels during puberty. We controlled for puberty level when considering relations, and thus the reported relations are not due to puberty level (and presumably the hormonal surge associated with puberty). We further tested whether relations between salivary immune parameters and child mental health were moderated by puberty level. Only 2 significant interactions were found, in comparison to several observed with sex, further confirming that observed interactions were not due to puberty. Nevertheless, the observed interactions with puberty were consistent with interactions found for sex, and we were not able to establish that sex continued to moderate associations after controlling for interactions with puberty. Another possibility is that that the effects of sex hormones on the immune system may emerge as a result of early (e.g., neonatal) organization effects rather than as the result of increasing sex hormone levels beginning around puberty [44]. Direct genetic effects on the immune system have also been documented [20]. Future research on immunology and developmental psychopathology should continue to explore differences in relations based on child sex and potential mediators of associations.
There are limitations to the current study. First, the study was cross-sectional in design, raising important questions about causality. However, activation of the immune system has been shown to cause alterations in human behavior, most notably increased sleepiness, reduced activity, and lower arousal [45]. These behaviors may develop into behavioral problems in the context of chronic immune activation [56]. Further, the immune system may affect the biological systems which support human behavior more generally [46]. Nevertheless, additional longitudinal research is needed to establish the direction of effects among mucosal immunity, child mental health, and child sex.
An additional limitation of the study is the use of healthy community children. As a result, caution should be taken when attempting to generalize findings to the context of chronic or acute illness. Maternal interview was used to screen for child health status, which is a limitation of this study and future research should use a physical and oral examination. It is possible that observed variation in immune parameters was due to oral health problems. However, periodontitis is exceptionally rare in children. Also, findings may not generalize to other developmental periods, and an exciting avenue for future research would include the consideration of relations in both younger (e.g., infancy and preschool) and older (e.g., adolescence) populations. Finally, the current study focused on two mucosal immune parameters: salivary IL-6 and SIgA. Future studies are needed to consider alternative immune parameters, including those derived from blood samples.
Despite these limitations, the current study has significantly advanced understanding of immunology and developmental psychopathology. Results indicate that mucosal immune parameters are consistently associated with girls’ risk for mental health problems and additional research on the effects and correlates of mucosal immunity should be encouraged. This is the first known study to extend findings of sex differences in relations between immune parameters and well-being to children.
Acknowledgments
This research was supported in part by AAES grant ALA080-049, a National Science Foundation Grant 0623936 and a National Institutes of Health Grant R01-HD046795. We are especially grateful to the students and laboratory staff who provided assistance, especially Lori Staton and Bridget Wingo. We would also like to thank the children and families who participated in this research.
Footnotes
Note: In the interest of full disclosure, Dr. Granger is the founder and Chief Scientific and Strategy Advisor of Salimetrics LLC (State College, PA).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Granger DA, Granger GA, Granger SW. Immunology and developmental psychopathology. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology, Vol II: Developmental neuroscience. 2. Hoboken, NJ: John Wiley & Sons; 2006. pp. 677–709. [Google Scholar]
- 2.Scannapieco FA. Position paper of the American Academy of Periodontology: Periodontal disease as a potential risk factor for systemic disease. Journal of Periodontology. 1998;69:841–50. [PubMed] [Google Scholar]
- 3.Kolenbrander PE, Anderson RN, Blehert DS, Egland PG, Foster JS, Palmer RJ. Communication among oral bacteria. Microbiology and molecular biology reviews. 2002;66:486–505. doi: 10.1128/MMBR.66.3.486-505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russell MW, Hajishengallis G, Childers NK, Mchalek SM. Secretory immunity in defense against cariogenic mutans streptococci. Caries Res. 1999;33:4–15. doi: 10.1159/000016490. [DOI] [PubMed] [Google Scholar]
- 5.Brandtzaeg P. Salivary immunoglobulins. In: Tenovuo JO, editor. Human saliva: Clinical chemistry and microbiology. Boca Raton, FL: CRC Press; 1989. pp. 1–54. [Google Scholar]
- 6.Quan CP, Berneman A, Pires R, Avrameas S, Bouvet JP. Natural polyreactive secretory immunoglobulin A autoantibodies as a possible barrier to infection in humans. Infect Immun. 1997;65:3997–4004. doi: 10.1128/iai.65.10.3997-4004.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. The New England Journal of Medicine. 1999;340:448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 8.Granger D, Kivlighan KT, Fortunato C, Harmon AG, Hibel EB, Whemboua GL. Integration of salivary biomarkers into developmental and behaviorally-oriented research: Problems and solutions for collecting specimens. Physiol Behav. 2007;92:583–90. doi: 10.1016/j.physbeh.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Tsuboi H, Hamer M, Tanaka G, Takagi K, Kinae N, Steptoe A. Responses of ultra-weak chemiluminescence and secretory IgA in saliva to the induction of angry and depressive moods. Brain, Behavior, and Immunity. 2008;22:209–14. doi: 10.1016/j.bbi.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Izawa S, Hirata U, Kodama M, Nomura S. The effects of daily events and moods on secretory immunoglobulin A in saliva. Japanese Journal of Physiological Psychology and Psychophysiology. 25:237–44. [Google Scholar]
- 11.Stone AA, Cox DS, Valdimarsdottir H, Jandorf L, Neale JM. Evidence that secretory IgA antibody is associated with daily mood. Journal of Personality and Social Psychology. 1987;52:988–93. doi: 10.1037//0022-3514.52.5.988. [DOI] [PubMed] [Google Scholar]
- 12.Takagi S, Ohira H. Effects of expression and inhibition of negative emotions on health, mood states, and salivary secretory immunoglobulin A in Japanese mildly depressed undergraduates. Perceptual and Motor Skills. 2004;98:1187–98. doi: 10.2466/pms.98.3c.1187-1198. [DOI] [PubMed] [Google Scholar]
- 13.Hewson-Bower B, Drummond PD. Psychological treatment for recurrent symptoms of colds and flu in children. Journal of Psychosomatic Research. 2001;51:369–77. doi: 10.1016/s0022-3999(01)00212-4. [DOI] [PubMed] [Google Scholar]
- 14.Gabbay V, Klein RG, Alonso CM, Babb JS, Nishawala M, De Jesus G, et al. Plasma immune dysregulation in adolescent major depressive disorder. Journal of Affective Disorders. 2009;115:177–82. doi: 10.1016/j.jad.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pervanidou P, Kolaitis G, Charitaki S, Margeli A, Ferentinos S, Bakoula C, et al. Elevated morning serum interleukin (IL)-6 or evening salivary cortisol concentrations predict posttraumatic stress disorder in children and adolescents six months after a motor vehicle accident. Psychoneuroendocrinology. 2007;32:991–99. doi: 10.1016/j.psyneuen.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Marsland AL, Prather AA, Peterson KL, Cohen S, Manuck SB. Antagonistic characteristics are positively associated with inflammatory markers independently of trait negative emotionality. Brain, Behavior, and Immunity. 2008;22:753–61. doi: 10.1016/j.bbi.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Human Reproduction Update. 2005;11:411–23. doi: 10.1093/humupd/dmi008. [DOI] [PubMed] [Google Scholar]
- 18.Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nature Reviews: Immunology. 2008;8:737–44. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Connor MF, Motivala SJ, Valladares EM, Olmstead R, Irwin MR. Sex differences in monocyte expression of IL-6: role of autonomic mechanisms. Am J Physiol Regul Integr Comp Physio. 2007;293:R145–51. doi: 10.1152/ajpregu.00752.2006. [DOI] [PubMed] [Google Scholar]
- 20.De Vries GJ. Sex steroids and sex chromosomes at odds? Endocrinology. 2005;146:3277–79. doi: 10.1210/en.2005-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lekander M, Elofsson S, Neve IM, Hansson LO, Unden AL. Self-rated health is related to levels of circulating cytokines. Psychosomatic Medicine. 2004;66:559–63. doi: 10.1097/01.psy.0000130491.95823.94. [DOI] [PubMed] [Google Scholar]
- 22.El-Sheikh M, Cummings EM, Kouros CD, Elmore-Staton L, Buckhalt J. Marital psychological and physical aggression and children’s mental and physical health: Direct, mediated, and moderated effects. Journal of Consulting and Clinical Psychology. 2008;76:138–48. doi: 10.1037/0022-006X.76.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Sheikh M, Buckhalt JA, Keller PS, Granger DA. Children’s objective and subjective sleep disruptions: Links with afternoon cortisol levels. Health Psychology. 2008;27:26–33. doi: 10.1037/0278-6133.27.1.26. [DOI] [PubMed] [Google Scholar]
- 24.Peterson AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–33. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 25.Granger DA, Kivlighan KT, El-Sheikh M, Gordis EB, Straud LR. Assessment of salivary alpha-amylase in biobehavioral research. In: Luecken LJ, Gallo LC, editors. Handbook of physiological research methods in health psychology. Thousand Oaks, CA: Sage Publications; 2008. pp. 95–113. [Google Scholar]
- 26.Lachar D, Gruber CP. PIC-2. 2. Los Angeles: Western Psychological Services; 2001. Personality Inventory for Children. [Google Scholar]
- 27.Wirt RD, Lachar D, Klinedinst JK, Seat PD. Multidimensional description of child personality: A manual for the Personality Inventory for Children. Los Angeles: Western Psychological Services; 1990. [Google Scholar]
- 28.Kovacs M. Children’s Depression Inventory. New York: Multi-Health Systems; 1992. [Google Scholar]
- 29.Carey MP, Faulstich ME, Gresham FM, Ruggiero L, Enyart P. Children’s Depression Inventory: Construct and discriminant validity across clinical and nonreferred (control) populations. Journal of Consulting and Clinical Psychology. 1987;55:755–61. doi: 10.1037//0022-006x.55.5.755. [DOI] [PubMed] [Google Scholar]
- 30.Cole DA, Hoffman K, Tram JM, Maxwell SE. Structural differences in parent and child reports of children’s symptoms of depression and anxiety. Psychological Assessment. 1998;12:174–85. doi: 10.1037//1040-3590.12.2.174. [DOI] [PubMed] [Google Scholar]
- 31.Kovacs M. The Children’s Depression Inventory: A self-rated depression scale for school-aged youngsters. University of Pittsburgh School of Medicine. 1983 Unpublished Manuscript. [Google Scholar]
- 32.Kovacs M. The Children’s Depression Inventory. North Tonawanda, NY: Multi-Health Systems; 1991. [Google Scholar]
- 33.Reynolds CR, Richmond BO. What I think and feel: A revised measure of children’s manifest anxiety. Journal of Abnormal Child Psychology. 1978;6:271–80. doi: 10.1007/BF00919131. [DOI] [PubMed] [Google Scholar]
- 34.Muris P, Merckelbach H, Ollendick T, King N, Bogie N. Three traditional and three new childhood anxiety questionnaires: Their stability and validity in a normal adolescent sample. Behavior Research and Therapy. 2002;40:753–72. doi: 10.1016/s0005-7967(01)00056-0. [DOI] [PubMed] [Google Scholar]
- 35.Seligman LD, Ollendick TH, Langley AK, Baldacci HB. The utility of measures of child and adolescent anxiety: A meta-analytic review of the Revised Children’s Manifest Anxiety Scale, the State-Trait Anxiety Inventory for Children, and the Child Behavior Checklist. Journal of Clinical Child and Adolescent Psychology. 2004;33:557–65. doi: 10.1207/s15374424jccp3303_13. [DOI] [PubMed] [Google Scholar]
- 36.Arbuckle JL. Analysis of Moment Structures 17.0.0. Crawfordville, FL: Amos Development Corporation; 2008. [Google Scholar]
- 37.Allison PD. Missing data techniques for structural equation modeling. Journal of Abnormal Psychology. 2003;112:545–57. doi: 10.1037/0021-843X.112.4.545. [DOI] [PubMed] [Google Scholar]
- 38.Woods A, Brull DJ, Humphries SE, Montgomery HE. Genetics of inflammation and risk of coronary artery disease: The central role of interleukin-6. European Heart Journal. 2000;21:1574–83. doi: 10.1053/euhj.1999.2207. [DOI] [PubMed] [Google Scholar]
- 39.Ader R. On the development of psychoneuroimmunology. European Journal of Pharmacology. 2000;405:167–76. doi: 10.1016/s0014-2999(00)00550-1. [DOI] [PubMed] [Google Scholar]
- 40.Deinzer R, Schuller N. Dynamics of stress-related decrease of salivary immunoglobulin A (SIgA): Relationship to symptoms of the common cold and studying behavior. Behavioral Medicine. 1998;23:161–69. doi: 10.1080/08964289809596372. [DOI] [PubMed] [Google Scholar]
- 41.Nozaki T, Hirao A, Nagasawa S, Sonomoto M, Nagata S, Daito M, et al. Utility of salivary SIgA as a marker for assessing stress caused by dental treatment. Journal of Oral Biosciences. 2007;49:128–35. [Google Scholar]
- 42.Foster JD, Trimm RF. On being eager and uninhibited: Narcissism and approach-avoidance motivation. Personality and Social Psychology Bulletin. 2008;34:1004–17. doi: 10.1177/0146167208316688. [DOI] [PubMed] [Google Scholar]
- 43.Kerr M, Tremblay RE, Pagani L, Vitaro F. Boys’ behavioral inhibition and the risk of later delinquency. Archives of General Psychiatry. 1997;54:809–16. doi: 10.1001/archpsyc.1997.01830210049005. [DOI] [PubMed] [Google Scholar]
- 44.Collaer ML, Hines M. Human behavioral sex differences: A role for gonadal hormones during early development? Psychological Bulletin. 1995;118:55–107. doi: 10.1037/0033-2909.118.1.55. [DOI] [PubMed] [Google Scholar]
- 45.Dantzer R. Cytokine-induced sickness behavior: Where do we stand? Brain, Behavior, and Immunity. 2001;15:7–24. doi: 10.1006/brbi.2000.0613. [DOI] [PubMed] [Google Scholar]
- 46.Dantzer R. Cytokine, sickness behavior, and depression. Immunology and Allergy Clinics of North America. 2009;29:247–64. doi: 10.1016/j.iac.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


