Abstract
Objective
Potential predictive/prognostic angiogenic markers were prospectively examined in a phase II trial of bevacizumab in epithelial ovarian cancer (EOC)/primary peritoneal cancer (PPC).
Methods
Recurrent/persistent EOC/PPC patients were treated with bevacizumab (15mg/kg IV q21days) until disease progression. Validated-immunohistochemistry (IHC) assays were performed on pre-cycle 1/4 tumor biopsies for CD31-microvessel density (MVD), VEGF-histoscore (HS), p53-HS, and TSP1 image analysis score (IA). Pre-cycle 1/4 serum and plasma VEGF were quantified using a validated-ELISA.
Results
CD31-MVD and serum VEGF, evaluated pre-cycle 1 in 41/61 and 51/61 eligible patients, respectively, did not appear to be correlated. High CD31-MVD, categorized at the median, appeared to be associated with tumor response, a 13-month shorter median survival, and an increased risk of death (unadjusted hazard ratio [HR]=2.2, 95% confidence interval [CI]=1.067–4.467). In addition, each standard deviation (SD) increase in CD31-MVD appeared to be associated with worse survival in unadjusted and adjusted analyses. IHC and plasma biomarkers did not change with bevacizumab treatment except for serum VEGF, which appeared to decrease during bevacizumab treatment. This decrease was not associated with response. High pre-cycle 1 serum VEGF, categorized at the median, was associated with 22-month shorter median survival and an increased risk of death (unadjusted HR=2.7, 95% CI=1.369–5.191). Categorized p53 appeared to be associated with unadjusted survival and each SD increase in TSP1-IA appeared to be associated with a decreased risk of progression in unadjusted and adjusted analyses.
Conclusions
Despite the limitations in sample size and exploratory nature of the study, angiogenic markers in tumor and serum may provide prognostic value in recurrent/persistent EOC/PPC, and are being prospectively evaluated in the GOG phase III trial of carboplatin, paclitaxel and bevacizumab/placebo in previously-untreated EOC/PPC.
Keywords: Ovarian Cancer, angiogenesis, bevacizumab, VEGF, CD31, biomarker
INTRODUCTION
Despite advances in the treatment of epithelial ovarian cancer (EOC), most women are expected to relapse and ultimately succumb to this disease [1]. New therapies are needed to improve patient survival and quality of life. Angiogenesis is one of the cardinal processes leading to invasion and metastasis of solid tumors [2] and appears to be an important target for cancer therapeutics. Recently, bevacizumab, a humanized monoclonal antibody that binds to vascular endothelial growth factor (VEGF), has shown clinical activity in patients with EOCs [3–5]. In these trials, patients with recurrent or persistent EOCs treated with bevacizumab either alone or in combination with other cytotoxic therapies have shown a 16–24% response rate, and 28–56% of patients demonstrated progression-free survival (PFS) ≥ 6-months. Further studies are needed to define the clinical factors and/or markers that predict treatment response and outcome to anti-angiogenic agents like bevacizumab [6].
We have previously noted that increased angiogenesis (high CD31 microvessel density (MVD) was associated with poor clinical outcomes, decreased thrombospondin-1 (TSP-1) levels and increased mutant p53 levels in prostate cancer [7]. CD31 is a pan-endothelial marker found on endothelial cells, endothelial and stromal precursors, macrophages and CD4+ B-cells and provides a histomorphometric measure of MVD in solid tumors [8]. VEGF, the target of bevacizumab, is a key pro-angiogenic factor that binds to a family of VEGF receptors and activates downstream pathways that stimulate endothelial cell growth, migration and survival, and regulate vascular permeability, mobilization of endothelial progenitor cells from bone marrow to distant sites of neovascularization, and tumor cell chemoresistance. TSP-1 is a complex protein that primarily functions as an endogenous angiogenesis inhibitor but can also stimulate angiogenesis via its 25-kDa heparin-binding domain and promote cell invasion by modulating extracellular proteases in later stages of cancer progression [9–14]. The p53 tumor suppressor was also examined in our study given the prevalence of p53 alterations (mutations and overexpression) previously described in EOC [15]. We report here the relationship of these angiogenic markers to clinical parameters in EOC patients treated with bevacizumab.
MATERIALS AND METHODS
Study Population and Clinical Samples
Sixty two patients with recurrent or persistent EOC/primary peritoneal cancer (PPC) patients were accrued from April 2002 to August 2004 from participating GOG institutions. These patients were treated with single agent bevacizumab (15mg/kg IV q21days) until disease progression [3]. As part of the planned translational research for this phase II trial, tumor tissue biopsies and serum/plasma samples were collected prior to the 1st and 4th cycle of bevacizumab treatment. In addition, serum/plasma samples were collected when the patients went off-treatment due to disease progression or toxicity.
Immunohistochemistry
Immunohistochemistry (IHC) assays were performed on formalin-fixed, paraffin-embedded tissue sections to detect CD31 (JC70A clone, Dako, Carpinteria, CA), TSP-1 (8A6B clone, Vision Biosystems, Norwell, MA), VEGF (Ab-1 clone, Lab Visions Corp, Fremont, CA) and p53 (DO-1 clone, Santa Cruz Biotechnology, Santa Cruz, CA). For each antibody, slides were organized in batches and staining was performed using an automated IHC stainer in two separate runs with appropriate positive and negative controls for each run. Antigen retrieval was performed using steam heat in buffer recommended by the manufacturer for each antibody. Antibodies were incubated for one hour at 23°C. EnVision Plus Detection system (DAKO, Carpinteria, CA) was used for antigen detection. Tissue sections were analyzed using standard light microscopy for stain intensity (0 to 4+) and identifying the percentage of positively stained cells. MVD counts were measured by counting CD31 IHC hot spots in three separate 400× fields [7]. Image analysis was performed for TSP-1. Photomicrographs of regions of IHC stained tissue sections were taken using a digital camera, and images were imported into Adobe Photoshop CS (Adobe, San Jose CA) and adjusted to “autolevels.” A saved color set containing the blue/green background colors was used to replace all background staining with white color (lightness = +100) such that only brown DAB staining remained in the image. The image was then imported into Kodak Image Station 2000 MM software, were the Auto ROI function was used to select the gray and black pixels. Area and intensity analysis were used to calculate average intensity per unit area and/or percent of area stained. For VEGF and p53, histoscores (HS) were determined by multiplying the percent of cells staining positive by the intensity of staining plus 1 (% positive × (intensity +1)) as described previously [7].
Enzyme-Linked Immunosorbent Assay
Circulating levels of VEGF were quantified in serum and plasma using an enzyme-linked immunosorbent assay (ELISA) validated by R&D Systems (Minneapolis, MN). Serum was prepared from blood drawn in a plain red top tube and centrifuged after a 30-minute incubation at room temperature to remove cells and the fibrin clot. Plasma was prepared from blood drawn into a purple top tube containing the anti-coagulant EDTA and centrifuged to remove cells. Serum and plasma were split into cryogenic vials, frozen and stored at ≤ −70°C prior to testing. All ELISA samples were run according to manufacturer’s specifications in triplicate and concentrations were interpolated from a VEGF standard curve.
Statistical Analysis
SAS version 9.1 (SAS Inc., 2003) was used to perform statistical analyses. Clinical response and PFS ≥ 6-months were considered as ordinal categorical variables. PFS was defined as the time period from study entry until disease progression, death, or date of last contact. Overall Survival (OS) was defined as the time period from study entry until death or date of last contact. Patients with indeterminate responses were excluded. Dichotomized variables were tabulated as high and low levels or positive and negative expression. Changes over time in continuously distributed biomarker levels were investigated with the sign’s test [16] because of the heavy degree of skewness observed in some of the marginal distributions. Associations between interval quality data with ordinal data were examined with Spearman’s correlation coefficient. Associations between dichotomized biomarkers among themselves and with ordinal patient characteristics such as tumor grade or performance status were characterized with Kendall’s tau-b correlation [17]. In cases where an ordering of categories were not apparent, odds ratios were calculated to characterize the degree of association between the two variables. The associations between markers and PFS or OS were assessed with Kaplan-Meier plots, stratified by the dichotomized biomarkers and with Cox proportional hazards models using univariate and multivariate models. Multivariate models incorporated the prognostic variables of age, performance status, and platinum sensitivity. The degree of association was characterized with hazard ratios and confidence intervals.
RESULTS
The GOG conducted a phase II evaluation of bevacizumab in the treatment of persistent or recurrent EOC or PPC. Translational research objectives were prospectively embedded into the protocol to explore potential predictive and/or prognostic relevance of a panel of angiogenic markers. Patient characteristics are shown in Table 1. There were 61 eligible and evaluable women treated with bevacizumab. This cohort had similar patient characteristics as the subset of 43 women with satisfactory pre-cycle 1 tumor biopsy specimens for IHC (see Supplemental Figure S1 and S2) and the subset of 52 women with satisfactory pre-cycle 1 serum or plasma specimen to quantify VEGF concentrations (Supplemental Figure S3). As validated cut-points have yet to be established for any of the angiogenic markers in ovarian cancer, each marker was categorized at the median and as a continuous variable for associations with response to bevacizumab.
Table 1.
Clinical characteristics for the GOG-0170D trial and the cohorts with any immunohistochemistry (IHC) assay or enzyme-linked immunosorbent assay (ELISA) data.
| Clinical Characteristics | Entire Cohort [n=61] |
Subset with IHC Data [n=43] |
Subset with ELISA Data [n=52] |
|---|---|---|---|
| Age | |||
| <50 | 18 (29.5) | 13 (30.2) | 17 (32.7) |
| 50–59 | 21 (34.4) | 16 (37.2) | 16 (30.8) |
| 60–69 | 13 (21.3) | 6 (14.0) | 11 (21.2) |
| 70–79 | 9 (14.8) | 8 (18.6) | 8 (15.4) |
| Race | |||
| Caucasian | 57 (93.4) | 40 (93.0) | 49 (94.2) |
| African American | 2 (3.3) | 2 (4.7) | 2 (3.8) |
| Asian | 2 (3.3) | 1 (2.3) | 1 (1.9) |
| GOG Performance Status | |||
| 0 Asymptomatic | 44 (72.1) | 31 (72.1) | 40 (76.9) |
| 1 Symptomatic | 17 (27.9) | 12 (27.9) | 12 (23.1) |
| Site of Disease | |||
| Ovary | 51 (83.6) | 37 (86.0) | 44 (84.6) |
| Primary Peritoneum | 10 (16.4) | 6 (14.0) | 8 (15.4) |
| Cell Type | |||
| Serous | 51 (83.6) | 34 (79.1) | 43 (82.7) |
| Mixed | 5 (8.2) | 4 (9.3) | 5 (9.6) |
| Endometrioid | 2 (3.3) | 2 (4.7) | 1 (1.9) |
| Clear | 2 (3.3) | 2 (4.7) | 2 (3.8) |
| Adenocarcinoma NOS | 1 (1.6) | 1 (2.3) | 1 (1.9) |
| Grade | |||
| 1 Well Differentiated | 4 (6.6) | 3 (7.0) | 4 (7.7) |
| 2 Moderately Differentiated | 29 (47.5) | 21 (48.8) | 24 (46.2) |
| 3 Poorly Differentiated | 25 (41.0) | 17 (39.5) | 21 (40.4) |
| Not Graded | 3 (4.9) | 2 (4.7) | 3 (5.8) |
| Prior chemotherapy | |||
| 1 regimen | 21 (34.4) | 16 (37.2) | 18 (34.6) |
| 2 regimens | 40 (65.6) | 27 (62.8) | 34 (65.4) |
| Prior hormonal therapy | |||
| None | 50 (82.0) | 35 (81.4) | 41 (78.8) |
| Yes | 11 (18.0) | 8 (18.6) | 11 (21.2) |
| Prior Radiation | |||
| None | 60 (98.4) | 42 (97.7) | 52 (100) |
| Yes | 1 (1.6) | 1 (2.3) | 0 (0) |
| Prior Immunotherapy | |||
| None | 57 (93.4) | 40 (93.0) | 48 (92.3) |
| Yes | 4 (6.6) | 3 (7.0) | 4 (7.7) |
| Prior Surgery | |||
| None | 2 (3.3) | 1 (2.3) | 2 (3.8) |
| Yes | 59 (96.7) | 42 (97.7) | 50 (96.2) |
Column percentages provided in parentheses.
NOS: not otherwise specified
Immunohistochemical Analysis of Angiogenesis Biomarkers in Recurrent/Persistent Tumors
There were 20/41 cases with matched pre-cycle 1 and 4 tumor biopsies. CD31-MVD and IHC levels of VEGF, TSP-1, and p53 did not appear to change following treatment with bevacizumab (see Supplemental Table 1). The distribution of pre-cycle 1 tumor biopsies with high versus low CD31-MVD, VEGF-HS, TSP-1 IA, or p53 was not notably different when cases were subgrouped by patient age group at enrollment, race, GOG performance status, histologic subtype, tumor grade, the number of prior chemotherapy regimens or proportion progression-free ≥ 6 months (data not shown).
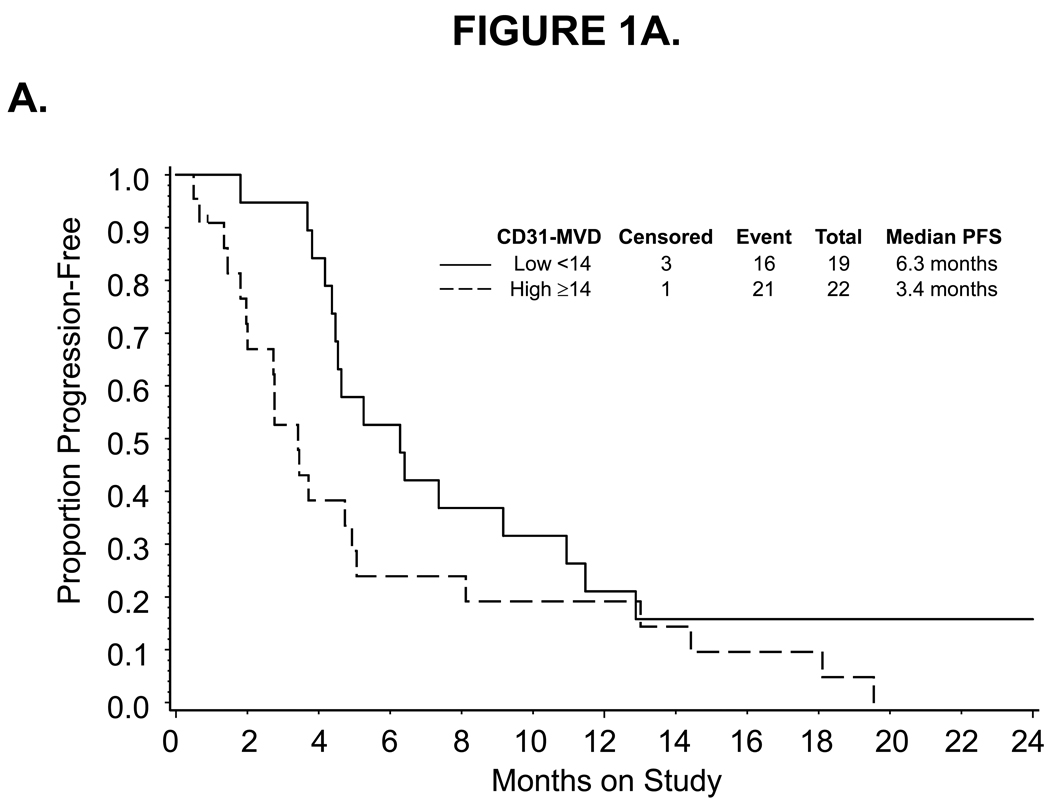
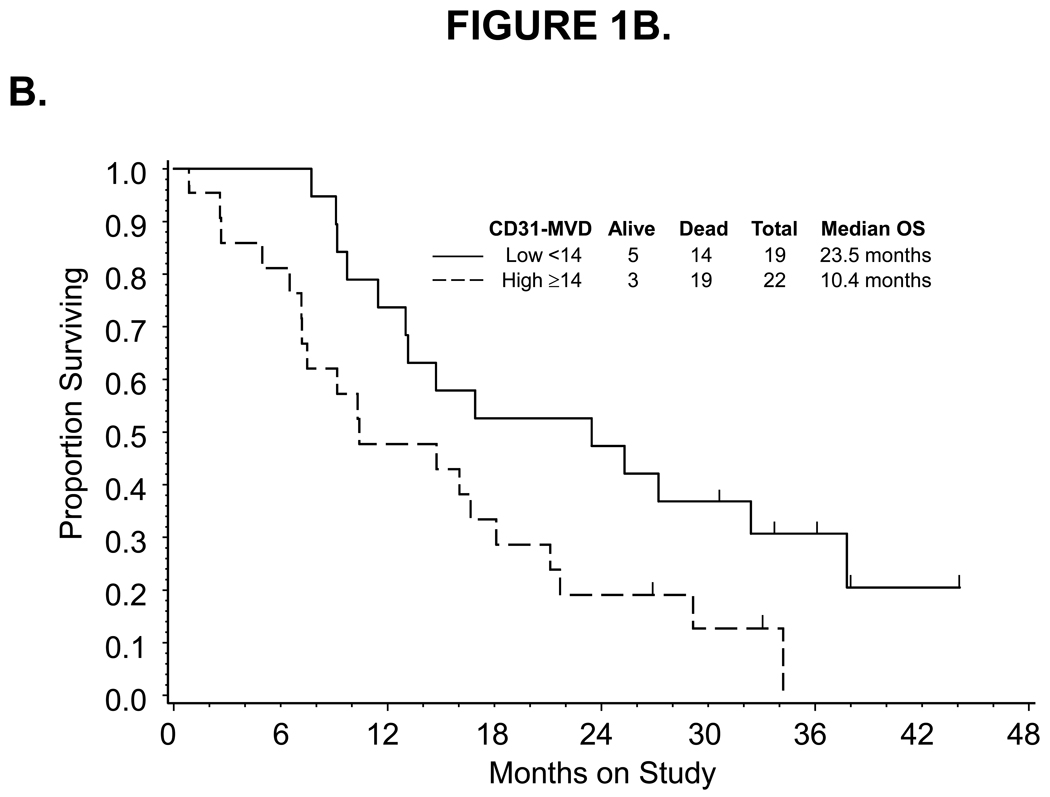
For high pre-cycle 1 CD31-MVD categorized at the median, a decreased response to bevacizumab was observed. Thirty-nine percent (7/18) and 14 % (3/22) of patients with low and high CD31-MVD had partial responses to bevacizumab, respectively (Table 2). Categorized CD31-MVD did not appear to be associated with PFS (Figure 1A) or risk of disease progression (Table 3). However, a 13-month shorter median survival (Figure 1B) and an increased risk of death (unadjusted hazard ratio [HR]=2.183, 95% confidence interval [CI]=1.067–4.467) was observed in women with high versus low CD31-MVD. After adjusting for prognostic variables, categorized CD31-MVD was not associated with an increased risk in death (Table 3). In contrast, each standard deviation (SD) increase in CD31-MVD was associated with worse survival using either unadjusted or adjusted Cox regression analyses (unadjusted HR=1.447, 95% CI=1.038–2.018; adjusted HR=1.550, 95% CI=1.073–2.238).
Table 2.
Associations between the baseline angiogenic markers and tumor response
| Partial Response | Tumor Response Stable Disease |
Increasing Disease | r | |
|---|---|---|---|---|
| CD31-MVD | ||||
| Low <14 | 7 | 10 | 1 | − 0.38‡ |
| High ≥14 | 3 | 11 | 8 | |
| Serum VEGF pg/ml | ||||
| Low <445.92 | 6 | 16 | 3 | − 0.16 |
| High ≥445.92 | 6 | 10 | 9 | |
| Plasma VEGF pg/ml | ||||
| Low <79.95 | 4 | 16 | 5 | 0.05 |
| High ≥79.95 | 8 | 10 | 7 | |
| TSP1-IA | ||||
| Low ≤63.43 | 3 | 12 | 3 | 0.10 |
| High>63.43 | 4 | 10 | 2 | |
| VEGF-HS | ||||
| Low ≤100 | 7 | 12 | 3 | − 0.23 |
| High >100 | 3 | 11 | 6 |
MVD: microvessel density; HS: histoscore; IA: image analysis; r: Kendall’s tau-b correlation coefficient.
Exploratory analysis using Kendall's tau-b test suggested that categorized CD31-MVD may be associated with tumor response. None of the other associations were notable.
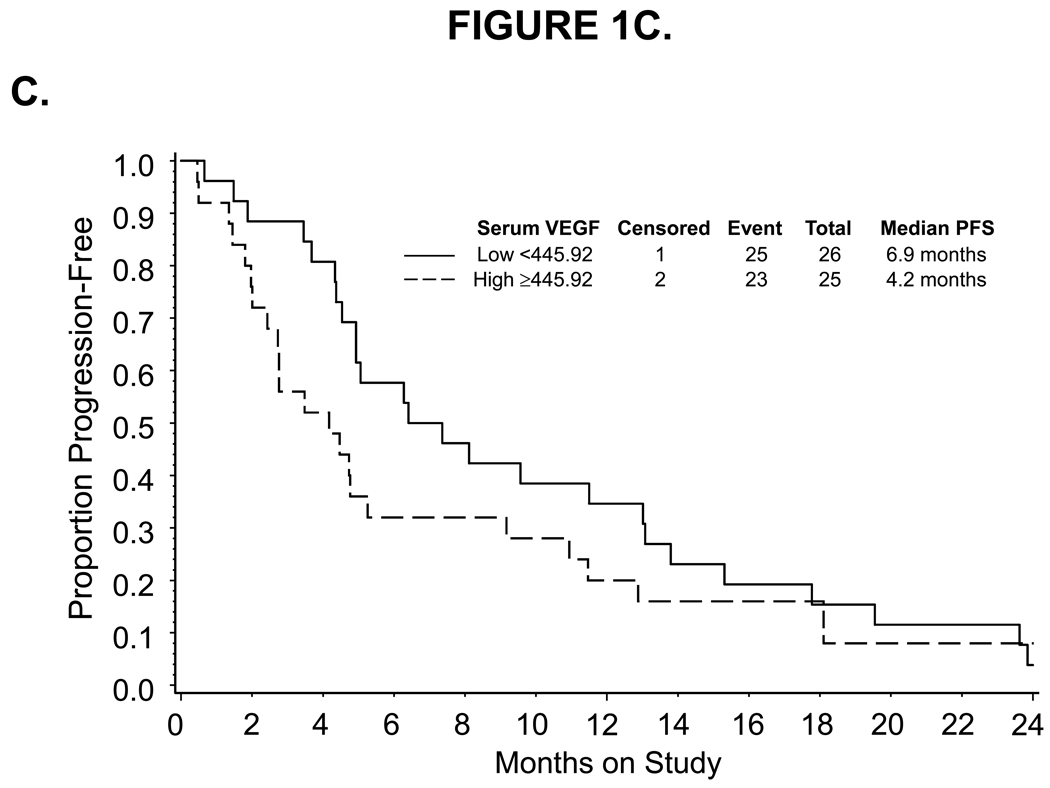
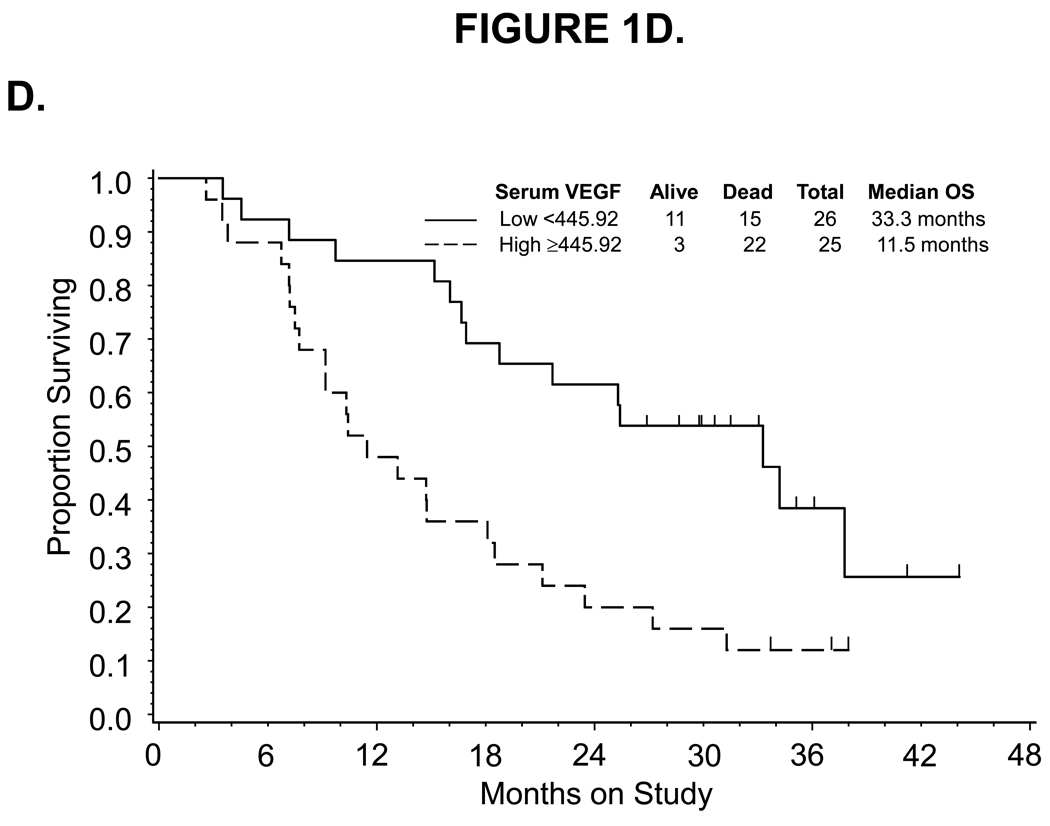
Figure 1.
Kaplan-Meier estimate of PFS (A,C) and OS (B,D) for women with low (<14) or high (≥14) CD31-MVD (A, B) and low (<445.92) or high (≥445.92) pre-cycle 1 serum VEGF concentration (C,D). Median PFS and OS provided in months from study enrollment. Logrank test was used to compare PFS and OS distributions by angiogenic marker overexpression and exploratory analysis suggests a potential association between CD31-MVD and either PFS or OS. Logrank test was used to compare PFS and OS distributions by categorized VEGF concentration and exploratory analysis suggests a potential association between pre-cycle 1 serum concentration of VEGF and OS. The figure represents unadjusted data for PFS and OS.
Table 3.
Associations between the categorized pre-cycle 1 level of CD31, TSP1, VEGF and p53 in tumor, plasma or serum with measures of clinical outcome including progression-free survival and overall survival
| Pre-Cycle 1 Biomarkers |
Progression-Free Survival | Overall Survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted Model † | Adjusted Model ‡ | Unadjusted Model † | Adjusted Model ‡ | |||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| CD31-MVD | ||||||||
| Low <14 | 1.000 | 1.000 | 1.000 | 1.000 | ||||
| High ≥14 | 1.923 | 0.997–3.711 | 1.794 | 0.886–3.630 | 2.183 | 1.067–4.467 | 1.822 | 0.854–3.888 |
| for each SD (9.43) change | 1.248 | 0.911–1.708 | 1.276 | 0.918–1.774 | 1.447 | 1.038–2.018 | 1.550 | 1.073–2.238 |
| Serum VEGF | ||||||||
| Low <445.92 | 1.000 | 1.000 | 1.000 | 1.000 | ||||
| High ≥445.92 | 1.372 | 0.776–2.424 | 1.453 | 0.694–3.041 | 2.666 | 1.369–5.191 | 2.231 | 0.905–5.500 |
| for each SD (362.18) change | 1.030 | 0.807–1.313 | 0.946 | 0.683–1.312 | 1.376 | 1.053–1.797 | 1.143 | 1.800–1.633 |
| Plasma VEGF | ||||||||
| Low <79.95 | 1.000 | 1.000 | 1.000 | 1.000 | ||||
| High ≥79.95 | 0.998 | 0.565–1.766 | 0.932 | 0.517–1.681 | 1.217 | 0.631–2.348 | 0.943 | 0.465–1.911 |
| for each SD (258.69) change | 1.212 | 0.926–1.586 | 1.229 | 0.907–1.664 | 1.289 | 0.941–1.765 | 1.166 | 0.820–1.658 |
| TSP1-IA | ||||||||
| Low ≤65.08 | 1.000 | 1.000 | 1.000 | 1.000 | ||||
| High >65.08 | 0.655 | 0.324–1.326 | 0.576 | 0.259–1.283 | 0.615 | 0.284–1.329 | 0.450 | 0.192–1.051 |
| for each SD (28.81) change | 0.629 | 0.427–0.927 | 0.544 | 0.343–0.863 | 0.719 | 0.463–1.116 | 0.603 | 0.365–0.998 |
| VEGF-HS | ||||||||
| Low ≤100 | 1.000 | 1.000 | 1.000 | 1.000 | ||||
| High >100 | 1.461 | 0.772–2.764 | 1.403 | 0.720–2.735 | 1.397 | 0.689–2.833 | 1.336 | 0.595–2.998 |
| for each SD (118.04) change | 1.049 | 0.773–1.423 | 1.076 | 0.765–1.514 | 0.972 | 0.695–1.359 | 0.992 | 0.670–1.467 |
| p53-HS | ||||||||
| Negative | 1.000 | 1.000 | 1.000 | 1.000 | ||||
| Positive | 0.872 | 0.428–1.779 | 0.968 | 0.449–2.086 | 0.419 | 0.188–0.935 | 0.612 | 0.260–1.442 |
| for each SD (194.49) change | 1.145 | 0.808–1.621 | 1.211 | 0.837–1.751 | 0.845 | 0.574–1.243 | 0.913 | 0.617–1.352 |
MVD: microvessel density; HS: histoscore; IA: image analysis; HR: hazard ratio; 95% CI: 95% confidence interval.
Hazard ratios listed in bold were notable for possible association with survival.
Exploratory Cox regression analysis.
Exploratory Cox regression analysis adjusted for patient age at enrollment, GOG performance status, and platinum sensitivity defined as platinum refractory (persistent disease during prior platinum therapy)/platinum resistant (complete response during prior platinum therapy followed by recurrence <6 months from last platinum dose) or platinum sensitive (complete response during prior platinum therapy followed by recurrence ≥6 months from last platinum dose).
Pre-cycle 1 VEGF-HS, categorized at the median or expressed continuously, was not associated with demographics, tumor characteristics, prior treatment, tumor response (Table 2, Supplemental Figure S2C), PFS (Table 3) or OS (Table 3). Although pre-cycle 1 TSP1-IA score, categorized at the median, did not appear to be associated with tumor response (Table 2), PFS (Table 3) or OS (Table 3), each SD increase in TSP1-IA was associated with a decreased risk of disease progression (unadjusted HR=0.63, 95% CI=0.43–0.93; adjusted HR=0.54, 95% CI=0.34–0.86; Table 3) and death only after adjusting for prognostic variables (adjusted HR=0.603, 95% CI=0.365–0.998), but not with tumor response (Supplemental Figure S2B). Pre-cycle 1 p53-HS, categorized as negative or positive or expressed continuously, did not appear to be associated tumor response (Table 2, Supplemental Figure S2D) or PFS (Table 3). A better OS and reduced risk of death (unadjusted HR=0.419, 95% CI=0.188–0.935) was observed in women with positive versus negative p53-HS. After adjusting for prognostic variables, p53-HS (categorized as positive versus negative or evaluated as a continuous variable) was not associated with an increased risk of death (Table 3).
Serum and Plasma VEGF Levels
Serum and plasma VEGF concentrations were quantified in 51 women pre-cycle 1, 34 (serum)/35 (plasma) women pre-cycle 4 and 21 women off-treatment due to disease progression or toxicity. Median serum VEGF concentrations were 446 pg/ml (18 to 1437 pg/ml) pre-cycle 1, 82 pg/ml (0 to 173 pg/ml) pre-cycle 4 and 106 pg/ml (41 to 224 pg/ml) off-treatment (Supplemental Figure S3A). For plasma VEGF, median pre-cycle 1, pre-cycle 4, and off-treatment concentrations were 80, 79 and 95 pg/ml, respectively, which were not significantly different (Supplemental Figure S3B). The distribution of pre-cycle 1 sera and plasma with high versus low VEGF was not notably different when cases were subgrouped by patient age at enrollment, race, histologic subtype, the number of prior chemotherapy regimens or proportion progression-free ≥ 6 months. Median pre-cycle 1 VEGF concentrations were approximately 5.6-fold higher in serum compared with plasma. When compared with pre-cycle 1 serum, the concentration of VEGF in pre-cycle 4 serum or off-treatment serum were notably lower.
For plasma VEGF, there was no evidence to suggest that pre-cycle 1 level was associated with tumor response (Table 2), PFS (Table 3) or OS (Table 3). Also, there was no evidence of an association between the observed decrease in serum VEGF following bevacizumab treatment and tumor response. Pre-cycle 1 serum VEGF, categorized at the median, appeared to be positively correlated with GOG performance status and negatively correlated with tumor grade, but did not appear to be associated with tumor response (Table 2), PFS (Table 3, Figure 1C) or risk of disease progression (Table 3). In addition, high serum VEGF, categorized at the median, was associated with a 22-month shorter median OS (Figure 1D) and a notable increased risk of death (unadjusted HR=2.666, 95% CI=1.369–5.191; Table 3). Each standard deviation (SD) increase in serum VEGF also appeared to be associated with worse survival (unadjusted HR=1.376, 95% CI=1.053–1.797). After adjusting for prognostic variables, serum VEGF (categorized at the median or expressed continuously) was not associated with an increased risk of death (Table 3).
DISCUSSION
We performed an exploratory analysis of various angiogenesis biomarkers on patient tumor and blood samples obtained prospectively from a positive phase II GOG study of recurrent/persistent EOC/PPC patients treated with single agent bevacizumab. We chose markers (CD31, TSP1, VEGF and p53) that have been well studied and carefully optimized with both positive and negative controls. Since a 21% response rate was noted with 52% of patients experiencing stable disease and 40% with PFS ≥ 6-months [3], the goal of this exploratory study was to screen a set of markers with potential prognostic and predictive value in EOC/PPC patients treated with bevacizumab.
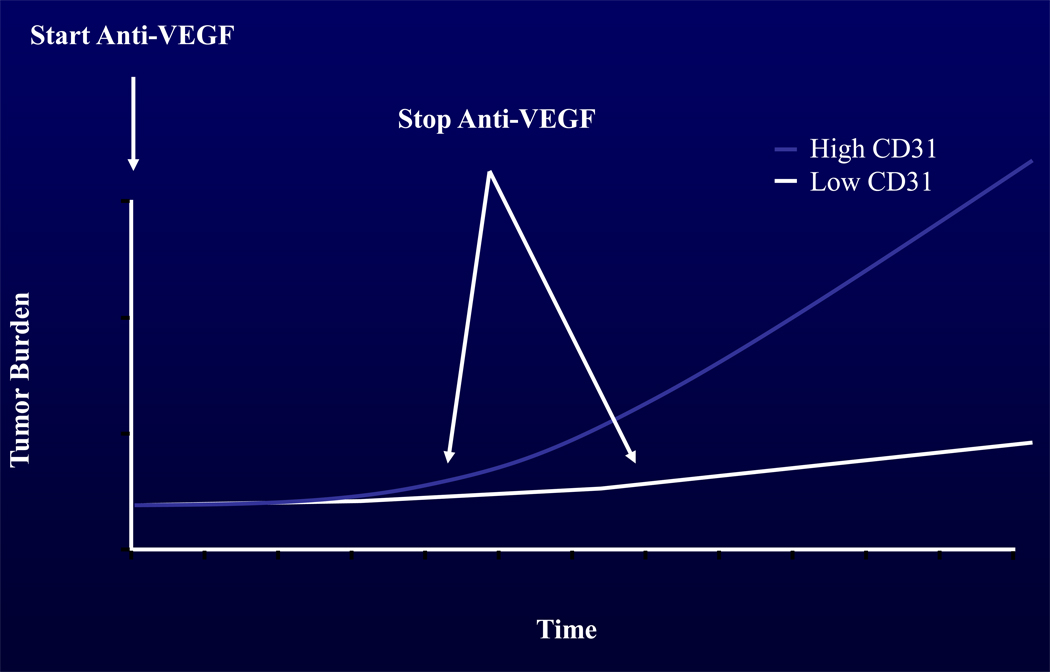
Despite the lack of tumor markers correlating to bevacizumab response in other trials, we observed that high CD31-MVD count was associated with poor tumor response to bevacizumab. Other investigators have looked at CD31-MVD and did not find any association with bevacizumab response in either breast or colon cancers [18, 19]. Our observation that high CD31-MVD was associated with poor tumor response to bevacizumab may reflect an effect specific to EOC/PPC. It is noteworthy that after discontinuation of bevacizumab therapy, patients with high CD31-MVD (had more rapid disease progression and death This observation coupled with the findings that high CD31-MVD patients had a statistically worse OS than patients with low CD31-MVD tumors but similar PFS curves, suggests the possibility that the patients with high CD31-MVD tumors benefit while on bevacizumab but that these tumors are intrinsically more aggressive and progress rapidly when the drug is discontinued. An idealized graphic of this phenomenon is presented in Figure 2. Ebos et al. [20] and Pàez-Ribes et al. [21], demonstrated that treatment with anti-angiogenic therapy may paradoxically induce adaptive evasive responses that accelerate invasion and metastasis and limit the long-term clinical benefit of anti-angiogenic therapies [20–23]. However, due to the study limitations, it is also possible that there may be intrinsic differences in behavior between more biologically aggressive, highly angiogenic tumors compared to indolent, less angiogenic tumors.
Figure 2.
Idealized model for tumor progression for the average patient with low CD31-MVD tumor and high CD31-MVD tumor on anti-VEGF therapy and after discontinuation of anti-VEGF therapy. Both representative cases began with the same degree of tumor burden that was noted on radiographic imaging and was monitored during the time of the study.
When we examined whether CD31-MVD, VEGF-HS and TSP1-IA were prognostic factors for persistent/recurrent EOC/PPC, we found that high pre-cycle 1 CD31-MVD count was associated with a 50% decrease in median PFS and OS. Also, unadjusted Cox proportional hazard modeling revealed that high pre-cycle 1 CD31-MVD was associated with a 2-fold higher risk of death. However, after adjusting for clinicopathologic factors, only a trend was noted. In contrast, when CD31-MVD was examined as a continuous variable, the association with OS was observed even after adjusting for prognostic variables. Taken together, these findings suggest that high CD31-MVD merits further investigation as a potential prognostic marker in women with EOC/PPC. Our findings are consistent with previously reported retrospective studies [24–26], but other studies have failed to show a relationship between CD31-MVD and survival in EOC patients [27–28].
High compared with low tumor VEGF-HS did not appear to be associated with either PFS or OS which agrees with that reported by Secord and coworkers [29]. In contrast, O’Toole et al. showed an association between high versus low tumor VEGF and PFS and OS [30]. Also, Duncan and colleagues [31] looked at 339 primary ovarian cancers on tissue microarrays and found that high tumor VEGF level was correlated with worsening survival and was an independent prognostic factor in multivariate analysis. High compared with low TSP-1 was associated with increased risk of disease progression and death in women with primary advanced stage EOC [32]. In contrast, we did not observe a notable association between TSP1-IA and either PFS or OS when categorized at the median, whereas each SD increase in TSP1-IA appeared to be associated with a decreased risk of disease progression and death only after adjusting for prognostic variables. There are a number of significant differences between these studies including the type of tumor tested (primary versus recurrent tumor), the method for evaluating immunochemical staining for individual angiogenic markers (semi-quantitative versus image analysis) and the type of treatment (cytotoxic chemotherapy versus anti-angiogenic therapy) which may explain at least in part the disparity in these findings.
In this cohort, women who were p53 positive in a pre-cycle 1 biopsy had a reduced risk of death but this association did not hold up after adjusting for prognostic variables or when this biomarker was evaluated as a continuous variable. A number of studies have shown that p53 mutations are often associated with poor prognosis in various cancers [33–36] but variable results have been published regarding the association between IHC staining of p53 and various measures of clinical outcome including PFS and OS (as referenced by Darcy et al) [15]. Interestingly, p53 mutation, but not p53 overexpression in primary epithelial ovarian cancers, has been associated with a time-dependent reduction in the risk of disease progression and death [36].
An association was also observed between pre-treatment serum VEGF and tumor grade as well as GOG performance status. Whether the observed increased risk of death is attributable directly to serum VEGF or indirectly due to platelets (a significant source of VEGF in serum [37–38]), tumor grade and/ or performance status will require additional studies. The lack of an association between plasma VEGF with OS may raise questions about the reliability of the observed association with serum VEGF and OS. Our results should be interpreted with caution as bevacizumab treatment may potentially interfere with the accurate measurement of VEGF levels in ELISA, which may be circumvented by immunodepletion of plasma samples and measuring free plasma VEGF as our plasma specimens were prepared from blood mixed with the anti-coagulant EDTA [39].
Despite limitations of this exploratory study which include small sample size, numerous analyses, findings due to chance, and lack of a control group for comparison, these correlative studies offer proof of principle that selected translational endpoints can be investigated in a meaningful way when material is prospectively obtained from a multi-institutional study of a molecular targeting agent. This phase II trial provides a series of testable hypotheses regarding CD31-MVD as a potential predictive marker for lack of response to bevacizumab, CD31-MVD and serum VEGF as potential prognostic factors for worse OS, and TSP1-IA and p53 HS as potential prognostic markers for improved OS. These markers are being prospectively tested in the GOG randomized, placebo control phase III front-line trial of carboplatin, paclitaxel and bevacizumab/placebo in advanced stage EOC/PPC patients which was recently reported [40].
Supplementary Material
Figure S1. Representative immunohistochemical expression of CD31 (A), TSP1 (B), VEGF (C) or p53 (D).
Figure S2. Scatter plots for CD31-microvessel density [MVD] (A), TSP1-image analysis [IA] score (B), VEGF-histoscore [HS] (C) and p53-HS (D) per patient in pre-cycle 1 and/or 4 tumor biopsies (A–D)
Figure S3. Scatter plots for serum (A) and plasma (B) VEGF concentration in pre-cycle 1, pre-cycle4 and/or off-treatment serum (A) or plasma (B).
Acknowledgments
This study was supported by a grant from the American Board of Obstetrics and Gynecology/American Association of Obstetricians and Gynecologists Foundation and National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA 27469), the GOG Tissue Bank (CA 27269, CA 11479) and the Gynecologic Oncology Group Statistical and Data Center (CA 37517). The following Gynecologic Oncology Group member institutions participated in this study: University of Alabama at Birmingham, Walter Reed Army Medical Center, University of California at Los Angeles, University of Washington, University of Iowa Hospitals and Clinics, University of California Medical Center at Irvine, Washington University School of Medicine, Columbus Cancer Council, and Community Clinical Oncology Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
REFERENCES
- 1.DiSaia PJ, Creasman WT. Epithelial ovarian cancer. In: DiSaia PJ, Creasman WT, editors. Clinical Gynecologic Oncology. 7th ed. St. Louis: Mosby-Year Book; 2007. pp. 313–367. [Google Scholar]
- 2.Kowanetz M, Ferrara N. Vascular endothelial growth factor signaling pathways: therapeutic perspective. Clin Cancer Res. 2006;12:5018–5022. doi: 10.1158/1078-0432.CCR-06-1520. [DOI] [PubMed] [Google Scholar]
- 3.Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2007;25:5165–5171. doi: 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]
- 4.Cannistra SA, Matulonis UA, Penson RT, Hambleton J, Dupont J, Mackey H, et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol. 2007;25:5180–5186. doi: 10.1200/JCO.2007.12.0782. [DOI] [PubMed] [Google Scholar]
- 5.Garcia AA, Hirte H, Fleming G, Yang D, Tsao-Wei DD, Roman L, et al. Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: a trial of the California, Chicago, and Princess Margaret hospital phase II consortia. J Clin Oncol. 2008;26:76–82. doi: 10.1200/JCO.2007.12.1939. [DOI] [PubMed] [Google Scholar]
- 6.Jubb AM, Oates AJ, Holden S, Koeppen H. Predicting benefit from anti-angiogenic agents in malignancy. Nat Rev Cancer. 2006;6:626–635. doi: 10.1038/nrc1946. [DOI] [PubMed] [Google Scholar]
- 7.Mehta R, Kyshtoobayeva A, Kurosaki T, Small EJ, Kim H, Stroup R, et al. Independent association of angiogenesis index with outcome in prostate cancer. Clin Cancer Res. 2001;7:81–88. [PubMed] [Google Scholar]
- 8.Sharma S, Sharma MC, Sarkar C. Morphology of angiogenesis in human cancers: a conceptual overview, histoprognostic perspective and significance of neoangiogenesis. Histopathology. 2005;46:481–489. doi: 10.1111/j.1365-2559.2005.02142.x. [DOI] [PubMed] [Google Scholar]
- 9.Wang TN, Qian X, Granick MS, Solomon MP, Rothman VL, Berger DH, et al. Thrombospondin-1 (TSP-1) promotes the invasive properties of human breast cancer. J Surg Res. 1996;63:39–43. doi: 10.1006/jsre.1996.0219. [DOI] [PubMed] [Google Scholar]
- 10.Taraboletti G, Morbidelli L, Donnini S, Parenti A, Granger HJ, Giavazzi R, et al. The heparin binding 25 kDa fragment of thrombospondin-1 promotes angiogenesis and modulates gelatinase and TIMP-2 production in endothelial cells. FASEB Jl. 2000;14:1674–1676. doi: 10.1096/fj.99-0931fje. [DOI] [PubMed] [Google Scholar]
- 11.Weinstat-Saslow DL, Zabrenetzky VS, VanHoutte K, Frazier WA, Roberts DD, Steeg PS. Transfection of thrombospondin 1 complementary DNA into a human breast carcinoma cell line reduces primary tumor growth, metastatic potential, and angiogenesis. Cancer Res. 1994;54:6504–6511. [PubMed] [Google Scholar]
- 12.Zabrenetzky V, Harris CC, Steeg PS, Roberts DD. Expression of the extracellular matrix molecule thrombospondin inversely correlates with malignant progression in melanoma, lung and breast carcinoma cell lines. Int J Cancer. 1994:59191–59195. doi: 10.1002/ijc.2910590209. [DOI] [PubMed] [Google Scholar]
- 13.Iruela-Arispe ML, Lombardo M, Krutzsch HC, Lawler J, Roberts DD. Inhibition of angiogenesis by thrombospondin-1 is mediated by 2 independent regions within the type 1 repeats. Circulation. 1999;100:1423–1431. doi: 10.1161/01.cir.100.13.1423. [DOI] [PubMed] [Google Scholar]
- 14.Kazerounian S, Yee KO, Lawler J. Thrombospondins in cancer. Cell Mol Life Sci. 2008;65:700–712. doi: 10.1007/s00018-007-7486-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darcy KM, Brady WE, McBroom JW, Bell JG, Young RC, McGuire WP, et al. Associations between p53 overexpression and multiple measures of clinical outcome in high-risk, early stage or suboptimally-resected, advanced stage epithelial ovarian cancers: A Gynecologic Oncology Group study. Gynecol Oncol. 2008;111:487–495. doi: 10.1016/j.ygyno.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollander M, Wolfe DA. Nonparametric Statistical Methods. New York: John Wiley & Sons; 1973. [Google Scholar]
- 17.Kendall MG. The treatment of ties in rank problems. Biometrika. 1945;33:239–251. doi: 10.1093/biomet/33.3.239. [DOI] [PubMed] [Google Scholar]
- 18.Wedam SB, Low JA, Yang SX, Chow CK, Choyke P, Danforth D, et al. Antiangiogenic and antitumor effects of bevacizumab in patients with inflammatory and locally advanced breast cancer. J Clin Oncol. 2006;24:769–777. doi: 10.1200/JCO.2005.03.4645. [DOI] [PubMed] [Google Scholar]
- 19.Jubb AM, Hurwitz HI, Bai W, Holmgren EB, Tobin P, Guerrero AS, et al. Impact of vascular endothelial growth factor-A expression, thrombospondin-2 expression, and microvessel density on the treatment effect of bevacizumab in metastatic colorectal cancer. J Clin Oncol. 2006;24:217–227. doi: 10.1200/JCO.2005.01.5388. [DOI] [PubMed] [Google Scholar]
- 20.Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steeg PS, Anderson RL, Bar-Eli M, Chambers AF, Eccles SA, Hunter K, et al. Preclinical drug development must consider the impact on metastasis. Clin Cancer Res. 2009;15:4529–4530. doi: 10.1158/1078-0432.CCR-09-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loges S, Mazzone M, Hohensinner P, Carmeliet P. Silencing or fueling metastasis with VEGF inhibitors: antiangiogenesis revisited. Cancer Cell. 2009;15:167–170. doi: 10.1016/j.ccr.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Hollingsworth HC, Kohn EC, Steinberg SM, Rothenberg ML, Merino MJ. Tumor angiogenesis in advanced stage ovarian carcinoma. Am J Pathol. 1995;147:9–19. [PMC free article] [PubMed] [Google Scholar]
- 25.Alvarez AA, Krigman HR, Whitaker RS, Dodge RK, Rodriquez GC. The prognostic significance of angiogenesis in epithelial ovarian carcinoma. Clin Cancer Res. 1999;5:587–591. [PubMed] [Google Scholar]
- 26.Goodheart MJ, Vasef MA, Sood AK, Davis CS, Buller RE. Ovarian cancer p53 mutation is associated with tumor microvessel density. Gynecol Oncol. 2002;86:85–90. doi: 10.1006/gyno.2002.6730. [DOI] [PubMed] [Google Scholar]
- 27.Ferrero A, Zola P, Mazzola S, Fuso L, Sarotto I, Ravarino N, et al. Pretreatment serum hemoglobin level and a preliminary investigation of intratumoral microvessel density in advanced ovarian cancer. Gynecol Oncol. 2004;95:323–329. doi: 10.1016/j.ygyno.2004.07.053. [DOI] [PubMed] [Google Scholar]
- 28.Rubatt JM, Darcy KM, Hutson A, Bean SM, Havrilesky LJ, Grace LA, et al. Independent prognostic relevance of microvessel density in advanced epithelial ovarian cancer and associations between CD31, CD105, p53 status, and angiogenic marker expression: A Gynecologic Oncology Group study. Gynecol Oncol. 2009;112:469–474. doi: 10.1016/j.ygyno.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 29.Secord AA, Darcy KM, Hutson A, Lee PS, Havrilesky LJ, Grace LA, et al. Co-expression of angiogenic markers and associations with prognosis in advanced epithelial ovarian cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2007;105:221–232. doi: 10.1016/j.ygyno.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 30.O’Toole SA, Sheppard BL, Laios A, O’Leary JJ, McGuinness EP, D’Arcy T, et al. Potential predictors of chemotherapy response in ovarian cancer: how do we define chemosensitivity? Gynecol Oncol. 2007;104:345–351. doi: 10.1016/j.ygyno.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 31.Duncan TJ, Al-Attar A, Rolland P, Scott IV, Deen S, Liu DT, et al. Vascular endothelial growth factor expression in ovarian cancer: a model for targeted use of novel therapies? Clin Cancer Res. 2008;14:3030–3035. doi: 10.1158/1078-0432.CCR-07-1888. [DOI] [PubMed] [Google Scholar]
- 32.Alvarez AA, Axelrod JR, Whitaker RS, Isner PD, Bentley RC, Dodge RK, et al. Thrombospondin-1 expression in epithelial ovarian carcinoma: association with p53 status, tumor angiogenesis, and survival in platinum-treated patients. Gynecol Oncol. 2001;82:273–278. doi: 10.1006/gyno.2001.6287. [DOI] [PubMed] [Google Scholar]
- 33.Grant SW, Kyshtoobayeva A, Kurosaki T, Jakowatz J, Fruehauf JP. Mutant p53 correlates with reduced expression of thrombospondin-1, increased angiogenesis, and metastatic progression in melanoma. Cancer Detect Prev. 1998;22:185–194. doi: 10.1046/j.1525-1500.1998.0oa18.x. [DOI] [PubMed] [Google Scholar]
- 34.Soussi T. The p53 tumor suppressor gene: from molecular biology to clinical investigation. Ann NY Acad Sci. 2000;910:121–137. doi: 10.1111/j.1749-6632.2000.tb06705.x. [DOI] [PubMed] [Google Scholar]
- 35.Steele RJ, Lane DP. P53 in cancer: a paradigm for modern management of cancer. Surgeon. 2005;3:197–205. doi: 10.1016/s1479-666x(05)80041-1. [DOI] [PubMed] [Google Scholar]
- 36.Havrilesky L, Darcy KM, Hamdan H, Priore RL, Leon J, Bell J, et al. Prognostic significance of p53 mutation and p53 overexpression in advanced epithelial ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3814–3825. doi: 10.1200/JCO.2003.11.052. [DOI] [PubMed] [Google Scholar]
- 37.Adams J, Carder PJ, Downey S, Forbes MA, MacLennan K, Allgar V, et al. Vascular endothelial growth factor (VEGF) in breast cancer: comparison of plasma, serum, and tissue VEGF and microvessel density and effects of tamoxifen. Cancer Res. 2000;60:2898–2905. [PubMed] [Google Scholar]
- 38.Banks RE, Forbes MA, Kinsey SE, Stanley A, Ingham E, Walters C, et al. Release of angiogenic cytokine vascular endothelial growth factor (VEGF) from platelets: significance for VEGF measurements and cancer biology. Br J Cancer. 1998;77:956–964. doi: 10.1038/bjc.1998.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loupakis F, Falcone A, Masi G, Fioravanti A, Kerbel RS, Del Tacca M, et al. Vascular endothelial growth factor levels in immunodepleted plasma of cancer patients as a possible pharmacodynamic marker for bevacizumab activity. J Clin Oncol. 2007;25:1816–1818. doi: 10.1200/JCO.2006.10.3051. [DOI] [PubMed] [Google Scholar]
- 40.Burger RA, Brady MF, Bookman MA, Walker JL, Homesley HD, Fowler J, et al. Phase III trial of bevacizumab (Bev) in the primary treatment of advanced epithelial ovarian cancer (EOC), primary peritoneal cancer (PPC), or fallopian tube cancer (FTC): a Gynecologic Oncology Group study. J Clin Oncol. 2010;28:18s. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Representative immunohistochemical expression of CD31 (A), TSP1 (B), VEGF (C) or p53 (D).
Figure S2. Scatter plots for CD31-microvessel density [MVD] (A), TSP1-image analysis [IA] score (B), VEGF-histoscore [HS] (C) and p53-HS (D) per patient in pre-cycle 1 and/or 4 tumor biopsies (A–D)
Figure S3. Scatter plots for serum (A) and plasma (B) VEGF concentration in pre-cycle 1, pre-cycle4 and/or off-treatment serum (A) or plasma (B).