Abstract
Objective
Endocervical curettage (ECC) specimens obtained during colposcopy can detect cervical cancer and precursors otherwise missed by biopsy alone; but the procedure can be painful and reduce compliance with needed follow-up. ECC is routinely performed in the Calgary Health Region colposcopy clinics, permitting a look at its real-world utility.
Study Design
We analyzed pathology and colposcopy reports from 2003–2007. We calculated the added diagnostic utility of ECC compared to cervical biopsy alone.
Results
ECC increased the diagnostic yield of cervical intraepithelial neoplasia grade 2 or worse (CIN2+) in 1.01% of 13,115 colposcopically-guided biopsy exams. Therefore, 99 ECC specimens were taken to detect one additional CIN2+. ECC detected 5.4% of 2,443 CIN2+ cases otherwise missed by biopsy alone. Utility was greatest among women aged 46+ referred after a high-grade cytology.
Conclusions
ECC is rarely informative when used routinely in colposcopic practice. Older women referred after high-risk cytology benefit most from ECC.
Keywords: cervical intraepithelial neoplasia, colposcopy, curettage, diagnosis, endocervical sampling
Introduction
Each year millions of women in the US and Canada are referred to colposcopy after abnormal cytology or positive HPV testing. The colposcopist will take biopsies of visualized lesions and possibly sample the endocervical canal using a curette to rule out the presence of hidden cervical intraepithelial neoplasia. This latter procedure, the endocervical curettage (ECC), involves circumferentially scraping the endocervical canal. Although several reviews have summarized the findings of numerous heterogeneous studies [1–6], the use of ECC remains controversial [7].
The most recent data indicate that ECC might increase the sensitivity of the colposcopic visit by detecting 2–6% of high-grade cervical intraepithelial neoplasia (CIN) or cancer that otherwise would have been missed by cervical biopsies alone [8, 9]. Analyzed from a related but different angle, these studies have also shown that ECC increases the diagnostic yield of colposcopic visits by 1–6% (increasing the total number of cases of high-grade CIN or cancer per colposcopic exam) [8, 10, 11]. Yet, ECC is often the most painful part of the colposcopy procedure and pathologic interpretation can be difficult due to small, fragmented and poorly oriented specimens with deficient stroma [2]. In addition, during the collection process the presence of an ectocervical lesion might contaminate the ECC specimen, resulting in more extensive treatment of a suspected endocervical lesion where none exists [7].
With the exception of its contraindication in pregnant women, the use of ECC varies widely among providers. Some colposcopists limit its use to indications for endocervical sampling recommended as acceptable or preferred by professional guidelines [12, 13] such as when managing a woman with: high-grade squamous intraepithelial lesion (HSIL) cytology, unsatisfactory colposcopy, atypical squamous cells of undetermined significance (ASC-US) or low-grade squamous intraepithelial lesion (LSIL), with or without visualized lesions, or suspicion of glandular extent [12]. Another option is to sample the endocervix using an endocervical brush instead of curettage. Still others uniformly collect ECC in every colposcopy exam of non-pregnant women, regardless of age, adequacy of colposcopy, or cytology result. Further identification of subgroups of women most likely to benefit from ECC is required but studies have lacked appropriate data or statistical power for such comparisons [5, 8–11, 14]. Evidence suggests that ECC is less advantageous in younger, nulliparous women referred for low grade abnormalities with satisfactory exams [2, 4, 8, 10, 14].
The Calgary Health Region and the Alberta Cervical Cancer Screening Program in Alberta, Canada have an extensive data collection system that records histopathology, cytopathology and colposcopic and patient characteristics for all colposcopy exams conducted. Because ECC is routinely taken at essentially all colposcopy exams in the outpatient colposcopy clinics, we had a rare opportunity to assess the benefit of ECC in thousands of colposcopic examinations. This large analysis provides many more outcomes than previous studies and permits careful stratification to identify subgroups of women for whom ECC is most valuable, without selection bias.
Materials and Methods
The Calgary Health Region provides services to a population of approximately 1.2 million. Colposcopy, cytopathology and histopathology are regionalized services with uniform practice guidelines and standards. De-identified pathology reports were obtained from Calgary Laboratory Services for histological specimens collected at colposcopy exams at Women’s Health Centre and Tom Baker Cancer Centre colposcopy clinics and read between January 1, 2003 and December 31, 2007. Using the patient number, records from the pathology database were linked with the colposcopic examination database to abstract the colposcopic impression and whether the examination was satisfactory, when available. In addition, personal characteristics of women were obtained including gravidity, parity, use of contraception and date of last menstrual period. The record review received human subjects research approval from the Conjoint Health Research Ethics Board Review, University of Calgary and Calgary Health Region and was considered exempt from review by the National Cancer Institute, National Institutes of Health.
The 60,537 histopathology specimens corresponded to 39,476 examinations where up to four types of specimens were collected. Types of specimens included: cervical biopsy, ECC, loop electrosurgical excision procedure (LEEP), endometrial biopsy, vulvar biopsy, vaginal biopsy, and cervical brush. If more than one of a specimen type was taken at the exam and analyzed (for example, multiple cervical biopsies), we considered the worst histopathology reading as a final diagnosis for that specimen type. If a specimen was read more than one time, we considered the final reading as the final diagnosis for that specimen.
This analysis was not conducted at the patient level, but we instead analyzed each examination where a cervical biopsy and ECC specimen was collected (n=13,476), under the assumption that the diagnostic utility of ECC did not depend on the women per se. Of note, we identified 19,372 examinations where only an ECC specimen was taken and 2,030 examinations where only a cervical biopsy specimen was taken (0.6% and 4.7% were diagnosed CIN3+ including 2 and 8 carcinomas, respectively). Each woman contributed on average 2.2 examinations to the dataset and women who contributed one examination did not differ than those contributing more than one with regards to age, parity or oral contraceptive use. Among these examinations we excluded those in which another type of histopathology specimen (e.g. endometrial biopsy) was taken (n=361, 2.7%) because the focus of this analysis was on the value added by ECC to colposcopically-guided biopsy for diagnosing cervical precancer and cancer. Our final analytic sample was 13,115 examinations.
Cytopathology records for specimens processed at Calgary Laboratory Services up to two years prior to the date of the reading of the histopathology specimen were retrieved. Cytology readings were standardized and categorized according to the Bethesda Classification System: ASC-US, ASC-H (atypical squamous cells, cannot rule out HSIL), AGUS (atypical glandular cells of undetermined significance), LSIL and HSIL. For this analysis, cytology interpretations of ASC-H were categorized with HSIL because of their rarity and the high-risk of cervical precancer and cancer associated with them [15, 16]. The colposcopy visit was considered a referral visit if a cytopathology result was within 270 days of the examination and the result was unsatisfactory or abnormal. The visit was considered follow-up to a previous diagnostic or treatment colposcopy visits and not a referral visit if no cytopathology result was identified for the patient, the cytopathology visit was >270 days before the histopathology reading, or the cytopathology result was normal.
We compared the final histopathology diagnosis for ECC specimen with the final histopathology diagnosis for the cervical biopsy specimens. Results were categorized as unsatisfactory, less than CIN grade 2 (CIN2), CIN2, CIN3, or cancer. For examinations where both ECC and cervical biopsy specimens were taken, our two primary outcomes were 1) the proportion of examinations where ECC detected CIN2 or worse (CIN2+) that would have been missed by cervical biopsy alone (diagnostic yield) and the number of needed to test (NNT) with ECC to detect one additional case of CIN2+ (calculated as the inverse of the diagnostic yield) and 2) the proportion of CIN2+ diagnoses that would have been diagnosed as <CIN2 based on cervical biopsy had ECC not been performed (additional case detection). We also considered CIN3 or worse (CIN3+) as a more scientifically rigorous precancerous endpoint and better surrogate of cervical cancer risk [17, 18].
Findings were stratified by the woman’s age at the examination, parity, method of contraception, referral cytology, and colposcopy impression when available. Because satisfactory visualization of the transformation zone is closely associated with menopausal status, we imputed this variable based upon their age and date of last menstrual period. Women under age 46 were classified as “premenopausal”, women age 46–59 with less than one year since last menstrual period were classified as “perimenopausal” and women either over age 60 or age 46–59 with one year or more since last menstrual period were classified as “postmenopausal”. Logistic regression and contingency analyses with chi-square statistics were used to evaluate the influence of potential confounders. All analyses were conducted using Stata 11.0 analytic software (StataCorp LP, College Station, TX).
Results
We identified 13,115 colposcopy examinations in which cervical biopsy and ECC specimens were collected and 79.4% had concordant diagnoses (Table 1). Compared to cervical biopsies, ECC specimens were more likely to be unsatisfactory (4.2% vs. 1.2%, McNemar’s Chi-square, p<.001 respectively) and less likely to be diagnosed as CIN2+ (4.3% vs. 17.6%, McNemar’s Chi-square, p<.001 respectively). Of the 16 cancers detected, 15 were diagnosed as CIN3+ by both cervical biopsy and ECC and one was diagnosed as <CIN2 by ECC.
Table 1.
Histopathology diagnoses for 13,115 colposcopically-guided biopsy examinations with both endocervical curettage (ECC) and cervical biopsy specimens processed at Alberta Hospital January 1, 2003-December 31, 2007*
| Histopathology result of cervical biopsy | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histopathology result of ECC |
Unsatisfactory | <CIN2 | CIN2 | CIN3 | Cancer | Total | ||||||
| N | col % | N | col % | N | col % | N | col % | N | col % | N | col % | |
| Unsatisfactory | 6 | 3.8 | 464 | 4.4 | 44 | 3.2 | 40 | 4.4 | 0 | 0.0 | 554 | 4.2 |
| <CIN2 | 150 | 94.3 | 10,052 | 94.4 | 1,184 | 85.9 | 612 | 66.7 | 1 | 6.7 | 11,999 | 91.5 |
| CIN2 | 3 | 1.9 | 86 | 0.7 | 127 | 8.3 | 44 | 4.8 | 0 | 0.0 | 260 | 2.0 |
| CIN3 | 0 | 0.0 | 43 | 0.4 | 23 | 1.5 | 221 | 24.1 | 7 | 46.7 | 294 | 2.2 |
| Cancer | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.1 | 7 | 46.7 | 8 | 0.1 |
| Total (row %) | 159 | 1.2 | 10,645 | 81.2 | 1,378 | 10.5 | 918 | 7.0 | 15 | 0.1 | 13,115 | 100.0 |
Lightly shaded cells indicate a more severe diagnosis based on ECC, and darkly shaded cells indicate a more severe diagnosis based on cervical biopsy, among women with satisfactory ECC and cervical biopsies.
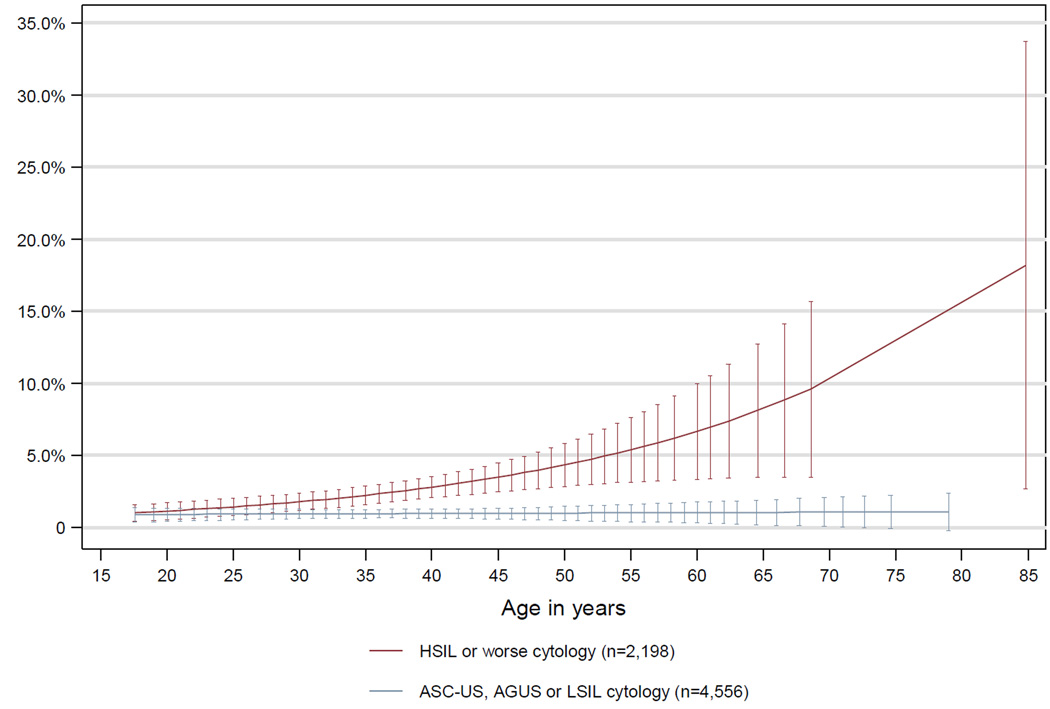
The diagnostic yield of ECC in all examinations was 1.01% (Tables 1 and 2). That is, in 132 of 13,115 examinations, ECC detected CIN2+ that would have otherwise been missed by colposcopically-guided biopsies. In these 132 cases the ECC diagnoses were CIN2 (n=86, 0.7%), CIN3 (n=34, 0.3%), or adenocarcinoma in situ/glandular dysplasia (n=9, 0.07%) (data not shown). The diagnostic yield of ECC was worse in examinations among women with a fully visible transformation zone (satisfactory colposcopy, p=.005) and associated characteristics such as age 45 or younger (p=.039), oral contraceptive use (p=.004), pre-menopausal status (p=.013, data not shown) and less than 4 live births (p=.085). For women attending a follow-up visit or referred for low grade cytologic interpretation, the diagnostic yield of ECC remained low across all ages (Figure 1). ECC conferred greatest diagnostic yield among women 46 years or older with 4 or more live births (p=.043), high grade or worse impression (p<.001) or HSIL or worse (HSIL+) referral cytology (p<.001) (Table 2).
Table 2.
Diagnostic yield among 13,115 colposcopically-guided biopsy examinations processed at Alberta Hospital 2003–2007: Percent of exams where endocervical curettage (ECC) detected CIN2* or worse that would have been missed by cervical biopsy alone (cbiopsy diagnosis less than CIN2), stratified by patient and colposcopy characteristics
| Total | Age 16–45 | Age 46–96 | |||||
|---|---|---|---|---|---|---|---|
| N | Column percent |
Percent diagnostic yield |
N | Percent diagnostic yield |
N | Percent diagnostic yield |
|
| TOTAL | 13115 | 100.0 | 1.0 | 10567 | 0.9 | 2548 | 1.4 |
| Age at admission (mean=35.6, median=33 years) | |||||||
| 17–25 | 2203 | 16.8 | 0.6 | ||||
| 26–35 | 5461 | 41.6 | 1.0 | ||||
| 36–45 | 2903 | 22.1 | 1.0 | ||||
| 46–55 | 1905 | 14.5 | 1.4 | ||||
| 56–94 | 643 | 4.9 | 1.2 | ||||
| p-valueα | .023 | ||||||
| Method of contraception | |||||||
| None | 2216 | 16.1 | 1.3 | 1423 | 1.2 | 693 | 1.4 |
| Pill | 4734 | 36.1 | 0.7 | 4543 | 0.6 | 191 | 1.1 |
| Other | 4112 | 31.4 | 1.2 | 3028 | 1.1 | 1084 | 1.5 |
| Missing | 2153 | 16.4 | 1.2 | 1573 | 1.2 | 580 | 1.2 |
| p-valueβ | .013 | .055 | .898 | ||||
| Number of live births | |||||||
| 0 | 5909 | 45.1 | 0.9 | 5618 | 0.8 | 291 | 1.4 |
| 1–3 | 4825 | 36.8 | 1.1 | 3219 | 1.0 | 1606 | 1.3 |
| 4–11 | 304 | 2.3 | 2.0 | 157 | 0.6 | 147 | 3.4 |
| Missing | 2077 | 15.8 | 1.1 | 1573 | 1.1 | 504 | 1.0 |
| p-valueαβ | .048 | .521 | .211 | ||||
| Colposcopy exam satisfactory | |||||||
| Yes | 8933 | 68.1 | 0.8 | 7512 | 0.7 | 1421 | 1.3 |
| No | 1685 | 12.9 | 1.5 | 1152 | 1.7 | 533 | 1.1 |
| Missing | 2497 | 19.0 | 1.3 | 1903 | 1.2 | 594 | 1.7 |
| p-valueβ | .005 | .001 | .711 | ||||
| Colposcopic impression | |||||||
| Normal | 724 | 5.5 | 0.7 | 453 | 0.2 | 271 | 1.5 |
| Atypia | 1089 | 8.3 | 0.7 | 675 | 0.9 | 414 | 0.5 |
| Low Grade | 7590 | 57.9 | 0.9 | 6376 | 0.8 | 1214 | 1.2 |
| High Grade/Cancer | 1892 | 14.4 | 1.6 | 1725 | 1.3 | 167 | 4.8 |
| Missing | 1820 | 13.9 | 1.2 | 1338 | 1.2 | 482 | 1.2 |
| p-valueαβ | .015 | .035 | .037 | ||||
| Referral cytology | |||||||
| ASC-US | 1471 | 11.2 | 0.8 | 1028 | 0.9 | 443 | 0.5 |
| AGUS | 145 | 1.1 | 1.4 | 89 | 1.1 | 56 | 1.8 |
| LSIL | 2940 | 22.4 | 1.0 | 2528 | 1.0 | 412 | 1.5 |
| HSIL | 2177 | 16.6 | 2.4 | 1873 | 1.9 | 304 | 5.3 |
| Cancer | 21 | 0.2 | 4.8 | 15 | 0.0 | 6 | 16.7 |
| Unsatisfactoryγ | 15 | 0.1 | 0.0 | 11 | 0.0 | 4 | 0.0 |
| Follow-up visit | 6346 | 48.4 | 0.6 | 5023 | 0.5 | 1323 | 0.7 |
| p-valueδ | <.001 | .003 | <.001 | ||||
Cervical intraepithelial neoplasia grade 2
Chi-square test for trend
Missing values excluded from statistical calculation of significance.
No CIN2+ was detected among women with an unsatisfactory referral cytology.
Chi-square test for trend excludes unsatisfactory and follow-up test results.
Figure 1.
Projected diagnostic yield in colposcopically-guided biopsy examinations processed at Alberta Hospital 2003–2007: Percent of exams where endocervical curettage (ECC) detected CIN2* or worse that would have been missed by cervical biopsy alone (cervical biopsy diagnosis less than CIN2) by patient age and referral cytology
*Cervical Intraepithelial Neoplasia Grade 2
Error bars indicate 95% confidence interval. Follow-up exams (n=6,346) are not presented as the diagnostic yield was 0.6% and not correlated with age (p=.99).
The additional case detection of CIN2+ by ECC was 5.4% (Tables 1 and 3). That is, of the 2,433 cases of CIN2+, 5.4% (the same 132 cases as a numerator of diagnostic yield, with a different denominator) would have been otherwise missed by colposcopically-directed biopsies if not for the ECC procedure. Similar to diagnostic yield, characteristics associated with less visibility of the transformation zone were associated with higher additional case detection: unsatisfactory colposcopy, p=.002) older age (p<.001), not using oral contraceptives (p<.001), post-menopausal status (p<.001, data not shown) and more than 4 live births (p=.060). Of note, ECC found the fewest additional cases of CIN2+ among women age 16–25 who used oral contraceptives (OC) as only 1.7% of 232 cases of CIN2+ in OC users was detected by ECC alone vs. 5.2% of 135 cases of CIN2+ in non-OC users (p=.061) (data not shown).
Table 3.
Additional case detection of ECC for 13,115 colposcopically-guided biopsy examinations processed at Alberta Hospital 2003–2007: Number of cases where either cervical biopsy or endocervical curettage (ECC) histopathology was CIN2* or worse and percent of cases detected by ECC alone (biopsy diagnosis was less than CIN2), stratified by patient and colposcopy characteristics
| Total cohort | Age 16–45 | Age 46–96 | ||||
|---|---|---|---|---|---|---|
| CIN2+ | % additional case detection |
CIN2+ | % additional case detection |
CIN2+ | % additional case detection |
|
| TOTAL | 2443 | 5.4 | 2175 | 4.5 | 268 | 13.1 |
| Age at admission (mean=35.6, median=33 years) | ||||||
| 17–25 | 404 | 3.5 | ||||
| 26–35 | 1208 | 4.5 | ||||
| 36–45 | 563 | 5.2 | ||||
| 46–55 | 220 | 12.3 | ||||
| 56–94 | 48 | 16.7 | ||||
| p-valueα | <.001 | |||||
| Method of contraception | ||||||
| None | 346 | 7.8 | 266 | 6.4 | 80 | 12.5 |
| Pill | 1002 | 3.1 | 969 | 3.0 | 33 | 6.1 |
| Other | 780 | 6.2 | 669 | 4.8 | 111 | 14.4 |
| Missing | 315 | 8.3 | 271 | 7.0 | 44 | 15.9 |
| p-valueβ | <.001 | .025 | .444 | |||
| Number of live birt | ||||||
| 0 | 1178 | 4.2 | 1133 | 4.1 | 45 | 8.9 |
| 1–3 | 898 | 5.9 | 728 | 4.4 | 170 | 12.4 |
| 4–11 | 57 | 10.5 | 41 | 2.4 | 16 | 31.3 |
| Missing | 310 | 7.4 | 273 | 6.6 | 37 | 13.5 |
| p-valueαβ | .018 | .939 | .062 | |||
| Colposcopy exam satisfactory | ||||||
| Yes | 1722 | 4.3 | 1559 | 3.5 | 163 | 11.7 |
| No | 311 | 8.4 | 258 | 7.8 | 53 | 11.3 |
| Missing | 410 | 7.8 | 358 | 6.2 | 52 | 19.2 |
| p-valueβ | .002 | .002 | .947 | |||
| Colposcopic impression | ||||||
| Normal | 56 | 8.9 | 45 | 2.2 | 11 | 36.4 |
| Atypia | 60 | 13.3 | 47 | 12.8 | 13 | 15.4 |
| Low Grade | 1082 | 6.2 | 974 | 5.3 | 108 | 13.9 |
| High Grade/Cancer | 969 | 3.1 | 873 | 2.5 | 96 | 8.3 |
| Missing | 276 | 8.0 | 236 | 6.8 | 40 | 15.0 |
| p-valueαβ | <.001 | .004 | .012 | |||
| Referral cytology | ||||||
| ASC-US | 146 | 7.5 | 124 | 7.3 | 22 | 9.1 |
| AGUS | 28 | 7.1 | 23 | 4.4 | 5 | 20.0 |
| LSIL | 535 | 5.6 | 491 | 4.9 | 44 | 13.6 |
| HSIL | 1142 | 4.6 | 1001 | 3.6 | 141 | 11.4 |
| Cancer | 16 | 6.3 | 11 | 0 | 5 | 20.0 |
| Unsatisfactory γ | - | - | - | - | - | - |
| Follow–up visit | 576 | 6.3 | 525 | 5.1 | 51 | 17.7 |
| p-valueδ | .132 | .044 | .933 | |||
Cervical intraepithelial neoplasia grade 2
Chi-square test for trend
Missing values excluded from statistical calculation of significance.
No CIN2+ was detected among women with an unsatisfactory referral cytology.
Chi-square test for trend excludes unsatisfactory and follow-up test results.
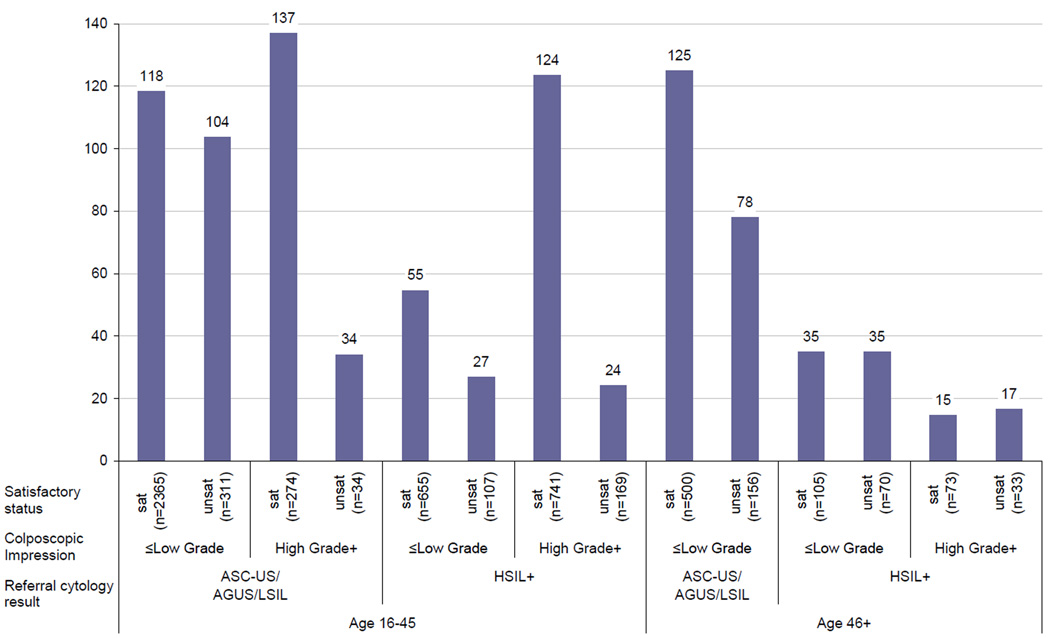
The overall NNT was 99 women: for every 99 colposcopically-guided biopsy exams, the ECC procedure would detect one additional case of CIN2+ (95% CI: 85–2,400, data not shown). For colposcopic examinations with a referral cytology (not a follow-up visit) we compared the NNT to detect an additional case of CIN2+ among subgroups of women by age (16–45 and 46+), referral cytology (ASC-US/AGUS/LSIL and HSIL+), colposcopic impression (≤low grade and high grade+), and satisfactory status (satisfactory and unsatisfactory) (Figure 2). Complete information was available for 5,621 of 6,754 (83.2%) exams. Among women age 16–45, the NNT was over 100 in half the categories. In addition, the NNT was high for women age 46+ with ASC-US, AGUS, or LSIL referral cytology, regardless of colposcopy impression or satisfactory status. The NNT was lowest (<35, meaning ECC was most predictive of CIN2+) for women with an unsatisfactory exam and either HSIL+ referral cytology or high grade or worse impression. Similar subgroups of 4,950 women attending colposcopy for a follow-up examination (as opposed to cytology referral) had a NNT ranging from 85–284 (data not shown).
Figure 2.
Number needed to test with endocervical canal curettage (ECC) to detect one additional case of cervical intraepithelial neoplasia grade 2 or worse (CIN2+) among 5,593 referral colposcopically-guided biopsy examinations processed at Alberta Hospital 2003–2007
* NNT was undefined as no CIN2+ was detected in examinations among women age 46+ with ASCUS/AGUS/LSIL cytology referral, high grade colposcopic impression (n=18 satisfactory colposcopy and n=10 with unsatisfactory colposcopy). These categories are excluded from the figure.
We noted generally similar patterns for our analysis using a CIN3+ endpoint instead of CIN2+ (Table 1). The diagnostic yield for CIN3+ was 0.50%, corresponding to a NNT of 200 women to detect one additional case of CIN3+. The additional case detection for 999 cases of CIN3+ was 6.6%. Of note, there was a greater additional case detection of CIN3+ among women referred for AGUS (12.5%), a cytologic diagnoses strongly associated with cervical precancer and cancer [19]. Also, because CIN3+ was uncommon among women age 16–25, the diagnostic yield of ECC was only 0.45% for CIN3+, although the additional detection for 99 cases of CIN3+ diagnosed in this age group was 10.1%.
Comment
Not all women equally benefited from the endocervical curettage procedure. Women received least benefit if they were: 1) attending colposcopy for a follow-up, 2) referred for ASC-US, AGUS or LSIL, 2) age 45 or younger with a minor cytologic abnormality and either satisfactory colposcopy and/or low grade impression. Women most likely to benefit had characteristics that would typically indicate an excisional procedure such as LEEP: 1) over age 45 with HSIL+ referral cytology or 2) age 45 or younger with unsatisfactory colposcopy and either a high grade or worse colposcopic impression or HSIL+ referral cytology.
Our results primarily present the diagnostic utility of ECC to detect CIN2+. Yet, the benefit of identifying an additional case of CIN2 which is subject to misclassification [17, 18] should be weighed with the morbidity associated with the ECC procedure. It is possible that other ectocervical sampling methods might better improve case detection in the colposcopically-guided biopsy procedure. For example, for lesions located on the ectocervix taking two or more biopsies instead of one has resulted in greater increased sensitivity of 15% for CIN2+ and 13% for CIN3+ [20, 21].
We noted that although for women age 16–25 the additional diagnostic yield of ECC was 0.45% for CIN3+, colposcopically-guided biopsies would have missed 10 of the 99 cases of CIN3+ in this age group. While it is possible that CIN3 when accurately identified in the endocervical canal has greater risk of invasive and subsequently undetected cervical disease, it is also likely that some CIN3 in this age group will regress [18, 22]. Given the low invasive potential of early cases of CIN3, this finding does not support routine use of ECC in this age group to improve sensitivity.
Our study does not estimate the sensitivity of either ECC or cervical biopsy because we did not definitively determine a woman’s disease status through pathology review or longitudinal record review. Instead we report the utility of endocervical curettage in “real-life” colposcopy practice where physicians with a variety of training levels routinely conduct ECC at all colposcopy exams. The findings lead us to question the ability of ECC to benefit the vast majority of colposcopic examinations in routine practice, especially among younger women and in women without a HSIL+ referral cytology. Over 4% of ECC specimens were inadequate. While it is generally agreed upon that ECC should not be performed in certain populations – adolescents, immunocompromised patients, and pregnant women, debate remains as to who should have an ECC [2, 6, 7]. Given that the few women likely to benefit from ECC are typically destined to have a LEEP or other excisional procedure, our findings do not support the widespread use of ECC in routine colposcopy practice.
Acknowledgements
Acknowledgement of financial support: Drs. Gage and Castle were supported by the Intramural Research Program of the NIH, National Cancer Institute.
The authors thank Calgary Laboratory Services for enabling the data extraction from their information.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abu J, Davies Q. Endocervical curettage at the time of colposcopic assessment of the uterine cervix. Obstet Gynecol Surv. 2005;60(5):315–320. doi: 10.1097/01.ogx.0000160774.92271.48. [DOI] [PubMed] [Google Scholar]
- 2.Driggers RW, Zahn CM. To ECC or not to ECC: the question remains. Obstet Gynecol Clin North Am. 2008;35(4):583–597. viii. doi: 10.1016/j.ogc.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Ferenczy A. Endocervical curettage has no place in the routine management of women with cervical intraepithelial neoplasia: debate. Clin Obstet Gynecol. 1995;38(3):644–648. doi: 10.1097/00003081-199509000-00024. [DOI] [PubMed] [Google Scholar]
- 4.Helmerhorst TJ. Clinical significance of endocervical curettage as part of colposcopic evaluation. A review. Int J Gynecol Cancer. 1992;2(5):256–262. doi: 10.1046/j.1525-1438.1992.02050256.x. [DOI] [PubMed] [Google Scholar]
- 5.Moniak CW, et al. Endocervical curettage in evaluating abnormal cervical cytology. J Reprod Med. 2000;45(4):285–292. [PubMed] [Google Scholar]
- 6.Noller KL. Endocervical curettage: a technique in search of an indication?: debate. Clin Obstet Gynecol. 1995;38(3):649–652. doi: 10.1097/00003081-199509000-00025. [DOI] [PubMed] [Google Scholar]
- 7.Agpar BS, Brotzman GL, Rubin MM. Chapter 6: Principles and Technique of Colposcopic Exam. In: Agpar BS, Brotzman GL, Spitzer M, editors. Colposcopy Principles and Practice, An Integrated Textbook and Atlas. Philadelphia: Saunders; 2008. p. 115. [Google Scholar]
- 8.Solomon D, et al. Diagnostic utility of endocervical curettage in women undergoing colposcopy for equivocal or low-grade cytologic abnormalities. Obstet Gynecol. 2007;110(2 Pt 1):288–295. doi: 10.1097/01.AOG.0000270154.69879.09. [DOI] [PubMed] [Google Scholar]
- 9.Pretorius RG, et al. Colposcopically directed biopsy, random cervical biopsy, and endocervical curettage in the diagnosis of cervical intraepithelial neoplasia II or worse. Am J Obstet Gynecol. 2004;191(2):430–434. doi: 10.1016/j.ajog.2004.02.065. [DOI] [PubMed] [Google Scholar]
- 10.Massad LS, Collins YC. Using history and colposcopy to select women for endocervical curettage. Results from 2,287 cases. J Reprod Med. 2003;48(1):1–6. [PubMed] [Google Scholar]
- 11.Irvin W, et al. Endocervical curettage. Does it contribute to the management of patients with abnormal cervical cytology? J Reprod Med. 2004;49(1):1–7. [PubMed] [Google Scholar]
- 12.Wright TC, Jr, et al. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197(4):346–355. doi: 10.1016/j.ajog.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 13.ACOG Practice Bulletin No. 99: management of abnormal cervical cytology and histology. Obstet Gynecol. 2008;112(6):1419–1444. doi: 10.1097/AOG.0b013e318192497c. [DOI] [PubMed] [Google Scholar]
- 14.Williams DL, Dietrich C, McBroom J. Endocervical curettage when colposcopic examination is satisfactory and normal. Obstet Gynecol. 2000;95(6 Pt 1):801–803. doi: 10.1016/s0029-7844(00)00821-8. [DOI] [PubMed] [Google Scholar]
- 15.Srodon M, Parry Dilworth H, Ronnett BM. Atypical squamous cells, cannot exclude high-grade squamous intraepithelial lesion: diagnostic performance, human papillomavirus testing, and follow-up results. Cancer. 2006;108(1):32–38. doi: 10.1002/cncr.21388. [DOI] [PubMed] [Google Scholar]
- 16.Barreth D, et al. Atypical squamous cells-cannot exclude high-grade squamous intraepithelial lesion (ASC-H): a result not to be ignored. J Obstet Gynaecol Can. 2006;28(12):1095–1098. doi: 10.1016/S1701-2163(16)32330-1. [DOI] [PubMed] [Google Scholar]
- 17.Sherman ME, et al. Histopathologic extent of cervical intraepithelial neoplasia 3 lesions in the atypical squamous cells of undetermined significance low-grade squamous intraepithelial lesion triage study: implications for subject safety and lead-time bias. Cancer Epidemiol Biomarkers Prev. 2003;12(4):372–379. [PubMed] [Google Scholar]
- 18.Castle PE, et al. Evidence for frequent regression of cervical intraepithelial neoplasia-grade 2. Obstet Gynecol. 2009;113(1):18–25. doi: 10.1097/AOG.0b013e31818f5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castle PE, et al. Relationship of atypical glandular cell cytology, age, and human papillomavirus detection to cervical and endometrial cancer risks. Obstet Gynecol. 115(2 Pt 1):243–248. doi: 10.1097/AOG.0b013e3181c799a3. [DOI] [PubMed] [Google Scholar]
- 20.Pretorius RG, et al. Inflation of sensitivity of cervical cancer screening tests secondary to correlated error in colposcopy. J Low Genit Tract Dis. 2006;10(1):5–9. doi: 10.1097/01.lgt.0000192694.85549.3d. [DOI] [PubMed] [Google Scholar]
- 21.Gage JC, et al. Number of cervical biopsies and sensitivity of colposcopy. Obstet Gynecol. 2006;108(2):264–272. doi: 10.1097/01.AOG.0000220505.18525.85. [DOI] [PubMed] [Google Scholar]
- 22.McCredie MR, et al. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008;9(5):425–434. doi: 10.1016/S1470-2045(08)70103-7. [DOI] [PubMed] [Google Scholar]